Abstract
Background and objectives
The antiproteinuric effect of a renin-angiotensin-aldosterone system blockade can be magnified by dietary salt restriction. This study sought to determine the effect of intensive low-salt diet education on BP and urine albumin excretion in nondiabetic patients with hypertension and albuminuria.
Design, setting, participants, & measurements
This study was conducted between March of 2012 and March of 2013 as an open-label, randomized, controlled trial. After a run-in period of 8 weeks, all patients received the angiotensin II receptor blocker olmesartan (40 mg daily). Patients were then divided into two groups. One group was treated for another 8 weeks with angiotensin II receptor blocker plus conventional low-salt diet education, and the other group was treated for 8 weeks with angiotensin II receptor blocker plus intensive low-salt diet education. The final analyses was performed with 245 completed patients.
Results
The amount of daily albuminuria was significantly decreased from 0 (566.0 [25.0–5398.6] mg/d) to 8 weeks (282.5 [16.1–4898.5] mg/d; P<0.001). From 8 to 16 weeks, the 24-hour urinary sodium excretion was decreased by 36.0±5.9 mmol/d in the intensive education group and 8.8±4.9 mmol/d in the conventional education group (interaction P<0.001). Patients who completed intensive low-salt diet education exhibited greater decreases in urinary albumin excretion than the control group (change in albuminuria from 8 to 16 weeks, −154.0 versus 0.4 mg/d; P=0.01). Urinary albumin excretion tended to decrease as the 24-hour urinary sodium excretion amount decreased (R=0.32; 95% confidence interval, 0.20 to 0.43; P<0.001).
Conclusions
The 24-hour urinary albumin excretion was decreased more in patients in the intensive low-salt diet education group than patients in the conventional education group. Weekly intensive education on a low-salt diet would be a suitable method for clinical practice.
Keywords: albuminuria, CKD, hypertension, nutrition, rennin, angiotensin system
Introduction
CKD is a potent risk factor for ESRD, cardiovascular disease (CVD), all-cause mortality, and hospitalization (1). CKD is also related to a decrease in quality of life and cognitive function (2). Hypertension and proteinuria promote CKD progression and increase the risk of CVD and mortality (3). Blockade of the renin-angiotensin-aldosterone system (RAAS) is well known to reduce urinary albumin excretion, and it is considered a mainstay of therapy in the prevention of ESRD (4,5). The antiproteinuric effect of the RAAS blockade can be magnified or reduced depending on the amount of dietary salt intake (6–11).
Low salt intake is associated with favorable effects on albuminuria, BP, and GFR (11–13). High salt intake enhances the generation of superoxide anion accompanied by enhanced renal expression and nicotinamide dehydrogenase activation. In addition, dietary salt increases the glomerular expression of TGF-β1 on renal tissue and also, augments nitric oxide production (14). High salt intake also induces the intrarenal aldosterone receptor and promotes renal fibrotic injury (15). Two recent studies identified that increased dietary salt intake promotes tissue inflammation by triggering IL-17–producing CD4+ T cell development (16,17).
Koreans are heavy consumers of sodium; with a dietary sodium level of 4790.7 mg/d, the country leads all other nations in terms of daily sodium intake (18). Nonmedical management with a low-salt diet (LSD) can reduce 24-hour urinary sodium excretion by 35–55 mEq/d (19). Several studies revealed an additive effect of dietary salt restriction with RAAS blockade on BP and proteinuria. However, most of these studies include a small number of patients (6,9–11,20), and there has been no randomized, controlled study in Korean populations. Most importantly, the methods for implementing LSDs or high-salt diets used in previous studies offered strongly controlled or modulated diets (6,11), or they provided salt tablets (9), which are not suitable for application in typical clinical practice.
The aim of this study was to determine the proteinuria-lowering effects of LSD education on urine protein excretion and BP in nondiabetic patients with hypertension and albuminuria who are already taking an RAAS blocker (olmesartan).
Materials and Methods
Study Population
This study was an open-label, case-control, randomized clinical trial (clinicaltrials.gov registration number NCT01552954). Patients were selected from the outpatient renal clinics of seven centers in Korea (Seoul National University Boramae Medical Center, Seoul National University Bundang Hospital, Seoul National University Hospital, Konkuk University Hospital, Dongguk University Ilsan Hospital, Kyung Hee University Medical Center, and Seoul St. Mary’s Hospital) and enrolled between March of 2012 and March of 2013. The investigators of each center had the responsibilities of recruitment, enrollment, and eligibility decision. The last included patient completed the study in September of 2013. All of the patients fulfilled the inclusion criteria, which were age of 19–75 years, the use of antihypertensive medication or a diagnosis of hypertension, Modification of Diet in Renal Disease study eGFR≥30 ml/min per 1.73 m2, random urine albumin-to-creatinine ratio ≥30 mg/g creatinine more than two times with a ≥1-week interval in the last 6 months, and an ability and willingness to provide written informed consent.
Patients with uncontrolled hypertension (BP>160/110 mmHg) at the time of screening, pregnant women, and patients with serum potassium >5.5 mEq/L, a malignancy, a diagnosis of CVD (cerebral infarction, hemorrhagic infarction, acute myocardial infarction or unstable angina, coronary angioplasty, or coronary artery bypass surgery) within the last 6 months, contraindication for angiotensin II receptor blockers (ARBs), and diabetes mellitus were excluded as well as continuous users of steroids or other immunosuppressive agents at the time of registration.
The sample size was calculated from reference of a specific article with proteinuria as an outcome (7). A two-sided 5% significance level, a power of 80%, and a sample size of 135 patients per group were necessary given an anticipated dropout rate of 20%. To recruit this number of patients, a 12-month inclusion period was anticipated.
All clinical investigations were conducted in accordance with the 2008 Declaration of Helsinki and the guidelines of good clinical practice. Informed written consent was obtained from each patient before inclusion.
