Abstract
The acute respiratory distress syndrome (ARDS) remains a major cause of morbidity and mortality in critically ill patients. Over the past several decades, alcohol abuse and cigarette smoke exposure have been identified as risk factors for the development of ARDS. The mechanisms underlying these relationships are complex and remain under investigation but are thought to involve pulmonary immune impairment as well as alveolar epithelial and endothelial dysfunction. This review summarizes the epidemiologic data supporting links between these exposures and ARDS susceptibility and outcomes and highlights key mechanistic investigations that provide insight into the pathways by which each exposure is linked to ARDS.
Keywords: Acute respiratory distress syndrome, Epidemiology, Modifiable risk factors, Alcohol abuse Cigarette smoking, Mechanisms, Future Interventions
The acute respiratory distress syndrome (ARDS) represents a significant health burden. Despite numerous efforts to identify effective treatments, few have been successful. As a result, considerable attention has now been given to the prevention of ARDS. Although many patients present with risk factors for ARDS, only a certain subset of these patients go on to develop it. While some of this phenomenon is likely explained by genetic factors, recent research has revealed that modifiable risk factors for ARDS exist as well. Alcohol use was the first major modifiable risk factor for ARDS to be identified. Significant details have since emerged over the past two decades about the mechanisms that underlie this relationship. These discoveries have spurred the search for additional risk factors. Further investigation has revealed smoking as an additional risk factor for ARDS. Although the data for this second association are newer and less developed, both of these relationships represent exciting discoveries in the quest to better understand, prevent and treat ARDS.
Alcohol Abuse
Alcohol is one of the most commonly used and abused drugs worldwide. In the United States, nearly 20 million adults annually meet the criteria for alcohol abuse or dependence.1,2 Alcohol is known to have numerous systemic health effects, including on the liver and central nervous system.3 From a respiratory standpoint, alcohol abuse has long been associated with an increased risk of pneumonia.4,5 More recently, alcohol abuse has been strongly linked in epidemiologic studies to development of ARDS in at-risk patients.
The first demonstration of an association between chronic alcohol abuse and ARDS was made by Moss et al, who retrospectively examined 351 patients at risk for ARDS.6 In this cohort, 43% of patients who chronically abused alcohol developed ARDS compared to only 22% of those who did not abuse alcohol, with the effect most pronounced in patients with sepsis. This study was limited by its retrospective design, particularly since this design required that alcohol use history be obtained by chart review and documented history; furthermore, this study did not adjust for concomitant cigarette smoking. Encouraged by these findings, Moss et al conducted a multicenter prospective study of 220 patients with septic shock to further assess this relationship. Methodologically, this study improved on its predecessor by using the Short Michigan Alcohol Screening Test (SMAST), which has previously been validated as a screening test for chronic alcohol abuse.7 A multivariate analysis again found that those who chronically abused alcohol developed ARDS more frequently than those who did not, 70% vs 31%, respectively.8 These two key studies thus served as the first major evidence that alcohol use was a risk factor for the development of ARDS.
Several studies have since reinforced the relationship between alcohol use and ARDS. Licker et al examined the incidence of ARDS in 879 non-small cell lung cancer patients undergoing thoracic surgery.9 Multivariate logistic regression found that preoperative chronic alcohol consumption was associated with increased odds of developing acute lung injury. In addition, two studies examining risk factors for transfusion-related acute lung injury (TRALI) found that chronic alcohol consumption was associated with the development of TRALI.10,11 Gajic et al found that patients who developed TRALI were more likely to be chronic alcohol users when compared to matched controls, 36.5% vs 17.6% respectively. More recently, Toy et al found that in a multivariate model, chronic alcohol use in patients receiving blood product transfusions significantly increased the odds of developing TRALI. A later study by Gajic et al that evaluated patients 5584 patients at risk for ARDS to determine a lung injury prediction score found alcohol to be a positive risk factor for the development of ARDS.12 These studies thus supported the prior observations and solidified the association between chronic alcohol use and ARDS (Table 1).
Table 1.
Studies evaluating the relationship between ARDS and alcohol use
| Author | Year | Study Size | Odds Ratio (history of alcohol abuse vs no abuse) |
P value |
|---|---|---|---|---|
| Moss et al6 | 1996 | 351 | 1.98* | < 0.001 |
| Moss et al8 | 2003 | 220 | 3.70 | < 0.001 |
| Licker et al9 | 2003 | 879 | 1.87 | 0.012 |
| Gajic et al10 | 2007 | 148 | ** | 0.006 |
| Gajic et al12 | 2011 | 5584 | ¶ | 0.028 |
| Toy et al11 | 2012 | 253 | 5.90 | 0.028 |
Relative Risk
No odds ratio or relative risk reported. 27 of 74 patients with acute lung injury had a history of alcohol abuse vs. 13 of 74 in matched controls.
No odds ratio or relative risk reported. 44 of 377 patients with acute lung injury had a history of alcohol abuse vs. 289 of 5,207 in patients without acute lung injury.
Although the relationship between chronic alcohol abuse and ARDS has been demonstrated numerous times, the effect of alcohol on ARDS outcomes has been less clear. Early studies that examined this relationship showed conflicting results. In a retrospective study, Moss et al found that amongst patients who developed ARDS, those with a history of chronic alcohol abuse had a significantly higher in-hospital mortality rate compared to those that did not abuse alcohol, 65% vs 36% respectively.6 However, a follow-up prospective study that used a more validated measure of alcohol abuse did not demonstrate any difference in mortality in ARDS patients when stratified by a history of alcohol abuse.8
In order to better evaluate the effect of alcohol use on ARDS outcomes, Clark et al performed a secondary analysis of patients enrolled in 3 ARDSnet trials: ALTA, EDEN and OMEGA, which examined the effects of aerosolized albuterol, omega-3 fatty acid supplementation and early vs delayed parenteral nutrition, respectively, in ARDS patients. Of note, all three studies were stopped early for futility. Participants enrolled in these trials (or their surrogates) completed the Alcohol Use Disorder Identification Test (AUDIT), a previously validated questionnaire 13 developed by the World Health Organization to stratify patients by level of alcohol consumption. In all, 1037 patients, representing 92% of all enrolled patients, had a completed AUDIT and were included in the secondary analysis performed by Clark et al. A multivariate analysis that adjusted for age, gender, severity of illness, history of smoking, ALI risk factor and baseline comorbidities found that a history of severe alcohol misuse was associated with an increased risk of death or persistent hospitalization at 90 days (OR = 1.78) compared to those with mild alcohol use. The authors used mild alcohol users rather than non-drinkers as the reference group since non-drinkers had poorer outcomes, thought to be due to comorbidities that discourage the consumption of alcohol. Thus, it appears likely that chronic alcohol abuse is associated with poor ARDS outcomes, though the data is less extensive for this association than for the association with susceptibility.
Mechanisms
Numerous studies have been performed both in animal models and humans in order to better understand the association between chronic alcohol use and ARDS. These studies have identified a central role for pulmonary immune dysfunction as well as alveolar epithelial dysfunction in the mechanistic link between alcohol and ARDS.
Pulmonary immune dysfunction
Both acute and chronic alcohol use can contribute to a dysfunctional pulmonary immune response. Acute alcohol use impairs neutrophil chemotaxis and function with subsequent decreased phagocytosis and bacterial killing.14–16 Chronic alcohol use is similarly associated with altered neutrophil function and decreased superoxide production.17 Interestingly, chronic alcohol use decreases levels of granulocyte/macrophage colony stimulating factor (GM-CSF) receptor and signaling in lung epithelium,18,19 which has been shown to result in defective alveolar macrophage maturation.20 The net effect of these abnormalities is an increased pulmonary bacterial burden.
