SUMMARY
The melanocortin-4 receptor (MC4R) is expressed in the brainstem and vagal afferent nerves, and regulates a number of aspects of gastrointestinal function. Here we show that the receptor is also diffusely expressed in cells of the gastrointestinal system, from stomach to descending colon. Furthermore, MC4R is the second most highly expressed GPCR in peptide YY (PYY) and glucagon-like peptide one (GLP-1) expressing enteroendocrine L cells. When vectorial ion transport is measured across mouse or human intestinal mucosa, administration of α-MSH induces a MC4R-specific PYY-dependent anti-secretory response consistent with a role for the MC4R in paracrine inhibition of electrolyte secretion. Finally, MC4R-dependent acute PYY and GLP-1 release from L cells can be stimulated in vivo by intraperitoneal administration of melanocortin peptides to mice. This suggests physiological significance for MC4R in L cells, and indicates a previously unrecognized peripheral role for the MC4R, complementing vagal and central receptor functions.
INTRODUCTION
The Melanocortin-4 Receptor (MC4R) is a 7-transmembrane (7TM) Gαs-coupled receptor that plays an integral role in energy homeostasis. Mutations causing loss-of-function of the MC4R result in severe obesity with hyperphagia, hyperinsulinemia, and increased somatic growth (Huszar et al., 1997). The MC4R is expressed in up to 150 brain regions, with particularly high expression in the paraventricular nucleus of the hypothalamus (PVN) as well as in the dorsal motor nucleus of the vagus (DMV) within the hindbrain (Mountjoy et al., 1994). Within these brain regions, the MC4R responds to its endogenous agonist, alpha-melanocyte stimulating hormone (α-MSH), and its endogenous antagonist, Agouti-Related Protein (AgRP), which are released from POMC neurons and AgRP/NPY neurons respectively. These inputs to MC4R neurons respond to a cascade of homeostatic cues either from the circulation or through vagal signals in order to regulate MC4R activity (Cone, 2005). Activation of the MC4R by α-MSH, or its synthetic analogues, generally leads to weight loss (Hsiung et al., 2005), due to a reduction in caloric intake and an increase in energy expenditure. Conversely, blockade of MC4R by AgRP results in robust increases in feeding as well as decreases in energy expenditure (Graham et al., 1997; Ollmann et al., 1997). The MC4R knockout (MC4R−/−) mouse expectedly exhibits severe obesity and hyperphagia (Huszar et al., 1997) with notable defects in acute responses to dietary fat and macronutrient preference (Butler et al., 2001; Panaro and Cone, 2013). Study of the MC4R has thus often focused on the mechanisms underlying the centrally mediated effects of MC4R on feeding behaviors.
The MC4R also regulates gastrointestinal (GI) function. The highest site of expression of the MC4R is in the DMV (Mountjoy et al., 1994), the site of the preganglionic parasympathetic vagal efferent nerves that regulate the GI system. Moderate levels are also seen at the primary site of receipt of vagal afferents in the nucleus of the solitary tract (NTS). Indeed, a third of all vagal afferents in the nodose ganglion express MC4R, and stomach and duodenum are innervated by MC4R positive vagal afferents and efferents (Gautron et al., 2010). While details of the neuroanatomy and function of MC4R in the DMV remain to be determined (e.g. see (Richardson et al., 2013), caudal brainstem administration of melanocortin agonists inhibits food intake (Grill et al., 1998; Williams et al., 2000), and injection of melanocortin agonists into either the DMV or NTS decreases phasic gastric contractions (Richardson et al., 2013). Stereotaxic injections of melanocortin agonists, MT-II or α-MSH into either the DMV or the NTS can modulate gastric activity via vagal outflow to the stomach. This effect was blocked by administration of melanocortin antagonist SHU9119, vagotomy, or knockout of the MC4R (Richardson et al., 2013). Thus, the melanocortin system is likely to affect food intake not only through effects on behavioral centers in the CNS, but also secondarily through MC4R signaling involved in the postprandial functions of the enteric nervous system (ENS) (Gautron et al., 2010).
In relation to food intake, the GI tract functions in part by releasing hormones that signal information about gut nutrient content to other peripheral organs and the brain. These hormones also function within the GI tract to regulate nutrient and electrolyte absorption. Prior to meal intake, P/D1 cells in the fundus of the stomach release ghrelin, a hormone that acts to initiate a feeding bout. Following a meal, several key peptides rise, including cholecystokinin (CCK) released from I cells in the duodenum (Lal et al., 2004), gastric inhibitory peptide (GIP) released from K cells in the duodenum and jejunum (Elliott et al., 1993), glucagon-like peptide 1 (GLP-1), and peptide YY (PYY) both released from L cells that predominate in the distal ileum and colon (Elliott et al., 1993; Pilichiewicz et al., 2006). Both CCK and PYY3-36, an active cleavage product of PYY, act as potent satiety factors that can reduce meal size acutely (Batterham et al., 2002; Pilichiewicz et al., 2006), and CCK requires brainstem MC4R signaling in order to reduce meal size (Fan et al., 2004).
Control of GI hormone release from enteroendocrine cells is mediated by a variety of signals, either apically-activated by luminal nutrients or basolaterally through stimulation by the ENS or from the circulation. Many G protein-coupled receptors (GPCRs) are expressed by enteroendocrine cells (Reimann et al., 2008; Samuel et al., 2008), and PYY secretion has been shown to be affected by GPCR activation, including the acylethanolamine receptor GPR119, expressed on enteroendocrine L cells. Apical or basolateral administration of a GPR119 agonist to mouse or human colon mucosa suppresses electrolyte secretion via endogenous PYY release and its subsequent binding to nearby epithelial Y1 receptors (Cox et al., 2010). A previous study detected MC4R mRNA by PCR in a mouse enterocyte preparation. In this study, reduced intestinal expression of microsomal triglyceride transfer protein (MTP) was seen in db/db and MC4R−/− mice (Iqbal et al., 2010). These data, along with the absence of effect of vagotomy on intestinal MTP expression, were used to infer functional activity of leptin and melanocortin signaling in intestinal epithelial cells. More recently, gastric ghrelin positive cells were shown to highly express several GPCRs, including the MC4R, with the potential to modulate hormone secretion in response to neural or endocrine signals (Engelstoft et al., 2013). The enrichment of GPCR expression in gastric ghrelin-positive cells suggests that the MC4R may also contribute directly to the regulation of hormone release via enteroendocrine cells. Given the discovery of MC4R in both vagal neurons and ghrelin cells, and the suggestion of broader MC4R expression along the length of the GI tract, we sought to characterize MC4R expression and function in enteroendocrine cells, a crucial site in gut-brain communication and energy homeostasis.
RESULTS
MC4R mRNA Expression is Enriched in Some Enteroendocrine Cell Populations
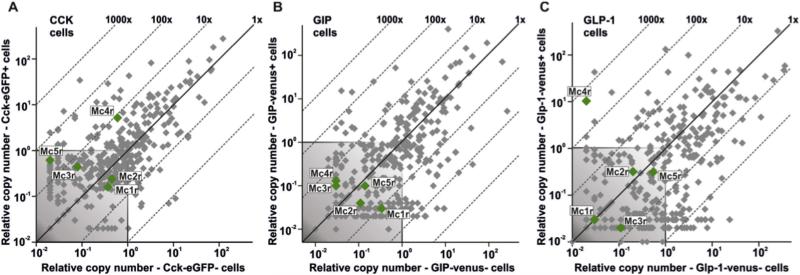
CCK-eGFP, GIP-venus, and GLP-1-venus positive cells were FACS-purified from single cell preparations of mucosal cells generated from the proximal small intestine of transgenic CCK-eGFP (Egerod et al., 2012), GIP-venus (Parker et al., 2009) or GLP-1-venus reporter mice (Reimann et al., 2008), respectively. cDNA from each of the purified enteroendocrine cell populations was analysed for melanocortin receptor expression by a qPCR array targeting 379 non-odorant 7TM receptors (Figure 1) as previously reported for gastric ghrelin cells (Engelstoft et al., 2013). Among the five melanocortin receptors, the MC4R was the only receptor expressed above background levels in CCK (Figure 1A) and GLP-1 cells (Figure 1C), whereas none of the melanocortin receptors were expressed above background levels in GIP cells (Figure 1B). MC4R mRNA was enriched 430-fold in the GLP-1 cells, thus being the second most enriched receptor expressed in these cells (Figure 1C). In CCK cells, MC4R mRNA was enriched 9-fold. Thus, MC4R is highly expressed in particular in the GLP-1 positive enteroendocrine cells.
