Abstract
Background and objectives
Little is known about the utility of self-rated general health assessments in persons with moderate-to-severe CKD. This study examined the ability of a single self-rated health measure to predict all-cause mortality and kidney disease progression in a cohort of 443 patients with stages 3–4 CKD, recruited between 2005 and 2011, and followed until the end of 2012. The performance of models incorporating self-rated health measures was compared with previously published predictive models and more complex models comprising a multibiomarker panel.
Design, setting, participants, & measurements
Participants were asked “In general, would you say your health is excellent, very good, good, fair, or poor?” Outcomes examined were time to all-cause mortality, kidney disease progression (initiation of RRT or 30% loss of eGFR), and a composite of these events. Model performances were compared using a nonparametric area under the curve (AUC) analysis.
Results
Over a median follow-up of 3.3 years, 118 (27%) participants died and 138 (31%) had progression of kidney disease. Fair-to-poor self-rated health status was associated with significantly greater risks of mortality (fully adjusted hazard ratio [HR] for relative to good-to-excellent self-rated health, 2.76; 95% confidence interval [95% CI], 1.28 to 5.89), kidney disease progression (HR, 1.94; 95% CI, 1.49 to 2.56), and the combined end point (HR, 2.21; 95% CI, 1.66 to 2.96). For 3-year mortality prediction, the self-rated health model (AUC, 0.80; 95% CI, 0.76 to 0.85) had significantly higher AUCs than the base model (AUC, 0.71; 95% CI, 0.66 to 0.76) and the multibiomarker panel model (AUC, 0.74; 95% CI, 0.68 to 0.80) (P=0.03 and P=0.04, respectively).
Conclusions
A single, easily obtained measure of self-rated health helps identify patients with CKD at high risk of mortality and kidney disease progression. Routine evaluation of self-rated health may help target individuals who might benefit from more intensive monitoring strategies.
Keywords: CKD, clinical epidemiology, mortality risk, ESRD, progression of chronic renal failure
Introduction
CKD, defined by an eGFR<60 ml/min per 1.73 m2 or the presence of albuminuria, is one of the fastest-growing chronic health conditions worldwide, particularly among older people (1,2). CKD now affects approximately one eighth of the United States population (2,3) and is associated with substantially increased risks of ESRD, cardiovascular events, and premature death (4–7).
However, predictive models based on multiple biomarkers are challenging to implement into clinical workflow due to their cost and complexity, the length of time required to obtain results, and the issue of how to manage missing information. Meanwhile, subjective health measures, including self-rated health assessment, are rapidly administered, widely used within clinical and epidemiologic research settings, and correlate well with important outcomes (8–10). Strong relationships between self-rated health measures and mortality have been reported in general adult and elderly populations (8–12).
The ability of self-rated health measures to accurately predict adverse clinical events has not been widely assessed in persons with CKD, who account for nearly 12% of United States adults. To address this knowledge gap, we examined the ability of a single self-rated health measure to predict all-cause mortality and kidney disease progression in a prospective cohort of patients with CKD. We were particularly interested in comparing the performance of models incorporating self-rated health measures to more complex models that incorporated biomarkers.
Materials and Methods
Study Population
The Seattle Kidney Study (SKS) is a nephrology clinic–based, prospective cohort study of 685 participants with CKD. Participant recruitment began in 2004 from outpatient nephrology clinics at the Veterans Affairs Puget Sound Health Care System (VAMC), followed by additional clinics at Harborview Medical Center (HMC) and the University of Washington (UW). Specific details of the SKS, including general inclusion and exclusion criteria, were previously described (13). This study focused on participants with moderate-to-severe CKD (stages 3 and 4; eGFR 15–59 ml/min per 1.73 m2) and thus we excluded 225 participants who had mild CKD (eGFR≥60 ml/min per 1.73 m2 with albuminuria) or highly advanced disease (eGFR<15 ml/min per 1.73 m2) at baseline. We further excluded 17 participants with missing self-rated health at baseline. The analytic cohort comprised the remaining 443 participants. The UW and VAMC institutional review boards approved the study protocol.
Assessment of Self-Rated Health
Study coordinators mailed the Kidney Disease Quality of Life (KDQOL) instrument (Rand) to participants before their scheduled study visits. All analyses presented here are based on the first question on the KDQOL instrument administered at baseline, which asks “In general, would you say your health is…?” followed by five answer choices ranging from excellent (score of 5) to very good, good, fair, or poor (score of 1). Based on previous studies of self-rated health and the limited number of participants who responded “excellent” (n=5), “very good” (n=20), or “poor” (n=30), we dichotomized responses into good to excellent versus fair to poor for regression analyses and prediction evaluation (14–17).
