Abstract
Purpose
In this paper, we examine the effectiveness of a variety of HIV diagnosis interventions that utilize the preventive potential of post-diagnosis behavior change (PDBC) in recently HIV-diagnosed men who have sex with men (MSM), as measured by reduction in number of new infections. Empirical evidence for PDBC was presented in the behavioral sub-study of the Southern California Acute Infection and Early Disease Research Program (AIEDRP). In previous modeling work, we demonstrated the existing preventive effects of PDBC. However, a large proportion of new infections among MSM are either undiagnosed or diagnosed late, and the preventive potential of PDBC is not fully utilized.
Methods
We derive empirical stochastic, network-based models to examine the effectiveness over a 10 year period of several diagnosis interventions that account for PDBC among MSM. These interventions involve tests with shorter detection windows, more frequent testing, and individualized testing regimens.
Results
We find that individualized testing interventions (i.e. testing individuals every 3 partners or 3 months, whichever is first, or every 6 partners or 6 months, whichever is first) result in significantly fewer new HIV infections than the generalized interventions we consider.
Conclusions
This work highlights the potential of individualized interventions for new public health policies in HIV prevention.
Introduction
Men who have sex with men (MSM) continue to be the population most affected by HIV in the United States (US) [1,2]. MSM account for an estimated 63% of all new HIV infections in the US [3]. Additionally, many infections in this population are either undiagnosed [3,4], or diagnosed late [5]. There are at least two public health benefits to early diagnosis: timely enrollment in treatment [6–8] and early adoption of behaviors to reduce risk of transmission to one’s partners; examples of the latter include partner reduction [9–11], serosorting [12–15], and increased condom use [10]. Multiple interventions to increase testing frequency, testing promptness after exposure, and test sensitivity, or to reduce the window period, are under consideration. The public health impacts of these interventions tend to be indirect, because cases averted are not among testers themselves but among their partners and partners’ partners. Empirically derived mathematical models and computational analyses can help assess the effectiveness of various interventions to reduce HIV incidence among MSM.
Early HIV diagnosis is a function of the “detection window” (time between infection and development of a positive test result) of the test used. HIV tests with shorter detection windows continue to be developed, and third- and fourth-generation enzyme immunoassays (EIA’s) have detection windows of 20–30 days and 15–20 days, respectively [16]. Fourth-generation EIAs are widely used in industrialized economies [17–19]. Rapid nucleic acid amplification tests (NAATs) are an alternative, as are antigen tests that can detect HIV as early as 10–15 days after infection [16,20]. However, NAATs are much more expensive than antibody tests [21]. Current recommendations in high-incidence populations include pooled NAAT or fourth-generation assays [22].
Early diagnosis also depends on MSM’s testing patterns and their risk behavior. Currently, the CDC recommends that all MSM test for HIV every year, and states that sexually active MSM may benefit from testing every 3 to 6 months [3], regardless of risk behavior including number of partners. Estimates from clinical data in four metropolitan US centers suggest that MSM tend to test on average about once a year [23], but data from the CDC’s National HIV Behavioral Surveillance (NHBS) system suggest that a high proportion of HIV-infected MSM (~44%) are undiagnosed [4]. As previously shown [24], these sources are difficult to reconcile; our knowledge of true testing frequencies and diagnosis levels for MSM remains imperfect.
Some health jurisdictions are exploring individually-tailored testing interventions. For example, the “Find Your Frequency” program, recently implemented in the Seattle metropolitan area, uses a website to encourage MSM to evaluate their risk based on the status and number of partners, type of sex, drug use and history of sexually transmitted diseases, and uses a simple algorithm to make recommendations to test every 3 or 12 months, contingent on individual risk characteristics [25]. Similarly, other health departments are interested in exploring an approach that proposes that MSM test after a certain number of partners or period of time, e.g. every 3 months or 3 partners, whichever comes first (known as “the oil change model”) [26]. Under this approach, testing recommendations are dependent on recent risk, with the hope that a tailored approach will result in more frequent testing among those at highest risk of HIV acquisition. Both these programs recognize that a single testing recommendation does not capture the diversity of risk among MSM.