Figure 1. Expression of Melanocortin Receptors in Enteroendocrine Cells.
Scattergrams exhibiting qPCR expression of the five melanocortin receptors (annotated green dots) among 379 7TM receptors (grey plus green dots) examined in FACS-purified cells from the proximal small intestine from transgenic (A) CCK-eGFP, (B) GIP-Venus, and (C) GLP-1-Venus reporter mice (X-axis) versus expression in non-fluorescent mucosal cells (Y-axis). The 45°-angled lines indicate the enrichment of expression in the fluorescent FACS-purified enteroendocrine cells versus the neighboring enterocytes. The grey shaded area in each of the scattergrams is considered as noise level.
L Cells Expressing GLP-1 or PYY Co-express a MC4R-GFP Marker
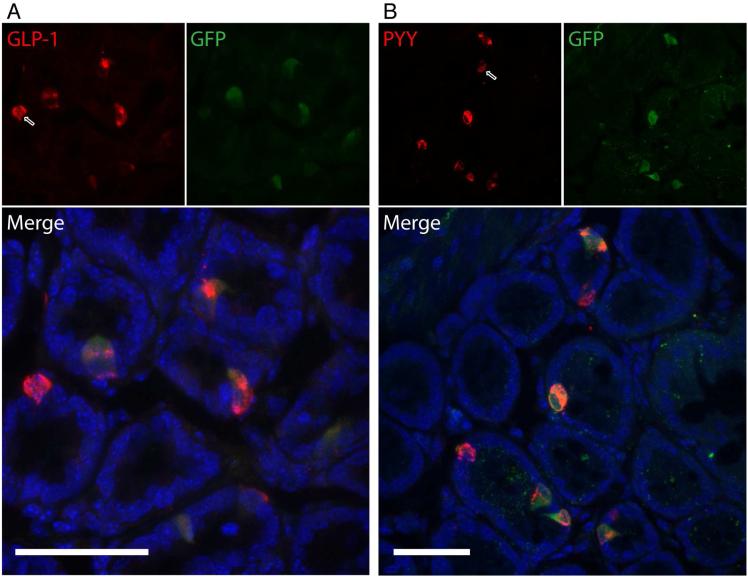
Tissue segments dissected from the GI tract of MC4R-Sapphire mice, which express green fluorescent protein (GFP) under the MC4R promoter (Liu et al., 2003), were collected to further characterize cells expressing MC4R by fluorescence immunohistochemistry, using antibodies to GFP, and GLP-1 or PYY, both of which are expressed and secreted by L cells within the gut. Segments representing the stomach, duodenum, jejunum, ileum, and colon were stained to detect co-expression of GFP and PYY or GLP-1. When viewing tissues within the gut mucosa, GFP positive cells were present in occasional cells throughout the GI tract from the stomach to the colon (Figure 2, and Figure S1). Also, L cells, marked by GLP-1 or PYY staining, are sparse in all regions of the gut (Figure S1), although they were most prominent in the colon (Figure 2), following the expected L cell distribution pattern. As indicated by the merged images, most cells positive for either GLP-1 (Figure 2A) or PYY (Figure 2B) also co-expressed GFP. Cells expressing GLP-1 or PYY, but lacking GFP, were also observed (white-lined arrows). Three dimensional reconstructions of the confocal data for both PYY and GLP-1 colocalization with MC4R-GFP can be viewed online in the Supplemental Data. Notably, in the stomach, jejunum, and ileum, there were GFP positive cells that did not express PYY or GLP-1, lending a potential role for MC4R in other populations aside from L cells, including potential non-epithelial cell types (Figure S1). These results suggest that MC4R is expressed peripherally in several GI cell populations, and most prominently in L cells.
Figure 2. Immunohistochemical Characterization of MC4R Expression in L Cells of the GI Tract.
MC4R-Sapphire mice, which express GFP under control of the MC4R promoter, were used to visualize MC4R-expressing cells in the mucosa of the colon. MC4R-positive cells are marked in green with an antibody against GFP; L cells are marked in red with antibodies against either A) GLP-1 or B) PYY. L cells are found throughout the GI tract, with highest frequency in the colon. Cells positive for both GLP-1 or PYY and GFP in the colon are visualized in the bottom panels. While many colonic L cells are also MC4R positive, there are L cells that do not co-express GFP, as marked with white-lined arrows. The images are 2-dimensional projections of 3-dimensional Z-stacks taken with a confocal microscope. The 3 dimensional renderings are available as supplemental videos. Scale bars are 40 μm. See also Figure S1 and Movies S1&S2.
α-MSH Stimulated PYY Response in Intestinal Mucosa is Mediated by MC4R
Efforts to detect functional MC4R responses in semi-purified ex vivo preparations were met with limited success. Melanocortin agonists induced ghrelin release from gastric mucosal preparations (Figure S2A), however, melanocortin agonists, such as α-MSH or LY2112688 were unable to induce GLP-1 release from mouse colonic crypt preparations (Figure S2B). In contrast, NDP-α-MSH and LY2112688 did elicit GLP-1 secretion from mouse distal small intestine in perfusion experiments, though the response was small compared to stimulation with neuromedin C (Figure S2C). In addition, GLUTag cells, a line derived from a GLP-1 positive L cell tumor (Drucker et al., 1994), exhibited a functional MC4R response to multiple melanocortin ligands, as measured using a phospho-ERK assay (Figure S2D).
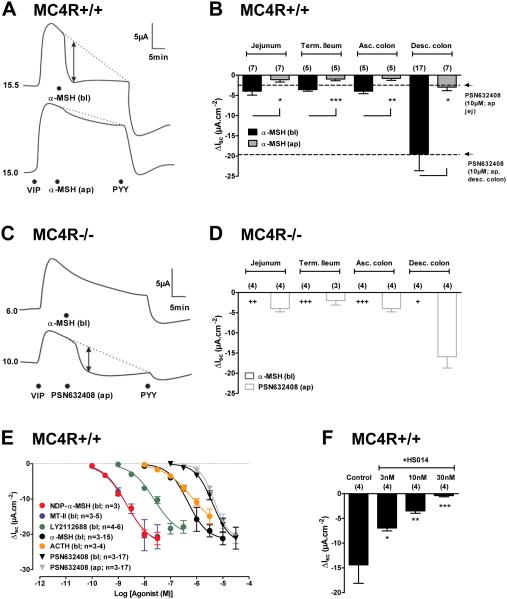
To further examine whether MC4R expression in L cells has functional significance, we next tested various MC4R agonists on intestinal mucosae from MC4R+/+ and MC4R−/− mice. α-MSH was applied either apically or basolaterally to different regions of the intestinal mucosae and changes in short-circuit current (ISC) were measured. This electrogenic ion transport (most likely apical Cl− secretion) was stimulated first by basolateral application of vasoactive inhibitory peptide (VIP), which binds its epithelial receptor, VPAC, and via Gαs-coupling propagates an increase in ISC. Anti-secretory effects after VIP pretreatment were measured as rapid reductions in ISC (relative to the slowly waning VIP response) and could result from locally released PYY (or NPY) stimulating Gαi-coupled epithelial Y1 receptors to ultimately reduce Cl− secretion (Cox et al., 2001; Cox et al., 2010). Basolateral application of α-MSH reduced ISC levels rapidly and to a significantly greater degree than apical peptide addition (Figure 3A) and consistently so in each of the four GI areas tested (Figure 3B). The remaining VIP-elevated ISC was abolished by subsequent addition of PYY as indicated in the representative traces (Figure 3A). The anti-secretory response profile to α-MSH exhibited a similar regional distribution to that described for L cell distribution in mice (Arantes and Nogueira, 1997). Furthermore, the α-MSH responses were of similar magnitude to those elicited by PSN632408 (PSN)-mediated GPR119 activation in the mouse jejunum and descending colon (dashed lines, Figure 3B), an effect described previously (Cox et al., 2010). When α-MSH was applied basolaterally to mucosae from MC4R−/− mice, the anti-secretory responses were absent. Importantly, responses to exogenous PYY (pooled data not shown) and PSN were present in these tissues, and were not significantly different from agonist responses in MC4R+/+ mucosae, suggesting otherwise normal mucosal transport and L cell function in the MC4R−/− mice (Figures 3C-D).
