Abstract
Background and objectives
Many children receiving extracorporeal membrane oxygenation develop AKI. If AKI leads to permanent nephron loss, it may increase the risk of developing CKD. The prevalence of CKD and hypertension and its predictive factors during long-term follow-up of children and adolescents previously treated with neonatal extracorporeal membrane oxygenation were determined.
Design, setting, participants, & measurements
Between November of 2010 and February of 2014, neonatal survivors of extracorporeal membrane oxygenation who visited the prospective follow-up program at 1, 2, 5, 8, 12, and 18 years of age were screened for CKD and hypertension (BP≥95th percentile of reference values). CKD was suspected in children with either an eGFR<90 ml/min per 1.73 m2 or proteinuria (urinary protein-to-creatinine ratio >0.50 for children ages ≤24 months and >0.20 at >24 months). The RIFLE classification (risk, injury, or failure as 150%, 200%, or 300% of serum creatinine reference values) was used to define AKI during extracorporeal membrane oxygenation without preemptive hemofiltration.
Results
Median follow-up of 169 screened participants was 8.2 years (interquartile range=5.2–12.1 years). Nine children had a lower eGFR, but all rates were >60 ml/min per 1.73 m2. Proteinuria was observed in 20 children (median=0.26 mg protein/mg creatinine; interquartile range=0.23–0.32 mg protein/mg creatinine), and 32 children had hypertension. Only history of AKI was associated with CKD (P=0.004). Children with RIFLE scores injury and failure had 4.3 times higher odds of CKD signs or hypertension than those without AKI (95% confidence interval, 1.6 to 12.1; P=0.004).
Conclusions
Altogether, 54 participants (32%) had at least one sign of CKD and/or hypertension. However, most values were marginally abnormal, with no immediate consequences for clinical care. Nevertheless, a prevalence of 32% clearly indicates that survivors of neonatal extracorporeal membrane oxygenation, especially those with AKI, are at risk of a more rapid decline of kidney function with increasing age. Therefore, screening for CKD development in adulthood is recommended.
Keywords: CKD, epidemiology and outcomes, children, acute renal, failure, clinical nephrology
Introduction
AKI is a frequent complication among critically ill children admitted to an intensive care unit (ICU). Reported incidences vary widely, between 4.5% and 82%, depending on the definition used and the population studied (1–6). Moreover, AKI is an independent risk factor for prolonged mechanical ventilation and pediatric ICU mortality (1,4). Recently, our group and other research groups reported high incidences of AKI in children treated with extracorporeal membrane oxygenation (ECMO), who tend to be the most critically ill admitted to an ICU (7–10).
Severe AKI can result in a substantial loss of functioning nephrons, which may lead to hyperfiltration in the spared ones (11). Single-nephron hyperfiltration, in turn, leads to glomerular sclerosis, interstitial fibrosis, and tubular atrophy. These pathologic changes may eventually lead to CKD, a condition characterized by a progressive decline in GFR over time, proteinuria, and/or systemic hypertension (12). Timely therapeutic interventions can slow the progression of renal injury, and medication should be titrated to actual clearance (13,14).
Over the past years, research has largely focused on short-term implications of AKI. The few follow-up studies in survivors of pediatric AKI in patients not treated with ECMO report prevalence of CKD from 6%–59% (15–19). This wide range may be partly explained by the heterogeneity of AKI and CKD definitions, the inclusion of patients with AKI of varying causes, including preexisting renal disease, and the limited sample sizes. Nonetheless, all studies clearly indicate that survivors of AKI during childhood are at risk of residual renal injury.
Several attempts have been made to evaluate renal recovery of survivors of pediatric ECMO. Two studies showed that 96% of survivors of pediatric ECMO who had received RRT recovered renal function before hospital discharge (20,21). Short-term recovery, however, does not rule out the development of CKD in the long run. To our knowledge, long-term follow-up of renal injury in children or adults treated with ECMO has not yet been addressed. The aim of our study was, therefore, to determine the prevalence of CKD and evaluate its predictive factors during long-term follow-up of children and adolescents previously treated with neonatal ECMO.
Materials and Methods
Setting and Participants
This cross-sectional study was conducted in our follow-up clinic between November 2010 and February 2014 and included children who had survived neonatal ECMO (ECMO onset ≤28 days after birth) between January 1, 1992, and December 31, 2012, at our hospital. We excluded children (1) with inadequate ECMO data, (2) already transferred to adult care, and (3) with congenital abnormalities of the kidneys and urinary tract. When children attended the follow-up clinic more than one time, only data of the latest available visit were used.
ECMO was initiated on reversible severe cardiorespiratory failure and an estimated mortality risk >80% using the criteria reported by Stolar et al. (22). The ECMO circuit was primed as described elsewhere (23). From 2005 onward, all patients received by protocol preemptive continuous hemofiltration (CH) by placement of a hemofilter (Multiflow 60; Hospal, Lyon, France) parallel to the ECMO circuit and distal to the roller pump. Pressure was measured proximal and distal to the filter. The pressure difference was kept constant at 40 mmHg. During CH, the predilution flow rate of the filtration fluid (HF-BIC32; Dirinco, Rosmalen, The Netherlands) was set at the default of 50 ml/kg per hour.
This study was part of a structured prospective post-ECMO follow-up program initiated in 2001, in which lung function, growth, and developmental parameters are routinely assessed at ages 1, 2, 5, 8, 12, and 18 years (24,25). The Medical Ethical Review Board of Erasmus Medical Center provided a waiver for ethics approval. On the basis of Dutch law, the study needs no approval, because it concerns only analyses of data collected in the context of clinical care. Also, no informed consent is needed for such studies (excerpt from the Ethical Review Board’s letter: “Medical Research in Human Subjects Act does not apply to this study, since subjects are not being submitted to any handling, nor are there rules of human behaviour being imposed”).
