Introduction
Transcaval (“caval-aortic”) access is a new approach to introduce large devices, such as transcatheter aortic valves, into the abdominal aorta in patients who otherwise lack access options. In this technique, coaxial catheters are introduced into the body from the femoral vein, and advanced into the abdominal aorta from the adjoining inferior vena cava (IVC) under fluoroscopic guidance. The aorto-caval fistula is closed using a nitinol cardiac occluder device after the therapeutic procedure (1). This paper describes how to plan transcaval access procedures using CT angiography.
Provide a final recommendation
After systematic image analysis (Figures 1–6), we provide favorability recommendations. “Unfavorable” indicates transcaval access is not recommended. “Feasible” suggests elevated risk over a favorable classification. Our current classification is summarized below.
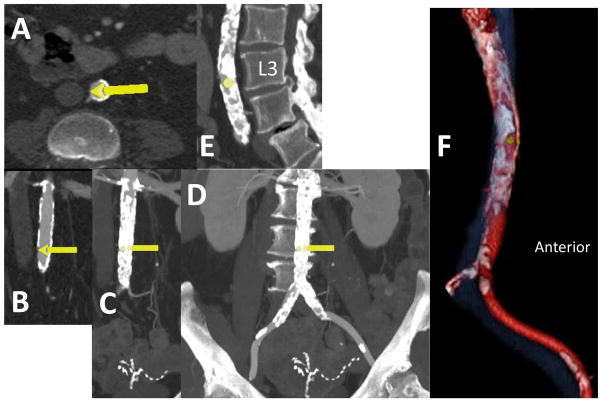
Figure 1. Find potential crossing targets.
Using CT analysis software, isolate (segment) aortoiliac arteries and IVC (from the level of the renal arteries to the femoral bifurcation) and find a calcium-free “target window” (wider than the proposed introducer sheath) on the right wall of the aorta close to the IVC. Heavily calcific targets are difficult to cross and may increase the risk of complications. Mark the proposed target with an arrow on a multi-planar CT reconstruction. Rotate coronal plane (panel A) obliquely to include the centers of the aorta and cava in axial and sagittal sections (panels B & C) at the proposed target, and generate volume-rendered images of the segmented aorta and cava (panel D, color aorta red, calcium white, cava blue with 30% transparency, and lumbar spine greyscale with 90% transparency for bony context). Manually assure that arterial segmentation includes “deep” or adventitial aortic calcification and includes all left renal veins. Cut away all visceral arteries (beyond a 1cm stump) and organs.
Figure 6. Post roadmap images in the procedure room to help guide operators.
(Panel A) Axial thin slice at the crossing target. (Panels B–D) Oblique-coronal views (rotated to match the recommended fluoroscopy projection angle, such as RAO 30o) at the crossing target. Supply three snapshots: (Panel B) thin to highlight the ideal crossing target, (Panel C) as thick as the aorta, to convey a maximum intensity projection of the entire aortic calcium pattern, (Panel D) thickest to depict aortic calcification in the context of the vertebral spine, to mimic the expected fluoroscopy pattern during the procedure. These are critical fiducial depictions of the target during crossing. (Panel E) Sagittal thin and/or thick slice along the vertebral column to indicate the crossing target. (Panel F) The en-face “hemi-aorta” view to depict the calcium-spared window on the aorta (yellow dot).
| Favorable |
ALL of the following
|
| Feasible: |
ANY of the following
|
| Unfavorable |
ANY of the following
|
Video 1. Animated volume renderings of the caval-aortic target and of the en face “hemi-aorta” views. We also recommend animated 3D volume renderings of the cava, aorta, and proposed targets, both full-volume and en face after “cutting away” the left half of the aorta.