Figure 3. α-MSH Activity in Mouse GI Mucosae Occurs via MC4R.
A: Representative α-MSH (1 μM) responses in MC4R+/+ mouse descending colon mucosa to apical (ap) or basolateral (bl) addition. Basal short-circuit current (ISC) values (in μA) are indicated to the left of each trace and the exposed mucosal area was 0.14 cm2. Mucosae were pre-stimulated with vasoactive intestinal polypeptide (VIP, bl, 10 nM) after which bl α-MSH inhibited the VIP-elevated ISC and subsequent bl addition of peptide YY (PYY, 10 nM) also inhibited ISC levels. The vertical arrow shows the point at which the α-MSH-induced ΔISC was measured from the extrapolated VIP response (dashed line). B: Sensitivity to α-MSH (1 μM) added to bl (black bars) or ap (grey bars) compartments bathing WT mucosae from different GI areas. Mucosae were prepared from jejunum, terminal (Term.) ileum, ascending (Asc.) colon and descending (Desc.) colon. The horizontal dashed lines represent responses to the GPR119 agonist PSN632408 (10 μM, ap) in jejunum (jej) and desc. colon. * P<0.05, ** P<0.01, *** P<0.001 comparing bl α-MSH with ap responses using 1-way ANOVA with Dunnett’s post-test. C: Representative traces showing loss of α-MSH response in MC4R−/− colon mucosa but normal GPR119 activity. Additions were in order, VIP (bl, 10 nM) α-MSH (bl, 1 μM) or GPR119 agonist PSN632408 (ap, 10 μM) and finally PYY (bl, 10 nM). D: Regional GI sensitivity to α-MSH and PSN632408 in MC4R−/− colon mucosa. Responses to α-MSH (1 μM) were absent while PSN632408 (ap, 10 μM) were normal. Statistical differences, + P<0.05, ++ P<0.01, +++ P<0.001 compare bl α-MSH responses in MC4R−/− with those in MC4+/+ colon (in B). E: Concentration-response curves in WT descending colon for NDP-α-MSH, MT-II, LY2112688, α-MSH and ACTH (all bl additions only) and for comparison PSN632408 added either ap (grey) or bl (black). EC50 values are quoted in Results text. F: MC4R antagonist HS014 inhibited α-MSH (1 μM) responses in WT mouse descending colon. Tissues were pre-treated with HS014 (at the concentrations shown) 20 min prior to α-MSH (bl, 1 μM). Bars and points are the mean ±1SEM with n values shown in parenthesis. * P<0.05, ** P<0.01, *** P<0.001 compare data points with control α-MSH responses using 1-way ANOVA with Dunnett’s post-test. See also Figure S2.
Concentration-response curves were constructed using a variety of basolaterally applied melanocortin receptor agonists, as well as apically and basolaterally applied GPR119 agonist PSN. Melanocortin receptor agonists exhibited between ~10 and ~1000 fold higher potency than PSN (Figure 3E). EC50 values (in nM) were; 2.0, 2.1, 23.9, 459.1 and 684.8, for MT-II, NDP-α-MSH, LY2112688, α-MSH and ACTH respectively, compared with 4.8 μM and 4.2 μM for apical and basolateral PSN. The rank order of potency of melanocortin peptides in the mucosal assay paralleled that seen in tissue culture expression systems (Mountjoy et al., 1994). Furthermore, pharmacological blockade of MC4R using the MC4R selective antagonist HS014 (Vergoni et al., 1998) was concentration-dependent (Figure 3F). Taken together, these results suggest that MC4R expression in the GI tract is functional, and has the potential to regulate epithelial Cl− secretion in a similar manner to that described for GPR119 agonism. Interestingly, this response appears to exhibit species-dependence, since melanocortin-induced PYY and GLP-1 release could not be detected in the rat, using either ISC analysis from isolated mucosal preparations, or perfused intestinal preparations (Figure S3).
Anti-secretory Effects of α-MSH are Y1 Receptor-mediated and Glucose-sensitive in Mouse and Human Colon
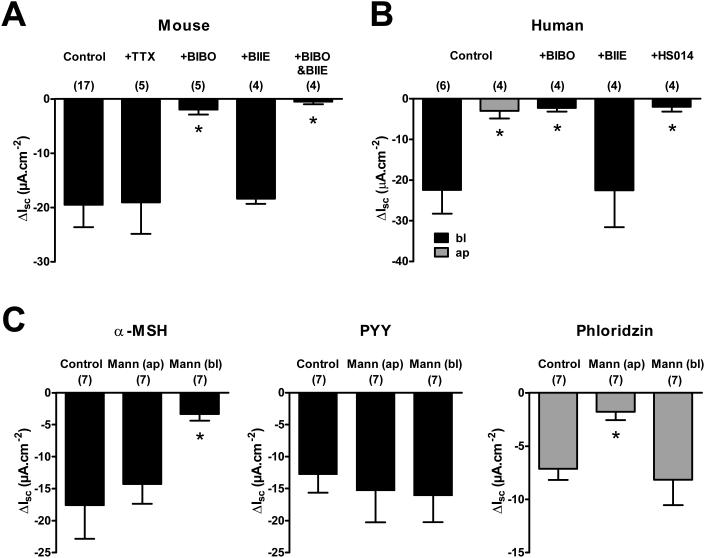
In mouse colon, α-MSH responses were not tetrodotoxin (TTX) sensitive, suggesting that the response to α-MSH is mediated directly at the epithelial cells and not via submucosal neuron stimulation (Figure 4A). In addition, pretreatment with the Y1 receptor antagonist BIBO3304 (BIBO) alone or together with the Y2 receptor antagonist BIIE0246 (BIBO&BIIE) almost completely blocked the effects of α-MSH (Figure 4A). Furthermore, testing of human colonic mucosa replicated the effects of basolaterally applied α-MSH on ISC and separately shows blockade by BIBO but not by BIIE alone (Figure 4B), consistent with the previous knowledge that Y2 receptors mediate neuronal inhibitory mechanisms in mouse and human colon (Hyland et al., 2003). The same Y1 receptor-mediation was seen for the more potent α-MSH agonists MT-II, NDP-α-MSH and LY2112688 (Figure S4). To ensure the specificity of MC4R in the effect seen in human tissues, pretreatment with HS014 virtually abolished the anti-secretory effects of α-MSH (Figure 4B). HS014 also antagonized MT-II, NDP-α-MSH and LY2112688 responses in C57BL/6J mouse colon, while not affecting PYY activity (Figure S4). Taken together, these results suggest that basolateral α-MSH reduces ISC via MC4R-stimulated release of PYY and activation of Y1 receptors, in both mouse and human colon.
Figure 4. α-MSH Activity in Mouse and Normal Human Colon Mucosa is Y1- and MC4R-mediated, and is Glucose-sensitive.