Outcome Ascertainment
Serum cystatin C levels were measured from frozen serum samples stored at −70°C using either a particle-enhanced immunonephelometric assay (Gentian A/S, Norway) with a clinical chemistry analyzer (DXC600; Beckman Coulter, Miami, FL) or a nephelometer (BNII; Siemens Healthcare Diagnostics Inc, Deerfield, IL). We certified the Gentian assay using cystatin C reference material (ERM-DAY7/IFCC) per its Certificate of Analysis and corrected by weight to yield a cystatin C concentration of 5.48 mg/L (uncertainty of 0.15 mg/L). We then calibrated the Siemens assay to the Gentian assay (gold standard) in a subset of 40 SKS participants who had serum samples simultaneously measured on both assays (r2=0.98). The assays are stable through several freeze-thaw cycles (18). Serum creatinine was measured by the modified rate Jaffe method with an assay traceable to isotope dilution mass spectrometry, respectively. We calculated eGFR annually using the combined cystatin and creatinine-based CKD Epidemiology Collaboration equation (19). Incident ESRD events, defined as the first occurrence of initiation of maintenance dialysis or receipt of a kidney transplant, were identified during twice-yearly surveillance calls and in-person follow-up examinations. Study coordinators specifically inquired about the initiation of dialysis since the previous study encounter.
The primary renal outcome was the time to first occurrence of >30% loss of baseline eGFR (20) or initiation of RRT (chronic dialysis or kidney transplantation) over follow-up.
Deaths were identified from proxies during surveillance calls and during scheduling calls for annual visits. Because kidney disease progression and mortality are competing outcomes in CKD patients, a combined adverse event end point (defined as the first occurrence of initiation of dialysis, kidney transplantation, loss of >30% of eGFR, or death from any cause during the study) was derived to evaluate potential informative censoring.
Covariate Ascertainment
Prevalent conditions were determined based on participant responses to questionnaires, and from hospitalizations that occurred after initial SKS enrollment but before initial assessment for this study, and were defined as previously described (13). Study staff assessed medication uses by computerized pharmacy records (VAMC) or by the inventory method (HMC and UW) (21).
Marital status, education attainment, work status, exercise frequency, smoking status, and alcohol use were determined via baseline lifestyle questionnaires. Serum, plasma, and urine samples were stored at −70°C until measurement. General chemistries were measured on a Beckman Coulter DxC autoanalyzer (Beckman Coulter, Brea, CA). Urine albumin and creatinine were measured in spot morning or overnight urine collections by a timed end point method and with the modified rate Jaffe method. Biomarkers were measured as follows: C-reactive protein (CRP) determined immunologically on a Behring Nephelometer II with commercially available reagents from Dade Behring (Dade Behring, Eschborn, Germany), 25-hydroxyvitamin D [25(OH)D] measured using high-performance HPLC–tandem mass spectrometry, parathyroid hormone (PTH) by the Beckman Coulter DxI automated immunoassay, intact fibroblast growth factor-23 (FGF-23) via ELISA (Kainos Laboratories, Tokyo, Japan), uric acid via enzymatic trinder (Beckman Coulter), serum sodium by indirect ion-selective electrode/light absorption spectroscopy glass membrane, and serum chloride by indirect ion-selective electrode/Ag/AgCl pellet. Owing to cost constraints, 25(OH)D, PTH, and FGF-23 were measured in a random subsample of the 443 participants (n=210).
Statistical Analyses
Baseline descriptive statistics on demographics, medical history, and clinical, laboratory, and lifestyle characteristics were examined by self-rated health levels. We report continuous variables as means±SDs or medians and interquartile ranges, and categorical variables as numbers and percentages.
To identify predictors of self-rated health, we assessed candidate patient characteristics for association with baseline self-rated health using forward stepwise logistic regression in which baseline self-rated health served as the dependent variable. Each characteristic (from Table 1) was regressed on self-rated health, and we ranked the characteristics from lowest to highest univariate Akaike information criterion (AIC) (22). Next, we combined patient characteristics into one model, adding each characteristic in the AIC-based order. We retained variables whose inclusion in the model reduced the AIC; if a variable did not reduce the AIC when it was added, it was dropped. Using the AIC has the appeal of not having to set arbitrary criteria for entering and removing variables. The likelihood ratio test comparing each successive model with the nested model without the parameter in question was used to obtain a P value.
Table 1.