In addition to its effects on neutrophils, alcohol use has a variety of effects on cytokine production in the lung. While acute alcohol use has been shown to impair production of proinflammatory cytokines such as TNF-α and IL-1β,21 which may predispose patients to pneumonia, chronic alcohol use has actually been associated with increased levels of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, in both human and animal studies.22–24 Recently, Burnham et al found elevated levels of CCL-5 (also known as RANTES), which is a chemoattractant for a variety of immune cells,25 in the BAL fluid of chronic alcoholics.26 This increase in inflammatory cytokines appears to have a significant effect on downstream inflammation, as IL-6 was recently shown to play a key role in the pulmonary inflammatory response of alcoholic mice in a burn injury model.25–27 This altered cytokine profile in conjunction with decreased neutrophil and alveolar macrophage function is thought to contribute to the development of ARDS in alcohol abusers.
Alveolar Epithelial Dysfunction
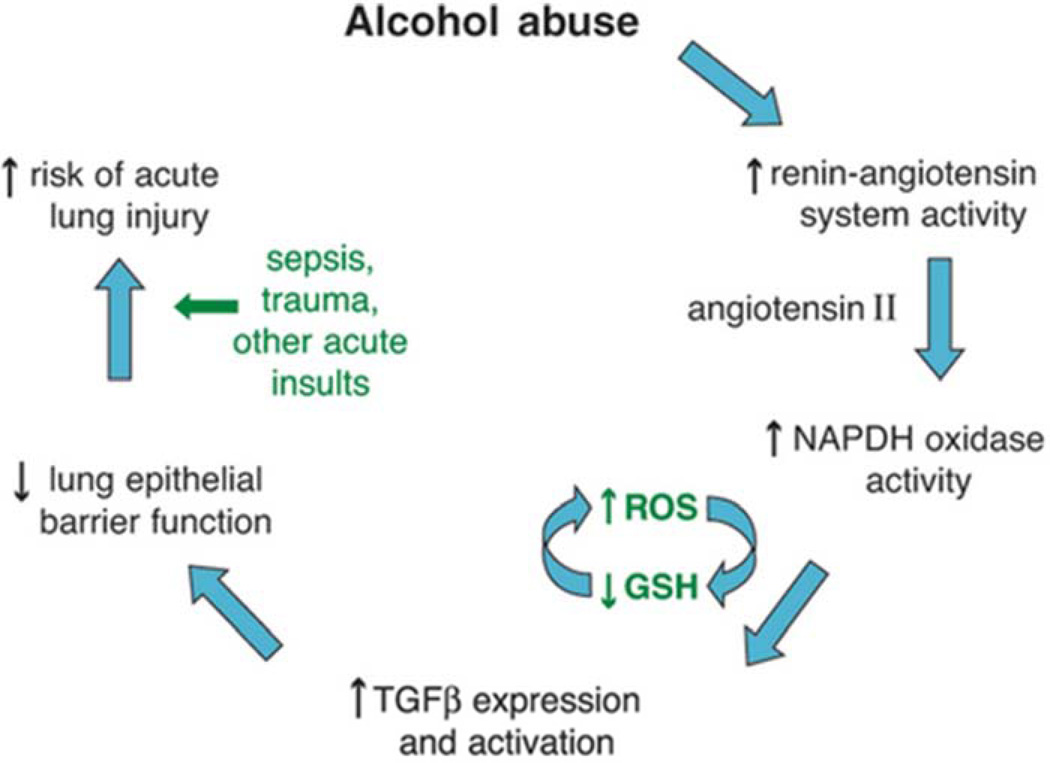
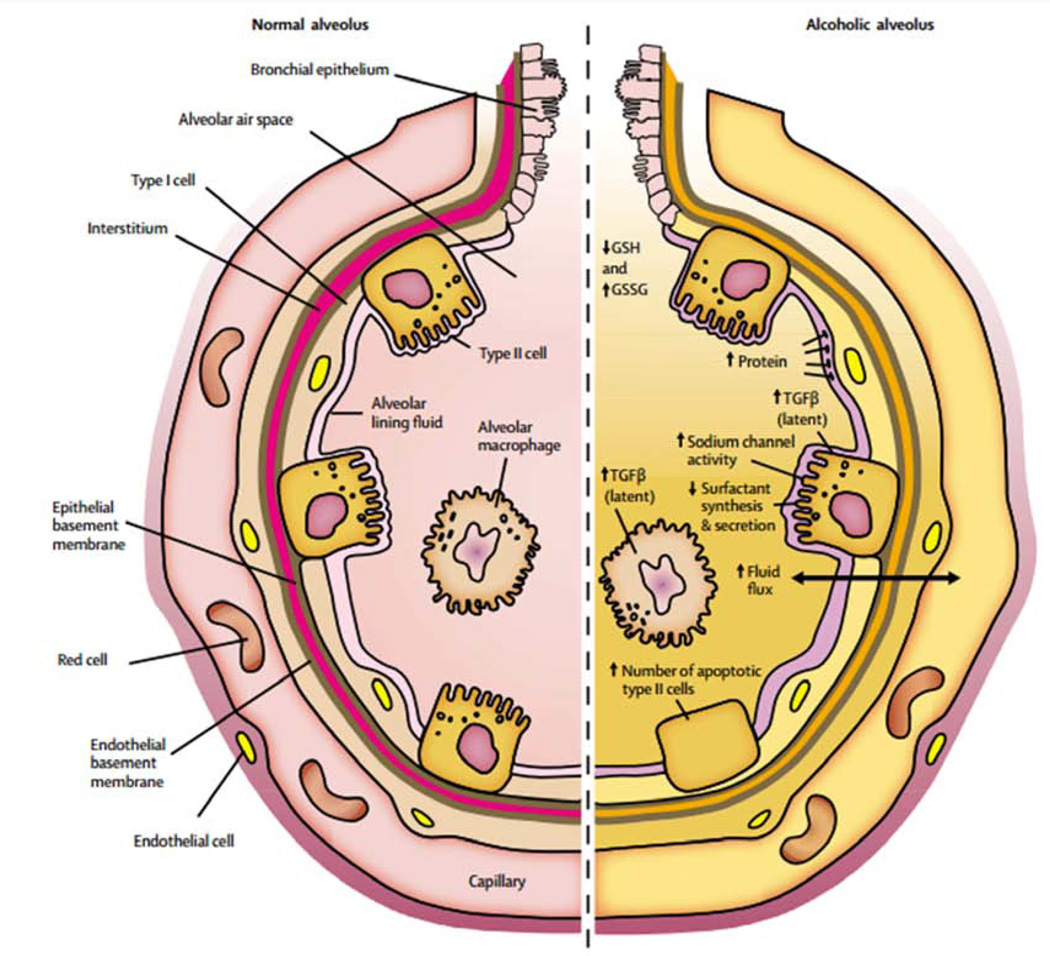
In addition to its effects on the lung inflammatory response, chronic alcohol use may also predispose to ARDS development by causing increased pulmonary oxidative stress and alveolar epithelial dysfunction. These effects are mediated in part via the renin-angiotensin system (Figure 1). Chronic alcohol use has long been known to increase activation of this system, resulting in elevated levels of angiotensin II in humans.28,29 Angiotensin II may contribute to alveolar epithelial dysfunction through a variety of mechanisms, including via systemic effects on vascular tone and fluid retention as well as via localized effects such as promoting apoptosis of alveolar epithelial cells.30 In addition, angiotensin II activates NADPH oxidase in the lung, resulting in elevated levels of reactive oxygen species.31,32 This increase in reactive oxygen species results in depletion in alveolar levels of the key antioxidant glutathione (GSH) and increases in alveolar oxidized glutathione (GSSG), a phenomenon seen both in animal models 33 and humans who abuse alcohol.34 Interestingly, patients with ARDS have been shown to demonstrate the same derangement with regards to pulmonary glutathione.35,36 This alteration in glutathione results in decreased antioxidant capacity in the lung and has further been linked to decreased surfactant synthesis37,38 and increased type II cell apoptosis.39 Additionally, the depletion of glutathione appears to increase levels of latent TGF-β, which subsequently contributes to baseline alveolar epithelial dysfunction, manifested by increased permeability and lung edema.40 The net result of increased activation of this pathway is an alveolar epithelium that is already dysfunctional and thus primed for developing ARDS when faced with an acute insult (Figure 2).