A: α-MSH (bl, 1 μM) in C57BL/6J mouse descending colon mucosa after vehicle (Control), tetrodotoxin (+TTX, bl, 100 nM) or the Y1 antagonist, BIBO3304 alone (+BIBO; bl, 300 nM) or together with the Y2 antagonist, BIIE0246 (+BIBO&BIIE; bl, 1μM), added 20 min prior to α-MSH. B: In human colon mucosa α-MSH responses (1 μM, bl) are also basolaterally-targeted (ap responses in grey), BIBO3304- and HS014-sensitive but not BIIE0246-sensitive (+BIBO; 300 nM, +BIIE; 1 μM, or +HS014; 100 nM, each added alone, 30 min prior to bl α-MSH). C: Responses to α-MSH (1 μM, bl) are glucose-dependent in WT mouse descending colon mucosa. In controls, KH buffer containing 11 mM glucose bathed mucosa on both sides, whereas 11 mM mannitol (Mann) replaced glucose on either ap or bl surface, and in the first histogram resultant α-MSH responses (bl, 1 μM) are shown. In the subsequent histograms, PYY (10 nM, bl) responses 20 min after α-MSH and finally the SGLT1 inhibitor, phloridzin (50 μM, ap, grey bars) was added 15 min after PYY. Ap mannitol only reduced apical SGLT1 activity and thus reduced the effect of apical phloridzin. Each bar is the mean -1SEM with n values in parenthesis. * P<0.05, compared to respective controls using 1-way ANOVA with Dunnett’s post-test. See also Figures S3-S6.
We characterized the glucose-sensitivity of the MC4R response by replacing glucose with mannitol on either the apical or basolateral side of mouse colonic mucosa. Responses to α-MSH were significantly inhibited when glucose was removed from the basolateral surface only, in contrast with PYY responses, which were not glucose-dependent (Figure 4C). Phloridzin inhibits the co-transport of Na+ and glucose across apical membranes via SGLT1 (i.e. it reduces ISC by inhibiting apical-to-basolateral Na+ movement) and thus replacing apical glucose only with mannitol rendered phloridzin significantly less active (Figure 4C).
Tissue resistances were the same in MC4R+/+ and MC4R−/− mucosa, however basal ISC levels were significantly elevated in MC4R−/− colon (P < 0.05) indicating loss of a mucosal anti-secretory agent but otherwise normal mucosal barrier function (Figure S5). Additionally, we observed that the competitive MC4R antagonist HS014 alone increased basal ISC levels in MC4R+/+ colon (3.6 ± 0.4 μA/cm2, n=31, at 30 nM) and in human colon (6.0 ± 2.4 μA/cm2, n=4, at 100 nM), indicating a degree of MC4R specific melanocortinergic tone in both tissues.
We next monitored fecal pellet transit in isolated colon from C57BL/6J mice in order to establish whether L cell activation results in slower colonic transit. In the presence of either α-MSH, LY2112688 or GPR119 agonist PSN at the same concentrations used in mucosal assays, colonic transit was inhibited significantly (Figure S6) suggestive of endogenous PYY (and possibly GLP-1) release resulting in slower colonic motility (Tough et al., 2011).
MC4R Activation Produces Acute Release of PYY and GLP-1 In Vivo
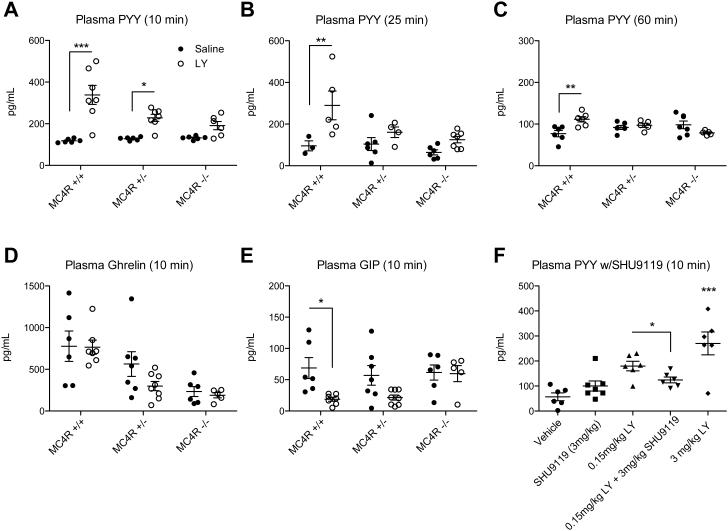
To test for functional effects of MC4R on L cells in vivo, we administered LY2112688 and then assayed acute changes in levels of circulating PYY. Mice were fasted for a minimum of 4 hours to attempt to avoid postprandial elevation of PYY. Furthermore, due to potential effects of stress, the mice were acclimated to handling with 7 days of vehicle IP injections prior to the study day. In order to assess the contribution of the MC4R to this response, we studied adult male MC4R+/+, MC4R+/−, and MC4R−/− mice. Animals were given IP injections of LY2112688 at a dose of 3 mg/kg of body weight, or an equal volume of saline (vehicle). At specific time points after the injection, blood was collected in order to assay plasma levels of PYY. At 10 minutes post-injection, there was a statistically significant 3-fold rise in plasma PYY in MC4R+/+ mice compared to saline controls, however that rise was blunted in MC4R+/− and MC4R−/− groups (Figure 5A). A similar rise was apparent in a different cohort at 25 minutes post-injection while the effect was blunted again in the MC4R+/− and MC4R−/− mice (Figure 5B). Lastly, in a cohort measured at 60 minutes post-injection, a statistically significant, though much lower rise in PYY still existed in MC4R+/+ compared with saline controls (Figure 5C). The increase in PYY following administration of LY2112688 in lean WT animals could also be blunted by the MC4R antagonist SHU9119 (Hruby et al., 1995), showing that either genetic or pharmacological blockade of the MC4R blunts the response (Figure 5F). Furthermore, intracerebroventricular (ICV) administration of two different potent MSH analogues, LY2112688 and MTII, at doses known to induce MC4R-mediated inhibition of food intake failed to induce a significant increase in serum PYY (Figure S7). Additionally, central administration of the MC4R antagonist, SHU9119, failed to block the increase in serum PYY induced by IP administration of LY2112688 (Figure S7). Together, these experiments argue that MC4R mediates a melanocortin-induced PYY release via a peripheral site of expression. No increase in GIP or ghrelin could be detected at 10 minutes post-LY injection, although there were decreases in GIP levels in MC4R+/+ mice and a trend towards lower ghrelin levels in MC4R−/− compared to MC4R+/+ mice regardless of treatment (Figure 5D-E). The lack of MC4R mediated release of other hormones under these conditions suggests an L cell specific function for MC4R.
Figure 5. Intraperitoneal Injection of LY2112688 (LY) Increases Circulating PYY in an MC4R-dependent Manner.
Fasting plasma PYY was assayed in adult male MC4R+/+, MC4R+/−, and MC4R−/− mice at A) 10 minutes, B) 25 minutes, and C) 60 minutes post IP injection of either vehicle or 3 mg/kg LY (A-C). The 10 minute samples were also analyzed for differences in D) Ghrelin and E) GIP across all genotypes. F) A sub-maximal dose of LY was inhibited by also injecting 3 mg/kg SHU9119. Closed symbols denote vehicle treatment; open symbols denote LY treatment (unless otherwise noted). Statistical significance was analyzed between treatments at each genotype using 2-way ANOVA (A-E) or 1-way ANOVA (F) with Bonferroni's post-test. *** P<0.001, ** P<0.01, * P<0.05. See also Figure S7.