Participant baseline characteristics (n=443)
| Variable | Self-Rated Health | |
|---|---|---|
| Good to Excellent | Fair to Poor | |
| Participants (n) | 207 | 236 |
| Age (yr) | 62.3±12.8 | 62.2±12.1 |
| Black race | 38 (18.3) | 64 (27.3) |
| Women | 36 (17.3) | 40 (17.0) |
| Body mass index (mg/kg2) | 30.4±6.6 | 32.4±7.6 |
| Systolic BP (mmHg) | 134.3±22.0 | 136.5±23.6 |
| Current smoking | 30 (14.7) | 44 (19.4) |
| Current alcohol use | 72 (36.9) | 57 (25.7) |
| Education status | ||
| Less than high school | 5 (2.6) | 15 (6.9) |
| Graduated high school | 132 (68.8) | 152 (69.7) |
| College degree or higher | 55 (28.7) | 51 (23.4) |
| Marital status | ||
| Married | 77 (39.1) | 85 (37.8) |
| Never married | 43 (21.8) | 48 (21.3) |
| Widowed | 15 (7.6) | 20 (8.9) |
| Divorced/separated | 62 (31.5) | 72 (32.0) |
| Work status | ||
| Employed full-time | 27 (19.2) | 12 (7.3) |
| Employed part-time | 17 (12.1) | 9 (5.5) |
| Unemployed | 28 (19.9) | 43 (26.2) |
| Retired | 68 (48.2) | 89 (54.3) |
| On disability | 1 (0.7) | 11 (6.7) |
| Exercise frequency in previous month | ||
| None | 28 (21.4) | 47 (31.3) |
| Once per week or less | 38 (29.0) | 37 (24.7) |
| More than once per week | 65 (49.7) | 66 (44.0) |
| Six-minute walk speed (m/s) | 0.11±0.03 | 0.09±0.04 |
| eGFR, CKD-EPI equation (ml/min per 1.73 m2) | 38.2±12.2 | 36.5±13.0 |
| Prevalent disease | ||
| Diabetes mellitus | 90 (43.3) | 152 (64.7) |
| Hypertension | 194 (93.3) | 234 (99.6) |
| Coronary artery disease | 80 (38.5) | 126 (53.6) |
| Medication use | ||
| Angiotensin-converting enzyme inhibitor use | 100 (48.1) | 136 (57.9) |
| Angiotensin II receptor blocker use | 67 (32.2) | 86 (36.6) |
| β-Blocker | 90 (43.3) | 159 (67.7) |
| Phosphate binder | 26 (12.5) | 31 (13.2) |
| Statin | 127 (61.1) | 147 (62.6) |
| Antidepressant | 58 (27.9) | 96 (40.9) |
| Laboratory measures | ||
| All participants | ||
| Albumin (g/dl) | 3.9±0.6 | 3.7±0.6 |
| Urine albumin to creatinine ratio (mg/g) | 86.3 (13.4, 603.0) | 204.9 (25.6, 1016.2) |
| Calcium (mg/dl) | 9.0±0.8 | 8.9±0.7 |
| Cystatin C (mg/dl) | 1.9±0.9 | 2.2±1.2 |
| Bicarbonate (mmol/L) | 24.1±3.5 | 24.3±3.8 |
| C-reactive protein (mg/L) | 2.1 (0.9, 6.4) | 3.2 (1.2, 6.9) |
| Uric acid (mg/dl) | 7.6±1.8 | 7.9±1.9 |
| Hemoglobin (g/dl) | 12.9±2.0 | 12.5±1.8 |
| Anion gap (mg/dl) | 11.4±3.7 | 11.2±2.3 |
| Phosphorus (mg/dl) | 3.6±0.6 | 3.8±0.8 |
| Random subset of 210 participants | ||
| Fibroblast growth factor-23 (pg/ml) | 61.7 (45.9, 93.5) | 65.0 (49.5, 101.5) |
| 25-Hydroxyvitamin D (ng/ml) | 31.2±15.8 | 27.8±13.6 |
| Parathyroid hormone (pg/ml) | 93.4±69.3 | 114.1±94.1 |
Data are presented as the mean±SD, n (%), or median (interquartile range), unless otherwise indicated. CKD-EPI, CKD Epidemiology Collaboration.
We calculated survival time as the time from the date of baseline examination until death, the kidney disease progression end point, or, for the combined end point, whichever occurred first. We censored participants when they became lost to follow-up (n=13) or at the end of the study period on February 27, 2013.
For the mortality end point, we calculated unadjusted incidence rates as the number of events divided by person-years at risk. For the kidney disease progression and combined end points, we calculated unadjusted incidence rates as the number of events divided by person-visits at risk. Cox proportional hazards regression and discrete-time proportional hazards models were used to estimate the relative hazard of mortality, and of kidney disease progression and the combined mortality/progression end point, respectively, after adjustment for covariates selected a priori, which we hypothesized might confound the association between self-rated health, the multibiomarker panel, or the traditional predictive risk factors and the outcomes of interest. We verified that the assumption of proportional hazards was satisfied.
