Abstract
Background and objectives
Cross-sectional studies have found that low serum bicarbonate is associated with slower gait speed. Whether bicarbonate levels independently predict the development of functional limitation has not been previously studied. Whether bicarbonate was associated with incident persistent lower extremity functional limitation and whether the relationship differed in individuals with and without CKD were assessed in participants in the Health, Aging, and Body Composition study, a prospective study of well functioning older individuals
Design, setting, participants, & measurements
Functional limitation was defined as difficulty in walking 0.25 miles or up 10 stairs on two consecutive reports 6 months apart in the same activity (stairs or walking). Kidney function was measured using eGFR by the Chronic Kidney Disease Epidemiology Collaboration creatinine equation, and CKD was defined as an eGFR<60 ml/min per 1.73 m2. Serum bicarbonate was measured using arterialized venous blood gas. Cox proportional hazards analysis was used to assess the association of bicarbonate (<23, 23–25.9, and ≥26 mEq/L) with functional limitation. Mixed model linear regression was performed to assess the association of serum bicarbonate on change in gait speed over time.
Results
Of 1544 participants, 412 participants developed incident persistent functional limitation events over a median 4.4 years (interquartile range, 3.1 to 4.5). Compared with ≥26 mEq/L, lower serum bicarbonate was associated with functional limitation. After adjustment for demographics, CKD, diabetes, body mass index, smoking, diuretic use, and gait speed, lower serum bicarbonate was significantly associated with functional limitation (hazard ratio, 1.35; 95% confidence interval, 1.08 to 1.68 and hazard ratio, 1.58; 95% confidence interval, 1.12 to 2.22 for bicarbonate levels from 23 to 25.9 and <23, respectively). There was not a significant interaction of bicarbonate with CKD. In addition, bicarbonate was not significantly associated with change in gait speed.
Conclusions
Lower serum bicarbonate was associated with greater risk of incident, persistent functional limitation. This association was present in individuals with and without CKD.
Keywords: acidosis, CKD, clinical epidemiology, geriatric, nephrology
Introduction
Individuals with CKD are at increased risk for developing functional limitation (1–3). One potential mechanism is acidosis, which is a known consequence of CKD. As part of metabolism, the body generates volatile acid, which is excreted as CO2 in the lungs, and nonvolatile acid, which is excreted by the kidney. Acidosis can produce muscle catabolism, leading to decreased muscle mass and decreased lower extremity strength (4–6). In a recent cross-sectional analysis of individuals over age 50 years participating in the National Health and Nutrition Examination Survey (NHANES), a low serum bicarbonate level (<23 mEq/L) was associated with slower gait speed and less quadriceps strength (7). Because slower gait speed is associated with an increased risk for developing disability (8), lower serum bicarbonate values may increase the risk of developing functional limitation. To our knowledge, this has not been studied prospectively.
We evaluated whether serum bicarbonate values were associated with the development of functional limitation or decline in gait speed over time in older individuals. We also assessed whether the relationship differed in those with and without CKD and whether the addition of bicarbonate to the model attenuated the relationship of CKD with functional limitation, suggesting that acidosis may be a mediator in the relationship of CKD with functional limitation.
Materials and Methods
Participants
The Health, Aging, and Body Composition (Health ABC) Study is a longitudinal study of changes in body composition and their effect on functional status and development of disability in older individuals. Participants were recruited from Medicare eligibility lists in Pittsburgh, Pennsylvania and Memphis, Tennessee between March of 1997 and July of 1998. Whites were recruited from a random sample of the lists; blacks were recruited from all age-eligible individuals in the regions. Eligibility criteria were age 70–79 years, no difficulty in performing activities of daily living, self-report of no difficulty walking 0.25 miles or up 10 stairs without resting, no report of the need of assistive devices to get around, no history of active treatment for cancer in the prior 3 years, and no plan to move out of the area in the next 3 years. The study was approved by the Institutional Review Boards at the University of Tennessee and the University of Pittsburgh. All participants provided written informed consent. At the year 3 visit, arterialized venous blood gas was obtained for measurement of pH, Pco2, and bicarbonate values. Individuals who did not have a measurement of bicarbonate or developed functional limitation before the year 3 visit were excluded.
Outcome
The primary outcome for this analysis is the development of persistent lower extremity functional limitation, which was the primary outcome of the Health ABC Study. Participants were contacted every 6 months and asked whether they had any difficulty or were not able to climb up 10 stairs and whether they had any difficulty or were not able to walk 0.25 miles. Persistent functional limitation was on the basis of two consecutive reports of any difficulty involving the same function (stairs or walking). Severe functional limitation was defined as a lot of difficulty or being unable to climb up 10 stairs or walk 0.25 miles. The time at risk was calculated from the date of the year 3 visit to the date of the first of two reports of disability.
Change in usual gait speed was evaluated as a secondary outcome. Gait speed was measured at annual clinic visits using a 20-m corridor walk. Individuals started from a standing stop and were asked to walk 20 m at their usual pace. Gait speed was calculated in meters walked per second. Gait speed was performed yearly. For this analysis, change in gait speed over years 3–6 of the study was used.
Measurements
To obtain arterialized venous blood gas, a cannula was placed in a hand or wrist vein, and then, the hand was placed in a warmer at 42°C and warmed for a minimum of 15 minutes before blood sampling. Samples were analyzed at the clinic site on the day drawn using an ABL5 Blood Gas Analyzer (Radiometer, Copenhagen, Denmark). The samples were analyzed in triplicate for pH and Pco2, and the bicarbonate value was calculated using the Henderson–Hasselbalch equation. The triplicate values were averaged.