Definitions of CKD Signs and Hypertension
CKD was defined as either an abnormal eGFR or proteinuria. During each visit, height, weight, and BP were measured, and blood and urine samples were taken and processed according to standardized protocols for determining serum creatinine (SCr) level (milligrams per deciliter) and urinary protein-to-creatinine (uP/C) ratio (milligrams of protein per milligram of creatinine).
Height was expressed in absolute value and SD score (SDS) for chronological age according to growth charts adjusted for country of origin (26). Body mass index (BMI) was defined as weight (kilograms)/height (meters2) and adjusted for age and sex to give a BMI SDS (27–29). Creatinine was assessed by an enzymatic assay (Creatinine Plus; Roche Diagnostics GmbH, Mannheim, Germany) on a Cobas 8000 Analyzer (Roche Diagnostics). Total urine protein concentration was determined by a turbidimetric assay on the Cobas 8000 Analyzer.
In all participants, GFR was estimated using the revised Schwartz Equation (0.413×height [centimeters]/SCr) (30). In participants ages >16 years, it was also estimated using the Modification of Diet in Renal Disease (MDRD) formula, because the cohorts used to develop and validate the Schwartz Equation all had very small proportions of adolescents (30–32). An eGFR<90 ml/min per 1.73 m2 was considered abnormal and staged (CKD stages G1–G5) according to the Kidney Disease Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guideline for the evaluation and management of CKD (33). Glomerular hyperfiltration was defined as an eGFR≥150 ml/min per 1.73 m2 (34).
Significant proteinuria was quantified as a uP/C ratio>0.50 mg protein/mg creatinine for children age ≤24 months and >0.20 mg protein/mg creatinine for children >24 months (35). If proteinuria was identified, urinalysis was repeated three times in a first morning sample to rule out an orthostatic effect. If all morning samples showed a normal uP/C ratio, midday proteinuria was considered orthostatic, and the participant was scored negative for proteinuria caused by CKD.
Arterial BP measurements (millimeters of Hg) were carried out with a cuff appropriate to the size of the child’s upper arm using an electronic device (Dynamap; Critikon, Tampa, FL) three times at 1-minute intervals with the child seated after 5 minutes of rest. The mean systolic and diastolic BPs of the second and third readings were calculated. Prehypertension and stages 1 and 2 hypertension were defined as a mean systolic and/or diastolic BP between 90th and 95th percentiles, BP≥95th and ≤99th percentiles, or BP>99th percentile, respectively, of reference values for sex, height SDS, and age (36).
Data Collection
The following data were collected from medical records: sex, gestational age, birth weight, use of nitric oxide ventilation, vasopressor drugs, and oxygenation index pre-ECMO. In addition, we collected data on the ECMO run and length of ICU stay in our hospital. Ultimately, for each patient, history of AKI was defined according to the maximal SCr-based RIFLE category obtained throughout ECMO: risk for kidney injury, injury to the kidney, and failure of kidney function (3). Because reliable SCr baseline concentrations pre-ICU admission were lacking in most patients, RIFLE categories risk, injury, and failure were defined as SCr>150%, >200%, and >300% of the median of age-specific SCr reference values, respectively (37). Urinary output criteria were not used to assign AKI severity stages. If CH during ECMO was provided preemptively, RIFLE scores could not be reliably estimated because of extrarenal SCr clearance and were scored as missing.
Statistical Analyses
Data are expressed as median values with interquartile ranges (IQRs) for continuous variables or numbers with percentages for categorical variables, unless indicated otherwise; 95% binomial confidence intervals (95% CIs) were calculated with the Agresti–Coull method. Univariate overall comparisons between groups using Mann–Whitney, Pearson chi-squared, or Fisher exact tests, as appropriate, were performed to detect differences between groups (participants versus nonparticipants and CKD versus no CKD) and determine the association between signs of CKD and hypertension. Linear-by-linear association chi-squared test (or Mantel–Haenszel test for linear association) was used to evaluate the association between history of AKI during ECMO (non-AKI, risk, injury, and failure) and CKD signs. Reported are odds ratios with 95% CIs. A two-sided P value=0.05 was considered the limit of significance in all analyses. Analyses were performed using SPSS, version 21.
Results
Study Population
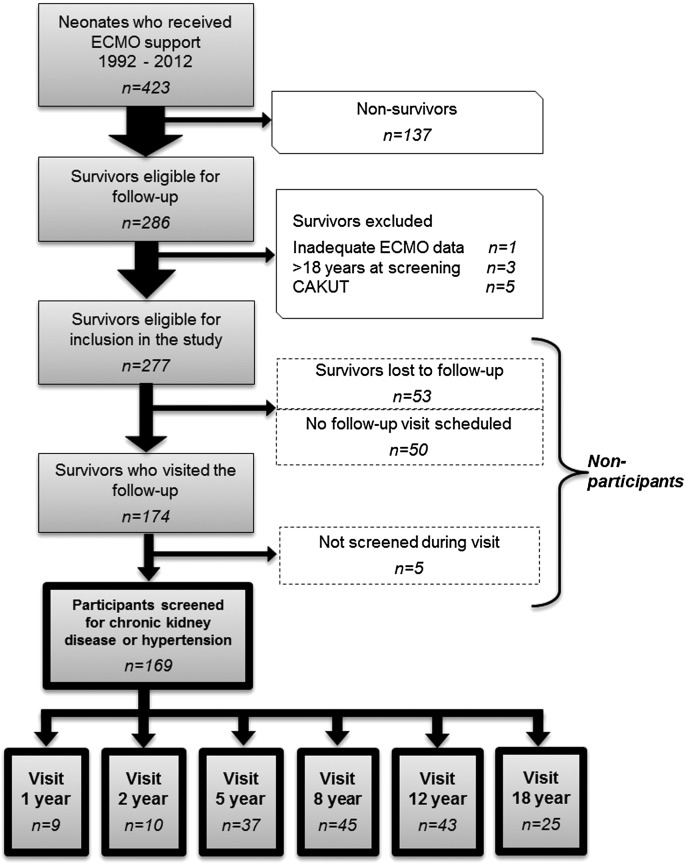
From 1992 to 2012, 277 of 423 (65%) neonates who received ECMO treatment survived and met eligibility criteria; 53 (19%) children were lost to follow-up for reasons such as nonresponse or refusal, 50 (18%) children had a scheduled follow-up visit within the study period, and neither SCr nor uP/C was assessed in five (2%) children who did visit the outpatient clinic. These 108 children are referred to as nonparticipants. Ultimately, the study cohort consisted of 169 participants (Figure 1).