The preventive potential of diagnosis-induced behavior change also depends upon its timing and magnitude. Interventions to maximize this prevention potential require knowledge of the network-level effects of such behavior change; network approaches for the implementation and assessment of interventions have traditionally been relatively uncommon, but have great potential [27]. Post-diagnosis behavior change (PDBC) in Southern Californian MSM was documented in the behavioral sub-study of the Southern California Acute Infection and Early Disease Research Program (AIEDRP) [9,10]. These studies demonstrated a statistically significant reduction in the mean number of partners and in the likelihood of unprotected anal intercourse (UAI) with the last partner of negative or unknown HIV status soon after a positive diagnosis [10]. In prior work, we demonstrated the epidemiological significance of PDBC; our findings indicated that without observed levels of PDBC, HIV prevalence in this population would be much higher [24].
How such behavior change can be used to better design interventions to impact incidence among MSM, however, remains largely unknown. The benefits of early diagnosis are recognized [28], and are being explored in modeling studies [29]. Much of this focus is on early initiation of treatment to prevent transmission through viral suppression (“treatment-as-prevention”) [30–33]. Relatively little attention is given to the preventive power of diagnosis-induced behavior change through changes in testing patterns (what we might call “testing-as-prevention”). In this paper, we develop network-based mathematical models, parameterized with detailed data on PDBC among US MSM, to examine the effectiveness of three types of HIV testing interventions to reduce incidence: 1) instituting tests with shorter detection windows, 2) increasing frequency of testing generally, and 3) increasing testing using risk-based individually-tailored algorithms.
Methods
We retain the model structure from our work on PDBC and HIV prevalence among Southern Californian MSM [24]. These models are derived from the exponential-family random graph model (ERGM) framework, which is increasingly used to model HIV transmission [34–36]. We incorporate numerous key processes: demographic (birth; death; and aging), epidemiological (testing behavior; treatment; methamphetamine use; circumcision), sexual network (partnership types, activity levels, concurrent partnerships of various types, sexual role heterogeneity; sero-adaptive behaviors) and biological (viral load trajectories; variable infectivity by stage; treatment status; and adherence). One key focus here is on the heightened risk of transmission due to acute infection during the first forty days after seroconversion [34].
As in our previous work, the behavioral data are primarily from the Southern California (Los Angeles and San Diego Counties) AIEDRP study [9,10], supplemented with published biological and demographic data (see the online appendix of our prior study [24]). Newly HIV-diagnosed men completed AIEDRP questionnaires via computer-assisted self-interviewing (CASI) at baseline; follow-up CASI interviews occurred at three months intervals. At baseline, respondents provided detailed information on their three most recent partners, and at follow-up, on the most recent partner, in addition to reporting total numbers of partners at baseline and follow-up [10]. The types of partnerships MSM engage in are complex [37], but for simplicity we dichotomize these partnerships as “main”, and “non-main” [24]. We model non-main partnerships as discrete unprotected anal intercourse (UAI) events, and main partnerships using temporally evolving networks, in which UAI episodes may occur on any given day. We do not consider protected anal intercourse, seropositioning (explicit adoption of roles by serostatus), or oral intercourse. Our baseline models assume a 22-day test detection window, consistent with third- and fourth-generation EIA’s, and other modeling work [34]. The key parameter sources are in Table 1.
Table 1.
Sources of key parameters. AIEDRP represents the Southern California sub-study of the Acute Infection and Early Disease Research Program; study details are in two prior publications [9,10]. Parameters marked PUMA are from the modeling component of the Prevention Umbrella for MSM in the Americas project [34]. Complete details for all our parameters are in the online appendix to a prior study [24].
| Source | Parameters |
|---|---|
| AIEDRP | Mean number of main and non-main partnerships, mixing by diagnosis status, daily probability of unprotected anal intercourse (UAI), mixing by age, mean duration of main partnerships, mean number of partnerships for methamphetamine users and non-users |
| PUMA | Detection window for HIV tests in baseline models, proportion of treated men who achieve partial, full, or no viral suppression, role versatility in main partnerships, all biological parameters (including evolution of viral load trajectories, adjustment parameters for acute and late-stage infection, and transmission) |
Our model is meant to represent only those MSM whose HIV risk is more than occasional, and not reflective of the unknown percentage of MSM who never engage in UAI, or whose lifetime UAI only occurs within concordant seronegative mutually monogamous main partnerships. This risk structure is reflected in the distribution of behaviors present in our baseline model. Of specific relevance to the testing interventions we consider is the fact that ~40% of HIV-uninfected men in the model average >3 UAI partners every 3 months or >6 UAI partners every 6 months.
We model three PDBC mechanisms (estimates in Table 2):
Table 2.