Pharmacological and Physiological Properties of MC4R-stimulated GI Peptide Release In Vivo
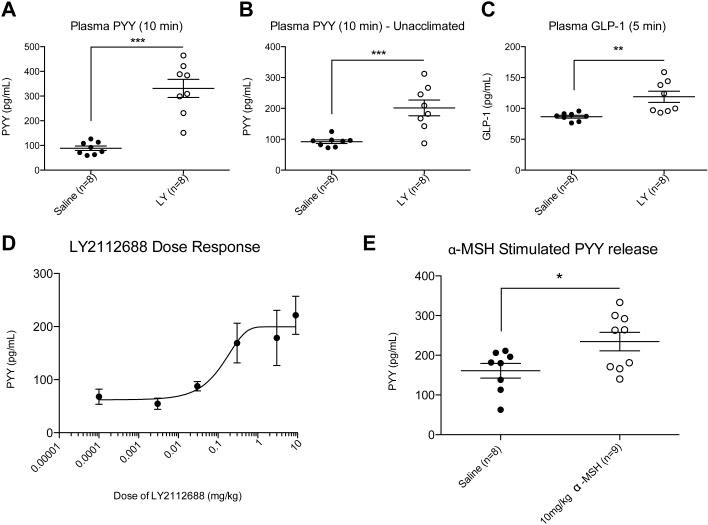
In order to determine the importance of acclimation in the detection of this response, PYY levels were measured in adult male C57BL/6J mice that were acclimated to handling and vehicle injections for up to 7 days, and in age-matched mice that were not. In the group that was acclimated, there was a statistically significant 3-4 fold rise in fasting plasma PYY at 10 minutes post-injection of 3 mg/kg LY2112688 compared to vehicle-injected controls (Figure 6A), consistent with that seen previously in the MC4R+/+ group (Figure 5A). In the group that was not acclimated, there was still a greater than 2 fold elevation in fasting plasma PYY following the injection of LY2112688 compared to the control group (Figure 6B), suggesting that the rise in fasting PYY levels in the circulation are only modestly affected by animal handling and the expected HPA axis activation. In addition to PYY release, L cells co-secrete the incretin GLP-1. Using plasma samples from an independent cohort of unacclimated mice, analyzed at 5 minutes post-treatment due to the extremely short half-life of GLP-1, we measured a significant 1.4 fold increase in GLP-1 in LY2112688 versus saline treated mice (Figure 6C).
Figure 6. Injection of LY2112688 Increases Plasma PYY in a Dose-dependent and Stress-independent Manner and also Affects Circulating GLP-1.
Fasting levels of plasma PYY and GLP-1 were assayed in adult male C57BL/6J mice that were either acclimated or unacclimated to handling and IP injections. A) Mice acclimated to daily handling and IP injections exhibit a significant increase in plasma PYY levels 10 minutes after injection of 3 mg/kg LY. B) IP injections to unacclimated mice also exhibit a significant increase in plasma PYY levels 10 minutes after injection of 3 mg/kg LY. C) GLP-1 levels 5 minutes after injection of 3 mg/kg LY compared to vehicle-injected mice. D) Naïve mice exhibit a dose-dependent increase in plasma PYY with injections of vehicle, 0.003 mg/kg LY, 0.03 mg/kg LY, 0.3 mg/kg LY, 3 mg/kg LY and 9 mg/kg LY. A near-maximal response was observed at a dose as low as 0.3 mg/kg LY. E) Fasting PYY levels exhibit a statistically significant rise in response to the endogenous melanocortin ligand α-MSH (10 mg/kg) in unacclimated mice at 10 minutes post-IP injection. Closed symbols denote vehicle treatment; open symbols denote LY treatment. Statistical significance was analyzed between groups using Student’s t-test. n=5 (saline) or 6 (drug), *** P<0.001, ** P<0.01, * P<0.05.
To further elaborate on MC4R-mediated gut peptide release, a dose-response experiment was conducted in which animals were injected with vehicle or varying doses of LY2112688 following a protocol with no acclimation period and a 4 hour fast (Figure 6D). The rise in PYY followed a dose-response relationship, and the rise in plasma PYY at 10 minutes post-injection was still robust at a dose as low as 0.3 mg/kg. Thus the release of PYY is sensitive to MC4R agonist concentrations in ranges used in prior pharmacological studies of the efficacy of melanocortin peptides in weight loss (Kievit et al., 2013). Treatment with the endogenous agonist α-MSH (10 mg/kg) also produced a statistically significant rise in plasma PYY relative to saline treatment (Figure 6E).
DISCUSSION
MC4R expression is found in many brain nuclei (Mountjoy et al., 1994), and these central MC4R sites control of multiple facets of feeding behavior and metabolism (Cone, 2005). Central melanocortin signaling has also been implicated in the actions of peripheral adipostatic, hunger, and satiety factors such as leptin (Balthasar et al., 2004), ghrelin (Shaw et al., 2005), leptin, and CCK (Fan et al., 2004). Additionally, data show that the central melanocortin system may also regulate GI function. MC4R expression in vago-vagal circuitry responsible for sensory and motor control of the GI tract (Gautron et al., 2010) suggest that peripheral MC4R functions deserve interrogation. In this study we demonstrate expression of MC4R-GFP immunoreactivity in GI cells from stomach to colon, and significant enrichment of MC4R in L cells. Indeed, MC4R was one of the most highly expressed GPCR in the GLP-1/PYY positive L cells, second only to the GPR119 receptor. This high level of enrichment of MC4R led to the hypothesis that peripheral MC4R may play an important role in the regulation of intestinal functions such as gut peptide release, and mucosal ion transport and motility, complimentary to previously characterized central MC4R mechanisms.
Enrichment of MC4R Expression in L cells
To further characterize MC4R expression in the gut, we focused on MC4R expression in enteroendocrine L cells. L cells, which are responsible for simultaneous secretion of PYY and GLP-1, express a high level of MC4R mRNA as evidenced by qRT-PCR analysis of 379 7TM receptors of FACS-purified L cell populations marked by a GLP-1 driven reporter mouse line (Figure 1C). MC4R expression was highest in the GLP-1 positive cells with a several hundred-fold enrichment relative to non-GLP-1 cells. Furthermore, we observed immunohistochemical colocalization of MC4R expressing cells with PYY and GLP-1 using a reporter mouse line that expresses GFP under the MC4R promoter (Liu et al., 2003) (Figure 2). Within the colon most GLP-1 and PYY immunoreactive L cells were labeled as MC4R-GFP positive; other cell types in the GI tract that appear to express MC4R in these studies remain to be identified.
Functional Activity of the MC4R in L Cells
In isolated preparations of murine intestinal mucosae, we observed robust MC4R anti-secretory responses upon stimulation with melanocortin agonists. This primarily basolateral response was indicative of paracrine PYY signaling, as it was mediated by Y1 receptors expressed on epithelial cells (Mannon et al., 1999). Importantly, a similar basolaterally-directed, Y1-dependence of MC4R anti-secretory activity was revealed in human colon mucosa, demonstrating conservation of this regulatory pathway in human large bowel. An absence of this response in rat intestine or colon was also noted. Blockade of intrinsic submucosal neuron activity was ineffective in blocking the α-MSH response, suggesting that functional contributions from putative neuronal MC4R were not mediating the response to exogenous α-MSH.
Removal of basolateral glucose significantly reduced the anti-secretory effects of α-MSH, and phloridzin's dependence on apical glucose for apically-targeted SGLT1 activity was evident. Thus, intestinal glucose-sensitive MC4R agonism is similar to that observed for glucose-sensitive intestinal GPR119 agonism (Cox et al., 2010) and compounds with clinical potential targeted at these receptors should therefore exhibit a reduced risk of hypoglycaemia.
Notably, MC4R activation also attenuated colonic transit time and to the same degree as GPR119 agonism, indicating the potential that L cell-derived PYY and GLP-1 mediate colonic brake and potentially more extensive inhibition such as ileal brake. Additionally, we showed that these intestinal mechanisms were operative in vivo, by treating mice with a number of peripherally delivered MC4R agonists. IP injections of LY2112688 or α-MSH were able to induce statistically significant, rapid rises in fasting plasma PYY and GLP-1 levels. Central administration of two different potent melanocortin peptides at doses known to inhibit food intake failed to produce a significant rise in serum PYY, while central administration of a potent MC4R antagonist failed to block the PYY rise induced by peripheral administration of the MC4R agonist LY2112688. Together, these data support the hypothesis that MC4R expression on L cells is the primary mediator of melanocortin-induced PYY release. Rises in plasma PYY traditionally occur in the post-prandial state and correlate with the activated cleavage product, PYY3-36, a potent satiety factor that acts to reduce meal size. While our assay did not distinguish between PYY and PYY3-36, these peptides have the potential to alter food intake through intestinal control and central action on satiety and reward (Batterham et al., 2002). Similarly, the α-MSH induced GLP-1 release would be expected to exert an incretin effect. Both the in vitro and the in vivo assays demonstrate the requirement of MC4R for peripheral stimulation of L cells as melanocortin agonists were ineffective in MC4R−/− tissues as well as in the MC4R−/− animal studies, and in vivo LY2112688-induced PYY release was blunted by administration of the MC4R antagonist, SHU9119. The lack of a similar effect of ICV administration of the potent α-MSH agonist MTII argues that CNS MC4R sites do not play a significant role in L cell peptide release in these assays. Taken together these data argue for melanocortin mediated PYY/GLP-1 release that results from activation of MC4R expressed specifically on L cells, without significant central or enteric neuron influence (Figure 7).