Regression models were constructed in order to progressively examine the confounding effects of patient characteristics on the association of self-rated health and time to death or kidney disease progression. Model one adjusted for age, sex, race/ethnicity, work status, and education attainment. Model two additionally included (1) covariates identified a priori based on suspicion that it may confound the association between self-rated health and mortality or kidney disease progression (body mass index, systolic BP, diabetes status, prevalent coronary artery disease status, smoking status, eGFR, and urine albumin to creatinine ratio [log-transformed]), and (2) additional characteristics independently associated with self-rated health, identified from stepwise regression methods (β-blocker use and current alcohol use).
Because previous studies found stronger associations of self-rated health with mortality among men and among younger individuals (8), we conducted sensitivity analyses stratified by sex and age group (<65 years versus ≥65 years). To examine whether associations varied by sex, age, and baseline eGFR, we used likelihood ratio tests comparing models with and without multiplicative interaction terms. P values from these tests were nonsignificant (all P values >0.40) indicating statistically similar prediction across sex, age, and baseline eGFR in this cohort. Therefore, we present results only for the complete study population.
Model Performance
To compare the discrimination performance of the models, receiver operating characteristic curves were obtained with unadjusted logistic regression models that examined all-cause mortality, kidney disease progression events, and the combined end point through 3 years of follow-up (23). A logistic regression procedure was used to determine test characteristics of each predictive model at the cut-point that predicted the probability of the outcome at ≥0.5. For analyses of predictive performance, three predictive models were examined for each outcome: kidney disease progression, all-cause mortality, and the combined event. Across outcomes, only the base models differed, with subsequent models being conserved. For prediction of the risk of all-cause mortality, the base model was composed of age, race, sex, eGFR, albuminuria, and the Framingham risk score (24). The Framingham risk score was derived for each individual using the sex-specific prediction formulas proposed by Wilson et al. based on traditional cardiovascular risk factors (age, total and HDL cholesterol categories, BP categories, diabetes mellitus, and smoking status) (24). For prediction of progression of CKD, the base model included age, sex, eGFR, albuminuria, serum calcium, serum phosphate, serum bicarbonate, and serum albumin as previously developed and validated by Tangri and collaborators in large cohorts of Canadian patients with stages 3–5 CKD (25). We incorporated race (white, black, or other) into this model, because previous studies have observed marked racial differences in risk of death and ESRD. For the combined end-point analyses, the base model comprehensively collected all factors from the base models for kidney disease progression and all-cause mortality. For all end points, the second model combined age, sex, race/ethnicity, eGFR, albuminuria, and added laboratory assessment of a multibiomarker panel. The multibiomarker panel included eight biomarkers from biologic pathways associated with increased morbidity and mortality: cystatin C (26), CRP (log-transformed) (27), 25(OH)D (28), PTH (29), FGF-23 (log-transformed) (30), hemoglobin (31), uric acid (32), and anion gap (33). The final predictive model combined age, sex, race, eGFR, albuminuria, and the participant’s response to the self-rated health question. Because it has been shown that self-rated health in combination with a multibiomarker panel predicts all-cause mortality better than either alone in the general population, we estimated the predictive performance of a model that combined the multibiomarker panel with the self-rated health model (11). Model performances were compared using a nonparametric area under the curve (AUC) analysis (34). Unadjusted associations between each biomarker and the self-rated health assessment were evaluated with Pearson’s correlation coefficients.
STATA software (version 13.0; College Station, TX) and the R 2.12.1 package were used for all analyses.
Results
Baseline Characteristics
At baseline, the mean age was 62 years and 17% of participants were women. Participants with lower (worse) self-rated health status were more likely to be African American, unemployed, and have diabetes mellitus or coronary artery disease (Table 1). Self-rated health status was not correlated with eGFR, nor was it related to serum calcium, bicarbonate, anion gap, or hemoglobin concentrations. Baseline characteristics that were independently associated with self-rated health were β-blocker use, prevalent diabetes, work status, body mass index, and current alcohol use (Table 2).
Table 2.
Independent correlates of fair or poor self-rated health among 443 participants with stages 3–4 CKD
| Variable | Odds Ratio (95% CI)a | Likelihood Ratio Test P value | Akaike Information Criterion |
|---|---|---|---|
| Empty model | — | — | 614.2 |
| Plus β-blocker use | 2.79 (1.96 to 4.69) | <0.001 | 588.4 |
| Plus prevalent diabetes | 2.23 (1.51 to 3.31) | <0.001 | 574.2 |
| Plus work status | |||
| Unemployed | 3.51 (1.68 to 7.32) | <0.001 | 389.1 |
| Retired | 1.98 (1.04 to 3.74) | ||
| On disability | 19.7 (2.30 to 168.4) | ||
| Plus body mass indexb | 1.05 (1.01 to 1.08) | 0.01 | 384.1 |
| Plus current alcohol use | 0.70 (0.41 to 1.19) | 0.07 | 382.7 |
95% CI, 95% confidence interval.
Odds of reporting fair-to-poor self-rated health relative to odds of reporting good-to-excellent self-rated health, adjusted for previous characteristics in the table.