Estimates of key input parameters that describe post-diganosis behavior change
| Parameter | Estimate | Source |
|---|---|---|
| Average reduction in mean number of non-main partnerships per individual for HIV-positive and undiagnosed MSM relative to HIV-negative or undiagnosed MSM | 25.6% | AIEDRP [10] |
| Average reduction in the number of sero-discordant partnerships with at least one HIV-positive and diagnosed individual, relative to the number expected by proportional mixing on diagnosis status | 40% | AIEDRP [10] |
| Reduction in daily probability of UAI within partnerships where at least one partner is HIV-positive and diagnosed relative to partnerships where both partners are HIV-negative or undiagnosed | 30% | PUMA [34] |
reduction in number of non-main UAI partnerships,
disproportionate selection of non-main partners by diagnosis status,
reduction in UAI within ongoing main partnerships.
Although this is not an explicit model of treatment-as-prevention interventions, treatment is initiated for most men when they have been diagnosed and infected long enough to have an expected CD4+ count that matches levels observed in practice at treatment initiation in the US [38,39] (as opposed to assuming that treatment guidelines are universally met). Thus, diagnosis is a necessary but not sufficient condition for initiating treatment. Further details are in Goodreau et al. [34].
We consider three baseline testing scenarios to account for the variation in existing reports of testing frequency and levels of diagnosis, summarized in Table 3. In all three, we assume that undiagnosed men have an equal and constant daily testing probability, independent of the time since last test and risk behavior. Our first baseline model is derived from a clinical study from four major US metropolitan centers [23]. This study reported a median inter-test interval (ITI) of 243 days, which corresponds to a mean ITI of 351 days, under our model’s assumption of memoryless (i.e. geometrically distributed) waiting times between tests. We then consider a second baseline testing frequency of one test on average every two years, consistent with a separate modeling study [13]. We call these baseline conditions the testing frequency (TF) scenarios, and label the former (mean 351 days) as “TF1”, and the latter (mean two years) as “TF2.” We then examine a separate scenario that matches the level of awareness (LOA) of infection from NHBS (55–60% of HIV-infected men are diagnosed) [4]. This condition yields a mean ITI of 4000 days (~10.9 years).
Table 3.
Salient features of each baseline scenario.
Since HIV among MSM in the US is a mature epidemic, we simulated each of the three baseline models until achieving stable equilibrium prevalence. Since our models are stochastic, prevalence does not achieve a single value at equilibrium, and was ~32–34% for the TF1 and TF2 baseline scenarios and ~44% for the LOA baseline scenario. Simulated prevalence outputs were used to calibrate the model; it must be noted that we see high values because our model represents only those MSM whose HIV risk is consequential (i.e. our denominator is smaller than in population-based estimates of HIV prevalence among MSM). Prevalence is higher in the LOA model because in the presence of PDBC, lower rates of testing at baseline are expected to yield higher prevalence.
We then model the effect of six interventions on each of our three baseline scenarios, as summarized in Table 4. Our first intervention considers a test with a 1-day detection window (abbreviated DW-1). This case is hypothetical, and provides a theoretical limit to the number of infections avertable by developing a test with the shortest possible detection window, without any change in baseline testing behavior. We then return to the baseline assumption of a 22-day detection window, and model generalized testing (GT) interventions where all MSM test every 6 months (GT-6) or every 3 months (GT-3). We then consider individualized testing (IT) interventions where MSM test every 6 months or 6 non-main UAI partners, whichever comes first (IT-6), or every 3 months or 3 non-main UAI partners, whichever comes first (IT-3). As a hypothetical scenario, we model a “best case” (BC) where every undiagnosed individual tests every day and the test has a 1-day detection window; this intervention helps us evaluate the theoretical limit of incidence reduction through diagnosis and PDBC alone. We also simulate a “control” setting with no changes to any baseline scenario. We assume that all MSM, including high-risk MSM, are equally reachable by the diagnosis interventions we consider. We assume complete uptake following notification; although we recognize this assumption is unrealistic, it allows us to consider the maximum potential of each intervention relative to one another.
Table 4.
Salient features of each intervention
| Intervention | Notation | Detection Window | Mean Inter-Test Interval |
|---|---|---|---|
| Baseline | BL | 22 days | As per scenario (TF1, TF2, LOA) under consideration |
| Shortened detection window | DW-1 | 1 day | Same as BL |
| Generalized testing | GT-6 | Same as BL | 6 months |
| GT-3 | Same as BL | 3 months | |
| Individualized testing | IT-6 | Same as BL | Every 6 partners or 6 months |
| IT-3 | Same as BL | Every 3 partners or 3 months | |
| Best Case | BC | 1 day | 1 day |
We model each intervention in daily time steps over 10 years, and perform 10 independent runs of each baseline/intervention combination. Our primary outcome is the number of new infections over those 10 years. We also consider the proportionate reduction in the number of new infections achieved through intervention relative to baseline, and relative to the maximum possible (defined as the difference in the mean number of infections between baseline and the hypothetical best case model).