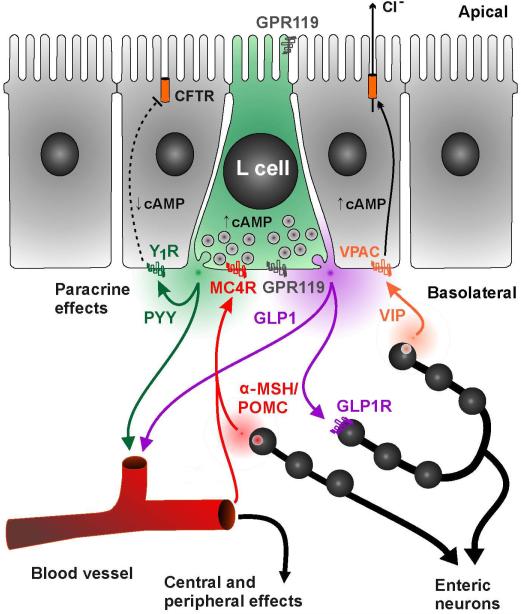
Figure 7. Regulation of GI Epithelial Function by MC4R Activation.
L cells receive basolateral regulatory input from enteric neurons, circulatory factors, and paracrine agents. MC4R, targeted primarily to the basolateral surface of L cells (as opposed to GPR119 which appears present on both domains), is capable of inducing release of PYY and GLP-1 in response to melanocortin peptides. The PYY release, up to 2-4 times above basal levels in vivo, is sufficient to decrease local intestinal epithelial Cl− secretion, shown here, and inhibit motility (an example of another peripheral effect). The physiological source of ligand remains to be determined but could be α-MSH or another POMC melanocortin derivative.
Physiological Relevance of MC4R in L Cells
Our studies highlight a robust hormonal response to MC4R activation that may have implications for behavioral and dietary control that are separate but complimentary to the roles of MC4R in the brain. Thus, α-MSH acting directly on the GI system could be expected to enhance the incretin response, inhibit GI functions such as ion transport and motility, and enhance the release of satiety factors acting both on vagal nerves and directly in the CNS. Our studies used exogenously applied MC4R agonists to activate this response, however analysis of the basal function of MC4R+/+ versus MC4R−/− colonic mucosa supports the notion of endogenous activators of this MC4R system as revealed by the higher basal ISC level of MC4R−/− mucosa and the acute pro-secretory effect of the MC4R antagonist, HS014 in mouse colon. However, the source and identity of the endogenous ligand that might activate MC4R in L cells remains unknown, and identification of the ligand is critical for understanding the physiological role of this system. Our studies indicate that MC4R is targeted to the basolateral domain of L cells located in the small and large intestine, arguing that the endogenous agonist is unlikely to activate the receptor from the intestinal lumen (Figure 7). There are a few possible sources for the endogenous ligand including hormones in the circulation, paracrine factors from the GI epithelium, and neuronal stimulation from the enteric nervous system. The most prominent circulating melanocortin agonist is ACTH, which is released from the pituitary gland and is capable of binding and activating MC4R in addition to MC2R. ACTH rises acutely with stress and could theoretically activate L cell MC4R to induce PYY release. However, no effect of a mild handling stressor on baseline PYY levels was observed (Fig. 6A-B). Further, a more potent 25 min restraint stressor also failed to alter serum PYY levels (data not shown). In the GI tract there have also been reports of immunoreactivity of POMC-derived proteins including γ-MSH, β-endorphin and ACTH within the gastric mucosa (Tanaka et al., 1982), and β-endorphin expression within mouse intestinal tuft cells (Gerbe et al., 2011) and these may activate mucosal MC4R as indicated in Figure 7. Future studies on immunohistochemical characterization of POMC expressing cells throughout the GI tract and enteric nervous system may thus ultimately uncover a new MC4R-mediated pathway that regulates GI function and feeding behaviors.
Pharmacological Relevance of Peripheral MC4R Expression
Due to its integral role in energy homeostasis and human syndromic obesity, the MC4R is a well-validated drug target. Orthosteric agonists of the MC4R, including the peptide LY2112688, failed in clinical trials due to deleterious target-mediated pressor effects despite successful reduction in body weight in multiple animal models (Greenfield et al., 2009). Interestingly, a near maximal PYY response was noted at LY2112688 doses as low as 0.3 mg/kg (Figure 6), similar to doses of melanocortin peptides previously shown to be sufficient to induce weight loss in primates (Kievit et al., 2013). More recently, the peptide agonist RM-493 has been described that causes significant weight loss in primates without a rise in blood pressure or heart rate following peripheral administration (Kievit et al., 2013). A speculative hypothesis is that the latter compound may have reduced brain penetrance, and that development of melanocortin agonists that lack extensive penetration of the blood-brain barrier may allow weight loss without unwanted pressor side-effects. If stimulation of MC4R in L cells and other peripherally located MC4R can mediate effects on energy homeostasis, this is an important shift in current thinking about the melanocortin system and drug development at the MC4R.
Finally, the rapid and robust in vivo release of PYY also represents a useful biomarker for MC4R receptor occupancy in vivo. At a timepoint only 10 minutes after injection of LY2112688, we consistently observed a MC4R-dependent 2-4 fold increase in plasma PYY. By simply assaying PYY levels, one can determine if MC4R is being activated in vivo without the time or expense associated with typical readouts like food intake and body weight changes. Such a facile bioassay is particularly useful for in vivo screening approaches that seek to rapidly verify receptor occupancy of MC4R targeted drugs. In conclusion, the signaling pathway elucidated here reveals a potentially significant peripheral MC4R activity on mouse and human GI function. Given the importance of MC4R function in a mouse model of bariatric surgery. The MC4R may thus also be included in the growing group of GPCRs with enriched expression on GI enteroendocrine cells with therapeutic potential in diabetes and obesity (Schwartz and Holst, 2010).
EXPERIMENTAL PROCEDURES
Fluorescence-assisted cell sorting (FACS) and qPCR
Single cell suspensions were made by mechanic and enzymatic disruption of small intestinal tissue from reporter mice and separated into fluorescence positive cells and fluorescence negative cells as described (Egerod et al., 2012; Parker et al., 2009; Reimann et al., 2008). cDNA from FACS purified cells were used to examine expression of melanocortin receptors using custom designed 384 well qPCR plates, as described (Engelstoft et al., 2013).
Immunohistochemistry
MC4R-Sapphire (MC4R-Sapp) C57BL/6J transgenic mice were used to detect MC4R expressing cells by fluorescent labeling of a tau-GFP fusion protein under control of the MC4R promoter (Liu et al., 2003). Males aged 7-8 weeks were subjected to a daytime fast of 4 hours in order to minimize the presence of intestinal food matter. The mice were deeply anesthetized using inhaled isoflurane and sacrificed by cervical dislocation. The peritoneal cavity was exposed and GI tract removed from stomach to rectum. 1 cm segments were removed from the glandular stomach (distal end), duodenum (adjacent to the pyloric sphincter), jejunum (halfway between the pyloric sphincter and the ileocecal valve), ileum (3 cm above the ileocecal valve), and colon (halfway between the cecocolic junction and anus). Segments were cleared by flushing with ice-cold PBS using a syringe and 12g needle until clean, then transferred to individual 15ml tubes filled with 4% PFA, pH 7.2, in PBS and left for 24 hours at 4°C. After fixation, the tissues were transferred to 20% sucrose in PBS for 48 hours at 4°C. Tissues were then dabbed of excess fluid, laid flat, and frozen in a base mold with O.C.T. compound (Tissue-Tek #4583) and stored at −80°C.