Study Protocol
We screened patients before 8 weeks of study initiation, and the protocol included a run-in period for antihypertensive medication adjustment. All of the participants had to stop all RAAS blocking agents or diuretic therapy and switch to antihypertensive agents of different categories during this period. At this point, the researchers gave the participants a list of food products that are commonly consumed in Korea together with their sodium content, which was identified by the Korean Dietetic Association. After an 8-week run-in period (0 weeks), the researchers conducted baseline laboratory examinations, and the patients completed a dish frequency questionnaire (DFQ; DFQ55). From 0 weeks, all of the enrolled patients were prescribed olmesartan medoxomil (Daewoong Pharmaceutical Co. Ltd./Daiichi Sankyo Korea Co. Ltd., Seoul, South Korea) at a 40-mg one time per day fixed dose until the end of the study. After another 8 weeks, the participants were randomly assigned to receive an LSD intervention after the second laboratory examination. The participants in the conventional education group received routine LSD education at an outpatient clinic. Otherwise, the participants in the intensive education group were closely supported by a dietary consultant and feedback by telephone for 30 minutes one time per week during the study period. The target amount of daily sodium intake was <100 mEq/d in the intensive education group, and a ≥25% reduction of salt intake was recommended in both groups. Before randomization, the participants who displayed poor medication adherence to olmesartan (used ≤60% of the prescribed medication) were removed from the study. After the 8-week study (16 weeks), the participants underwent a third laboratory examination and completed the DFQ55 again. We assessed drug compliance using pill counts and evaluated the adequacy of 24-hour urine samples with correction by calculating the predicted daily creatinine excretion (men: −12.63×age+15.12×body weight+7.39×height×79.9 mg/d; women:−4.72×age+8.58×body weight+5.09×height−74.5 mg/d) (21). For safety assessments, researchers assessed self-reported hypotension by giving participants an autosphygmomanometer. Adverse effects of olmesartan were assessed by measuring aspartate aminotransferase, alanine aminotransferase, BP, and serum creatinine.
Randomization and Masking
Eligible participants were randomly assigned (1:1) to either conventional education or intensive education for LSD with computerized block randomization (block size six) and balanced according to institution and sex. It was not feasible to mask participants to allocation, but clinicians were masked to group assignment.
Outcome Measures
The primary end point was the decrement of 24-hour urine albumin levels after LSD implementation. The secondary end points were the decrement of albuminuria with olmesartan treatment and the decrement of BP and 24-hour urine sodium excretion after LSD education. At 0, 8, and 16 weeks, on the day before each visit, the patients were told to collect 24-hour urine samples to assess proteinuria and urinary sodium, urea, potassium, and creatinine excretion.
Statistical Analyses
All of the analyses and calculations were performed using SPSS Statistics V21.0 (IBM Corporation, Armonk, NY). Continuous variables were expressed as means±SDs or medians (interquartile ranges), and categorical variables were expressed as a frequency (percentage). The independent t test or Mann–Whitney U test was used to compare continuous variables between the groups according to the normality assumption. The chi-squared test was used to analyze the categorical variables. To assess the effect of intensive LSD education for urine sodium and albumin excretion over time, we used a linear mixed effect model (LMM) for repeated measures. Before analysis, variables deviating from the normal distribution were logarithm-transformed. The model considered the baseline level of specific variables (age, randomization group, time, and randomization group by time) as fixed effects. Data from the LMM were expressed by least squares means±SEMs. Correlation analysis was used to assess linear relationships between changes in variables with reduction of salt consumption. Differences with two-tailed P value <0.05 were considered statistically significant.
Results
Patient Characteristics
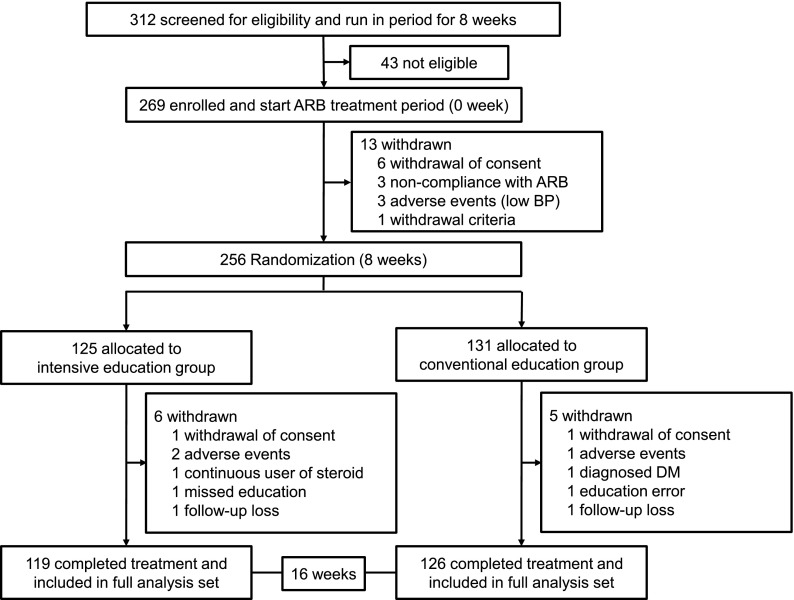
In total, 312 patients were screened, and 269 patients were enrolled. Of 269 patients, 24 patients dropped out during the study period, and the 245 patients who completed the trial were included in the analysis (Figure 1). The percentage of compliance to olmesartan treatment was 98.0 (92.0–100.0) %, and there were no significant differences between the groups at both weeks 8 (P=0.29) and 16 (P=0.42). One and three patients at weeks 0 and 16, respectively, did not collect 24-hour urine. The percentages of compliance for 24-hour urines were 95.0%, 91.5%, and 88.0%. We only presented the results from the original data, because there were no differences in results when corrected values were used.
Figure 1.
Study flow chart. ARB, angiotensin II receptor blocker; DM, diabetes mellitus.