Figure 1.
Potential mechanism by which alcohol primes the lung for Acute Respiratory Distress Syndrome. From Kershaw et al, Alcoholic Lung Disease. Alcohol Res Health. 2008 Sep;31(1):66–75; with permission.
Figure 2.
Baseline dysfunction in the alcoholic alveolus.
From The Lancet, Vol. 368, Marc Moss & Ellen Burnham, Alcohol abuse in the critically ill patient, 2231-42, 2006, with permission.
Future Interventions
The high prevalence of alcohol abuse worldwide and its association with ARDS present a unique opportunity to improve patient outcomes. While decreasing the prevalence of alcohol abuse remains an important goal, in addition, the unique mechanistic abnormalities involved in this relationship provide several potentially exciting therapeutic targets for alcoholic patients either at risk for or with ARDS.
One potential therapeutic intervention would be to attempt to increase glutathione levels, which play such a key role in the alveolar epithelial dysfunction observed with alcohol use. The use of N-acetyl-cysteine (NAC), a glutathione precursor, is one potential approach. In both endotoxin and microembolism rat models of ARDS, pretreatment with IV NAC attenuated lung injury.41,42 Prior small clinical trials have shown that administration of IV NAC increases glutathione levels in patients with ARDS, although no significant improvement in outcomes was observed (ventilator free days or mortality).43–45 However, these studies included small heterogeneous samples of patients with ARDS, rather than focusing on only those with a history of alcohol abuse. It remains unclear whether administration of glutathione would be a successful therapeutic strategy in alcoholic patients either at risk for or who have already developed ARDS.
Given the role angiotensin II seems to play in altering glutathione levels, the renin-angiotensin system also presents a potential therapeutic target for alcoholic patients with ARDS. Interestingly, some data suggests that angiotensin converting enzyme (ACE) polymorphisms that increase angiotensin II levels may affect the risk of developing ARDS and mortality. Marshall et al found that an ACE genotype that causes increased ACE activity was more prevalent in Caucasian ARDS patients compared with other ICU patients or the general population.46 Furthermore, amongst patients with ARDS, this genotype was associated with increased mortality. A later study by Villar et al in Spanish patients did not find a similar relationship.47 However, a recent meta-analysis by Matsuda et al of nearly 5000 patients with ARDS, including Caucasian and Asian ethnicities, found that ACE genotypes associated with increased activity were associated with an increased risk of mortality from ARDS in Asian populations.48 These findings, in conjunction with studies in mice that show decreased ACE activity to be protective in animal models of acid aspiration and sepsis-induced ARDS,49 make the renin-angiotensin system an intriguing therapeutic target. To date, there has been no formal evaluation of the role of ACE-Inhibitors (ACE-I) or Angiotensin-Receptor Blockers (ARB) in ARDS patients, including alcoholics. Although the utility of these agents may be limited because patients with ARDS also often have shock or renal failure, further studies would be required to determine any potential benefits.
The GM-CSF depleted state that is induced by chronic alcohol use also serves as a potential therapeutic target for patients with ARDS. Treatment of alcohol fed rats with GM-CSF has been shown to improve not only alveolar macrophage function,19 but also decreased alveolar permeability and increased lung edema fluid clearance.50 Likewise, elevated levels of bronchoalveolar lavage fluid GM-CSF are associated with improved mortality in patients with ARDS.51 A Phase II trial that randomized ARDS patients to GM-CSF vs placebo showed improved oxygenation with no adverse effects.52 However, this study as well as a larger Phase II randomized clinical trial of GM-CSF vs placebo found no improvement in outcomes such as ventilator free days, organ failure-free days or mortality.52,53 Whether GM-CSF would improve outcomes in ARDS patients with a history of chronic alcohol use remains unknown.
Smoking
Smoking remains a global epidemic. While anti-smoking efforts in the United States continue to slowly decrease the rate of smoking amongst adults (currently 18.1%),54 tobacco use continues to be the leading cause of preventable death both in the US and worldwide, killing nearly 6 million people annually.55 Although many harmful effects of smoking, particularly on the lung, have been known for quite some time, the link between ARDS and smoking has been established only recently.
Early studies investing the relationship between smoking history and ARDS suggested a possible association, though the findings were inconsistent. Christenson et al studied nearly 4000 patients undergoing cardiac surgery and found in multivariable analysis that a clinical history of being an active smoker was associated with an increased risk of developing ARDS.56 However, this study did not address or adjust for alcohol use. A later study by Iribarren et al retrospectively studied a large cohort of patients in a single health plan network, 56 of whom went on to develop ARDS.57 Multivariate analysis showed that a history of active cigarette smoking was associated with increased odds of developing ARDS. Interestingly, increased amounts of smoking (> 20 cigarettes per day) were associated with an even greater odds of developing ARDS. While this study did adjust for chronic alcohol use amongst patients, it was limited by its retrospective nature and the use of diagnostic coding, which detects a low prevalence of ARDS. In contrast to these positive studies, a multicenter observational study by Gajic et al of 5584 patients at risk for ARDS did not find cigarette smoking to be a predictive risk factor for developing ARDS.12 The conflicting findings of these studies may be due in large part to reliance on smoking history. Recent studies have determined that biomarkers of tobacco use, such as plasma cotinine, are significantly more sensitive for tobacco exposure in critically ill patients compared to self-reported histories.58
To further investigate this possible association, Calfee et al prospectively measured plasma cotinine levels in blunt trauma patients at risk for ARDS. Additionally, alcohol exposure was measured by both clinical history and AUDIT surveys. Increasing levels of plasma cotinine were associated with an increased risk of developing ARDS. Interestingly, in a multivariate model, including adjustments for alcohol use, both active smoking as well as moderate to severe passive smoke exposure predicted the development of ARDS.59 These findings were the first to link smoking to ARDS using biomarkers and also to identify secondhand smoke as a potential risk factor for ARDS development. If confirmed, these findings may have important public health implications, particularly with regards to public smoking bans. Despite the strengths of this study, its findings were limited by its homogenous study population, all of whom were victims of severe blunt trauma enrolled at a single center.
Since then, studies in different patient populations have provided additional evidence in support of an association between smoking and ARDS. Toy et al found that active smoking was associated with an increased risk of TRALI, after adjustment for other predictors.11 Likewise, Diamond et al conducted a multicenter study of 1255 lung transplant patients to identify risk factors for primary graft dysfunction (PGD), a form of acute lung injury that occurs within 72 hours of lung transplant.60 In this analysis, donor smoking was associated with increased odds of developing PGD, a finding that was robust to adjustment for other predictors. These studies add to the growing body of literature that supports an association between smoking and ARDS (Table 2).
Table 2.