Figure 1.
Flowchart of patient recruitment. Flowchart detailing inclusion and exclusion criteria for children who survived neonatal ECMO support, which resulted in the final study cohort. CAKUT, congenital abnormality of the kidneys and urinary tract; ECMO, extracorporeal membrane oxygenation.
Baseline Characteristics
Participants versus nonparticipants did not differ in baseline or clinical characteristics (Table 1). The most common diagnoses to initiate ECMO were meconium aspiration syndrome (52%) and congenital diaphragmatic hernia (21%). Median ECMO duration was 119 hours (IQR=84–156 hours). Of all participants, 31 (18%) participants initially received venovenous ECMO. Of 107 participants who received ECMO without preemptive CH, evidence of AKI during ECMO was present in 64 patients; 34 (32%) patients were classified as risk, 25 (23%) patients were classified as injury, and five (5%) patients were classified as failure. Of 62 participants who received ECMO with preemptive CH, 12 (19%) participants had SCr levels indicating AKI; however, all were scored as missing.
Table 1.
Baseline and clinical characteristics of participants versus nonparticipants
| Characteristics | Participants (n=169) | Nonparticipants (n=108) | P |
|---|---|---|---|
| Sex (girls) | 75 (44) | 49 (45) | 0.90a |
| Gestational age, wk | 40.0 (38.6–41.3) | 39.7 (39.0–40.9) | 0.78b |
| Birth weight, kg | 3.4 (3.0–3.8) | 3.4 (2.9–3.8) | 0.99b |
| Diagnosis | |||
| Meconium aspiration syndrome | 87 (52) | 50 (46) | |
| Congenital diaphragmatic hernia | 36 (21) | 13 (12) | |
| Isolated persistent pulmonary | 21 (12) | 14 (13) | 0.09a |
| Sepsis | 9 (5) | 16 (15) | |
| Other | 16 (10) | 15 (14) | |
| Pre-ECMO | |||
| Age at start ECMO, h | 41 (28–73) | 42 (28–68) | 0.84b |
| Vasopressor drugs (yes) | 161 (95) | 102 (94) | 0.77a |
| Nitric oxide ventilation (yes) | 133 (79) | 84 (78) | >0.99a |
| Highest oxygenation index | 42 (31–55) | 42 (31–56) | 0.74b |
| During ECMO | |||
| ECMO mode (VV) | 27 (16) | 10 (9) | 0.26a |
| Conversion (VV to VA) | 4 (2) | 3 (3) | |
| Preemptive continuous hemofiltration (yes) | 62 (37) | 32 (30) | 0.24a |
| RIFLE scores | 107/169 (63) | 76/108 (70) | |
| Missing RIFLE scores because of preemptive continuous hemofiltration | 62 (37) | 32/108 (30) | |
| Maximum RIFLE scorea | |||
| No AKI | 43/107 (40) | 32/76 (42) | |
| Risk | 34/107 (32) | 25/76 (33) | |
| Injury | 25/107 (23) | 17/76 (22) | 0.92a |
| Failure | 5/107 (5) | 2/76 (3) | |
| RRT | 2/5 (40) | 1/2 (50) | |
| ECMO duration, h | 119 (84–156) | 129 (86–187) | 0.16b |
| ICU admission, d | 10 (6–26) | 9 (6–17) | 0.20b |
RIFLE categories risk, injury, and failure were defined as serum creatinine >150%, >200%, and >300% of the median of age-specific reference values, respectively. RIFLE scores were only assessed among patients treated with neonatal ECMO without preemptive continuous hemofiltration. Continuous data are expressed as median (interquartile range), and categorical data are expressed as number (percentage). ECMO, extracorporeal membrane oxygenation; VV, venovenous; VA, venoarterial; ICU, intensive care unit.
Assessment of intergroup differences (that is, participants versus nonparticipants) using the Pearson chi-squared or Fisher exact test.
Assessment of intergroup differences (that is, participants versus nonparticipants) using the Mann–Whitney test.
Screening
The median time until screening was 8.2 years (IQR=5.2–12.1 years) after neonatal ECMO. The median SDS height and BMI were −0.40 (IQR=−1.05–0.51) and −0.05 (IQR=−0.75–0.77), respectively.