Sodium DFQ55, which was assessed at 0 and 8 weeks, showed significant group-by-time interaction effect (P<0.001) and revealed a significant reduction in salt intake in the intensive education group (from 2579.0±222.0 to 1468.1±202.7 mg/d; P<0.001), contrary to the findings in the conventional education group (from 2262.5±215.7 to 2128.8±197.0 mg/d; P=0.51). The sodium DFQ55 of each week correlated with the corrected 24-hour urinary sodium excretion value (0 weeks [n=244]: R=0.15, P=0.02; 16 week [n=242]: R=0.12, P=0.06 by Pearson correlation analysis).
Table 1 shows the baseline characteristics of the entire population and each group. The mean age of the participants was 49.5 years, and 49.8% of the patients were men. The demographics were not significantly different between the two groups, including BP, creatinine, and eGFR, with the exception of the BUN levels and the proportion of patients who exercised. The mean 24-hour urinary sodium and albumin excretions at baseline were 155.2±70.0 mEq/d and 566.0 (248.0–1286.9) mg/d, respectively.
Table 1.
Baseline characteristics and parameters
| Category | Intensive Education (n=119) | Conventional Education (n=126) | Total (n=245) | P Value |
|---|---|---|---|---|
| Characteristics and lifestyle | ||||
| Characteristics | ||||
| Age (yr) | 48.2±12.2 | 50.7±14.3 | 49.5±13.3 | 0.14a |
| Men | 57 (47.9) | 65 (51.6) | 122 (49.8) | 0.61b |
| Weight (kg) | 68.1±13.5 | 67.5±13.5 | 67.8±13.5 | 0.74a |
| Height (cm) | 163.8±8.9 | 162.8±8.9 | 163.3±8.9 | 0.99a |
| Systolic BP (mmHg) | 131.0±11.0 | 130.8±12.4 | 130.9±11.8 | 0.88a |
| Diastolic BP (mmHg) | 79.7±8.2 | 79.2±9.9 | 79.4±9.1 | 0.65a |
| Pulse rate (beats/min) | 77.5±11.5 | 77.0±12.5 | 77.2±12.0 | 0.72a |
| Menopause in women | 34 (44.2) | 36 (50.7) | 70 (47.3) | 0.51b |
| Lifestyle | ||||
| Smoker: never/ex/current | 89 (74.8)/20 (16.8)/10 (8.4) | 83 (65.9)/24 (19.0)/19 (15.1) | 172 (70.2)/44 (18.0)/29 (11.8) | 0.21b |
| Smoking amount (pack-yr) | 0.0 (0.0–1.5) | 0.0 (0.0–6.5) | 0.0 (0.0–4.0) | 0.28c |
| Drinker: never/ex/current | 66 (55.5)/9 (7.6)/44 (37.0) | 61 (48.4)/12 (9.5)/53 (42.1) | 127 (51.8)/21 (8.6)/97 (39.6) | 0.53b |
| Drinking amount (bottle/wk) | 0.0 (0.0–0.5) | 0.0 (0.0–1.0) | 0.0 (0.0–0.6) | 0.36c |
| Exercise | 51 (42.9) | 77 (61.1) | 128 (52.2) | 0.005b |
| Exercise amount (min/wk) | 0.0 (0.0–180.0) | 120.0 (0.0–240.0) | 60.0 (0.0–210.0) | 0.001c |
| Blood and urine measurements | ||||
| Blood measurements | ||||
| White blood cells (per mm3) | 6270 (5200–7380) | 6225 (5118–7038) | 6250 (5130–7225) | 0.29c |
| Hemoglobin (g/dl) | 14.2 (12.7–15.4) | 13.8 (12.7–15.0) | 14.1 (12.7–15.0) | 0.27c |
| Hematocrit | 41.3 (38.3–44.2) | 40.2 (37.7–43.5) | 41.0 (38.0–43.8) | 0.26c |
| Platelet (×1000/mm3) | 235 (204–277) | 225.0 (194.8–277.0) | 230 (199–277) | 0.33c |
| BUN (mg/dl) | 15.0 (12.0–20.0) | 17.0 (14.0–21.0) | 16.0 (13.0–20.0) | 0.01c |
| Creatinine (mg/dl) | 1.05 (0.83–1.31) | 1.08 (0.84–1.48) | 1.06 (0.84–1.40) | 0.39c |
| eGFR (ml/min per 1.73 m2)d | 68.4±23.6 | 66.2±25.6 | 67.3±24.6 | 0.47a |
| Cholesterol (mg/dl) | 179 (159–204) | 179 (156–203) | 179 (158–203) | 0.82c |
| Uric acid (mg/dl) | 6.0 (4.9–7.6) | 6.3 (5.0–7.8) | 6.2 (5.0–7.6) | 0.43c |
| Aspartate aminotransferase (IU/L) | 21 (18–28) | 22 (19–26) | 22 (18–28) | 0.37c |
| Alanine aminotransferase (IU/L) | 20 (15–29) | 20 (16–27) | 20 (15–28) | 0.79c |
| Na+ (mEq/L) | 141 (139–142) | 141 (139–142) | 141 (139–142) | 0.61c |
| K+ (mEq/L) | 4.4 (4.1–4.6) | 4.4 (4.1–4.6) | 4.4 (4.1–4.6) | 0.44c |
| Cl− (mEq/L) | 104 (103–106) | 104 (103–106) | 104 (103–106) | 0.79c |