Studies examining the relationship between smoking and ARDS
| Author | Year | Study Size | Odds Ratio (active smokers vs nonsmokers) |
P value |
|---|---|---|---|---|
| Christenson et al56 | 1996 | 3,848 | 2.01* | < 0.001 |
| Iribarren et al57 | 2000 | 121,012 | 2.85 (< 1 pack/day)* 4.59 (≥ 1 pack/day)* |
< 0.05 <0.05 |
| Gajic et al12 | 2011 | 5,584 | ** | NS |
| Calfee et al59 | 2011 | 144 | 2.77 | 0.01 |
| Toy et al11 | 2012 | 253 | 3.40 | 0.02 |
| Diamond et al60 | 2013 | 1,255 | 1.80 | 0.002 |
Relative Risk
No odds ratio or relative risk reported. 107 of 377 patients with acute lung injury had a history of active smoking vs. 1,239 of 5,207 in patients without acute lung injury
There are limited data on the outcomes of smokers who develop ARDS. One small study examined 47 patients with ARDS and found that non-survivors were more likely to be smokers than survivors.61 A recent study by Hsieh et al sought to better evaluate this question using 381 patients with ARDS from the ALTA and OMEGA ARDS Network randomized controlled trials.62 Urine NNAL (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol), a well validated biomarker of tobacco use with a 2 week half-life,63 was used to stratify patients by smoking exposure status. Although active smokers were found to be younger, with significantly lower severity of illness scores and fewer comorbidities, they had a similar severity of lung injury as measured by either the Berlin Definition or Murray Lung Injury Score, raising the possibility that smokers may be more prone to developing ARDS with a lower severity of illness. While smoking was associated with lower mortality in unadjusted analysis, a multivariate analysis that controlled for the disparities in age, comorbidities and severity of illness between smokers and nonsmokers showed no association between smoking status and 60 day mortality.
Mechanism
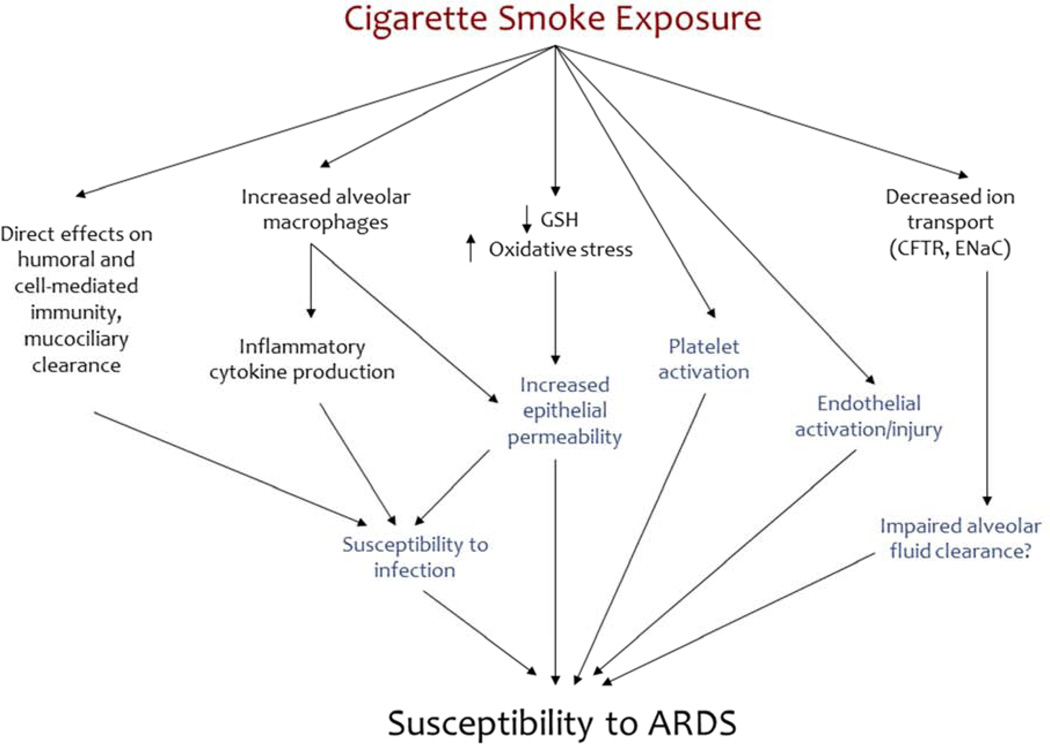
The mechanisms through which smoking contributes to the development of ARDS remain under investigation (Figure 3). In contrast to alcohol, there are relatively few lab-based studies explicitly evaluating the relationship between smoking and ARDS; thus, inferences about the potential mechanisms of association between smoking and ARDS must largely be extrapolated from studies on smoking’s effects on the lungs in other experimental settings. With this caveat, the mechanisms linking smoking and ARDS likely involve pulmonary immune dysfunction (as with chronic alcohol use) as well as dysfunction of both the alveolar epithelium and endothelium.
Figure 3.
Mechanisms through which smoking may prime the lung for ARDS
Pulmonary Immune Dysfunction
Smoking impairs pulmonary immune function though a variety of pathways. Smoking has numerous direct effects on innate and adaptive immunity that increase the risk of infection.64 These effects include impaired mucociliary function, decreased surfactant production, altered T cell responses, depressed NK cell function and decreased immunoglobulin levels.65,66 Additionally, cigarette smoke has been shown to lower the rate of bacterial clearance by alveolar macrophages.67–69 This decrease in bacterial clearance in turn is thought to result in an influx of neutrophils into surrounding tissues, with an associated increase in proinflammatory cytokines and an elevated proteolytic burden.67 Furthermore, smoking promotes biofilm formation,70 which plays a role in the increased risk of respiratory infection in smokers.71,72 This impairment in immunity and predisposition to infection is one potential mechanism by which smoking may increase the risk of ARDS.
Alveolar Epithelial Dysfunction
Since the 1980s, studies have demonstrated increased alveolar epithelial permeability in smokers compared to non-smokers,73 mimicking a key pathophysiologic feature of ARDS. This effect on alveolar permeability may be related to the neutrophil influx observed with smoking, though studies on this mechanistic link have reported conflicting findings. Animal studies by Bhalla et al found that reducing pulmonary neutrophils improved alveolar permeability.74 However, in similar animal studies, Kleeberger et al found that reducing this neutrophil influx did not attenuate epithelial permeability.75 A more recent study by Li et al also found that neutrophil depletion did not improve epithelial permeability,76 suggesting that other mechanisms must be playing a role as well.
Since then, several studies have shown that the profound oxidant effect of smoking77 may be one of the major contributors to the alveolar epithelial dysfunction seen in smokers. Li et al demonstrated that intratracheal inhalation of cigarette smoke in rats resulted in decreased levels of total BAL fluid glutathione with increases in the oxidized form, GSSG.76 In animal models, this phenomenon has been linked to increases in alveolar epithelial permeability, while increasing intracellular glutathione has been shown to ameliorate this effect.78 These findings are remarkably similar to those seen in the setting of alcohol abuse, although the timing of the effects are different: specifically, pulmonary glutathione depletion in alcohol users appears to be more of a chronic phenomenon, while in animal models of smoking, the effect is acute, lasting only 6 hours. In an attempt to replicate these findings in humans, Morrison et al performed lung scans to measure alveolar permeability in human subjects. They found that chronic smokers had increased alveolar permeability compared to non-smokers, and that permeability increased even further after chronic smokers acutely smoked a cigarette.79 However, unlike in animal models, this study did not find any statistically significant difference in BAL fluid glutathione levels between smokers or nonsmokers, suggesting that other mechanisms likely contribute to this phenomenon. Recent evidence shows that cigarette smoke likely disrupts tight junction integrity through an epidermal growth factor receptor (EGFR) pathway,80,81 which could help explain the increased alveolar permeability seen in smokers. Additionally, cigarette smoke decreases the expression of the primary ion channels responsible for resolving alveolar edema,82,83 which likely further contributes to baseline epithelial dysfunction.