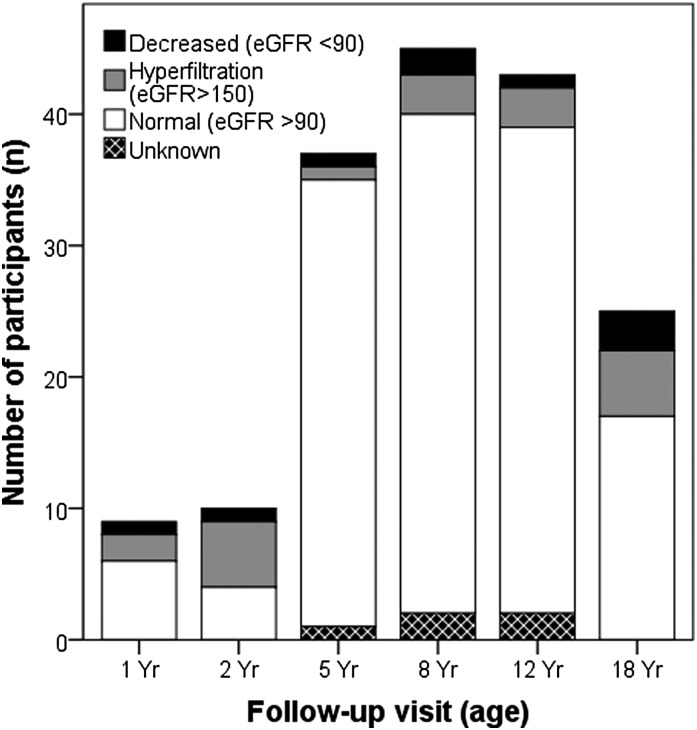
SCr levels for eGFR were measured in 164 (97%) participants. The median eGFR was 117 ml/min per 1.73 m2 (IQR=104–133 ml/min per 1.73 m2). In nine (5%) children, the eGFR was between 90 and 60 ml/min per 1.73 m2, which was classified as CKD stage G2. None of the participants had an eGFR<60 ml/min per 1.73 m2. Two participants ages >16 years and initially staged as CKD stage G2 using the Schwartz Equation had an eGFR>90 ml/min per 1.73 m2 (CKD stage G1) using the MDRD formula. In contrast, 19 (12%) children had an eGFR≥150 ml/min per 1.73 m2, suggesting hyperfiltration (Figure 2).
Figure 2.
GFR screening results for all patients stratified by age group. GFR was estimated using the revised Schwartz Equation (0.413×height [centimeters]/serum creatinine). An eGFR<90 ml/min per 1.73 m2 was considered abnormal. Glomerular hyperfiltration was defined as an eGFR≥150 ml/min per 1.73 m2. SCr, serum creatinine.
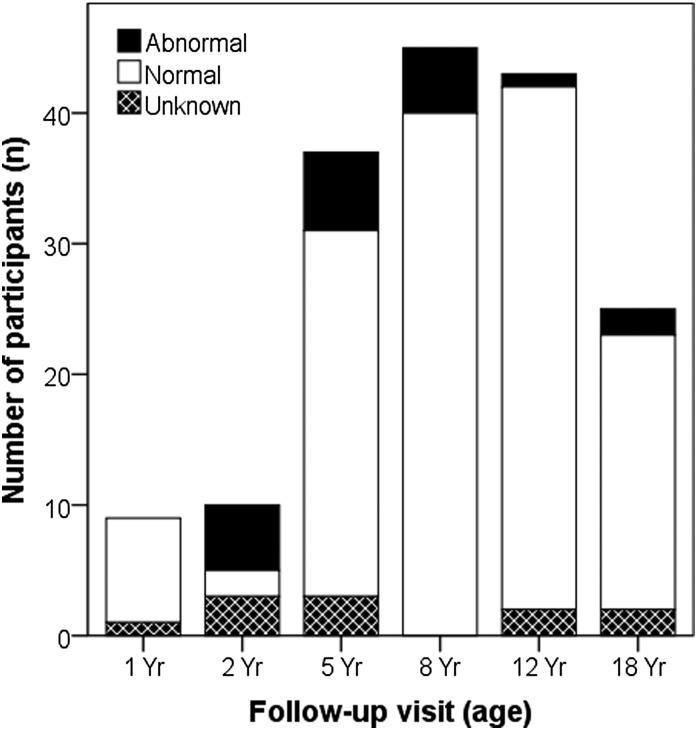
Urine samples were collected in 158 (93%) participants. uP/C ratio was higher in 24 (15%) children, of whom one child was <24 months old (uP/C ratio=0.59 mg protein/mg creatinine) and 23 children were >24 months old (median=0.26 mg protein/mg creatinine; IQR=0.27–0.39 mg protein/mg creatinine). Also, four participants had orthostatic proteinuria. Hence, 20 (12%) children had persistent proteinuria on repeated testing (median=0.26 mg protein/mg creatinine; IQR=0.23–0.32 mg protein/mg creatinine) (Figure 3).
Figure 3.
Urinary protein-to-creatinine screening results for all patients stratified by age group. Significant proteinuria was quantified as urinary protein-to-creatinine ratio >0.50 mg protein/mg creatinine for children ages ≤24 months and >0.20 mg protein/mg creatinine for children ages >24 months. If proteinuria was identified, urinalysis was repeated three times in a first morning sample to rule out an orthostatic effect. In case of orthostatic proteinuria, the participant was scored negative for proteinuria. uP/C ratio, urinary protein-creatinine ratio.
