Abstract
The melanocortin-4 receptor (MC4R) is essential for control of energy homeostasis in vertebrates. MC4R interacts with melanocortin receptor accessory protein 2 (MRAP2) in vitro, but its functions in vivo are unknown. We found that MRAP2a, a larval form, stimulates growth of zebrafish by specifically blocking the action of MC4R. In cell culture, this protein binds MC4R and reduces the ability of the receptor to bind its ligand, α–melanocyte-stimulating hormone (α-MSH). A paralog, MRAP2b, expressed later in development, also binds MC4R but increases ligand sensitivity. Thus, MRAP2 proteins allow for developmental control of MC4R activity, with MRAP2a blocking its function and stimulating growth during larval development, whereas MRAP2b enhances responsiveness to α-MSH once the zebrafish begins feeding, thus increasing the capacity for regulated feeding and growth.
The melanocortin-4 receptor (MC4R), a G protein–coupled receptor (GPCR), plays a central role in energy homeostasis (1–4) and somatic growth (1, 2, 5). Mutations in the gene encoding MC4R are the most common monogenic cause of severe early-onset obesity in humans (1). In the zebrafish, as in mammals, MC4R is prominently involved in the regulation of energy homeostasis and somatic growth (6). Dominant negative mutations in MC4R are a natural cause of increased growth rate and final size in some teleost species (5). An artificially induced increase in MC4R activity early in the development of the zebrafish embryo causes a decrease in growth, a decrease in growth hormone gene expression, and a compensatory increase in growth hormone–releasing hormone (ghrh) gene expression (6), thus providing quantitative assays for MC4R activity in vivo. The melanocortin receptors have been shown to interact with the melanocortin receptor accessory proteins MRAP1 and MRAP2 (7–13), which are single-transmembrane proteins that form unusual antiparallel homo- and heterodimers (7–9). Whereas MRAP1 is essential for adrenocorticotropic hormone receptor (MC2R) trafficking to the plasma membrane, ligand binding, and downstream signaling (7, 8, 11), the functions of MRAP2 remain unclear. In the zebrafish, MRAP2 exists in two isoforms, a and b (14). Here, we investigated the role of MRAP2a and MRAP2b in the regulation of MC4R activity in vivo in the zebrafish and in vitro in human embryonic kidney (HEK) 293T cells.
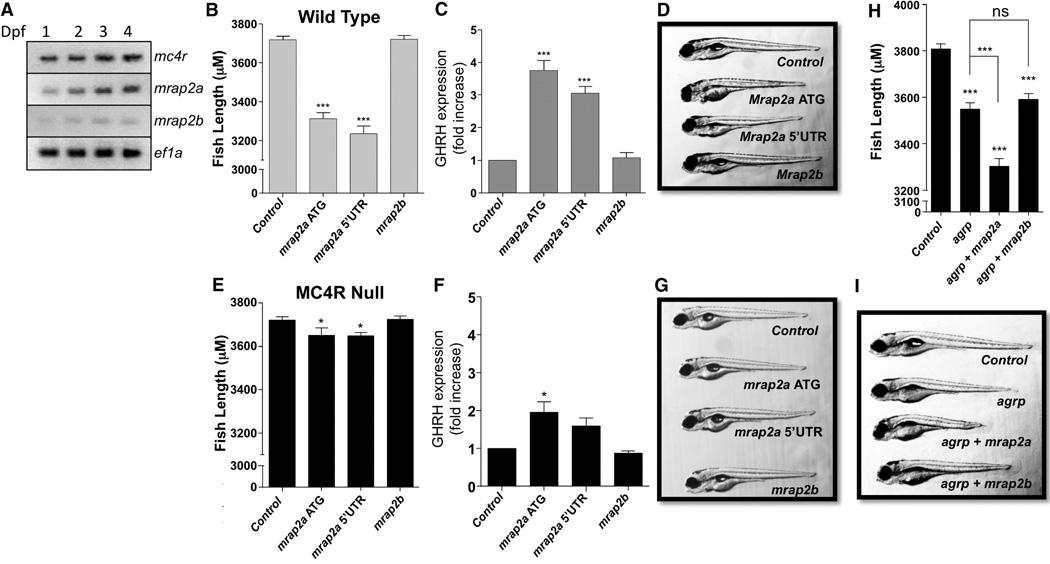
We first characterized the distribution and developmental expression kinetics of mc4r, mrap2a, and mrap2b gene expression in the zebrafish embryo at 1, 2, 3, or 4 days post-fertilization (dpf) by reverse transcription polymerase chain reaction (RT-PCR) (Fig. 1A). mc4r and mrap2a mRNA were detectable from 1 dpf and their expression increased every day until 4 dpf, whereas mrap2b was hardly detectable. To identify the larval tissue distribution of mrap2 mRNAs, we performed whole-mount in situ hybridization on zebrafish embryos at 5 dpf. mrap2a was ubiquitously expressed, whereas no mrap2b-specific staining was detected (fig. S1).