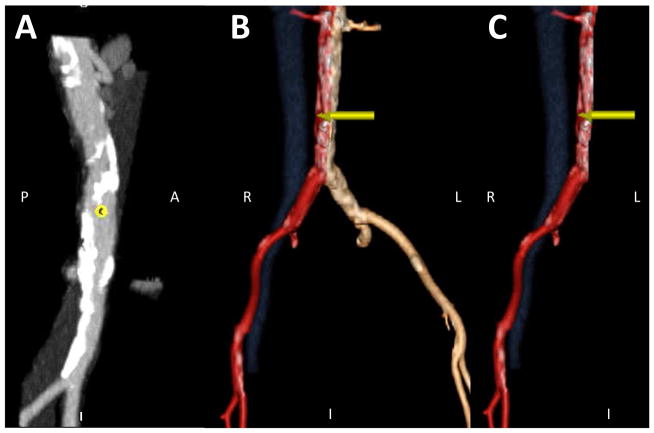
Figure 2. Refine the proposed crossing target using an en-face “hemi-aorta” view.
The en face view (panel A) depicts calcium-free windows on the right aortic wall as seen from the cava. Isolate the right half of the aorta to depict mural aortic calcium (panel B & C) by slicing away the entire left half (panel C).
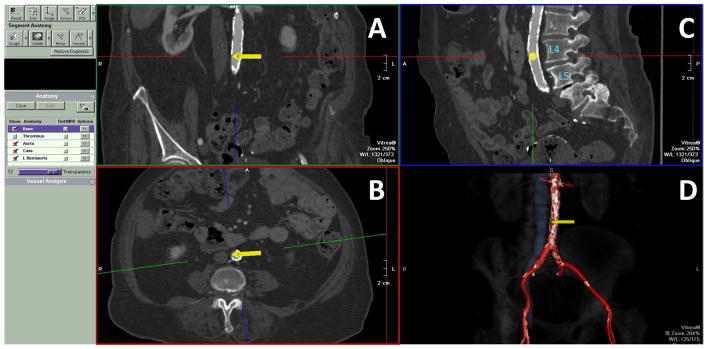
Figure 3. Further refine the proposed target to avoid hazards.
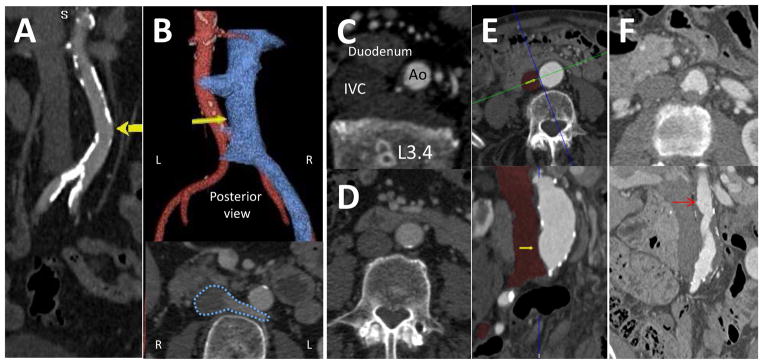
The target should not be far from the cava (panel A), but should be sufficiently far from the right renal arteries, left renal veins, and the aortoiliac bifurcation (>10mm) to avoid closure device encroachment (Panel 4D). Retroaortic veins (panel B, white arrow and dotted blue outline) should be identified and avoided. There should be no important interposed structures, such as bowel (panel C). More often, bowel is “draped” across the cava and aorta (panel D), and this needs the operator to aim posteriorly. Aortic aneurysm (panel E) and atheroma (panel F) do not disqualify crossing; pouch-shaped targets (yellow and red arrows, respectively) appear to seat closure devices well. However, intraluminal aortic pathology cephalad to the crossing target, such as aortic aneurysm/thrombus, atheroma, or dissection, may constitute hazardous sheath trajectories. Lumbar artery ostia should be avoided. However, lumbar venous plexus structures are redundant and should not disqualify a transcaval trajectory.
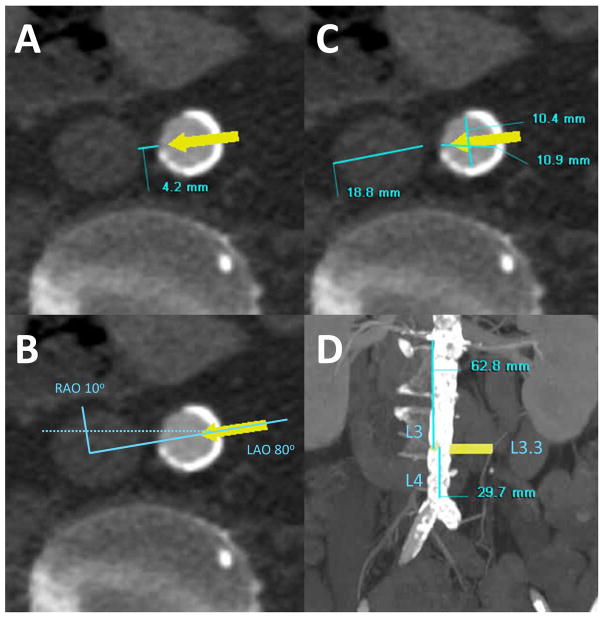
Figure 4. Obtain key measurements.