Body mass index (in kilograms per meter squared) modeled continuously.
Self-Rated Health, Mortality, and ESRD Events
During a median follow-up of 3.3 years (interquartile range, 1.9–4.6 years),118 participants died and 138 participants had progressive kidney disease (66 initiated dialysis, one received a kidney transplant, and 71 experienced a >30% loss of eGFR) (Table 3). Forty-one participants reached the kidney disease progression end point before death during the study.
Table 3.
Associations of self-rated health with all-cause mortality, kidney disease progression, and the combined end point of mortality or kidney disease progression
| End Point | Self-Rated Health | |
|---|---|---|
| Good to Excellent | Fair to Poor | |
| All-cause mortality | ||
| Events, n (IR) | 30 (37.6) | 88 (109.2) |
| HR (95% CI) | ||
| Model 1 | 1.0 (ref) | 3.39 (1.70 to 6.75) |
| Model 2 | 1.0 (ref) | 2.76 (1.28 to 5.89) |
| Kidney disease progression | ||
| Events, n (IR) | 50 (89.3) | 88 (193.8) |
| HR (95% CI) | ||
| Model 1 | 1.0 (ref) | 2.38 (1.20 to 4.70) |
| Model 2 | 1.0 (ref) | 1.94 (1.49 to 2.56) |
| Combined end point | ||
| Events, n (IR) | 68 (136.3) | 147 (300.1) |
| HR (95% CI) | ||
| Model 1 | 1.0 (ref) | 2.49 (1.94 to 3.26) |
| Model 2 | 2.21 (1.66 to 2.96) | |
Incidence rates are expressed per 1000 person-years. Model 1 includes age, sex, race (white, black, or other), work status (full-time, part-time, unemployed, retired, or on disability), education attainment (some high school or less, high school, or college or more). Model 2 includes model 1 and adds body mass index, systolic BP, diabetes status, prevalent coronary artery disease, current smoking status, eGFR, urine albumin to creatinine ratio (log-transformed), β-blocker use, and current alcohol use. IR, incident rate; HR, hazard ratio; ref, reference value.
Mortality rates and incidence rates of kidney disease progression were higher among participants with fair-to-poor health status, compared with participants with good-excellent self-rated health (Table 3). Fair-to-poor self-rated health status was associated with a significantly higher risk of mortality (hazard ratio [HR], 2.76; 95% confidence interval [95% CI], 1.28 to 5.89), kidney disease progression (HR, 1.94; 95% CI, 1.49 to 2.56), and the combined end point (HR, 2.21; 95% CI, 1.66 to 2.96) (Table 3), relative to good-to-excellent self-rated health, after full adjustment for covariates.
Prediction of Adverse Events
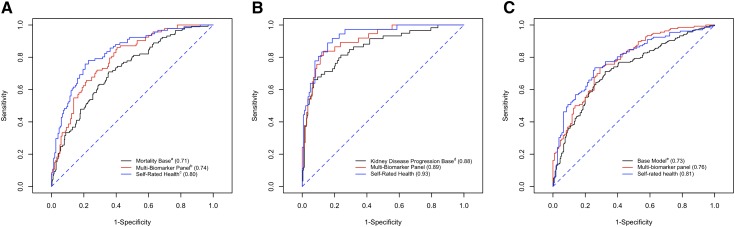
Sensitivity and specificity for the base model, multibiomarker panel, and self-rated health model to predict kidney disease progression and mortality at 3 years were assessed across a range of cutoff values (Figure 1). For 3-year mortality prediction, the self-rated health model (AUC, 0.80; 95% CI, 0.76 to 0.85) had significantly higher AUCs than the base model (AUC, 0.71; 95% CI, 0.66 to 0.76) and the multibiomarker panel model (AUC, 0.74; 95% CI, 0.68 to 0.80) (P=0.03 and P=0.04, respectively) (Table 4).
Figure 1.
Receiver operating characteristic curve for all-cause mortality, incident kidney disease progression, and the combined end point prediction at 3 years. All-cause mortality (A); incident kidney disease progression (B); and combined end point (C). The axes display the model name and accompanying area under the curve for prediction of event. αSensitivity and specificity estimated at the cut-point that predicted the probability of the outcome at ≥0.5. aMortality base model composed of age, race, sex, eGFR, albuminuria, and the Framingham risk score (n=443). bMultibiomarker panel composed of age, race, sex, eGFR, albuminuria, cystatin C, C-reactive protein (log-transformed), 25-hydroxyvitamin D, parathyroid hormone, fibroblast growth factor-23 (log-transformed), hemoglobin, uric acid, and anion gap (n=210). cSelf-rated health model combined age, sex, race, eGFR, albuminuria and the participant’s response to the self-rated health question (n=443). dThe kidney disease progression base model included age, sex, eGFR, albuminuria, serum calcium, serum phosphate, serum bicarbonate, and serum albumin (n=443). eThe combined end point base model includes all characteristics from the mortality base model and the kidney disease progression base model (n=443).