Fig. 1. Regulation of MC4R by MRAP2a in the zebrafish embryo.
(A) RT-PCR showing the level of mRNA expression for mc4r, mrap2a, and mrap2b in the first 4 days of zebrafish development. (B to G) Length [(B) and (E)], ghrh expression [(C) and (F)], and representative pictures [(D) and (G)] of 5 dpf wild-type or mc4r-null zebrafish injected with the indicated morpholinos. (H and I) Length (H) and representative picture (I) of zebrafish embryos injected with (top to bottom) control morpholino or morpholino targeting agrp, mrap2a, or both. *P < 0.05, ***P < 0.001; ns, not significant.
To determine whether MRAP2a was modulating MC4R in vivo, we knocked down mrap2a expression by using two distinct antisense morpholino oligonucleotides targeting two different sites of the mrap2a transcript. Relative to fish injected with a nontargeting morpholino, embryos injected with either of the mrap2a morpholinos showed a significant decrease in linear growth [11% with the morpholino targeting the start of the coding sequence, ATG, and 13% with the morpholino targeting the 5′ untranslated region (5′UTR)] (Fig. 1, B and D) and a factor of 3 to 4 increase in ghrh expression (Fig. 1C), consistent with an increase in MC4R activity. In contrast, when the same experiment was conducted in mc4r-null fish, only a small decrease in linear growth and small increase in ghrh were observed (Fig. 1, E to G). The specificity of the morpholinos was demonstrated by inhibition of a green fluorescent protein reporter plasmid containing the target sequence, and by the rescue of both length and ghrh expression phenotype by co-injection of mrap2a mRNA lacking the morpholino target sequences (fig. S2, A to C). Overall, these results indicate that MRAP2a suppresses MC4R signaling in the larval zebrafish. The use of morpholinos to down-regulate mrap2b did not have any impact on the size of the zebrafish or ghrh expression, regardless of genotype (Fig. 1); this result was expected because mrap2b is not expressed in the embryo.
We previously reported that MC4R is constitutively inhibited by high levels of agouti-related protein (AgRP), the endogenous MC4R inverse agonist, in the zebrafish embryo (6). To determine whether both AgRP and MRAP2a contribute to the silencing of MC4R, we injected wild-type zebrafish zygotes with control morpholinos or morpholinos targeting agrp alone, agrp and mrap2a, or agrp and mrap2b. As previously reported, agrp morpholino caused a measurable decrease (7%) in fish growth by increasingMC4R activity, as measured at 5 dpf (6). When co-injected, agrp and mrap2a morpholinos caused a more profound impairment of growth (13%) than agrp morpholino alone (Fig. 1, H and I), which suggests that AGRP and MRAP2a both participate in keeping MC4R inactive in the zebrafish embryo. Co-injection of agrp and mrap2b morpholinos had the same effect as agrp alone.