| Total carbon dioxide (mEq/L) | 26.0 (24.3–28.9) | 26.1 (24.5–28.0) | 26.0 (24.5–28.1) | 0.74c |
| Urine measurements | ||||
| Random urine Na+ (mEq/L) | 90.3±43.1 | 85.9±40.8 | 88.0±41.9 | 0.41a |
| 24-h Urine Na+ (mEq/d) | 154.5±69.6 | 155.8±70.6 | 155.2±70.0 | 0.89a |
| Random urine K+ (mEq/L) | 56.0±27.4 | 57.4±31.6 | 56.7±29.6 | 0.71a |
| 24-h Urine K+ (mEq/d) | 53.1±21.4 | 55.5±22.3 | 54.4±21.9 | 0.39a |
| Random urine creatinine (mg/dl) | 114.4 (78.5–181.0) | 118.0 (79.9–163.0) | 115.9 (79.7–174.6) | 0.57c |
| 24-h Urine creatinine (mg/d) | 1160 (930–1488) | 1184 (927–1520) | 1170 (929–1500) | 0.93c |
| Albumin/creatinine ratio (mg/g creatinine) | 454.8 (135.7–890.7) | 394.1 (199.8–919.6) | 396.2 (166.0–899.0) | 0.70c |
| 24-h Urine albumin (mg/d) | 715.2 (259.4–1348.5) | 507.9 (230.3–1285.7) | 566.0 (248.0–1286.9) | 0.41c |
| Creatinine clearance (ml/min) | 82.0±31.9 | 79.7±36.1 | 80.8±34.1 | 0.61a |
| Comorbidities and medications | ||||
| Comorbidities | ||||
| Hypertension | 119 (100.0) | 125 (100.0) | 245 (100.0) | >0.99b |
| Dyslipidemia | 66 (56.4) | 69 (58.5) | 135 (57.4) | 0.79b |
| Angina | 0 (0.0) | 1 (0.8) | 1 (0.4) | 0.37b |
| Stroke | 2 (1.7) | 4 (3.2) | 6 (2.4) | 0.68b |
| Medications | ||||
| Angiotensin-converting enzyme inhibitor | 7 (5.9) | 10 (7.9) | 17 (6.9) | 0.62b |
| Angiotensin II receptor blocker | 94 (79.0) | 91 (72.2) | 185 (75.5) | 0.24b |
| β-Blocker | 25 (21.0) | 23 (18.3) | 48 (19.6) | 0.63b |
| Calcium channel blocker | 64 (53.8) | 67 (53.2) | 131 (53.5) | >0.99b |
| Diuretics | 13 (10.9) | 15 (11.9) | 28 (11.4) | 0.84b |
| Other hypertension medications | 9 (7.6) | 9 (7.1) | 18 (7.3) | >0.99b |
| Statin | 60 (50.4) | 60 (47.6) | 120 (49.0) | 0.70b |
| Fibrates | 3 (2.5) | 2 (1.6) | 5 (2.0) | 0.68b |
| Omega-3 | 29 (24.4) | 33 (26.2) | 62 (25.3) | 0.77b |
| Other dyslipidemia medications | 12 (10.1) | 7 (5.6) | 19 (7.8) | 0.23b |
| Aspirin | 37 (31.1) | 42 (33.3) | 79 (32.2) | 0.79b |
| Clopidogrel | 1 (0.8) | 3 (2.4) | 4 (1.6) | 0.62b |
| Cilostazol | 3 (2.5) | 5 (4.0) | 8 (3.3) | 0.72b |
| Other antiplatelet agents | 11 (9.2) | 15 (11.9) | 26 (10.6) | 0.54b |
| Steroids | 3 (2.5) | 4 (3.2) | 7 (2.9) | >0.99b |
| Immunosuppressive agents | 1 (0.8) | 3 (2.4) | 4 (1.6) | 0.62b |
| Nonsteroidal anti-inflammatory drugs | 5 (4.2) | 3 (2.4) | 8 (3.3) | 0.49b |
| Hormone replacement therapy in women | 2 (2.7) | 2 (2.9) | 4 (2.8) | >0.99b |
Data are presented as means±SDs, medians (interquartile ranges), or number (percentage).
P value estimated by independent t test.
P value estimated by χ2 test.
P value estimated by Mann–Whitney U test.
eGFR was calculated using the Isotope Dilution Mass Spectrometry traceable modified Modification of Diet in Renal Disease equation (milliliters per minute per 1.73 m2).
Primary and Secondary End Points
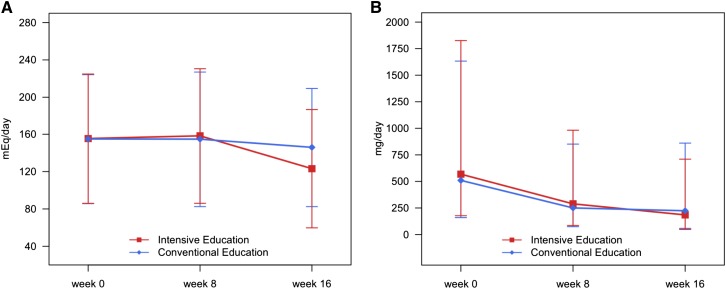
After olmesartan treatment for 8 weeks, the daily urinary albumin excretion amount was decreased significantly (Figure 2). We compared the 24-hour urine profiles between 8 and 16 weeks using the LMM. The results showed significant group-by-time interaction effects on the 24-hour urinary sodium and albumin excretions (interaction P<0.001) (Table 2), which meant that there were different tendencies across time between the two education groups.
Figure 2.
Change in 24-hour urine sodium and albumin excretions by randomization group. (A) Change in the mean 24-hour urine sodium by group. (B) Change in the mean albumin excretion amounts by group. Mean values of albumin were calculated from logarithm-transformed data and after that, recalculated to raw scale.
Table 2.