Table 4.
Overall performance of prediction models
| Outcome | Predictive Model | AUC (95% CI) | Sensitivity | Specificity |
|---|---|---|---|---|
| All-cause mortality | Mortality base modela | 0.71 (0.66 to 0.76) | 48.5 | 80.8 |
| Multibiomarker panelb | 0.74 (0.68 to 0.80) | 83.3 | 56.8 | |
| Self-rated health modelc | 0.80 (0.76 to 0.85) | 60.6 | 81.7 | |
| Multibiomarker panel plus self-rated health | 0.78 (0.72 to 0.84) | 76.9 | 67.3 | |
| Kidney disease progression | Kidney disease progression base modeld | 0.88 (0.83 to 0.92) | 57.7 | 93.9 |
| Multibiomarker panel | 0.89 (0.85 to 0.94) | 61.3 | 90.3 | |
| Self-rated health model | 0.93 (0.88 to 0.97) | 60.0 | 94.7 | |
| Multibiomarker panel plus self-rated health | 0.90 (0.83 to 0.96) | 76.2 | 94.0 | |
| Combined end point | Combined base modele | 0.73 (0.69 to 0.78) | 74.1 | 58.6 |
| Multibiomarker panel | 0.76 (0.70 to 0.82) | 87.2 | 50.0 | |
| Self-rated health model | 0.81 (0.76 to 0.84) | 71.3 | 79.3 | |
| Multibiomarker panel plus self-rated health | 0.77 (0.72 to 0.81) | 67.3 | 74.8 |
Sensitivity and specificity estimated at the cut-point that predicted the probability of the outcome at ≥0.5. Pearson’s correlation coefficients between the self-rated health assessment and each of the biomarkers from the multibiomarker panel are as follows (higher value refers to worse self-rated health): r=0.14 for cystatin C, r=0.08 for logCRP, r=−0.16 for 25-hydroxyvitamin D, r=0.10 for parathyroid hormone, r=0.11 for fibroblast growth factor-23 (log-transformed), r=−0.12 for hemoglobin, r=0.09 for uric acid, and r=0.03 for anion gap. AUC, area under the curve; CRP, C-reactive protein.
The mortality base model was composed of age, race, sex, eGFR, albuminuria, and the Framingham Risk Score (n=443).
The multibiomarker panel included cystatin C, CRP (log-transformed), 25-hydroxyvitamin D, parathyroid hormone, fibroblast growth factor-23 (log-transformed), hemoglobin, uric acid, and anion gap (n=210).
The self-rated health model combined age, sex, race, eGFR, albuminuria, and the participant’s response to the self-rated health question (n=443). The multibiomarker panel plus self-rated health model comprised 210 participants.
The kidney disease progression base model included age, sex, eGFR, albuminuria, serum calcium, serum phosphate serum bicarbonate, and serum albumin (25) (n=443).
The kidney disease progression plus mortality base model (combined base model) includes all characteristics from the mortality base model and the kidney disease progression base model.
Similarly, for kidney disease progression prediction, both the multibiomarker panel (AUC, 0.89; 95% CI, 0.85 to 0.94) and the self-rated health models (AUC, 0.93; 95% CI, 0.88 to 0.97) had higher predictive performance than the base model (AUC, 0.88; 95% CI, 0.83 to 0.92) (P=0.04 and P=0.03, respectively), yet there was no significant difference between the multibiomarker panel and the self-rated health models (P=0.08). A model that included both the biomarker panel and the self-rated health model did not improve the area under the receiver operating characteristic curve, across any end point.
Discussion
In this clinic-based cohort study of participants with moderate-to-severe CKD, we found that lower self-rated health was significantly associated with greater risks of all-cause mortality and kidney disease progression, independent of traditional risk factors. Furthermore, the single self-rated health question predicted mortality and kidney disease progression more precisely than previously published predictive models. Improvements in risk prediction using self-rated health were similar or greater than improvements in prediction garnered from a panel of biomarkers.
Little published data exist on the association of self-rated health and adverse clinical outcomes among patients with moderate-to-severe CKD. Our study’s risk estimates for mortality were similar to those previously reported in other (less diverse) populations (35,36). Among 1443 incident dialysis patients in The Netherlands, poor and fair self-rated health were associated with greater risks of death, similar in magnitude to those observed in our study, with adjusted HRs of 3.56 (95% CI, 1.71 to 7.42) for poor and 2.09 (95% CI, 1.06 to 4.12) for fair. In the largest study to date, with nearly 30 years of follow-up, Bopp et al. observed an independent, graded association of self-rated health with mortality among 8000 community-dwelling participants from the Swiss National Research Program (37).