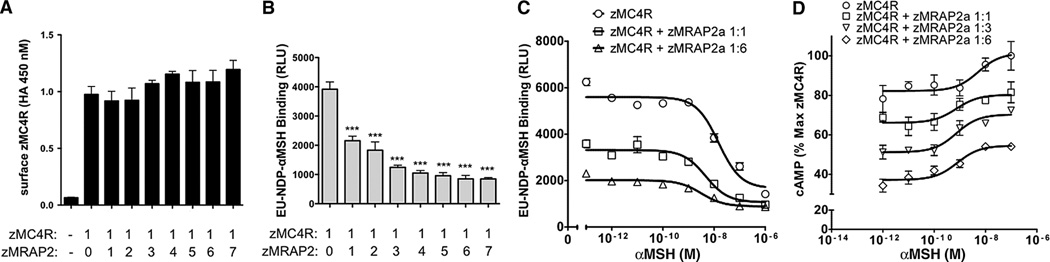
We next investigated the mechanism by which MRAP2a regulates MC4R. Surface expression of MC4R at the plasma membrane of transfected HEK293T cells was not changed by MRAP2a expression (Fig. 2A). However, because the number of receptors at the plasma membrane may not reflect the number of receptors that are competent to bind ligand and signal, we measured the effect of MRAP2a on the density of high-affinity binding sites for the MC4R agonist NDP–α-MSH. MRAP2a caused up to an 80% decrease in europium-labeled NDP–α-MSHbinding toMC4R (Fig. 2B). MRAP2a did not significantly change the affinity of MC4R for α-MSH, as measured in a competition binding assay (median inhibitory concentration IC50 = 45 ± 15 nM) (Fig. 2C). These results suggest that MRAP2a reduces ligand binding by decreasing the number of binding sites but not by altering affinity. Both constitutive activity and α-MSH–inducible activity were suppressed in parallel by increasing expression of MRAP2a (Fig. 2D),which suggests that MRAP2a stabilizes an inactive conformation of MC4R. We performed a coimmunoprecipitation assay and found that MC4R and MRAP2a are part of the same complex; a large fraction of the receptor copurified with MRAP2a (fig. S3A), and conversely, a large fraction of MRAP2a coprecipitated with MC4R (fig. S3B).
Fig. 2. Regulation of MC4R byMRAP2a.
(A) Surface expression of MC4R measured by whole-cell enzyme-linked immunosorbent assay (ELISA) in nonpermeabilized HEK293T cells transfected with mc4r and increasing amounts of mrap2a. (B) Europium-labeled NDP–α-MSH binding in HEK293T cells transfected with mc4r and increasing amounts of mrap2a. (C) Competition binding assay in HEK293T cells transfected with mc4r without or with mrap2a at mc4r/mrap2a ratios of 1:1 or 1:6. (D) Concentration-response curves of α-MSH–induced cAMP production in HEK293T cells expressing the CRE-luciferase reporter, mc4r, and different amounts of mrap2a. ***P < 0.001.
As mentioned previously, the zebrafish has two mrap2 genes, mrap2a and mrap2b. As shown above, mrap2b expression was barely detectable in the embryo (Fig. 1A). To determine whether its expression increased later in the life of the zebrafish, we harvested RNA from the brain of adult fish and measured the expression of mc4r, mrap2a, and mrap2b, using RT-PCR and quantitative PCR (qPCR). The expression of all three genes increased in the adult brain relative to embryos (Fig. 3, A and B). Most notably, the expression of mrap2b increased in the adult by a factor of 10, raising the possibility that MRAP2b regulates MC4R function in the adult fish.
Fig. 3. Regulation of MC4R by MRAP2b.
(A) RT-PCR depicting the expression of mc4r, mrap2a, mrap2b, and the housekeeping gene ef1a in zebrafish adult brain. (B) qPCR measuring the change in expression of mc4r, mrap2a, and mrap2b in the adult brain relative to the 4 dpf embryo. (C) qPCR (top) and RT-PCR (bottom) depicting the expression of mouse MRAP2 at different embryonic and postnatal stages; E, embryonic day. (D) Surface expression of MC4R measured by whole-cell ELISA in nonpermeabilized HEK293T cells transfected with mc4r and different amounts of mrap2b. (E) Competition binding assay in HEK293T cells transfected with mc4r alone or with mrap2b at the indicated ratio. (F) Concentration-response curves of α-MSH–induced cAMP production in HEK293T cells transfected with mc4r and the indicated amount of mrap2b or mouse MRAP2. ***P < 0.001.