Attained clinical parameters according to randomized groups
| Variables | Interaction P Value | Week 8 (n=245) | Week 16a (n=242) | P Value (16 Versus 8) |
|---|---|---|---|---|
| 24-h Urine albumin (mg/d)b | <0.001 | |||
| Intensive education (n=119) | 276.5±1.1 | 178.0±1.1 | <0.001 | |
| Conventional education (n=126) | 258.5±1.1 | 231.1±1.1 | 0.06 | |
| 24-h Urine Na+ (mEq/d) | <0.001 | |||
| Intensive education (n=119) | 158.2±5.7 | 122.2±5.0 | <0.001 | |
| Conventional education (n=126) | 155.1±5.5 | 146.3±4.9 | 0.10 | |
| Systolic BP (mmHg) | 0.56 | |||
| Intensive education (n=119) | 122.4±1.2 | 121.2±1.3 | 0.38 | |
| Conventional education (n=126) | 122.7±1.2 | 122.6±1.3 | 0.95 | |
| Diastolic BP (mmHg) | 0.43 | |||
| Intensive education (n=119) | 73.9±0.9 | 73.6±0.9 | 0.80 | |
| Conventional education (n=126) | 74.1±0.9 | 74.8±0.9 | 0.37 | |
| Serum creatinine (mg/dl)b | 0.17 | |||
| Intensive education (n=119) | 1.13±1.01 | 1.15±1.01 | 0.01 | |
| Conventional education (n=126) | 1.12±1.01 | 1.13±1.01 | 0.56 | |
| eGFR (ml/min per 1.73 m2)c | 0.06 | |||
| Intensive education (n=119) | 64.9±0.8 | 63.4±1.0 | 0.06 | |
| Conventional education (n=126) | 64.5±0.8 | 65.1±0.9 | 0.42 | |
| Creatinine clearance (ml/min) | 0.40 | |||
| Intensive education (n=119) | 79.1±1.7 | 75.4±1.8 | 0.04 | |
| Conventional education (n=126) | 76.1±1.7 | 74.5±1.8 | 0.35 |
Data are expressed by least squares means±SEMs from a linear mixed effects model.
The 24-hour urine collection at the 16th week was omitted in three of 245 patients (one patient for the intensive education group and two patients for the conventional education group).
P value was from the result of a logarithm-transformed variable because of skewed distribution of albumin excretion.
eGFR was calculated using the Isotope Dilution Mass Spectrometry traceable modified Modification of Diet in Renal Disease equation (milliliters per minute per 1.73 m2).
Specific results within group comparisons were as follows. With the ARB treatment for 8 weeks, the 24-hour urinary sodium excretion amount was significantly decreased between 8 and 16 weeks in the intensive education group (change in sodium excretion: 36.0 mEq/d; P<0.001). However, the significant reduction was not identified in the conventional education group (change in sodium excretion: 8.8 mEq/d; P=0.10) (Figure 2A). Similarly, recipients in the intensive education group exhibited greater decreases in urinary albumin excretion (from 276.5 to 178.0 mg/d; P<0.001) than those in the conventional education group (from 258.5 to 231.1 mg/d; P=0.06) after randomization (Figure 2B).
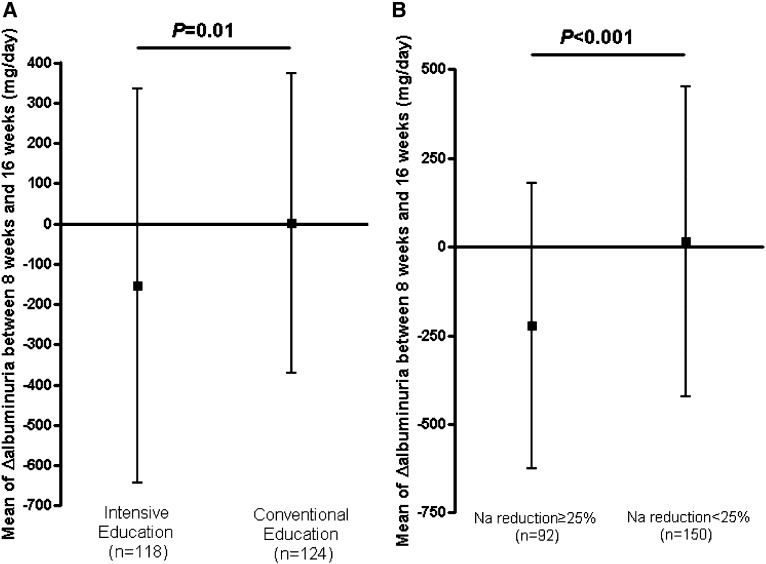
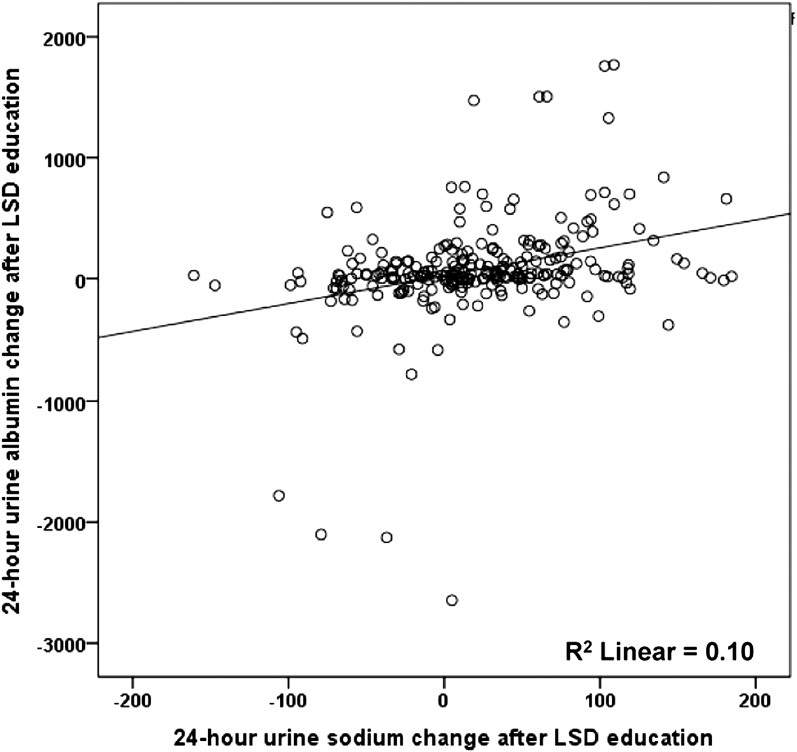
Regarding change in albuminuria, recipients in the inensive LSD education group exhibited greater decreases in urinary albumin excretion than those in the conventional education group (−154.0 versus 0.4 mg/d; P=0.01) (Figure 3A). In all participants, urinary albumin excretion tended to decline as the 24-hour urinary sodium excretion decreased (Pearson correlation analysis, R=0.32; 95% confidence interval, 0.20 to 0.43; P<0.001) (Figure 4). Systolic and diastolic BPs were decreased by olmesartan treatment between 8 and 16 weeks of the study, but the changes were not significantly different between the two groups.