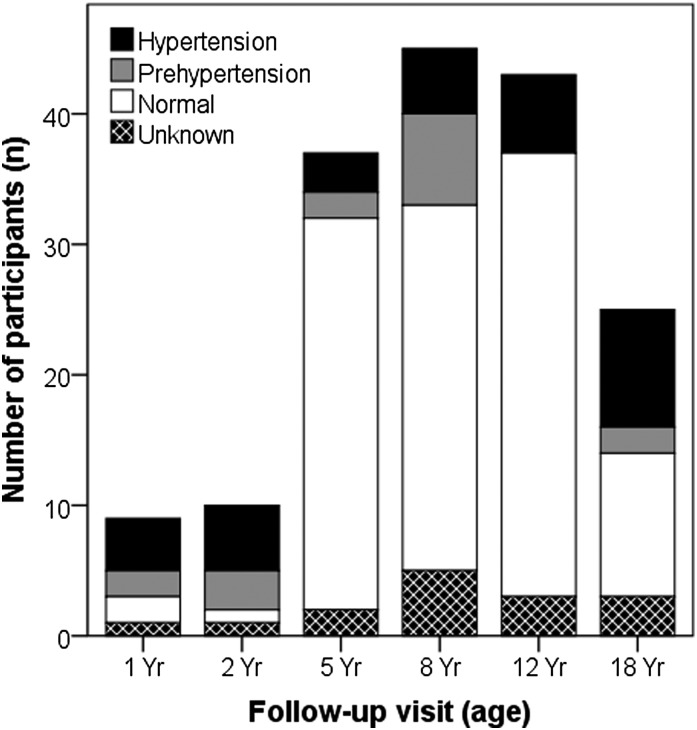
None of the participants screened had a history of hypertension or were receiving antihypertensive medication. BP was validly measured in 154 (91%) participants. In the remaining 15 participants, measurements were unreliable because of agitation. Sixteen (10%) participants met criteria for prehypertension. Twenty-two (14%) participants met criteria of stage 1 hypertension on the basis of either systolic BP (n=16) or diastolic BP (n=6). Ten (7%) participants met criteria of stage 2 hypertension on the basis of either systolic BP (n=8) or diastolic BP (n=2). Only one participant had hypertension with both systolic and diastolic BP>99th percentile (Figure 4).
Figure 4.
BP screening results for all patients stratified by age group. Prehypertension and stages 1 and 2 hypertension were defined as a mean systolic and/or diastolic BP between 90th and 95th percentiles, BP≥95th and ≤99th percentiles, or BP>99th percentiles of reference values for sex, height SD score, and age, respectively.
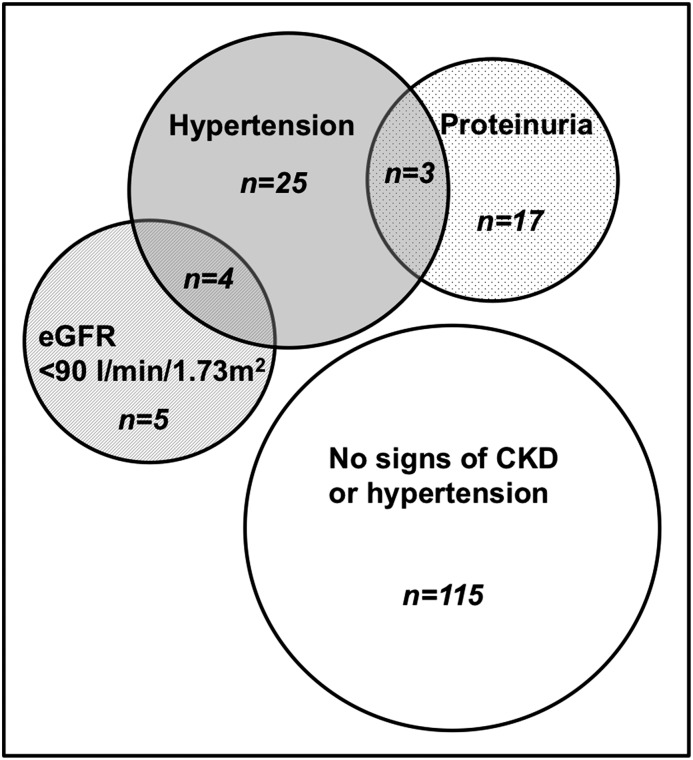
Altogether, signs of CKD, including either a lower eGFR or higher uP/C ratio, were present in 29 (17%; 95% CI, 12 to 24) participants, whereas 32 (19%; 95% CI, 14 to 26) participants had stage 1 or 2 hypertension. Moreover, four participants with a lower eGFR and three participants with proteinuria also presented with hypertension (Figure 5). CKD signs were not associated with hypertension (Fisher exact test, P>0.99 for both proteinuria and lower eGFR). None of the children presented with abnormal test results on all three parameters. Also, zero of eight children with BMI>2 SDS had hypertension.
Figure 5.
The primary outcome of all participants screened. GFR was estimated using the revised Schwartz Equation (0.413×height [centimeters]/serum creatinine). An eGFR<90 ml/min per 1.73 m2 was considered abnormal. Significant proteinuria was quantified as a urinary protein-to-creatinine ratio >0.50 mg protein/mg creatinine for children ages ≤24 months and >0.20 mg protein/mg creatinine for children ages >24 months. Prehypertension and stages 1 and 2 hypertension were defined as a mean systolic and/or diastolic BP between 90th and 95th percentiles, BP≥95th and ≤99th percentiles, or BP>99th percentiles of reference values for sex, height SD score, and age, respectively.
Associations with CKD
Univariate analysis explored the association between CKD signs or hypertension at follow-up and clinical variables during ICU admission, including diagnosis, nitric oxide ventilation pre-ECMO, ECMO mode and duration, preemptive CH during ECMO, and history of AKI during ECMO (Table 2). The clinical variable history of AKI during ECMO was explored solely among 107 participants having received ECMO without preemptive CH. A higher proportion of participants with AKI according to the RIFLE criteria had CKD signs or hypertension (22 of 64 participants; 34%) compared with the non-AKI group (nine of 43 participants; 21%; linear-by-linear association chi-squared test, P=0.004). Children with RIFLE scores injury and failure had 4.3 times higher odds of CKD signs or hypertension than those without AKI (95% CI, 1.6 to 12.1; P=0.004). None of the other examined variables were associated with CKD (all P>0.05) (Table 2).