MRAP2 expression in the mouse is also not seen during embryogenesis and appears only after birth, reaching maximal levels close to the time of weaning, at day 18 (Fig. 3C). This suggests a conservation of the expression kinetics for the mouseMRAP2 and teleostMRAP2b proteins. In contrast to MRAP2a, which had no effect on the trafficking of MC4R, MRAP2b caused a modest increase in MC4R surface expression in transfected HEK293T cells (Fig. 3D). MRAP2b also caused a dose-dependent increase in maximal binding, probably due to the increased number of MC4 receptors at the cell surface; however, MRAP2b did not change the affinity of MC4R for α-MSH (IC50 = 50 ± 15 nM) (Fig. 3E).
Remarkably,MRAP2b suppressed the constitutive activity of MC4R by 93% and shifted the dose-response curve for agonist, increasing α-MSH potency by a factor of 17 (MC4R alone, median effective concentration EC50 = 140 ± 60 nM; MC4R+MRAP2b,EC50 = 8.3± 1.7 nM).MRAP2b also amplified the maximal cAMP response to α-MSH stimulation, from a factor of 1.5 in its absence to a factor of 17.5 in its presence. The mouse MRAP2 replicated all of the signaling effects caused by MRAP2b on the zebrafish MC4R (Fig. 3F), thereby confirming that MRAP2b is the homologous isoform to the mammalian MRAP2. After expression in HEK293T cells, a coimmunoprecipitation assay showed that MC4R copurified with MRAP2b (fig. S4A) and that MRAP2b copurified withMC4R (fig. S4B), which suggests that those proteins are in the same complex.
For MRAP2a or MRAP2b to have direct effects on the pharmacology and physiology ofMC4R in vivo, they must be expressed in the same cells. We used in situ hybridization to localize mc4r and mrap2 mRNAs in adult zebrafish brain slices. mc4r and mrap2a were largely colocalized, as were mc4r and mrap2b (fig. S5). This result supports the concept that the diverse effects of MRAP2s on MC4R signaling observed in vitro are relevant in vivo and may play amajor role in energy homeostasis.
The broad tissue expression of MRAP2 proteins in vertebrates, and our observation that mrap2a blockade continues to have a small effect in mc4r mutant fish, together suggest that the MRAP2 proteins regulate multiple GPCRs. Nonetheless, the MRAP2-GPCR interaction appears highly selective. MRAP2a and MRAP2b did not modify zebrafish MC3R signaling (fig. S7A), and mouse MRAP2 had no effect on the function of the mouse corticotropin-releasing hormone 1 and 2 receptors or the mouse neuropeptide Y2 and Y5 receptors (fig. S7, B to E). A small change in efficacy was observed in the presence ofMRAP2 at the mouse glucagon-like peptide–1 receptor and β2-adrenergic receptor (fig. S7, F and G).
Our findings reveal a new level of complexity in the regulation ofMC4R signaling by uncovering the ability of MRAP2 proteins to modify the pharmacology and physiology of MC4R in zebrafish. The zebrafish MC4R displays an atypical signaling profile with very high constitutive activity and a modest cyclic adenosine monophosphate (cAMP) response to its agonist α-MSH. In the embryo, the MC4R inverse agonist AgRP is expressed at a high level (6). We show here that mrap2a is also expressed in the embryo and inhibits MC4R signaling. AgRP and MRAP2a collaborate to stabilize MC4R in an inactive state, inhibiting both constitutive activity and ligand-induced signaling, and thus maximizing growth during the larval period.
mrap2b is expressed at high levels only in adult fish, where it could act to reduce the constitutive activity of MC4R and simultaneously sensitizes the receptor to agonist. In this manner, MRAP2b would convert the adult zebrafish MC4R from a constitutively active to a ligand-dependent receptor. Additionally, mrap2b expression kinetics matches that of the mouse MRAP2 and is functionally homologous.MRAP2a and MRAP2b proteins share a strong homology with each other and with mammalian MRAP2 in most of the N-terminal region and the transmembrane domain, whereas theN-terminal 15 amino acids and C terminus of these proteins are vastly divergent (fig. S6). Interestingly, the N-terminal and transmembrane domain of MRAP1 are sufficient for full activity of the mammalian MC2R (15). The first 15 amino acids of MRAP2a and MRAP2b could thus represent an important regulatory region of MRAP2s, possibly responsible for the differential regulation of MC4R.