Figure 3.
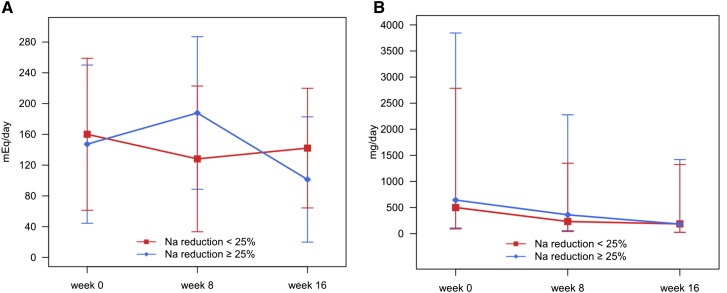
Change of 24-hour urine albumin excretion after low-salt diet education expressed by mean of the change in albuminuria between 8 and 16 weeks. (A) Recipients in the intensive low-salt diet education group displayed a larger decrease in urinary albumin excretion than those in the conventional education group (change in albuminuria, −154.0 versus 0.4 mg/d expressed by mean±SD; P=0.01). (B) The subgroup analysis according to the decrement of urinary sodium excretion after randomization. The Na reduction ≥25% group exhibited a marked reduction of urinary albumin excretion (change in albuminuria, −221.8 versus 15.2 mg/d expressed by mean±SD; P<0.001).
Figure 4.
Correlation between the reduction of 24-hour urine sodium and albumin excretion after low-salt diet (LSD) education. Urinary albumin excretion decreased as the 24-hour urinary sodium amount decreased (Pearson correlation analysis, R=0.32; 95% confidence interval, 0.20 to 0.43; P<0.001).
Subgroup Analyses
We performed subgroup analysis according to the decrement of urinary sodium excretion after randomization: Na reduction <25% (poor compliance to LSD) and Na reduction ≥25% (good compliance to LSD). In the subgroup analysis, the participants in the Na reduction ≥25% group showed the greater reduction in sodium and albumin excretions (Figure 5). In LMM, group-by-time interaction effect of urinary sodium profiles at 8 and 16 weeks was significantly different between the compliance groups (interaction P<0.001) (Table 3). Within subgroup comparison, the interweek variation of the Na reduction<25% subgroup in both education groups showed increase in urine sodium excretion (P=0.01 for the intensive education group; P<0.001 for the conventional education group), which indicated poor compliance to LSD.
Figure 5.
Change in 24-hour urine sodium and albumin excretions by the compliance group. Na reduction <25% was poor compliance to the low-salt diet, and Na reduction ≥25% was good compliance to the low-salt diet. (A) Change in the mean 24-hour urine sodium excretion amounts according to the extent of 24-hour urine sodium amount reduction between the 8th and 16th weeks. (B) Change in the mean 24-hour urine albumin excretion amounts according to the extent of 24-hour urine sodium amount reduction between the 8th and 16th weeks. Mean values of albumin were calculated from logarithm-transformed data and then, recalculated to raw scale.
Table 3.
Parameters depending on the reduction in 24-hour urine sodium content between weeks 8 and 16
| Variables | Interaction P Value | Week 8 | Week 16 | P Value (16 Versus 8) |
|---|---|---|---|---|
| Intensive education | ||||
| 24-h Urine albumin (mg/d)a | <0.001 | |||
| 8th–16th 24-h Na reduction<25% (n=60) | 259.0±1.1 | 212.4±1.1 | 0.02 | |
| 8th–16th 24-h Na reduction>25% (n=58) | 319.9±1.1 | 161.5±1.1 | <0.001 | |
| 24-h Urine Na+ (mEq/d) | <0.001 | |||
| 8th–16th 24-h Na reduction<25% (n=60) | 125.9±7.3 | 139.1±5.9 | 0.01 | |
| 8th–16th 24-h Na reduction>25% (n=58) | 190.3±7.3 | 103.8±6.0 | <0.001 | |
| Systolic BP (mmHg) | 0.89 | |||
| 8th–16th 24-h Na reduction<25% (n=60) | 123.2±1.7 | 121.5±1.9 | 0.36 | |
| 8th–16th 24-h Na reduction>25% (n=58) | 121.9±1.8 | 120.6±1.9 | 0.49 | |
| Diastolic BP (mmHg) | 0.54 | |||
| 8th–16th 24-h Na reduction<25% (n=60) | 73.7±1.3 | 73.9±1.3 | 0.91 | |
| 8th–16th 24-h Na reduction>25% (n=58) | 74.2±1.3 | 73.3±1.4 | 0.46 | |
| Conventional education | ||||
| 24-h Urine albumin (mg/d)a | 0.03 | |||
| 8th–16th 24-h Na reduction<25% (n=60) | 241.9±1.1 | 234.4±1.1 | 0.66 | |
| 8th–16th 24-h Na reduction>25% (n=58) | 276.4±1.1 | 196.9±1.2 | 0.004 | |
| 24-h Urine Na+ (mEq/d) | <0.001 | |||
| 8th–16th 24-h Na reduction<25% (n=60) | 147.5±6.1 | 162.8±5.5 | <0.001 | |
| 8th–16th 24-h Na reduction>25% (n=58) | 177.7±9.8 | 104.9±9.0 | <0.001 | |
| Systolic BP (mmHg) | 0.76 | |||
| 8th–16th 24-h Na reduction<25% (n=60) | 123.2±1.5 | 122.5±1.4 | 0.68 | |
| 8th–16th 24-h Na reduction>25% (n=58) | 122.1±2.4 | 122.4±2.3 | 0.91 | |
| Diastolic BP (mmHg) | 0.28 | |||
| 8th–16th 24-h Na reduction<25% (n=60) | 73.3±1.0 | 74.8±1.1 | 0.16 | |
| 8th–16th 24-h Na reduction>25% (n=58) | 75.9±1.6 | 75.1±1.7 | 0.68 |
Data are expressed by least squares means±SEMs from a linear mixed effects model.