Slide mounted tissue (10 μm) sections were permeabilized for 30 minutes with 0.5% Triton-X 100 in PBS at room temperature (RT). Non-specific binding was blocked with 10% normal donkey serum in PBS containing 0.1% Triton-X-100 for one hour at RT. Sections were then incubated in primary antibody in PBS containing 0.1% Triton-X-100 for 2-3 days at 4°C, followed by 4 × 10 minute PBS washes. Sections were then incubated overnight at 4°C with secondary antibodie s in PBS containing 0.1% Triton-X-100. Finally, sections were washed with PBS (4 × 10 minutes) with DAPI included in the first wash (1:50K) and then coverslipped with PolyAquamount (Polysciences). All staining steps except for washes were done in a humidified chamber with 100-200 μl of reagent per slide, each slide temporarily covered with a glass coverslip during incubation steps.
Primary antibodies used included chicken anti-GFP (1:1K, Abcam #ab13970), rabbit anti-GLP-1 (1:1K, Phoenix Pharmaceuticals #H-028-13) and rabbit anti-PYY (1:1K, Abcam #ab22663). Secondary antibodies included donkey anti-rabbit Alexa 546 (1:1K, Invitrogen) and donkey anti-chicken cy5 (1:1K, Jackson ImmunoResearch). Control slides without primary antibodies were used in addition to immunostaining for GFP in littermate wild-type mice.
Images of fluorescently labeled gut sections were taken with a Zeiss 710 confocal microscope equipped with a 63X/1.4 N.A. Plan Apochromat oil lens. Excitation/emission wavelengths (nm) for each fluorophor were; DAPI, 405/410-505; Alexa 546, 561/568-610; cy5, 633/638-759. Image analysis was done with Imaris (version 7.6.0).
Measurement of ISC
GI tissue from adult (12 - 20 week old) male C57BL/6J, MC4R+/+ or MC4R−/− mice or clinical specimens were dissected free of overlying smooth muscle and mucosae placed between 2 halves of Ussing chambers exposing an area of 0.14cm2. Mucosae were bathed both sides with Krebs-Henseleit (KH) buffer of following composition (in mM: 117 NaCl, 24.8 NaHCO3, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2 and 11.1 D-glucose) aerated with 95% O2/5% CO2 (pH 7.4) and voltage-clamped at 0 mV as described (Cox et al., 2001; Cox and Tough, 2002). Resultant changes in ISC were measured as μA/cm2 once preparations had stabilized (15 min). Mouse mucosae were pretreated with secretagogue, VIP (10 nM, basolateral) and once elevated ISC had reached its peak and was declining at a constant rate, α-MSH was added to either the apical or basolateral reservoir to determine the sidedness of these peptide responses. Subsequently a concentration of 1 μM α-MSH (basolateral) was selected as the MC4R stimulus because it elicited near-maximal responses in mouse colon. To ascertain endogenous peptides stimulated by basolateral α-MSH, mucosa were pretreated with Y1 antagonist (BIBO3304, 300 nM), Y2 antagonist (BIIE0246, 1 μM) or MC4R antagonist (HS014, 30 nM for mouse, and 100 nM for human mucosa) each added basolaterally, and 20 min later PYY (10 nM) was added as a control Y1/Y2 receptor stimulus.
To test glucose-sensitivity of α-MSH (1 μM) responses, mouse colon was bathed with KH buffer containing glucose (11.1 mM) in one reservoir and mannitol (11.1 mM) in place of glucose in the other. PYY (10 nM) responses were measured 15-min after α-MSH addition and apical SGLT1 inhibitor, phloridzin (50 μM, apical) was added (15-min after PYY) to confirm the requirement of glucose for this inhibitory activity.
ISC responses in μA are expressed as the mean ±SEM per unit area (cm2). Single comparisons (using GraphPad Prism) were performed using Student's unpaired t-test while multiple comparisons utilized 1-way ANOVA with Dunnett's post-test and P ≤ 0.05 were considered statistically significant.
Plasma Hormone Measurements
Except when indicated, experimental mice (C57BL/6J; MC4R+/+, +/−, and −/− ) were acclimated to handling and injections for up to 7 days prior to blood collection. The mice were scruffed, then injected with 100-200 μL of saline, each day in order to minimize stress during final blood collection. On the day of blood collection, mice were subjected to a 4-hour daytime fast to reduce postprandial hormones to baseline levels. Mice were then injected with the indicated dose of LY2112688, α-MSH, or vehicle (saline), in 100-200ul volumes, according to body weight. Blood was collected from mice at times indicated either by decapitation under deep anesthesia from inhaled isofluorane or by submandibular bleeding in conscious mice. Repeated sampling by submandibular bleeding was done at least 2 weeks apart to allow for complete recovery from blood loss. Blood was collected into tubes containing appropriate volumes of EDTA, Protease Inhibitor Cocktail (Sigma, P8340), and DPP-IV Inhibitor (Millipore, Cat. No. DPP4) and kept on ice. Upon completion of blood collection, the tubes containing blood, EDTA, and protease inhibitors were spun at 3000 X G at 4°C fo r 30 minutes. Plasma was removed and spun at 10000 × G at 4°C for 1 minute to pellet remaining blood cells. Plasma was frozen at −80°C until use. Plasma hormones were ass ayed using the MilliplexMAP Mouse Metabolic Hormone - Magnetic Bead Panel Immunoassay (Millipore MMHMAG-44K), with duplicate 10 μL samples of undiluted plasma to detect any combination of hormones including PYY (total), GLP-1, ghrelin, amylin (active), GIP, insulin, and leptin. The assay was read on a Luminex 100 analyzer. Total GLP-1 levels were also assayed separately with greater sensitivity using an ELISA kit (Millipore EZGLP1T-36K). Values were plotted in GraphPad Prism and statistical analyses were conducted using ANOVA with Bonferroni post-test between all experimental groups, or by t-test when only 2 groups were available.
Intracerebroventricular Injections
Mice (9 week old C57BL/6J) with third ventricle intracerebroventricular (ICV) cannulation were obtained from Jackson Labs. After acclimatization to handling and manipulation of the cannula for several days, mice were fasted (4hrs). Each mouse was then given an ICV injection (10 nmol SHU9119 or LY2112688 in saline) in a volume of 2 μL, followed by an IP injection (3 mg/kg LY2112688 or saline) in a volume of 100 μL. Submandibular bleeds were performed 10 minutes later. In a parallel study, the same group of mice received an ICV injection (3nmol MTII or saline), followed by an IP injection of saline. Submandibular bleeds were performed after 10 minutes. Plasma was assayed for PYY levels as before.