We hypothesize the rank of effectiveness of each of these interventions a priori, as: BC > IT-3 > IT-6 > GT-3 > GT-6 > DW-1. Because our simulations are stochastic, two scenarios with similar true impact may yield point estimates in the reverse order from logical expectations. Thus, we perform two-sample t-tests (at the 0.05-level) to make pairwise comparisons of the number of infections produced by each intervention in this sequence. The choice of t-test was based on our limited number of runs per scenario (10), which was in turn based on the computational complexity of the models; this test is conservative, and any results that are significant with this number of repetitions will remain so at larger numbers of model runs.
Results
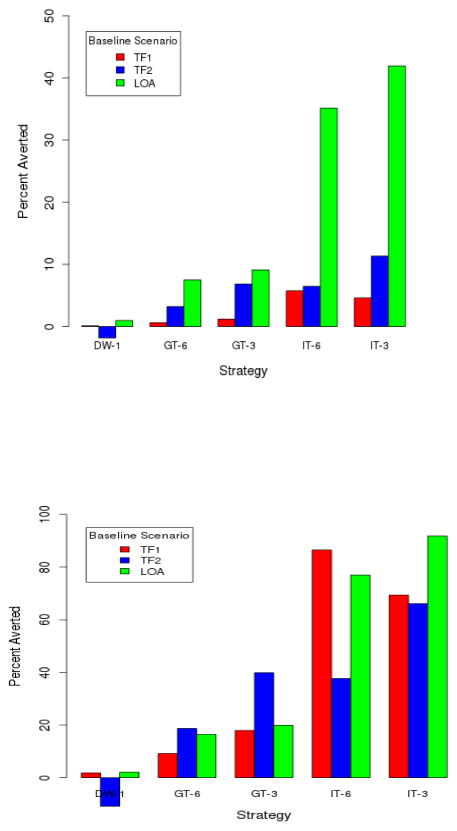
We summarize the mean number of infections for each baseline scenario, and for each intervention from each baseline in Table 5, along with the results of the two-sampled t-tests. The number of infections averted relative to baseline, and relative to the maximum possible, are in Figure 1. Since we assume a very infrequent rate of testing in the LOA model to match the empirical proportion of HIV-infected individuals who are unaware of their status, any intervention produces a larger effect in the baseline LOA scenario relative to the baseline TF1 or TF2 scenarios. Our interest is in comparing incidence between interventions within a baseline scenario.
Table 5.
Number of new infections produced with different interventions in each baseline scenario. The 95% confidence interval about the mean is computed using the t-distribution across 10 repetitions of each intervention.1 The numbers in parentheses are p-values from two-sample t-tests of sequential comparison of number of new infections between the testing interventions that appear in consecutive rows, within each testing scenario (i.e. column). The order of effectiveness of interventions was hypothesized a priori. Comparisons significant at the 0.05-level are in boldface.
| Model | TF1 | TF2 | LOA |
|---|---|---|---|
| BL | 738.9±20.1 | 790.2±22.9 | 955.3±21.1 |
|
| |||
| DW-1 | 738.0±10.2 (0.94) | 804.9±22.3 (0.41) | 944.1±25.7 (0.62) |
|
| |||
| GT-6 | 734.4±3.4 (0.52) | 764.9±8.6 (0.007) | 881.8±18.5 (0.002) |
|
| |||
| GT-3 | 730.1±11.6 (0.54) | 736.2±12.3 (0.002) | 866.6±10.3 (0.21) |
| IT-6 | 696.5±10.9 (0.001) | 739.2±14.3 0.77 |
618.2±35.5 (<0.001) |
| IT-3 | 704.9±21.3 (0.53) | 705.8±16.7 (0.01) | 553.6±15.2 (0.009) |
|
| |||
| BC | 689.9±10.1 (0.26) | 654.8±6.6 (<0.0001) | 517.9±10.8 (0.002) |
In the TF1 and TF2 scenarios, we assume a mean inter-test interval of 351 days and two years respectively, and the LOA scenario is set up to match 55–60% of infected diagnosed at equilibrium prevalence.1
Figure 1.