Endothelial and Platelet Dysfunction
The damage caused by cigarette smoke on the lungs is not limited to the alveolar epithelium. Smoking also causes vascular endothelial injury and alters endothelial function, a key pathophysiologic change that is also seen in ARDS. Early animal models demonstrated that cigarette smoke increases pulmonary endothelial permeability.84 Since then, further study has confirmed that cigarette smoke increases lung vascular permeability and worsens LPS-induced lung edema.85,86 Interestingly, the increased endothelial permeability observed with exposure to cigarette smoke seems to be at least in part mediated by increased levels of reactive oxygen species in the lung and furthermore is attenuated by NAC.85,86 This increase in ROS seems to have a number of downstream targets including inhibition of Rho A85 and activation of mitogen-activated protein kinases (MAPK)86 that ultimately result in changes to the cytoskeleton resulting in endothelial barrier dysfunction. It is notable that reactive oxygen species seem to play a key role in both the epithelial and endothelial dysfunction caused by smoking.
Like endothelial dysfunction, to which it is closely linked, platelet dysfunction has long been noted to be a characteristic feature of ARDS.87,88 Patients with ARDS have been observed to have increased procoagulant and decreased fibrinolytic activity in the alveolar lining layer and microvasculature.89 These abnormalities promote pulmonary fibrin deposition90 and can result in microthrombi in small vessels, as pulmonary arterial thrombi and distal filling defects of the microvasculature have been detected in patients with ARDS.91 These factors likely contribute significantly to gas exchange abnormalities seen in patients with ARDS.87 Cigarette smoke has been noted to have similar effects on platelets. Both active and passive smoking have been observed to increase platelet activation, predisposing to thrombus.92 Additionally, platelet activation also results in damage to the endothelium, which can result in vasoconstriction, further prothrombotic and proinflammatory states and cell proliferation in the vessel walls.92 Interestingly, the effects of second hand smoke on endothelial and platelet dysfunction are approximately 90% of those of active smoking.92 Thus, the effects of cigarette smoking on platelets likely plays a key role in contributing to endothelial dysfunction, which may further predispose smokers to ARDS.
Future Directions
Because the mechanistic links between smoking and ARDS are less well-defined than those between alcohol and ARDS, the identification of potential targeted therapies is more challenging. One potential area of intervention is the increased oxidative stress caused by cigarettes, which seems to play a key role in both the alveolar epithelial and endothelial abnormalities associated with smoking. Given that chronic alcohol use seems to further affect the antioxidant system and frequently co-exists with cigarette smoking in patients, the antioxidant system becomes an even more exciting source for potential intervention. As mentioned previously, studies that examined the use of NAC to replenish the antioxidant system did not show any improvement in outcomes in patients with ARDS. However, this specific population, which may have decreased antioxidant function at baseline, may merit further study. Furthermore, it may be useful to assess the effect of treating this population with NAC while they are at risk for lung injury, as opposed to afterwards once the inflammatory response is well-established. Further investigation is clearly needed to better understand the relationship between smoking and ARDS in order to identify additional potential therapeutic targets. Meanwhile, continued public health interventions, such as anti-smoking campaigns and public smoking bans, may help decrease the burden of smoking associated ARDS.
Air Pollution
Air pollution has been associated with a variety of adverse health outcomes, including all-cause mortality.93 This phenomenon is thought to be driven primarily by an increase in cardiorespiratory events. Several epidemiologic studies have shown that air pollution is associated with an increased risk of myocardial infarction and cardiovascular disease mortality.94–97 The association between air pollution and respiratory mortality is less clear, with some studies showing an increase in respiratory mortality,93,98,99 while other studies have found no such relationship.100–102 Although the association between air pollution and respiratory mortality is not entirely clear, air pollution has been associated with respiratory morbidities including increased susceptibility to airway infection103 and decreased lung function.104 However, there are no epidemiologic studies that examine the relationship between air pollution and ARDS.
Despite the lack of epidemiologic studies involving a possible association between air pollution and ARDS, there are several reasons to hypothesize that such a relationship may exist. First, cigarette smoke and ambient air pollution share many of the same compounds, such as ozone and particulate matter < 2.5 µm (PM2.5). Given that cigarette smoke has previously been shown to be a risk factor for ARDS,11,56,57,59,60 it is plausible that air pollution may pose a similar risk. Second, air pollution and its constituents have been associated with changes in the lung that mimic those of ARDS. Studies in humans demonstrate that air pollution is associated with increased pulmonary inflammation, oxidative stress105 and endothelial dysfunction106 while ozone exposure has been associated with increased epithelial permeability.107 These findings suggest that like cigarette smoke, air pollution may prime the lung to develop ARDS by causing increased baseline inflammation as well as epithelial and endothelial dysfunction. However, additional studies are needed to better examine the potential relationship between air pollution and ARDS in humans.
Conclusion
Significant progress has been made since the search for environmental risk factors for ARDS began nearly two decades ago. Chronic alcohol use and smoking have been identified in numerous studies to independently increase the risk of developing of ARDS and potentially affect the outcomes of patients who go on to develop the disease. These findings have important implications for public health and for ARDS prevention. Additionally, scientific studies have yielded tremendous insight into many of the mechanisms involved in the relationship between chronic alcohol use and ARDS, and numerous potential viable therapeutic targets have been identified that may enable clinicians to better treat chronic alcohol users with ARDS. Mechanistic studies into the relationship between smoking and ARDS are less developed and represent an important area for future investigation. Future studies are also needed to define the overlap or potential synergy between these two exposures, since smoking and alcohol use often coexist in patients. Finally, further epidemiologic study is needed to determine if there are additional environmental factors, such as air pollution, that may also be associated with an increased risk of developing ARDS.
KEY POINTS.
Multiple observational studies have demonstrated that chronic alcohol use is a risk factor for the development of ARDS.
Alcohol use may promote development of ARDS via increased angiotensin II, producing increasing oxidative stress which creates baseline alveolar epithelial dysfunction and primes the lung for developing non-cardiogenic pulmonary edema.
Although less studied than alcohol use, cigarette smoke exposure also appears likely to be a risk factor for ARDS.