During zebrafish embryonic development, all the energy consumed is obtained from the yolk sac. Our findings suggest that the embryo benefits from havingMC4R locked in an inactive state by the joint actions of AgRP and MRAP2a. Activation of MC4R at this stage would slow the rapid maturation to the mobile free-feeding juvenile stage reached at 5 dpf. Upon maturation and depletion of the yolk sac, the zebrafish must regulate nutrient intake. Appropriate behavioral response to diurnal, seasonal, and other inputs requires a functional adipostat and energy balance sensor. This switch is aided by MRAP2b, which forms a complex with MC4R and renders it highly sensitive to α-MSH.
MRAP2 introduces a previously unappreciated level of complexity in the control of MC4R, with developmentally regulated paralogs in the fish that can either inhibit (MRAP2a) or stimulate (MRAP2b) ligand-mediated receptor activation (fig. S8). A component of this complexity is retained in mammals: Asai et al. (16) show that, likeMRAP2b, mouseMRAP2 expression is activated proximal to weaning and increases the responsiveness ofMC4R to α-MSH. Their observation that MRAP2 deletion causes an obesity syndrome in the mouse can likely be attributed, in part, to reduced function ofMC4R (16). However, given the ubiquitous expression of MRAP2 proteins, we hypothesize that these proteins also modulate the activity of GPCRs and perhaps other membrane proteins as well.
Supplementary Material
Acknowledgments
Supported by NIH grants DK075721 and DK070332 (R.D.C.), DK19974 (P.M.H.), and F23DK091055 (J.A.S.); United States–Israel Binational Agricultural Research and Development Fund (BARD) research grant IS-4489-12 (R.D.C. and C.Z.); and Vanderbilt Diabetes Research and Training Center grant DK020593 (R.D.C.). All data and methods are publicly available in the supplementary materials.
Footnotes
Supplementary Materials
www.sciencemag.org/cgi/content/full/341/6143/278/DC1
Materials and Methods
Figs. S1 to S8
References (17–19)
References and Notes
- 1.Farooqi IS, et al. N. Engl. J. Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 2.Huszar D, et al. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 3.Vaisse C, Clement K, Guy-Grand B, Froguel P. Nat. Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 4.Yeo GS, et al. Nat. Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 5.Lampert KP, et al. Curr. Biol. 2010;20:1729–1734. doi: 10.1016/j.cub.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C, Forlano PM, Cone RD. Cell Metab. 2012;15:256–264. doi: 10.1016/j.cmet.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sebag JA, Hinkle PM. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20244–20249. doi: 10.1073/pnas.0708916105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebag JA, Hinkle PM. J. Biol. Chem. 2009;284:22641–22648. doi: 10.1074/jbc.M109.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sebag JA, Hinkle PM. Sci. Signal. 2010;3:ra28. doi: 10.1126/scisignal.2000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinkle PM, et al. Eur. J. Pharmacol. 2011;660:94–102. doi: 10.1016/j.ejphar.2010.10.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metherell LA, et al. Nat. Genet. 2005;37:166–170. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- 12.Chan LF, et al. Proc. Natl. Acad. Sci. U.S.A. 2009;106:6146–6151. doi: 10.1073/pnas.0809918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy S, Rached M, Gallo-Payet N. Mol. Endocrinol. 2007;21:1656–1669. doi: 10.1210/me.2007-0041. [DOI] [PubMed] [Google Scholar]
- 14.Agulleiro MJ, et al. Mol. Cell. Endocrinol. 2010;320:145–152. doi: 10.1016/j.mce.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 15.Sebag JA, Hinkle PM. J. Biol. Chem. 2009;284:610–618. doi: 10.1074/jbc.M804413200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asai M, et al. Science. 2013;341:275–278. doi: 10.1126/science.1233000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.