(Top) Number of infections prevented by each intervention relative to baseline. (Bottom) Number of infections prevented by each intervention relative to maximum possible, defined as the difference between the mean number of infections in the baseline and best case interventions. In each case, results are averaged across 10 stochastic model runs.
For all baseline scenarios, instituting a test with a 1-day detection window does not significantly reduce the number of new infections. Generalized testing every 3 months and 6 months significantly reduces the number of infections in TF2 only; thus the effectiveness of generalized interventions appears contingent upon the baseline assumptions that define each scenario. In the TF1 scenario, the IT-6 intervention performs significantly better than generalized interventions that involve more frequent testing but there is no significant difference between the IT-3 and IT-6 interventions. In the TF2 and LOA scenarios, the IT-3 intervention performs significantly better than the IT-6. Thus, one or both IT interventions are significantly more effective (as measured by incidence) in all baseline scenarios. For two of the three scenarios, the IT-6 intervention is significantly more effective than GT-3, despite less testing by most MSM in the former. This is because the impact of that reduction is more than offset by greater testing among the most highly active MSM.
Discussion
Among the implementable interventions we consider, individualized testing is most effective in preventing new infections over a 10-year period. In all scenarios, an intervention that only uses a test with the shortest possible detection window without any change in testing behavior does not make a significant difference in the number of new infections. The effectiveness of the generalized testing interventions (whether every 6 or 3 months) depends on the baseline assumptions that define the scenario. At one extreme, the TF1 scenario assumed approximately annual testing on average (and resulted in approximately 95% of infections diagnosed); at the other, the LOA scenario was set to match 55–60% of diagnoses among those infected at equilibrium (and resulted in testing on average once every 10.9 years). The truth is likely somewhere between these two extremes, and investigating these distinct baseline conditions leads us to conclude that individualized testing interventions are significantly more effective under all baseline testing assumptions.
The IT interventions account for men who have three or six partners, and those who have significantly more partners in a short period. The success of these IT interventions, however, is contingent upon continual risk assessment, and adherence to testing regimens.
While overall reductions in incidence might seem low, we must note that these interventions only consider testing and diagnosis. In practice, interventions may involve a combination of testing, prevention through antiretroviral treatment (ART), and pre-exposure prophylaxis (PrEP). Future modeling work should consider the interaction of these interventions. We only focused on testing due to its relatively low implementation cost, and because there is a public health infrastructure devoted to its delivery, especially in large urban settings with higher populations of MSM. This work helps isolate the benefits of testing and diagnosis interventions, all else being equal.
Our study has several limitations. We assume optimistically that all MSM, even high risk MSM, are equally reachable by these interventions. Implicitly we also assume that risky men will be open to testing, and will not suffer from “testing fatigue”. Our baseline models also did not consider dependence between the timing of risk behavior and testing behavior. Studies have shown that some men test after specific risk episodes [40–42]. Additionally, a test with a shorter detection window could cause men to interpret partners’ test results as more reliable, and increase levels of serosorting. This could yield a greater impact of tests with short window periods than our results suggest. We also assume that within each intervention and baseline scenario, individual-level risk behaviors do not change in the absence of an HIV diagnosis; future empirical work should focus on risk behaviors in the absence of testing and diagnosis.
Our intervention models assume that all diagnosed individuals are notified, and they modify their behavior immediately in accordance with the specified rules. Violations of this assumption are likely for non-rapid tests. Therefore, efforts to notify all diagnosed individuals quickly must be a priority. Home-testing may be helpful in increasing testing among MSM, and reduce the barrier between diagnosis and PDBC.
Additionally, our IT intervention models were restricted to non-main partners, as a computational simplification. Counting main partners also may reduce the time between tests, and increase the effectiveness of these interventions. IT interventions require MSM to account for partner numbers and time since their last test; while accounting for both is difficult, the increased use of online and app-based dating and hookup sites in MSM communities may make this process easier, especially with the development of an app that includes sexual and testing history.
Our data on PDBC are from urban Southern California, and other parameters are from a broader array of urban US settings. The relevance of these results to particular communities in the US or elsewhere depends upon the resemblance between that setting and our source data. Identifying the public health impact of individualized interventions will require continued analyses of feasibility and acceptability among MSM, and a full cost-benefit analysis to evaluate implementation challenges and goals.