Cigarette smoke may prime the lung to develop ARDS by creating baseline epithelial and endothelial injury, likely through direct exposure to powerful oxidants contained in cigarettes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug and alcohol dependence. 2004 Jun 11;74(3):223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. Journal of studies on alcohol. 1997 Sep;58(5):464–473. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- 3.Moss M, Burnham EL. Alcohol abuse in the critically ill patient. Lancet. 2006 Dec 23;368(9554):2231–2242. doi: 10.1016/S0140-6736(06)69490-7. [DOI] [PubMed] [Google Scholar]
- 4.Almirall J, Bolibar I, Serra-Prat M, et al. New evidence of risk factors for community-acquired pneumonia: a population-based study. The European respiratory journal. 2008 Jun;31(6):1274–1284. doi: 10.1183/09031936.00095807. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Sola J, Junque A, Estruch R, Monforte R, Torres A, Urbano-Marquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Archives of internal medicine. 1995 Aug 7–21;155(15):1649–1654. doi: 10.1001/archinte.1995.00430150137014. [DOI] [PubMed] [Google Scholar]
- 6.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA : the journal of the American Medical Association. 1996 Jan 3;275(1):50–54. [PubMed] [Google Scholar]
- 7.Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) Journal of studies on alcohol. 1975 Jan;36(1):117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- 8.Moss M, Parsons PE, Steinberg KP, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Critical care medicine. 2003 Mar;31(3):869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 9.Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesthesia and analgesia. 2003 Dec;97(6):1558–1565. doi: 10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- 10.Gajic O, Rana R, Winters JL, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. American journal of respiratory and critical care medicine. 2007 Nov 1;176(9):886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012 Feb 16;119(7):1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. American journal of respiratory and critical care medicine. 2011 Feb 15;183(4):462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donovan DM, Dunn CW, Rivara FP, Jurkovich GJ, Ries RR, Gentilello LM. Comparison of trauma center patient self-reports and proxy reports on the Alcohol Use Identification Test (AUDIT) The Journal of trauma. 2004 Apr;56(4):873–882. doi: 10.1097/01.ta.0000086650.27490.4b. [DOI] [PubMed] [Google Scholar]
- 14.Boe DM, Nelson S, Zhang P, Bagby GJ. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. The Journal of infectious diseases. 2001 Nov 1;184(9):1134–1142. doi: 10.1086/323661. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson E, Lindstrom P, Patarroyo M, et al. Ethanol impairs certain aspects of neutrophil adhesion in vitro: comparisons with inhibition of expression of the CD18 antigen. The Journal of infectious diseases. 1991 Mar;163(3):591–597. doi: 10.1093/infdis/163.3.591. [DOI] [PubMed] [Google Scholar]
- 16.MacGregor RR, Spagnuolo PJ, Lentnek AL. Inhibition of granulocyte adherence by ethanol, prednisone, and aspirin, measured with an assay system. The New England journal of medicine. 1974 Sep 26;291(13):642–646. doi: 10.1056/NEJM197409262911302. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg SS, Zhao X, Hua L, Wang JF, Nelson S, Ouyang J. Ethanol inhibits lung clearance of Pseudomonas aeruginosa by a neutrophil and nitric oxide-dependent mechanism, in vivo. Alcoholism, clinical and experimental research. 1999 Apr;23(4):735–744. [PubMed] [Google Scholar]
- 18.Joshi PC, Applewhite L, Mitchell PO, et al. GM-CSF receptor expression and signaling is decreased in lungs of ethanol-fed rats. American journal of physiology. Lung cellular and molecular physiology. 2006 Dec;291(6):L1150–L1158. doi: 10.1152/ajplung.00150.2006. [DOI] [PubMed] [Google Scholar]
- 19.Joshi PC, Applewhite L, Ritzenthaler JD, et al. Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. Journal of immunology (Baltimore, Md.) 2005 Nov 15;175(10):6837–6845. doi: 10.4049/jimmunol.175.10.6837. [DOI] [PubMed] [Google Scholar]
- 20.Dranoff G, Crawford AD, Sadelain M, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science (New York, N.Y.) 1994 Apr 29;264(5159):713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 21.Standiford TJ, Danforth JM. Ethanol feeding inhibits proinflammatory cytokine expression from murine alveolar macrophages ex vivo. Alcoholism, clinical and experimental research. 1997 Oct;21(7):1212–1217. [PubMed] [Google Scholar]
- 22.Deviere J, Content J, Denys C, et al. High interleukin-6 serum levels and increased production by leucocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokines production. Clinical and experimental immunology. 1989 Aug;77(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- 23.Crews FT, Bechara R, Brown LA, et al. Cytokines and alcohol. Alcoholism, clinical and experimental research. 2006 Apr;30(4):720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 24.McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology (lBaltimore, Md.) 1989 Mar;9(3):349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- 25.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990 Oct 18;347(6294):669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 26.Burnham EL, Kovacs EJ, Davis CS. Pulmonary cytokine composition differs in the setting of alcohol use disorders and cigarette smoking. American journal of physiology. Lung cellular and molecular physiology. 2013 Jun 15;304(12):L873–L882. doi: 10.1152/ajplung.00385.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen MM, Bird MD, Zahs A, et al. Pulmonary inflammation after ethanol exposure and burn injury is attenuated in the absence of IL-6. Alcohol (Fayetteville, N.Y.) 2013 May;47(3):223–229. doi: 10.1016/j.alcohol.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puddey IB, Beilin LJ, Vandongen R. Regular alcohol use raises blood pressure in treated hypertensive subjects. A randomised controlled trial. Lancet. 1987 Mar 21;1(8534):647–651. doi: 10.1016/s0140-6736(87)90413-2. [DOI] [PubMed] [Google Scholar]
- 29.Linkola J, Fyhrquist F, Ylikahri R. Renin, aldosterone and cortisol during ethanol intoxication and hangover. Acta physiologica Scandinavica. 1979 May;106(1):75–82. doi: 10.1111/j.1748-1716.1979.tb06372.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang R, Ramos C, Joshi I, et al. Human lung myofibroblast-derived inducers of alveolar epithelial apoptosis identified as angiotensin peptides. The American journal of physiology. 1999 Dec;277(6 Pt 1):L1158–L1164. doi: 10.1152/ajplung.1999.277.6.L1158. [DOI] [PubMed] [Google Scholar]
- 31.Bechara RI, Pelaez A, Palacio A, et al. Angiotensin II mediates glutathione depletion, transforming growth factor-beta1 expression, and epithelial barrier dysfunction in the alcoholic rat lung. American journal of physiology. Lung cellular and molecular physiology. 2005 Sep;289(3):L363–L370. doi: 10.1152/ajplung.00141.2005. [DOI] [PubMed] [Google Scholar]
- 32.Polikandriotis JA, Rupnow HL, Elms SC, et al. Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung. American journal of respiratory cell and molecular biology. 2006 Mar;34(3):314–319. doi: 10.1165/rcmb.2005-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guidot DM, Modelska K, Lois M, et al. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. American journal of physiology. Lung cellular and molecular physiology. 2000 Jul;279(1):L127–L135. doi: 10.1152/ajplung.2000.279.1.L127. [DOI] [PubMed] [Google Scholar]
- 34.Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. American journal of respiratory and critical care medicine. 2000 Feb;161(2 Pt 1):414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 35.Pacht ER, Timerman AP, Lykens MG, Merola AJ. Deficiency of alveolar fluid glutathione in patients with sepsis and the adult respiratory distress syndrome. Chest. 1991 Nov;100(5):1397–1403. doi: 10.1378/chest.100.5.1397. [DOI] [PubMed] [Google Scholar]
- 36.Bunnell E, Pacht ER. Oxidized glutathione is increased in the alveolar fluid of patients with the adult respiratory distress syndrome. The American review of respiratory disease. 1993 Nov;148(5):1174–1178. doi: 10.1164/ajrccm/148.5.1174. [DOI] [PubMed] [Google Scholar]