P value was from the result of a logarithm-transformed variable because of skewed distribution of albumin excretion.
Conversely, the Na reduction ≥25% subgroup displayed a marked reduction of urinary sodium excretion, regardless of the randomization group (P<0.001). This result means that, after LSD education, there was a significant reduction in albuminuria in the Na reduction ≥25% group. In albuminuria, 61.9% of participants in the intensive education group (73 of 118 patients) and 38.7% of participants in the conventional education group (48 of 124 patients) achieved the target of ≥25% reduction. The patients who achieved an Na reduction ≥25% were 26.3% more likely to also achieve ≥25% reduction in albuminuria. Additionally, although the effect of LSD on BP was examined in the subgroup analysis, it was not significant (Table 3).
Adverse Effects
There was no significant adverse event related with olmesartan, and no serious adverse events were reported. The LSD could cause hypotension, but there were no participants who dropped out because of hypotension after randomization. Two and one participants in the intensive and conventional education groups, respectively, withdrew their consent because of minor adverse events: two participants in the intensive education group dropped out because of elevated serum creatinine levels, and one participant in the conventional education group dropped out because of headache. During the study period, hyperkalemia (range of 5.6–6.2 mEq/L) was detected in five participants (2.0%) after olmesartan treatment. However, all of the hyperkalemic events were developed before randomization and corrected with dietary potassium restriction and cation exchange resin.
Discussion
The results of this study showed that the reduction in the 24-hour urinary albumin excretion amount achieved with olmesartan therapy could be further enhanced with intensive LSD education. Without a reduction of salt intake, the additional antiproteinuric effect was attenuated or annihilated.
The recommended dietary salt intake for patients with CKD and hypertension is <5–6 g/d (2.4 g/d for sodium; approximately equal to 100 mEq/d sodium excretion) (22,23). According to the sodium DFQ55 in this study, participants exhibited relatively lower levels of sodium consumption (105.1 mEq at week 0 and 78.6 mEq at week 16, which are equivalent to sodium intakes of 2.4 and 1.8 g/d, respectively) than typically consumed by Korean populations or reported in previous studies (24–26). These underestimated DFQ55 values may reflect the inexactitude of sodium intake estimation only by dietary recall. Moreover, this could explain why the correlation coefficient between the DFQ55 value and the 24-hour urine sodium amount was low. In addition, the finding that the baseline 24-hour urinary sodium excretion amount was relatively low compared with the mean value of the Korean population on the basis of the 2011 Korea Health Statistics (18) can be explained by the possibility that somewhat routine LSD education was already applied and emphasized in study populations according to the guidelines for patients with hypertension and proteinuria.
There were several short-term studies on the effect of restricting salt intake on BP and albuminuria reduction during RAAS blockade (6,9,10). Enhanced treatment effects of LSD education on albuminuria were observed in our study. These effects are in line with previous research and support the analysis that LSDs potentiate the effects of ARBs. However, there was no additional effect on BP in our participants. According to a meta-analysis of randomized, controlled trials, reducing sodium intake by 50 mEq/d decreases systolic and diastolic BPs by means of 3.6 and 1.9 mmHg, respectively, in subjects with hypertension and decreases systolic and diastolic BPs by means of 1.8 and 0.9 mmHg, respectively, in subjects who are normotensive (27). Our results illustrated that intensive education reduced 24-hour urinary sodium excretion by 36.0 mEq/d, systolic BP by 1.7 mmHg, and diastolic BP by 0.5 mmHg. These results are intermediate between the values for subjects with hypertension and subjects who are normotensive as estimated by the BP decrement on the basis of sodium reduction. After the run-in period, the participants of our study exhibited a mean BP at baseline within the normal range with previously prescribed antihypertensive medication. After 8 weeks of olmesartan treatment, systolic and diastolic BPs were further reduced to 122.1 and 73.6 mmHg, respectively. After that time, participants were randomized to receive conventional or intensive LSD education. That could be the reason that the effect of LSD on BP was insufficient to have statistical significance after randomization.
We estimated sodium intake from the 24-hour urinary sodium excretion amount. This is the gold standard measurement for monitoring salt intake and known to be more reliable than food questionnaires. Furthermore, we evaluated the compliance to 24-hour urine collection by calculating the predicted urinary creatinine excretion amount to account for collection errors. The comparative findings were not altered after correcting urinary sodium and albumin values (data not shown), which confirmed the accuracy of the results. The fact that the data were obtained from a homogenous population that was prospectively followed in the setting of a randomized, controlled clinical study is another strength of this study.