Table 2.
Association between clinical variables and the presence of CKD signs or hypertension (n=169)
| Clinical Variable | Non-CKD (n=115) | CKD or Hypertension (n=54) | P |
|---|---|---|---|
| Gestational age, wk | 40.0 (38.6–41.1) | 39.7 (39.0–41.0) | 0.63a |
| Diagnosis | |||
| Meconium aspiration syndrome | 62 (54) | 25 (46) | 0.87b |
| Congenital diaphragmatic hernia | 22 (19) | 14 (26) | |
| Isolated persistent pulmonary | 14 (12) | 7 (13) | |
| Sepsis | 6 (5) | 3 (6) | |
| Other | 11 (10) | 5 (9) | |
| Pre-ECMO | |||
| Age at start ECMO, h | 41 (26–74) | 43 (31–74) | 0.50a |
| Vasopressor drugs (yes) | 108 (94) | 53 (98) | 0.23b |
| Nitric oxide ventilation (yes) | 92 (80) | 41 (76) | 0.55b |
| Highest oxygenation index | 41 (31–53) | 44 (30–58) | 0.50a |
| During ECMO | |||
| ECMO mode (VV) | 20 (17) | 11 (20) | 0.72b |
| Preemptive continuous hemofiltration (yes) | 39 (34) | 23 (43) | 0.28b |
| Patients on ECMO without preemptive continuous hemofiltration | 76 | 31 | |
| Maximum RIFLE scoreb | |||
| No AKI | 34 (44) | 9 (29) | |
| Risk | 28 (37) | 6 (19) | |
| Injury | 12 (16) | 13 (42) | 0.004b |
| Failure | 2 (3) | 3 (10) | |
| ECMO duration, h | 120 (89–159) | 105 (74–155) | 0.37a |
RIFLE categories risk, injury, and failure were defined as serum creatinine>150%, >200%, and >300% of the median of age-specific reference values, respectively. RIFLE scores were only assessed among patients treated with neonatal ECMO without preemptive continuous hemofiltration. Continuous data are expressed as median (interquartile range), and categorical data are expressed as number (percentage).
Assessment of intergroup differences (that is, non-CKD versus CKD) using the Mann–Whitney test.
Assessment of intergroup differences (that is, non-CKD versus CKD) using the Pearson chi-squared or linear-by-linear association chi-squared test.
Discussion
This is the first study, to our knowledge, prospectively evaluating the prevalence of CKD signs and hypertension during long-term follow-up in a large cohort of children previously treated with neonatal ECMO. A homogeneous study group was created by enrolling only those children who received neonatal ECMO support and excluding children with congenital abnormalities of the kidneys and urinary tract. Given that 54 (32%) participants fulfilled the predefined criteria for CKD or hypertension, this study shows that survivors of ECMO are at increased risk of developing CKD or hypertension, irrespective of AKI history. Still, most values were mildly abnormal, and clinical interventions were not yet needed apart from lifestyle advice or monitoring by a general practitioner. Because CKD progresses very slowly, more advanced CKD may not occur until adulthood. Nevertheless, one must realize that there may be limited renal reserve in these children.
Thirty-two (19%) participants had hypertension, which is considerably higher than a prevalence of 0.3% that was shown in a recent community-based study (38). Because in our study, BP readings were performed during a single visit instead of repetitively, we may have overestimated the hypertension prevalence. Of the clinical variables examined, only history of AKI during ECMO was significantly associated with CKD signs or hypertension. Thus, participants with positive markers of CKD did not differ by diagnosis or other tested clinical variables.
A surprising finding is the high occurrence of hyperfiltration (12%; 19 of 169 participants). This may partially be explained by the relationship between SCr levels and muscle mass. Considering that, in an earlier study from our group, neonatal survivors who had been treated with ECMO showed a significantly reduced exercise capacity, participants may have reduced creatinine generation proportional to a low muscle mass (39). In these patients, creatinine clearance tends to overestimate GFR, whereas the protein-to-creatinine ratio will underestimate protein excretion. Thus, the actual prevalence of CKD may be underestimated in this study.
Prior follow-up studies in critically ill patients not treated with ECMO reported CKD prevalence ranging from 6% to 59% (15–19). The highest incidence (17 of 29 patients; 59%), which was reported by Askenazi et al. (15), in children surviving AKI of varying causes may partly be explained by the inclusion of hyperfiltration in their definition of CKD. Mammen et al. (17), in contrast, used a stricter CKD definition (albuminuria and/or eGFR<60 ml/min per 1.73 m2) and found an incidence of 10% after a median follow-up of 1.1 years among 126 patients surviving AKI in the pediatric ICU. When applying this strict definition to our cohort, the CKD incidence would have been similar (12%; 19 of 162 participants). Ultimately, Mammen et al. (17) reported that one of six (16.6%) patients treated with ECMO surviving AKI had CKD but did not provide detailed clinical data.
Major strengths of our study include the large number of participants and the long follow-up time until screening. The follow-up time in previous studies in pediatric ICU survivors was no longer than 3 years, thereby potentially underestimating the true burden of CKD (17). This was not an issue in our study, because 40% of all participants were at least 12 years old. In addition, we used a standard definition to systematically classify AKI during ECMO to explore the potential association with CKD. Children were assigned RIFLE scores during ECMO using SCr values compared with age-appropriate SCr reference values. These SCr reference values were obtained from children without kidney dysfunction daily shortly after birth and at increasing intervals thereafter (37). This method, therefore, allowed us to reliably identify various degrees of AKI during neonatal ECMO support, despite the maternal SCr influence and rapidly changing renal function after birth as well as the absence of baseline SCr concentrations, which was the case with the majority of neonates requiring ECMO.