Finally, we reiterate that the focus of this work is on increasing testing to further leverage the behavior change that already appears to follow HIV diagnosis for many men (i.e. stand-alone “testing-as-prevention”). We do not consider treatment-as-prevention interventions, which have been explored by other models; that work, however, does not generally consider existing PDBC in as much detail as we do. Future work should consider the synergistic interactions of the two approaches. Nevertheless, even in the absence of treatment-as-prevention, we find convincing evidence that individually tailored testing interventions could have significantly more public health impact than traditional, “one-size-fits-all” HIV testing recommendations for MSM.
Acknowledgments
This research was supported by NIH Grants R01 DA 022116, R01 AI 083060, R01 HD 068395 and R01 DA 033875. Partial support for this research came from a Eunice Kennedy Shriver National Institute of Child Health and Human Development research infrastructure Grant, R24 HD 042828, to the Center for Studies in Demography & Ecology at the University of Washington. Additional support was provided by NIH Grants P01 AI 074621, R24 AI 106039, and U01 AI 043638, and California HIV/AIDS Research Program ID01-SDSU-056. The two lead authors also acknowledge the support of the interdisciplinary graduate program in Quantitative Ecology and Resource Management (QERM), the Center for Quantitative Science (CQS), the Network Modeling Group at the University of Washington, the STATNET Development Team, and the International Clinical Research Center, Department of Global Health, University of Washington.
List of Abbreviations
- AIEDRP
Acute Infection and Early Disease Research Program
- BC
Best Case
- CDC
Centers for Disease Control and Prevention
- CASI
Computer-Assisted Self Interview
- DW-1
Detection window of 1 day
- EIA
Enzyme immuno-assay
- GT
Generalized testing
- GT-3
Generalized testing every 3 months
- Gt-6
Generalized testing every 6 months
- HIV
Human immunodeficiency virus
- ITI
Inter-test interval
- IT
Individualized testing
- IT-3
Individualized testing every 3 partners or every 3 months
- IT-6
Individualized testing every 6 partners or 6 months
- LOA
Level of Awareness
- MSM
Men who have sex with men
- NAAT
Rapid nucleid acid amplification tests
- NHBS
National HIV Behavioral Sureveillance
- PDBC
Post-diagnosis behavior change
- TF
Testing Frequency
- TF1
Testing at average interval of 351 days
- TF2
Testing at average interval of 730 days
- UAI
Unprotected anal intercourse
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wejnert C, Le B, Rose CE, Oster AM, Smith AJ, Zhu J. HIV Infection and Awareness among Men Who Have Sex with Men-20 Cities, United States, 2008 and 2011. PLoS One. 2013;8:e76878. doi: 10.1371/journal.pone.0076878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finlayson TJ, Le B, Smith A, Bowles K, Cribbin M, Miles I, et al. HIV risk, prevention, and testing behaviors among men who have sex with men--National HIV Behavioral Surveillance System, 21 U.S. cities, United States, 2008. MMWR Surveill Summ. 2011;60:1–34. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Department of Health and Human Services. HIV among Gay and Bisexual Men. 2014 May; Retrieved Sept 2, 2014 from World Wide Web http://www.cdc.gov/hiv/pdf/msm_fact_sheet_final_2014.pdf.
- 4.Centers for Disease Control and Prevention Department of Health and Human Services. . Prevalence and Awareness of HIV Infection Among Men Who Have Sex With Men - 21 cities, United States, 2008. Morb Mortal Wkly Rep. 2010;59:1201–7. [PubMed] [Google Scholar]
- 5.Department of Health and Human Services Centers for Disease Control and Prevention. Vital Signs: HIV Testing and Diagnosis Among Adults - United States, 2001–2009. Morb Mortal Wkly Rep. 2010;59:1550–5. [PubMed] [Google Scholar]
- 6.Hamlyn E, Jones V, Porter K, Fidler S. Antiretroviral treatment of primary HIV infection to reduce onward transmission. Curr Opin HIV AIDS. 2010;5:283–90. doi: 10.1097/COH.0b013e32833a6b11. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368:218–30. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorbach PM, Drumright LN, Daar ES, Little SJ. Transmission behaviors of recently HIV-infected men who have sex with men. J Acquir Immune Defic Syndr. 2006;42:80–5. doi: 10.1097/01.qai.0000196665.78497.f1. [DOI] [PubMed] [Google Scholar]