The major limitation of this study is that we used a 24-hour volume urine collected at a single time point for each follow-up visit. Urine excretion can be influenced by the sodium intake of individual patients at certain points. In addition, our analysis did not consider a hard end point, such as ESRD. The effects of intensive LSD education by this method on renoprotection, long-term urinary protein excretion, and other determinants of renal function deterioration also need to be identified. Additionally, because of the relatively low sodium intake (3569 mg/d by 24-hour urine analysis) at baseline, these findings may not be generalizable to a diverse population or a nonclinical trial. Although the difference in albuminuria reduction between groups was statistically significant, the magnitude was modest compared with the reduction with RAAS blockade. Therefore, other interventions, such as RAAS blockades, optimal BP control, and appropriate use of diuretics, should be emphasized in clinical practice.
In conclusion, despite similar effects on BP control and eGFR, 24-hour urinary albumin excretion was decreased more in patients in the intensive LSD education group than the conventional education group. This result has two key points. First, LSD effectively reduced proteinuria in nondiabetic patients with hypertension who received ARB therapy. Second, feedback and education by telephone for 30 minutes weekly would be a suitable method for educating on the LSD in actual clinical practice. These findings are relevant to health care providers, because applicable LSD education methods in clinical practice settings effectively reduce proteinuria.
Disclosures
None.
Acknowledgments
The authors thank Sohee Oh at the Department of Biostatistics, Seoul National University Boramae Medical Center for her valuable contribution to the statistical analyses. We thank all of the investigators and their staffs for their participation.
This study was funded by Daiichi Sankyo Korea Co. Ltd. and Daewoong Pharmaceutical Co. Ltd. Olmesartan medoxomil was provided by the same pharmaceutical companies for all participants.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Madan P, Kalra OP, Agarwal S, Tandon OP: Cognitive impairment in chronic kidney disease. Nephrol Dial Transplant 22: 440–444, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Locatelli F, Marcelli D, Comelli M, Alberti D, Graziani G, Buccianti G, Redaelli B, Giangrande A, Northern Italian Cooperative Study Group : Proteinuria and blood pressure as causal components of progression to end-stage renal failure. Nephrol Dial Transplant 11: 461–467, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Balamuthusamy S, Srinivasan L, Verma M, Adigopula S, Jalandhara N, Hathiwala S, Smith E: Renin angiotensin system blockade and cardiovascular outcomes in patients with chronic kidney disease and proteinuria: A meta-analysis. Am Heart J 155: 791–805, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Casas JP, Chua W, Loukogeorgakis S, Vallance P, Smeeth L, Hingorani AD, MacAllister RJ: Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: Systematic review and meta-analysis. Lancet 366: 2026–2033, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G: Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 19: 999–1007, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swift PA, Markandu ND, Sagnella GA, He FJ, MacGregor GA: Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives: A randomized control trial. Hypertension 46: 308–312, 2005 [DOI] [PubMed] [Google Scholar]
- 8.He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN, MacGregor GA: Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension 54: 482–488, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Ekinci EI, Thomas G, Thomas D, Johnson C, Macisaac RJ, Houlihan CA, Finch S, Panagiotopoulos S, O’Callaghan C, Jerums G: Effects of salt supplementation on the albuminuric response to telmisartan with or without hydrochlorothiazide therapy in hypertensive patients with type 2 diabetes are modulated by habitual dietary salt intake. Diabetes Care 32: 1398–1403, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slagman MC, Waanders F, Hemmelder MH, Woittiez AJ, Janssen WM, Lambers Heerspink HJ, Navis G, Laverman GD, HOlland NEphrology STudy Group : Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: Randomised controlled trial. BMJ 343: d4366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houlihan CA, Allen TJ, Baxter AL, Panangiotopoulos S, Casley DJ, Cooper ME, Jerums G: A low-sodium diet potentiates the effects of losartan in type 2 diabetes. Diabetes Care 25: 663–671, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Allen TJ, Waldron MJ, Casley D, Jerums G, Cooper ME: Salt restriction reduces hyperfiltration, renal enlargement, and albuminuria in experimental diabetes. Diabetes 46: 19–24, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Vegter S, Perna A, Postma MJ, Navis G, Remuzzi G, Ruggenenti P: Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol 23: 165–173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ying WZ, Sanders PW: Dietary salt enhances glomerular endothelial nitric oxide synthase through TGF-beta1. Am J Physiol 275: F18–F24, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND: Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol 28: 158–167, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA: Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496: 518–522, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK: Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496: 513–517, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Health and Welfare : Korea Health Statistics 2011: Korea National Health and Nutrition Examination Survey (KNHANES V-2), Seoul, South Korea, Ministry of Health and Welfare, 2012 [Google Scholar]
- 19.Geleijnse JM, Kok FJ, Grobbee DE: Blood pressure response to changes in sodium and potassium intake: A metaregression analysis of randomised trials. J Hum Hypertens 17: 471–480, 2003 [DOI] [PubMed] [Google Scholar]
- 20.McMahon EJ, Bauer JD, Hawley CM, Isbel NM, Stowasser M, Johnson DW, Campbell KL: A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol 24: 2096–2103, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawasaki T, Uezono K, Itoh K, Ueno M: Prediction of 24-hour urinary creatinine excretion from age, body weight and height of an individual and its application. Nippon Koshu Eisei Zasshi 38: 567–574, 1991 [PubMed] [Google Scholar]
- 22.Conlin PR, Chow D, Miller ER, 3rd, Svetkey LP, Lin PH, Harsha DW, Moore TJ, Sacks FM, Appel LJ: The effect of dietary patterns on blood pressure control in hypertensive patients: Results from the Dietary Approaches to Stop Hypertension (DASH) trial. Am J Hypertens 13: 949–955, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee : The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 24.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) : Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 25.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G: Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 354: 359–364, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Lambers Heerspink HJ, Holtkamp FA, Parving HH, Navis GJ, Lewis JB, Ritz E, de Graeff PA, de Zeeuw D: Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int 82: 330–337, 2012 [DOI] [PubMed] [Google Scholar]
- 27.He FJ, MacGregor GA: How far should salt intake be reduced? Hypertension 42: 1093–1099, 2003 [DOI] [PubMed] [Google Scholar]