Several limitations can be identified. First, not all survivors of ECMO could be included, because some were lost to follow-up or not scheduled for follow-up within the study period. Nonetheless, baseline characteristics of the participants did not differ from those of the nonparticipants. Hence, we feel that our results can be interpreted without significant selection bias. Second, only a global assessment of chronic kidney damage was performed: GFR was estimated by a formula instead of measured by plasma clearance studies, BP was measured incidentally at the outpatient clinic and not by ambulant BP measurement at home, and no ultrasound study of the kidneys was performed. The methods applied were those that could be relatively easy implemented in our follow-up program. On the basis of these results, future research should include more rigorous methods to gain a more in depth insight on CKD. Third, proteinuria was evaluated according to a uP/C ratio. On one hand, if the denominator (urine creatinine concentration) is low, which might be the case in individuals with a reduced muscle mass, the ratio may easily overestimate proteinuria (40,41). On the other hand, the prevalence of proteinuria was so much higher than reported in healthy school-aged children and adolescents who were born preterm that we feel this finding cannot fully be explained by overestimation because of reduced muscle mass (42,43). Fourth, the association between history of AKI during ECMO and CKD signs or hypertension was solely explored among participants who received neonatal ECMO without CH. The reason is that extrarenal elimination of creatinine precludes the use of SCr levels for AKI assessment during CH, more so because we exclusively used SCr without urine criteria for grading AKI severity (44). Fifth, in contrast to the United Kingdom Collaborative ECMO Trial, our study was not a randomized study with controls suffering from severe neonatal cardiorespiratory failure and treated conventionally (45). With only two ECMO centers in The Netherlands, the large majority of neonates with similar severity of illness is treated with ECMO. Therefore, we could not include a sufficient number of children who survived without ECMO to serve as a control group, because use of historical data is not desirable.
Altogether, this study shows that, after a median follow-up of 8 years, almost one third of all neonatal survivors treated with ECMO have at least one sign of CKD and/or hypertension. To date, the immediate clinical implications are still limited given the only slightly abnormal screening results. Nevertheless, a prevalence of 32%, even higher in children with a prior history of AKI, clearly indicates that these survivors are at risk of developing CKD or hypertension, necessitating adequate treatment given the limited renal reserve. Therefore, even longer-term follow-up, including wide-interval screening for signs of chronic kidney damage in adulthood, is recommended in all patients treated with ECMO.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the Dutch National ECMO Follow-Up Team and, specifically, Marjolein Spoel (physician) for her contribution to this study. Eiske M. Dorresteijn (pediatric nephrologist) critically reviewed the manuscript. The authors also thank Ko Hagoort for editorial assistance.
This work was supported by an unrestricted grant from the Sophia Foundation for Scientific Research (633).
The data from this study were presented in part at the 16th Triannual Congress of the International Pediatric Nephrology Association, August 30–September 3, 2013, in Shanghai, China.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02890314/-/DCSupplemental.
References
- 1.Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, Phan V, Zappitelli M: Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: A two-center retrospective cohort study. Crit Care 15: R146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey D, Phan V, Litalien C, Ducruet T, Mérouani A, Lacroix J, Gauvin F: Risk factors of acute renal failure in critically ill children: A prospective descriptive epidemiological study. Pediatr Crit Care Med 8: 29–35, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL: Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71: 1028–1035, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Schneider J, Khemani R, Grushkin C, Bart R: Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med 38: 933–939, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Plötz FB, Bouma AB, van Wijk JA, Kneyber MC, Bökenkamp A: Pediatric acute kidney injury in the ICU: An independent evaluation of pRIFLE criteria. Intensive Care Med 34: 1713–1717, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Koralkar R, Ambalavanan N, Levitan EB, McGwin G, Goldstein S, Askenazi D: Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res 69: 354–358, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Zwiers AJ, de Wildt SN, Hop WC, Dorresteijn EM, Gischler SJ, Tibboel D, Cransberg K: Acute kidney injury is a frequent complication in critically ill neonates receiving extracorporeal membrane oxygenation: A 14-year cohort study. Crit Care 17: R151, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadepalli SK, Selewski DT, Drongowski RA, Mychaliska GB: Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: An insidious problem. J Pediatr Surg 46: 630–635, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Smith AH, Hardison DC, Worden CR, Fleming GM, Taylor MB: Acute renal failure during extracorporeal support in the pediatric cardiac patient. ASAIO J 55: 412–416, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Askenazi DJ, Ambalavanan N, Hamilton K, Cutter G, Laney D, Kaslow R, Georgeson K, Barnhart DC, Dimmitt RA: Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatr Crit Care Med 12: e1–e6, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Brenner BM, Mackenzie HS: Nephron mass as a risk factor for progression of renal disease. Kidney Int Suppl 63: S124–S127, 1997 [PubMed] [Google Scholar]