- 10.Gorbach PM, Weiss RE, Jeffries R, Javanbakht M, Drumright LN, Daar ES, et al. Behaviors of Recently HIV-Infected Men Who Have Sex With Men in the Year Postdiagnosis: Effects of Drug Use and Partner Types. J Acquir Immune Defic Syndr. 2011;56:176–82. doi: 10.1097/QAI.0b013e3181ff9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallabhaneni S, McConnell JJ, Loeb L, Hartogensis W, Hecht FM, Grant RM, et al. Changes in seroadaptive practices from before to after diagnosis of recent HIV infection among men who have sex with men. PLoS One. 2013;8:e55397. doi: 10.1371/journal.pone.0055397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golden MR, Stekler J, Hughes JP, Wood RW. HIV Serosorting in Men Who Have Sex With Men: Is It Safe? (vol 49, pg 212, 2008) Jaids-Journal Acquir Immune Defic Syndr. 2008;49:464. doi: 10.1097/QAI.0b013e31818455e8. [DOI] [PubMed] [Google Scholar]
- 13.Cassels S, Menza TW, Goodreau SA, Golden MR. HIV serosorting as a harm reduction strategy: evidence from Seattle, Washington. AIDS. 2009;23:2497–506. doi: 10.1097/QAD.0b013e328330ed8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golden MR, Dombrowski JC, Kerani RP, Stekler JD. Failure of serosorting to protect African American men who have sex with men from HIV infection. Sex Transm Dis. 2012;39:659–64. doi: 10.1097/OLQ.0b013e31825727cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen NE, Meyer JP, Bollinger R, Page KR. HIV testing behaviors among Latinos in Baltimore City. J Immigr Minor Heal. 2012;14:540–51. doi: 10.1007/s10903-012-9573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Branson BM, Stekler JD. Detection of acute HIV infection: We can’t close the window. J Infect Dis. 2012;205:521–4. doi: 10.1093/infdis/jir793. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N Engl J Med. 2011;364:1943–54. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Branson BM. State of the art for diagnosis of HIV infection. Clin Infect Dis. 2007;45 (Suppl 4):S221–225. doi: 10.1086/522541. [DOI] [PubMed] [Google Scholar]
- 19.Eshleman SH, Khaki L, Laeyendecker O, Piwowar-Manning E, Johnson-Lewis L, Husnik M, et al. Detection of individuals with acute HIV-1 infection using the ARCHITECT HIV Ag/Ab Combo assay. J Acquir Immune Defic Syndr. 2009;52:121–4. doi: 10.1097/QAI.0b013e3181ab61e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilcher CD, Christopoulos KA, Golden M. Public Health Rationale for Rapid Nucleic Acid or p24 Antigen Tests for HIV. J Infect Dis. 2010;201:S7–S15. doi: 10.1086/650393. [DOI] [PubMed] [Google Scholar]
- 21.Kelly JA, Morin SF, Remien RH, Steward WT, Higgins JA, Seal DW, et al. Lessons Learned about Behavioral Science and Acute/Early HIV Infection. The NIMH Multisite Acute HIV Infection Study: V. AIDS Behav. 2009;13:1068–74. doi: 10.1007/s10461-009-9579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’neal JD, Golden MR, Branson BM, Stekler JD. HIV Nucleic Acid Amplification Testing Versus Rapid Testing: It Is Worth the Wait. Testing Preferences of Men Who Have Sex With Men. J Acquir Immune Defic Syndr. 2012;60:e119–122. doi: 10.1097/QAI.0b013e31825aab51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helms DJ, Weinstock HS, Mahle KC, Bernstein KT, Furness BW, Kent CK, et al. HIV Testing Frequency Among Men Who Have Sex With Men Attending Sexually Transmitted Disease Clinics: Implications for HIV Prevention and Surveillance. J Acquir Immune Defic Syndr. 2009;50:320–6. doi: 10.1097/QAI.0b013e3181945f03. [DOI] [PubMed] [Google Scholar]
- 24.Khanna AS, Goodreau SM, Gorbach PM, Daar E, Little SJ. Modeling the Impact of Post-Diagnosis Behavior Change on HIV Prevalence in Southern California Men Who Have Sex with Men (MSM) AIDS Behav. 2014;18:1523–31. doi: 10.1007/s10461-013-0646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Public Health Departments in King, Pierce, and Snohomish Counties and the Washington State Department of Health. 2012 Retrieved September 2 2014 from the World Wide Web: http://www.findyourfrequency.com/. Find Your Frequency.