We found that self-rated health better predicted kidney disease progression than total mortality. The degree to which self-rated health can act as a predictor of events depends on the comprehensiveness and accuracy of the information that the individual incorporates into his or her self-rating. Several studies have shown that the association of self-rated health with mortality is stronger when the cause of death is a condition of which the respondent is likely to have known when giving the health assessment (38,39). Thus, participants in our study may have been more acutely aware of risks for progressing to ESRD than dying.
The link between self-rated health and adverse outcomes, although well established, is poorly understood. Two small trials, of a dance intervention in adolescent girls (40) and a combined physical activity/dietary modification program in community-dwelling adults in Japan (41), have shown potential modifiability of self-rated health. In population studies, self-rated health is a comprehensive, inclusive, and informative measure of health status (42). The most plausible explanation for the strong independent association of self-rated health with adverse events is that self-reported measures reflect aspects of health and illness not fully captured by common health indicators such as comorbid medical diagnoses, laboratory tests, or clinician assessments of health status. Self-rated health can reflect a holistic self-perception of an individual’s health that blends prior health history, current kidney disease and comorbid illness burden, and expectations for future health, which may be involved in the biologic pathways underlying adverse clinical outcomes.
Our study was strengthened by a well characterized, prospective sample of patients with CKD. Our study also has several limitations. The study cohort was limited to participants from a clinic-based study of CKD; compared with the general CKD population, these individuals may be likely to be more health-conscious, more likely to incorporate relevant information into their self-rated health, and more aware of their risks of clinical outcomes. This may limit the generalizability of our findings. The preponderance of male participants in our study, stemming from the inclusion of the VAMC study site, may have interfered with our ability to detect potential sex-related differences in the associations of self-rated health and important outcomes. Broadly inclusive future studies are warranted. The use of a single annual eGFR is expected to lead to misclassification in the outcome of decline in kidney function. However, misclassification in this study is likely to be nondifferential, leading to a bias of results toward the null. Finally, although poor self-rated health appears to identify patients at high risk of adverse health outcomes, it may not readily identify the most appropriate treatments to mitigate these risks.
In conclusion, a single, easily obtained measure of self-rated health helps to identify patients with CKD at high risk of mortality and kidney disease progression. Self-rated health assessment could be incorporated into patient registration, nursing intake, or clinical evaluations to provide a rapid, inexpensive, and efficient tool for identifying patients who might benefit from more intensive surveillance and/or risk factor management. Further research is needed to determine the optimal way to incorporate assessment of self-reported health into clinical practice, as well as to investigate whether interventions that optimize measures of self-reported health translate into improved outcomes for patients.
Disclosures
None.
Acknowledgments
The Seattle Kidney Study was originally supported by a grant from the Northwest Kidney Center Foundation. This research was also supported by the National Institutes of Health National Heart, Lung, and Blood Institute (Grant 2R01-HL070938 [to J.H.]).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J: Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305: 2532–2539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Renal Data Service : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, Newman AB, Siscovick D, Shlipak MG, Cardiovascular Health Study : Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med 142: 497–505, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 352: 2049–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B: Cardiovascular mortality risk in chronic kidney disease: Comparison of traditional and novel risk factors. JAMA 293: 1737–1745, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Hillege HL, Janssen WM, Bak AA, Diercks GF, Grobbee DE, Crijns HJ, Van Gilst WH, De Zeeuw D, De Jong PE, Prevend Study Group : Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med 249: 519–526, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Idler EL, Benyamini Y: Self-rated health and mortality: A review of twenty-seven community studies. J Health Soc Behav 38: 21–37, 1997 [PubMed] [Google Scholar]
- 9.DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P: Mortality prediction with a single general self-rated health question. A meta-analysis. J Gen Intern Med 21: 267–275, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franks P, Gold MR, Fiscella K: Sociodemographics, self-rated health, and mortality in the US. Soc Sci Med 56: 2505–2514, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Haring R, Feng YS, Moock J, Völzke H, Dörr M, Nauck M, Wallaschofski H, Kohlmann T: Self-perceived quality of life predicts mortality risk better than a multi-biomarker panel, but the combination of both does best. BMC Med Res Methodol 11: 103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorr DA, Jones SS, Burns L, Donnelly SM, Brunker CP, Wilcox A, Clayton PD: Use of health-related, quality-of-life metrics to predict mortality and hospitalizations in community-dwelling seniors. J Am Geriatr Soc 54: 667–673, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Roshanravan B, Khatri