- 37.Holguin F, Moss I, Brown LA, Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. The Journal of clinical investigation. 1998 Feb 15;101(4):761–768. doi: 10.1172/JCI1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velasquez A, Bechara RI, Lewis JF, et al. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis-mediated lung dysfunction in ethanol-fed rats. Alcoholism, clinical and experimental research. 2002 Aug;26(8):1245–1251. doi: 10.1097/01.ALC.0000024269.05402.97. [DOI] [PubMed] [Google Scholar]
- 39.Brown LA, Harris FL, Bechara R, Guidot DM. Effect of chronic ethanol ingestion on alveolar type II cell: glutathione and inflammatory mediator-induced apoptosis. Alcoholism, clinical and experimental research. 2001 Jul;25(7):1078–1085. [PubMed] [Google Scholar]
- 40.Bechara RI, Brown LA, Roman J, Joshi PC, Guidot DM. Transforming growth factor beta1 expression and activation is increased in the alcoholic rat lung. American journal of respiratory and critical care medicine. 2004 Jul 15;170(2):188–194. doi: 10.1164/rccm.200304-478OC. [DOI] [PubMed] [Google Scholar]
- 41.Wegener T, Sandhagen B, Saldeen T. Effect of N-acetylcysteine on pulmonary damage due to microembolism in the rat. European journal of respiratory diseases. 1987 Apr;70(4):205–212. [PubMed] [Google Scholar]
- 42.Davreux CJ, Soric I, Nathens AB, et al. N-acetyl cysteine attenuates acute lung injury in the rat. Shock (Augusta, Ga.) 1997 Dec;8(6):432–438. [PubMed] [Google Scholar]
- 43.Suter PM, Domenighetti G, Schaller MD, Laverriere MC, Ritz R, Perret C. N-acetylcysteine enhances recovery from acute lung injury in man. A randomized, double-blind, placebo-controlled clinical study. Chest. 1994 Jan;105(1):190–194. doi: 10.1378/chest.105.1.190. [DOI] [PubMed] [Google Scholar]
- 44.Bernard GR, Wheeler AP, Arons MM, et al. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest. 1997 Jul;112(1):164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- 45.Domenighetti G, Suter PM, Schaller MD, Ritz R, Perret C. Treatment with N-acetylcysteine during acute respiratory distress syndrome: a randomized, double-blind, placebo-controlled clinical study. Journal of critical care. 1997 Dec;12(4):177–182. doi: 10.1016/s0883-9441(97)90029-0. [DOI] [PubMed] [Google Scholar]
- 46.Marshall RP, Webb S, Bellingan GJ, et al. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2002 Sep 1;166(5):646–650. doi: 10.1164/rccm.2108086. [DOI] [PubMed] [Google Scholar]
- 47.Villar J, Flores C, Perez-Mendez L, et al. Angiotensin-converting enzyme insertion/deletion polymorphism is not associated with susceptibility and outcome in sepsis and acute respiratory distress syndrome. Intensive care medicine. 2008 Mar;34(3):488–495. doi: 10.1007/s00134-007-0937-z. [DOI] [PubMed] [Google Scholar]
- 48.Matsuda A, Kishi T, Jacob A, Aziz M, Wang P. Association between insertion/deletion polymorphism in angiotensin-converting enzyme gene and acute lung injury/acute respiratory distress syndrome: a meta-analysis. BMC medical genetics. 2012;13:76. doi: 10.1186/1471-2350-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005 Jul 7;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pelaez A, Bechara RI, Joshi PC, Brown LA, Guidot DM. Granulocyte/macrophage colony-stimulating factor treatment improves alveolar epithelial barrier function in alcoholic rat lung. American journal of physiology. Lung cellular and molecular physiology. 2004 Jan;286(1):L106–L111. doi: 10.1152/ajplung.00148.2003. [DOI] [PubMed] [Google Scholar]
- 51.Matute-Bello G, Liles WC, Radella F, 2nd, et al. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Critical care medicine. 2000 Jan;28(1):1–7. doi: 10.1097/00003246-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Presneill JJ, Harris T, Stewart AG, Cade JF, Wilson JW. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. American journal of respiratory and critical care medicine. 2002 Jul 15;166(2):138–143. doi: 10.1164/rccm.2009005. [DOI] [PubMed] [Google Scholar]
- 53.Paine R, 3rd, Standiford TJ, Dechert RE, et al. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Critical care medicine. 2012 Jan;40(1):90–97. doi: 10.1097/CCM.0b013e31822d7bf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vital signs: current cigarette smoking among adults aged >/=18 years--United States, 2005–2010. MMWR. Morbidity and mortality weekly report. 2011 Sep 9;60(35):1207–1212. [PubMed] [Google Scholar]
- 55.WHO report on the global tobacco epidemic. Geneva, Switzerland: World Health Organization; [Google Scholar]
- 56.Christenson JT, Aeberhard JM, Badel P, et al. Adult respiratory distress syndrome after cardiac surgery. Cardiovascular surgery (London, England) 1996 Feb;4(1):15–21. doi: 10.1016/0967-2109(96)83778-1. [DOI] [PubMed] [Google Scholar]
- 57.Iribarren C, Jacobs DR, Jr., Sidney S, Gross MD, Eisner MD. Cigarette smoking, alcohol consumption, and risk of ARDS: a 15-year cohort study in a managed care setting. Chest. 2000 Jan;117(1):163–168. doi: 10.1378/chest.117.1.163. [DOI] [PubMed] [Google Scholar]
- 58.Hsieh SJ, Ware LB, Eisner MD, et al. Biomarkers increase detection of active smoking and secondhand smoke exposure in critically ill patients. Critical care medicine. 2011 Jan;39(1):40–45. doi: 10.1097/CCM.0b013e3181fa4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calfee CS, Matthay MA, Eisner MD, et al. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. American journal of respiratory and critical care medicine. 2011 Jun 15;183(12):1660–1665. doi: 10.1164/rccm.201011-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. American journal of respiratory and critical care medicine. 2013 Mar 1;187(5):527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ando K, Doi T, Moody SY, Ohkuni Y, Sato S, Kaneko N. The effect of comorbidity on the prognosis of acute lung injury and acute respiratory distress syndrome. Internal medicine (Tokyo, Japan) 2012;51(14):1835–1840. doi: 10.2169/internalmedicine.51.6434. [DOI] [PubMed] [Google Scholar]
- 62.Hsieh SJ, Zhuo H, Benowitz NL, et al. Prevalence and Impact of Active and Passive Cigarette Smoking in Acute Respiratory Distress Syndrome. Critical care medicine. 2014 doi: 10.1097/CCM.0000000000000418. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goniewicz ML, Eisner MD, Lazcano-Ponce E, et al. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2011 Mar;13(3):202–208. doi: 10.1093/ntr/ntq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edwards D. Immunological effects of tobacco smoking in “healthy” smokers. Copd. 2009 Feb;6(1):48–58. doi: 10.1080/15412550902724206. [DOI] [PubMed] [Google Scholar]
- 65.Sopori M. Effects of cigarette smoke on the immune system. Nature reviews. Immunology. 2002 May;2(5):372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 66.Mehta H, Nazzal K, Sadikot RT. Cigarette smoking and innate immunity. Inflammation research : official journal of the European Histamine Research Society … [et al.] 2008 Nov;57(11):497–503. doi: 10.1007/s00011-008-8078-6. [DOI] [PubMed] [Google Scholar]
- 67.Drannik AG, Pouladi MA, Robbins CS, Goncharova SI, Kianpour S, Stampfli MR. Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection. American journal of respiratory and critical care medicine. 2004 Dec 1;170(11):1164–1171. doi: 10.1164/rccm.200311-1521OC. [DOI] [PubMed] [Google Scholar]
- 68.Phipps JC, Aronoff DM, Curtis JL, Goel D, O’Brien E, Mancuso P. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Infection and immunity. 2010 Mar;78(3):1214–1220. doi: 10.1128/IAI.00963-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marti-Lliteras P, Regueiro V, Morey P, et al. Nontypeable Haemophilus influenzae clearance by alveolar macrophages is impaired by exposure to cigarette smoke. Infection and immunity. 2009 Oct;77(10):4232–4242. doi: 10.1128/IAI.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mutepe ND, Cockeran R, Steel HC, et al. Effects of cigarette smoke condensate on pneumococcal biofilm formation and pneumolysin. The European respiratory journal. 2013 Feb;41(2):392–395. doi: 10.1183/09031936.00213211. [DOI] [PubMed] [Google Scholar]
- 71.Sanz Herrero F, Blanquer Olivas J. Microbiology and risk factors for community-acquired pneumonia. Seminars in respiratory and critical care medicine. 2012 Jun;33(3):220–231. doi: 10.1055/s-0032-1315634. [DOI] [PubMed] [Google Scholar]
- 72.Fung HB, Monteagudo-Chu MO. Community-acquired pneumonia in the elderly. The American journal of geriatric pharmacotherapy. 2010 Feb;8(1):47–62. doi: 10.1016/j.amjopharm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Jones JG, Minty BD, Lawler P, Hulands G, Crawley JC, Veall N. Increased alveolar epithelial permeability in cigarette smokers. Lancet. 1980 Jan 12;1(8159):66–68. doi: 10.1016/s0140-6736(80)90493-6. [DOI] [PubMed] [Google Scholar]