Materials
BIBO3304 and BIIE0246 (Tocris Bioscience) were dissolved in 10% DMSO (at 1 mM) and stored at −20°C. Peptide stocks of LY211688 (Bachem Laboratories), α-MSH (Abcam), HS014 (Tocris Bioscience), NDP-α-MSH, ACTH and MT II (Phoenix Pharmaceuticals), VIP and PYY (Cambridge Bioscience) were dissolved in water, and aliquots stored at −20°C. PSN632408 (Cayman Chemical) was dissolved in 95% ethanol. All other compounds were of analytical grade (Sigma-Aldrich).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Heidi Moreno (Vanderbilt) for excellent technical assistance and Baljit Gill-Barman (Guy's & St Thomas' NHS Hospitals Trust) for assisting with human tissue specimens. This work was supported by research funds to RDC (NIH R24DK096527, RO1DK070332), FMG and FR (WT088357/Z/09/Z, WT084210/Z/07/Z), and from the Novo Nordisk Foundation (HMC). RDC also receives support from the Vanderbilt Diabetes Research and Training Center (DK20593) and Digestive Disease Research Center (P30DK058404). The Novo Nordisk Foundation Center for Basic Metabolic Research (www.metabol.ku.dk) is supported by an unconditional grant from the Novo Nordisk Foundation (TWS, JJH, BH, BS, MSE). CLM is an employee and shareholder of Novo Nordisk A/S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, 7 figures, and 2 movies, and may be found online at…
REFERENCES
- Arantes RM, Nogueira AM. Distribution of enteroglucagon- and peptide YY-immunoreactive cells in the intestinal mucosa of germ-free and conventional mice. Cell Tissue Res. 1997;290:61–69. doi: 10.1007/s004410050908. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr., Elmquist JK, et al. Leptin Receptor Signaling in POMC Neurons Is Required for Normal Body Weight Homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4:605–611. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cox HM, Pollock EL, Tough IR, Herzog H. Multiple Y receptors mediate pancreatic polypeptide responses in mouse colon mucosa. Peptides. 2001;22:445–452. doi: 10.1016/s0196-9781(01)00355-2. [DOI] [PubMed] [Google Scholar]
- Cox HM, Tough IR. Neuropeptide Y, Y1, Y2 and Y4 receptors mediate Y agonist responses in isolated human colon mucosa. Br J Pharmacol. 2002;135:1505–1512. doi: 10.1038/sj.bjp.0704604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox HM, Tough IR, Woolston AM, Zhang L, Nguyen AD, Sainsbury A, Herzog H. Peptide YY is critical for acylethanolamine receptor Gpr119-induced activation of gastrointestinal mucosal responses. Cell Metab. 2010;11:532–542. doi: 10.1016/j.cmet.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ, Jin T, Asa SL, Young TA, Brubaker PL. Activation of proglucagon gene transcription by protein kinase-A in a novel mouse enteroendocrine cell line. Mol Endocrinol. 1994;8:1646–1655. doi: 10.1210/mend.8.12.7535893. [DOI] [PubMed] [Google Scholar]
- Egerod KL, Engelstoft MS, Grunddal KV, Nohr MK, Secher A, Sakata I, Pedersen J, Windelov JA, Fuchtbauer EM, Olsen J, et al. A Major Lineage of Enteroendocrine Cells Coexpress CCK, Secretin, GIP, GLP-1, PYY, and Neurotensin but Not Somatostatin. Endocrinology. 2012;153:5782–95. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. The Journal of endocrinology. 1993;138:159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- Engelstoft SM, Park W, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pederson MH, Nohr MK, et al. Seven transmembrane G protein-coupled receptor repertoire of gastrin ghrelin cells. Molecular Metabolism. 2013;2:376–392. doi: 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci. 2004;7:335–336. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J. Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions. J Comp Neurol. 2010;518:6–24. doi: 10.1002/cne.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. The Journal of cell biology. 2011;192:767–780. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M, Shuttre JR, Sarmiento U, Sarosi I, Stark KL. Overexpression of Agrt leads to obesity in transgenic mice. Nature Genetics. 1997;17:273–274. doi: 10.1038/ng1197-273. [DOI] [PubMed] [Google Scholar]
- Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J. Neurosci. 1998;18:10128–10135. doi: 10.1523/JNEUROSCI.18-23-10128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby VJ, Lu D, Sharma SD, Castrucci AL, Kesterson RA, Al-Obeidi FA, Hadley ME, Cone RD. Cyclic lactam α-melanotropin analogues of Ac-Nle4-c[Asp4,D-Phe7, Lys10]α-MSH(4-10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J. Med. Chem. 1995;38:3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- Hsiung HM, Hertel J, Zhang XY, Smith DP, Smiley DL, Heiman ML, Yang DD, Husain S, Mayer JP, Zhang L, et al. A novel and selective beta-melanocyte-stimulating hormone-derived peptide agonist for melanocortin 4 receptor potently decreased food intake and body weight gain in diet-induced obese rats. Endocrinology. 2005;146:5257–5266. doi: 10.1210/en.2005-0177. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Hyland NP, Sjoberg F, Tough IR, Herzog H, Cox HM. Functional consequences of neuropeptide Y Y 2 receptor knockout and Y2 antagonism in mouse and human colonic tissues. Br J Pharmacol. 2003;139:863–871. doi: 10.1038/sj.bjp.0705298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J, Li X, Chang BH, Chan L, Schwartz GJ, Chua SC, Jr., Hussain MM. An intrinsic gut leptin-melanocortin pathway modulates intestinal microsomal triglyceride transfer protein and lipid absorption. J Lipid Res. 2010;51:1929–1942. doi: 10.1194/jlr.M005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P, Pranger L, Cowley MA, Grove KL, Culler MD. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62:490–497. doi: 10.2337/db12-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal S, McLaughlin J, Barlow J, D'Amato M, Giacovelli G, Varro A, Dockray GJ, Thompson DG. Cholecystokinin pathways modulate sensations induced by gastric distension in humans. Am J Physiol Gastrointest Liver Physiol. 2004;287:G72–79. doi: 10.1152/ajpgi.00351.2003. [DOI] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannon PJ, Kanungo A, Mannon RB, Ludwig KA. Peptide YY/neuropeptide Y Y1 receptor expression in the epithelium and mucosal nerves of the human colon. Regul Pept. 1999;83:11–19. doi: 10.1016/s0167-0115(99)00035-x. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol. Endo. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang Y-K, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–137. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Panaro BL, Cone RD. Melanocortin-4 receptor mutations paradoxically reduce preference for palatable foods. Proc Natl Acad Sci U S A. 2013;110:7050–7055. doi: 10.1073/pnas.1304707110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009;52:289–298. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilichiewicz AN, Little TJ, Brennan IM, Meyer JH, Wishart JM, Otto B, Horowitz M, Feinle-Bisset C. Effects of load, and duration, of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am J Physiol Regul Integr Comp Physiol. 2006;290:R668–677. doi: 10.1152/ajpregu.00606.2005. [DOI] [PubMed] [Google Scholar]
- Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J, Cruz MT, Majumdar U, Lewin A, Kingsbury KA, Dezfuli G, Vicini S, Verbalis JG, Dretchen KL, Gillis RA, et al. Melanocortin signaling in the brainstem influences vagal outflow to the stomach. J Neurosci. 2013;33:13286–13299. doi: 10.1523/JNEUROSCI.0780-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TW, Holst B. An enteroendocrine full package solution. Cell Metab. 2010;11:445–447. doi: 10.1016/j.cmet.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Shaw AM, Irani BG, Moore MC, Haskell-Luevano C, Millard WJ. Ghrelin-induced food intake and growth hormone secretion are altered in melanocortin 3 and 4 receptor knockout mice. Peptides. 2005;26:1720–1727. doi: 10.1016/j.peptides.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Tanaka I, Nakai Y, Nakao K, Oki S, Masaki N, Ohtsuki H, Imura H. Presence of immunoreactive gamma-melanocyte-stimulating hormone, adrenocorticotropin, and beta-endorphin in human gastric antral mucosa. J Clin Endocrinol Metab. 1982;54:392–396. doi: 10.1210/jcem-54-2-392. [DOI] [PubMed] [Google Scholar]
- Tough IR, Forbes S, Tolhurst R, Ellis M, Herzog H, Bornstein JC, Cox HM. Endogenous peptide YY and neuropeptide Y inhibit colonic ion transport, contractility and transit differentially via Y(1) and Y(2) receptors. Br J Pharmacol. 2011;164:471–484. doi: 10.1111/j.1476-5381.2011.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergoni AV, Bertolini A, Mutulis F, Wikberg JE, Schioth HB. Differential influence of a selective melanocortin MC4 receptor antagonist (HS014) on melanocortin-induced behavioral effects in rats. Eur. J. Pharmacol. 1998;362:95–101. doi: 10.1016/s0014-2999(98)00753-5. [DOI] [PubMed] [Google Scholar]
- Williams DL, Kaplan JM, Grill HJ. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology. 2000;141:1332–1337. doi: 10.1210/endo.141.4.7410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.