M, Robinson-Cohen C, Levin G, Patel KV, de Boer IH, Seliger S, Ruzinski J, Himmelfarb J, Kestenbaum B: A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis 60: 912–921, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mewton L, Andrews G: Poor self-rated health and its associations with somatisation in two Australian national surveys. BMJ Open 3: 3, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawachi I, Kennedy BP, Glass R: Social capital and self-rated health: A contextual analysis. Am J Public Health 89: 1187–1193, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marmot MG, Fuhrer R, Ettner SL, Marks NF, Bumpass LL, Ryff CD: Contribution of psychosocial factors to socioeconomic differences in health. Milbank Q 76: 403–448, 305, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Power C, Matthews S, Manor O: Inequalities in self rated health in the 1958 birth cohort: Lifetime social circumstances or social mobility? BMJ 313: 449–453, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finney H, Newman DJ, Gruber W, Merle P, Price CP: Initial evaluation of cystatin C measurement by particle-enhanced immunonephelometry on the Behring nephelometer systems (BNA, BN II). Clin Chem 43: 1016–1022, 1997 [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS, CKD Prognosis Consortium : Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith NL, Psaty BM, Heckbert SR, Tracy RP, Cornell ES: The reliability of medication inventory methods compared to serum levels of cardiovascular drugs in the elderly. J Clin Epidemiol 52: 143–146, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Shtatland ES, Cain E, Barton MB: The perils of stepwise logistic regression and how to escape them using information criteria and the output delivery system. In: Proceedings of the Twenty-Sixth Annual SAS Users Group International Conference. Cary, NC, SAS Institute Inc, 2001 [Google Scholar]
- 23.Cook NR: Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 115: 928–935, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB: Prediction of coronary heart disease using risk factor categories. Circulation 97: 1837–1847, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RT, CKD Prognosis Consortium : Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 369: 932–943, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuang CH, Lee YY, Sheu BF, Hsiao CT, Loke SS, Chen JC, Li WC: Homocysteine and C-reactive protein as useful surrogate markers for evaluating CKD risk in adults. Kidney Blood Press Res 37: 402–413, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Chartsrisak K, Vipattawat K, Assanatham M, Nongnuch A, Ingsathit A, Domrongkitchaiporn S, Sumethkul V, Distha-Banchong S: Mineral metabolism and outcomes in chronic kidney disease stage 2-4 patients. BMC Nephrol 14: 14, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asche CV, Marx SE, Kim J, Unni SK, Andress D: Impact of elevated intact parathyroid hormone on mortality and renal disease progression in patients with chronic kidney disease stages 3 and 4. Curr Med Res Opin 28: 1527–1536, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, Djurdjev O: Left ventricular mass index increase in early renal disease: Impact of decline in hemoglobin. Am J Kidney Dis 34: 125–134, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Navaneethan SD, Beddhu S: Associations of serum uric acid with cardiovascular events and mortality in moderate chronic kidney disease. Nephrol Dial Transplant 24: 1260–1266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abramowitz MK, Hostetter TH, Melamed ML: The serum anion gap is altered in early kidney disease and associates with mortality. Kidney Int 82: 701–709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44: 837–845, 1988 [PubMed] [Google Scholar]
- 35.Hayashino Y, Fukuhara S, Akiba T, Akizawa T, Asano Y, Saito S, Kurokawa K: Low health-related quality of life is associated with all-cause mortality in patients with diabetes on haemodialysis: The Japan Dialysis Outcomes and Practice Pattern Study. Diabet Med 26: 921–927, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Thong MS, Kaptein AA, Benyamini Y, Krediet RT, Boeschoten EW, Dekker FW, Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Study Group : Association between a self-rated health question and mortality in young and old dialysis patients: A cohort study. Am J Kidney Dis 52: 111–117, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Bopp M, Braun J, Gutzwiller F, Faeh D, Swiss National Cohort Study Group : Health risk or resource? Gradual and independent association between self-rated health and mortality persists over 30 years. PLoS ONE 7: e30795, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benjamins MR, Hummer RA, Eberstein IW, Nam CB: Self-reported health and adult mortality risk: An analysis of cause-specific mortality. Soc Sci Med 59: 1297–1306, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Fernández-Ruiz M, Guerra-Vales JM, Trincado R, Fernández R, Medrano MJ, Villarejo A, Benito-León J, Bermejo-Pareja F: The ability of self-rated health to predict mortality among community-dwelling elderly individuals differs according to the specific cause of death: data from the NEDICES cohort. Gerontology 59: 368–377, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duberg A, Hagberg L, Sunvisson H, Möller M: Influencing self-rated health among adolescent girls with dance intervention: A randomized controlled trial. JAMA Pediatr 167: 27–31, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Kimura M, Moriyasu A, Kumagai S, Furuna T, Akita S, Kimura S, Suzuki T: Community-based intervention to improve dietary habits and promote physical activity among older adults: A cluster randomized trial. BMC Geriatr 13: 8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada C, Moriyama K, Takahashi E: Self-rated health as a comprehensive indicator of lifestyle-related health status. Environ Health Prev Med 17: 457–462, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]