- 26.Wohlfeiler D, Goodreau SM, Kern D. Do we need an “Oil-Change” model of STD/HIV Testing?. National Coalition of STD Directors Annual Meeting; Albuquerque, NM. November 14, 2013. [Google Scholar]
- 27.Wohlfeiler D, Potterat JJ. Using gay men’s sexual networks to reduce sexually transmitted disease (STD)/human immunodeficiency virus (HIV) transmission. Sex Transm Dis. 2005;32:48–52. doi: 10.1097/01.olq.0000175394.81945.68. [DOI] [PubMed] [Google Scholar]
- 28.Valdiserri RO, Holtgrave DR, West GR. Promoting early HIV diagnosis and entry into care. AIDS. 1999;13:2317–30. doi: 10.1097/00002030-199912030-00003. [DOI] [PubMed] [Google Scholar]
- 29.Powers KA, Ghani AC, Miller WC, Hoffman IF, Pettifor AE, Kamanga G, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–68. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnighausen T, Becker S, Bendavid E, Bershteyn A, Blandford J, Boily MC, et al. HIV treatment as prevention: models, data, and questions--towards evidence-based decision-making. PLoS Med. 2012;9:e1001259. doi: 10.1371/journal.pmed.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delva W, Eaton JW, Meng F, Fraser C, White RG, Vickerman P, et al. HIV treatment as prevention: optimising the impact of expanded HIV treatment programmes. PLoS Med. 2012;9:e1001258. doi: 10.1371/journal.pmed.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eaton JW, Johnson LF, Salomon JA, Barnighausen T, Bendavid E, Bershteyn A, et al. HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa. PLoS Med. 2012;9:e1001245. doi: 10.1371/journal.pmed.1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cremin I, Alsallaq R, Dybul M, Piot P, Garnett G, Hallett TB. The new role of antiretrovirals in combination HIV prevention: a mathematical modelling analysis. AIDS. 2013;27:447–58. doi: 10.1097/QAD.0b013e32835ca2dd. [DOI] [PubMed] [Google Scholar]
- 34.Goodreau SM, Carnegie NB, Vittinghoff E, Lama JR, Sanchez J, Grinsztejn B, et al. What Drives the US and Peruvian HIV Epidemics in Men Who Have Sex with Men (MSM)? PLoS One. 2012;7:e50522. doi: 10.1371/journal.pone.0050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris M, Kurth AE, Hamilton DT, Moody J, Wakefield S. Concurrent Partnerships and HIV Prevalence Disparities by Race: Linking Science and Public Health Practice. Am J Public Health. 2009;99:1023–31. doi: 10.2105/AJPH.2008.147835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodreau SM, Cassels S, Kasprzyk D, Montano DE, Greek A, Morris M. Concurrent Partnerships, Acute Infection and HIV Epidemic Dynamics Among Young Adults in Zimbabwe. AIDS Behav. 2012;16:312–22. doi: 10.1007/s10461-010-9858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorbach PM, Holmes KK. Transmission of STIs/HIV at the partnership level: beyond individual-level analyses. J Urban Heal. 2003;80:15–25. doi: 10.1093/jurban/jtg079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swindells S, Cobos DG, Lee N, Lien EA, Fitzgerald AP, Pauls JS, et al. Racial/ethnic differences in CD4 T cell count and viral load at presentation for medical care and in follow-up after HIV-1 infection. AIDS. 2002;16:1832–4. doi: 10.1097/00002030-200209060-00020. [DOI] [PubMed] [Google Scholar]
- 39.Moreira RI, Luz PM, Struchiner CJ, Morgado M, Veloso VG, Keruly JC, et al. Immune status at presentation for HIV clinical care in Rio de Janeiro and Baltimore. J Acquir Immune Defic Syndr. 2011;57 (Suppl 3):S171–178. doi: 10.1097/QAI.0b013e31821e9d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White EW, Lumley T, Goodreau SM, Goldbaum G, Hawes SE. Stochastic models to demonstrate the effect of motivated testing on HIV incidence estimates using the serological testing algorithm for recent HIV seroconversion (STARHS) Sex Transm Infect. 2010;86:506–11. doi: 10.1136/sti.2009.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White E, Goldbaum G, Goodreau S, Lumley T, Hawes SE. Interpopulation variation in HIV testing promptness may introduce bias in HIV incidence estimates using the serologic testing algorithm for recent HIV seroconversion. Sex Transm Infect. 2010;86:254–7. doi: 10.1136/sti.2009.037291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Remis RS, Palmer RW. Testing bias in calculating HIV incidence from the Serologic Testing Algorithm for Recent HIV Seroconversion. AIDS. 2009;23:493–503. doi: 10.1097/QAD.0b013e328323ad5f. [DOI] [PubMed] [Google Scholar]