- 12.Metcalfe W: How does early chronic kidney disease progress? A background paper prepared for the UK Consensus Conference on early chronic kidney disease. Nephrol Dial Transplant 22[Suppl 9]: ix26–ix30, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Zandi-Nejad K, Brenner BM: Primary and secondary prevention of chronic kidney disease. J Hypertens 23: 1771–1776, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL: 3-5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69: 184–189, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Buysse CM, Raat H, Hazelzet JA, Hulst JM, Cransberg K, Hop WC, Vermunt LC, Utens EM, Maliepaard M, Joosten KF: Long-term health status in childhood survivors of meningococcal septic shock. Arch Pediatr Adolesc Med 162: 1036–1041, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG: Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: A prospective cohort study. Am J Kidney Dis 59: 523–530, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Slack R, Hawkins KC, Gilhooley L, Addison GM, Lewis MA, Webb NJ: Long-term outcome of meningococcal sepsis-associated acute renal failure. Pediatr Crit Care Med 6: 477–479, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Abitbol CL, Bauer CR, Montané B, Chandar J, Duara S, Zilleruelo G: Long-term follow-up of extremely low birth weight infants with neonatal renal failure. Pediatr Nephrol 18: 887–893, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Meyer RJ, Brophy PD, Bunchman TE, Annich GM, Maxvold NJ, Mottes TA, Custer JR: Survival and renal function in pediatric patients following extracorporeal life support with hemofiltration. Pediatr Crit Care Med 2: 238–242, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Paden ML, Warshaw BL, Heard ML, Fortenberry JD: Recovery of renal function and survival after continuous renal replacement therapy during extracorporeal membrane oxygenation. Pediatr Crit Care Med 12: 153–158, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stolar CJ, Snedecor SM, Bartlett RH: Extracorporeal membrane oxygenation and neonatal respiratory failure: Experience from the extracorporeal life support organization. J Pediatr Surg 26: 563–571, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Blijdorp K, Cransberg K, Wildschut ED, Gischler SJ, Jan Houmes R, Wolff ED, Tibboel D: Haemofiltration in newborns treated with extracorporeal membrane oxygenation: A case-comparison study. Crit Care 13: R48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nijhuis-van der Sanden MW, van der Cammen-van Zijp MH, Janssen AJ, Reuser JJ, Mazer P, van Heijst AF, Gischler SJ, Tibboel D, Kollée LA: Motor performance in five-year-old extracorporeal membrane oxygenation survivors: A population-based study. Crit Care 13: R47, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanekamp MN, Mazer P, van der Cammen-van Zijp MH, van Kessel-Feddema BJ, Nijhuis-van der Sanden MW, Knuijt S, Zegers-Verstraeten JL, Gischler SJ, Tibboel D, Kollée LA: Follow-up of newborns treated with extracorporeal membrane oxygenation: A nationwide evaluation at 5 years of age. Crit Care 10: R127, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fredriks AM, van Buuren S, Burgmeijer RJ, Meulmeester JF, Beuker RJ, Brugman E, Roede MJ, Verloove-Vanhorick SP, Wit JM: Continuing positive secular growth change in The Netherlands 1955-1997. Pediatr Res 47: 316–323, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Fredriks AM, van Buuren S, Wit JM, Verloove-Vanhorick SP: Body index measurements in 1996-7 compared with 1980. Arch Dis Child 82: 107–112, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fredriks AM, van Buuren S, Jeurissen SE, Dekker FW, Verloove-Vanhorick SP, Wit JM: Height, weight, body mass index and pubertal development references for children of Moroccan origin in The Netherlands. Acta Paediatr 93: 817–824, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Fredriks AM, van Buuren S, Jeurissen SE, Dekker FW, Verloove-Vanhorick SP, Wit JM: Height, weight, body mass index and pubertal development reference values for children of Turkish origin in the Netherlands. Eur J Pediatr 162: 788–793, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staples A, LeBlond R, Watkins S, Wong C, Brandt J: Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol 25: 2321–2326, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Kidney Disease: Improving Global Outcomes (KDIGO) : KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2: 337–414, 2012 [Google Scholar]
- 34.Piepsz A, Tondeur M, Ham H: Revisiting normal (51)Cr-ethylenediaminetetraacetic acid clearance values in children. Eur J Nucl Med Mol Imaging 33: 1477–1482, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Hogg RJ, Portman RJ, Milliner D, Lemley KV, Eddy A, Ingelfinger J: Evaluation and management of proteinuria and nephrotic syndrome in children: Recommendations from a pediatric nephrology panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE). Pediatrics 105: 1242–1249, 2000 [DOI] [PubMed] [Google Scholar]
- 36.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents : The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[Suppl 4th Report]: 555–576, 2004 [PubMed] [Google Scholar]
- 37.Boer DP, de Rijke YB, Hop WC, Cransberg K, Dorresteijn EM: Reference values for serum creatinine in children younger than 1 year of age. Pediatr Nephrol 25: 2107–2113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo JC, Sinaiko A, Chandra M, Daley MF, Greenspan LC, Parker ED, Kharbanda EO, Margolis KL, Adams K, Prineas R, Magid D, O’Connor PJ: Prehypertension and hypertension in community-based pediatric practice. Pediatrics 131: e415–e424, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Cammen-van Zijp MH, Gischler SJ, Hop WC, de Jongste JC, Tibboel D, Ijsselstijn H: Deterioration of exercise capacity after neonatal extracorporeal membrane oxygenation. Eur Respir J 38: 1098–1104, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Schwartz GJ, Furth SL: Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol 22: 1839–1848, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, Heilberg IP: Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol 3: 348–354, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vehaskari VM, Rapola J: Isolated proteinuria: Analysis of a school-age population. J Pediatr 101: 661–668, 1982 [DOI] [PubMed] [Google Scholar]
- 43.Keijzer-Veen MG, Schrevel M, Finken MJ, Dekker FW, Nauta J, Hille ET, Frölich M, van der Heijden BJ, Dutch POPS-19 Collaborative Study Group : Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J Am Soc Nephrol 16: 2762–2768, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Wlodzimirow KA, Abu-Hanna A, Slabbekoorn M, Chamuleau RA, Schultz MJ, Bouman CS: A comparison of RIFLE with and without urine output criteria for acute kidney injury in critically ill patients. Crit Care 16: R200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.UK Collaborative ECMO Trail Group : UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Lancet 348: 75–82, 1996 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.