(Panel A) The lumen-to-lumen distance between the aorta and inferior vena cava at the target, measured horizontally (include interposed aortic atheroma or thrombus). The commercially available nitinol occluder devices have a neck length of 7–8 mm. Tracts longer than 8–12mm may be less favorable and distances exceeding 12mm are unfavorable for transcaval procedures using currently available occluder devices. (Panel B) The recommended working fluoroscopic projection angles for crossing and corresponding orthogonal projection for pointing at the aortic snare. The angle is measured between the cava and aortic centers while the patient is lying flat. (Panel C) The diameter of the inferior vena cava and aorta at the target. This is measured along the line connecting the cava and aortic centers, and is used to choose a suitable caval guiding catheter and choose an oversized snare device that will seat properly to create a “bullseye” target during transcaval crossing. (Panel D) The lumbar spine landmark corresponding to the proposed crossing target. Depict these landmarks in a coronal oblique view at the recommended projection angle, and in a straight sagittal view. We report a semi-quantitative location score, where the mid-body of the 3rd lumbar vertebra is called L3.0, and the middle of the intervertebral disc between L3 and L4 is called L3.5. We identify bailout options including the diameter of the aorta above and below the target (typically ± 3cm), and the “best” iliofemoral artery side to be used in case emergency endograft therapy is required. These measurements inform the choice of endograft device. The distance of the proposed target from the right renal artery and the top of the aorto-iliac bifurcation. This helps the operator assure the closure device will not interfere with either vascular structure. The trajectory distance from the femoral vein skin puncture into the aortic target must be significantly less (by approximately 7cm) than the working length of the selected introducer sheath, even in obese patients.
Figure 5. Classify the aortic calcification pattern.
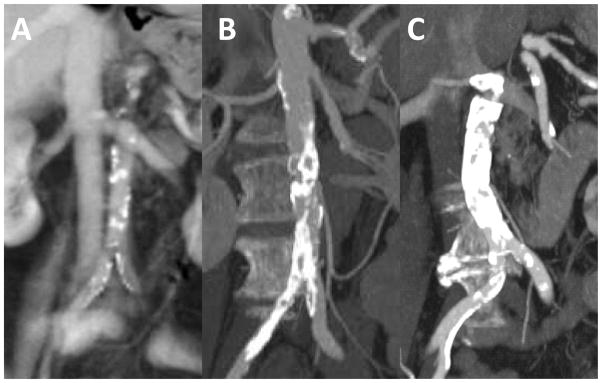
We use the following grades: 0 = no calcium; 1 = scattered calcium that is not confluent (panel A); 2 = multifocal calcification having a calcium-spared “window” at a proposed crossing target (panel B); 3 = “porcelain” abdominal aorta, which at present we consider a contraindication for this procedure (panel C).
Acknowledgments
Sources of Funding: Supported by the Division of Intramural Research, National Heart Lung and Blood Institute, National Institutes of Health (Z01-HL005062 and Z01-HL006040 to RJL).
We thank Janet F. Wyman and Christina Nelson at Henry Ford Health System.
Abbreviations
- CTA
Computed tomographic angiography
Footnotes
Competing interests: RJL, TR, and ABG are inventors on patent applications, assigned to NIH and HFHS respectively, on devices for transcaval access and closure.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenbaum AB, O’Neill WW, Paone G, et al. Caval-aortic access to allow transcatheter aortic valve replacement in patients otherwise ineligible: Initial human experience. J Am Coll Cardiol. 2014;63:2795–2804. doi: 10.1016/j.jacc.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]