- 74.Bhalla DK, Daniels DS, Luu NT. Attenuation of ozone-induced airway permeability in rats by pretreatment with cyclophosphamide, FPL 55712, and indomethacin. American journal of respiratory cell and molecular biology. 1992 Jul;7(1):73–80. doi: 10.1165/ajrcmb/7.1.73. [DOI] [PubMed] [Google Scholar]
- 75.Kleeberger SR, Hudak BB. Acute ozone-induced change in airway permeability: role of infiltrating leukocytes. Journal of applied physiology (Bethesda, Md. : 1985) 1992 Feb;72(2):670–676. doi: 10.1152/jappl.1992.72.2.670. [DOI] [PubMed] [Google Scholar]
- 76.Li XY, Rahman I, Donaldson K, MacNee W. Mechanisms of cigarette smoke induced increased airspace permeability. Thorax. 1996 May;51(5):465–471. doi: 10.1136/thx.51.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environmental health perspectives. 1985 Dec;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li XY, Donaldson K, Rahman I, MacNee W. An investigation of the role of glutathione in increased epithelial permeability induced by cigarette smoke in vivo and in vitro. American journal of respiratory and critical care medicine. 1994 Jun;149(6):1518–1525. doi: 10.1164/ajrccm.149.6.8004308. [DOI] [PubMed] [Google Scholar]
- 79.Morrison D, Rahman I, Lannan S, MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. American journal of respiratory and critical care medicine. 1999 Feb;159(2):473–479. doi: 10.1164/ajrccm.159.2.9804080. [DOI] [PubMed] [Google Scholar]
- 80.Petecchia L, Sabatini F, Varesio L, et al. Bronchial airway epithelial cell damage following exposure to cigarette smoke includes disassembly of tight junction components mediated by the extracellular signal-regulated kinase 1/2 pathway. Chest. 2009 Jun;135(6):1502–1512. doi: 10.1378/chest.08-1780. [DOI] [PubMed] [Google Scholar]
- 81.Heijink IH, Brandenburg SM, Postma DS, van Oosterhout AJ. Cigarette smoke impairs airway epithelial barrier function and cell-cell contact recovery. The European respiratory journal. 2012 Feb;39(2):419–428. doi: 10.1183/09031936.00193810. [DOI] [PubMed] [Google Scholar]
- 82.Xu H, Ferro TJ, Chu S. Cigarette smoke condensate inhibits ENaC alpha-subunit expression in lung epithelial cells. The European respiratory journal. 2007 Oct;30(4):633–642. doi: 10.1183/09031936.00014107. [DOI] [PubMed] [Google Scholar]
- 83.Cantin AM, Hanrahan JW, Bilodeau G, et al. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. American journal of respiratory and critical care medicine. 2006 May 15;173(10):1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 84.Holden WE, Maier JM, Malinow MR. Cigarette smoke extract increases albumin flux across pulmonary endothelium in vitro. Journal of applied physiology (Bethesda, Md.: 1985) 1989 Jan;66(1):443–449. doi: 10.1152/jappl.1989.66.1.443. [DOI] [PubMed] [Google Scholar]
- 85.Lu Q, Sakhatskyy P, Grinnell K, et al. Cigarette smoke causes lung vascular barrier dysfunction via oxidative stress-mediated inhibition of RhoA and focal adhesion kinase. American journal of physiology. Lung cellular and molecular physiology. 2011 Dec;301(6):L847–L857. doi: 10.1152/ajplung.00178.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schweitzer KS, Hatoum H, Brown MB, et al. Mechanisms of lung endothelial barrier disruption induced by cigarette smoke: role of oxidative stress and ceramides. American journal of physiology. Lung cellular and molecular physiology. 2011 Dec;301(6):L836–L846. doi: 10.1152/ajplung.00385.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ware LB, Matthay MA. The acute respiratory distress syndrome. The New England journal of medicine. 2000 May 4;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 88.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. The Journal of clinical investigation. 2009 Nov;119(11):3450–3461. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gunther A, Mosavi P, Heinemann S, et al. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia. Comparison with the acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2000 Feb;161(2 Pt 1):454–462. doi: 10.1164/ajrccm.161.2.9712038. [DOI] [PubMed] [Google Scholar]
- 90.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Critical care medicine. 2003 Apr;31(4 Suppl):S213–S220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 91.Greene R. Pulmonary vascular obstruction in the adult respiratory distress syndrome. Journal of thoracic imaging. 1986 Jul;1(3):31–38. doi: 10.1097/00005382-198607000-00006. [DOI] [PubMed] [Google Scholar]
- 92.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005 May 24;111(20):2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 93.Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax. 2014 Apr 4; doi: 10.1136/thoraxjnl-2013-204492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mustafic H, Jabre P, Caussin C, et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA : the journal of the American Medical Association. 2012 Feb 15;307(7):713–721. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 95.Pope CA, 3rd, Burnett RT, Krewski D, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009 Sep 15;120(11):941–948. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- 96.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002 Oct 19;360(9341):1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 97.Brunekreef B, Beelen R, Hoek G, et al. Effects of long-term exposure to traffic-related air pollution on respiratory and cardiovascular mortality in the Netherlands: the NLCS-AIR study. Research report (Health Effects Institute) 2009 Mar;(139):5–71. discussion 73–89. [PubMed] [Google Scholar]
- 98.Beelen R, Hoek G, van den Brandt PA, et al. Long-term effects of traffic-related air pollution on mortality in a Dutch cohort (NLCS-AIR study) Environmental health perspectives. 2008 Feb;116(2):196–202. doi: 10.1289/ehp.10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dong GH, Zhang P, Sun B, et al. Long-term exposure to ambient air pollution and respiratory disease mortality in Shenyang, China: a 12-year population-based retrospective cohort study. Respiration international review of thoracic diseases. 2012;84(5):360–368. doi: 10.1159/000332930. [DOI] [PubMed] [Google Scholar]
- 100.Dockery DW, Pope CA, 3rd, Xu X, et al. An association between air pollution and mortality in six U.S. cities. The New England journal of medicine. 1993 Dec 9;329(24):1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 101.Pope CA, 3rd, Thun MJ, Namboodiri MM, et al. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. American journal of respiratory and critical care medicine. 1995 Mar;151(3 Pt 1):669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- 102.Dimakopoulou K, Samoli E, Beelen R, et al. Air Pollution and Nonmalignant Respiratory Mortality in 16 Cohorts within the ESCAPE Project. American journal of respiratory and critical care medicine. 2014 Mar 15;189(6):684–696. doi: 10.1164/rccm.201310-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Noah TL, Zhou H, Zhang H, et al. Diesel exhaust exposure and nasal response to attenuated influenza in normal and allergic volunteers. American journal of respiratory and critical care medicine. 2012 Jan 15;185(2):179–185. doi: 10.1164/rccm.201103-0465OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schultz ES, Gruzieva O, Bellander T, et al. Traffic-related air pollution and lung function in children at 8 years of age: a birth cohort study. American journal of respiratory and critical care medicine. 2012 Dec 15;186(12):1286–1291. doi: 10.1164/rccm.201206-1045OC. [DOI] [PubMed] [Google Scholar]
- 105.Huang W, Wang G, Lu SE, et al. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. American journal of respiratory and critical care medicine. 2012 Dec 1;186(11):1150–1159. doi: 10.1164/rccm.201205-0850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bind MA, Baccarelli A, Zanobetti A, et al. Air pollution and markers of coagulation, inflammation, and endothelial function: associations and epigene-environment interactions in an elderly cohort. Epidemiology (Cambridge, Mass.) 2012 Mar;23(2):332–340. doi: 10.1097/EDE.0b013e31824523f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Que LG, Stiles JV, Sundy JS, Foster WM. Pulmonary function, bronchial reactivity, and epithelial permeability are response phenotypes to ozone and develop differentially in healthy humans. Journal of applied physiology (Bethesda, Md.: 1985) 2011 Sep;111(3):679–687. doi: 10.1152/japplphysiol.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]