Serum creatinine was measured at the University of Vermont on an Ektachem 700 (Eastman Kodak, Rochester, NY) using an isotope dilution mass spectrometry traceable assay. CKD was defined as an eGFR<60 ml/min per 1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration equation (9). Race and smoking status (never, current, or former) were by self-report. Participants were asked to bring in all prescription and nonprescription medications that they had taken in the previous 2 weeks; the medications were recorded by study personnel. Diabetes was defined as self-report of diabetes, use of diabetic medication, or fasting blood glucose ≥126 mg/dl. At year 2 (clinic visit 1 year before), dietary intake was assessed using a semiquantitative food frequency questionnaire (10). Net endogenous acid production (NEAP) was estimated using the formula: 54.5 (protein intake/potassium intake)−10.2 (11).
Weight was measured by a standard balance beam scale to the nearest 0.1 kg. Body height was measured to the nearest millimeter using a wall-mounted stadiometer. Body mass index was calculated as weight divided by the square of the height.
Statistical Analyses
Serum bicarbonate was analyzed both as a continuous variable and using clinical categories (<23, 23–25.9, and ≥26 mEq/L). Characteristics by bicarbonate category were assessed by linear regression with linear contrast for trend for continuous variables. For categorical variables, test of trend was assessed using the Cochran–Armitage test. The association of smoking with bicarbonate category was tested using a chi-squared test. A secondary analysis evaluated pH as <7.40 versus ≥7.40.
A survival curve was generated using Kaplan–Meier methodology, and the differences between bicarbonate categories was tested using a log rank test. The association of serum bicarbonate or low pH with lower extremity functional limitation was examined in a series of Cox proportional hazards models using variables that were selected a priori as factors that might confound the relationship of bicarbonate with functional limitation. The models examined bicarbonate alone as well as adjusted for age, race, and sex (model 2); additionally adjusted for CKD (model 3); additionally adjusted for diuretic use, diabetes, smoking, and body mass index (model 4); and additionally adjusted for baseline gait speed (model 5). Individuals were censored for mortality. We examined the interactions of bicarbonate with CKD to assess whether the relationship varied in individuals with and without CKD. The maximum follow-up was 5 years after the year 3 clinic visit.
Changes in gait speed at years 3–6 were analyzed using mixed models with repeated measures using unstructured covariance.
SAS V9.3 (SAS Institute, Cary, NC) was used for the analyses. P values <0.05 were considered statistically significant in all analyses, including interaction terms.
Results
Of 3075 individuals enrolled in the Health ABC Study, 2275 individuals had a serum bicarbonate value measured at the year 3 exam. Of these individuals, 600 individuals were excluded, because they had developed functional limitation before the measurement of serum bicarbonate; 129 individuals were eliminated for missing creatinine values, and another two individuals were eliminated for missing smoking information. This left 1544 individuals for the analysis. The mean age of the study sample was 75.4±2.8 years; 47.0% were women, and 34.7% were black. The mean eGFR was 76.7±15.3 ml/min per 1.73 m2, and 213 (13.8%) participants had an eGFR<60 ml/min per 1.73 m2. Participant characteristics by bicarbonate category are shown in Table 1. Individuals with lower bicarbonate values were more likely to be men, have CKD and diabetes, be current smokers, have higher NEAP, and they were less likely to be on diuretics. Individuals with CKD were more likely to have a bicarbonate value <23 (17.8% versus 8.3%) and a pH<7.4 (8.0% versus 1.5%); Pco2 values were similar in those with and without CKD.
Table 1.
Participant characteristics by bicarbonate level
| Characteristic | Bicarbonate Category (mEq/L) | P for Trend | ||
|---|---|---|---|---|
| <23 (n=149) | 23–25.9 (n=814) | ≥26 (n=581) | ||
| Age, yr | 75.5±2.7 | 75.4±2.8 | 75.5±2.9 | 0.80 |
| Race, black (%) | 51 (34.2) | 263 (32.3) | 221 (38.0) | 0.08 |
| Sex, women (%) | 60 (40.3) | 366 (45.0) | 299 (51.5) | 0.003 |
| CKD (%) | 38 (25.5) | 111 (13.6) | 64 (11.0) | <0.001 |
| Diabetes (%) | 37 (25.8) | 140 (17.2) | 93 (16.0) | 0.04 |
| BMI, kg/m2 | 26.3±4.4 | 26.8±4.1 | 26.6±4.7 | 0.44 |
| Smoking (%) | <0.001a | |||
| None | 50 (33.6) | 364 (44.6) | 294 (50.6) | |
| Former | 72 (48.3) | 392 (48.2) | 261 (44.9) | |
| Current | 27 (18.1) | 58 (7.1) | 26 (4.5) | |
| Diuretic use (%) | 20 (13.4) | 135 (16.6) | 218 (37.5) | <0.001 |
| Usual gait speed, m/s | 1.17±0.20 | 1.22±0.20 | 1.19±0.19 | 0.40 |
| pH (range) | 7.40±0.04 (7.31–7.57) | 7.41±0.02 (7.31–7.48) | 7.41±0.03 (7.31–7.59) | <0.001 |
| Pco2, mmHg | 35.2±3.2 | 39.2±2.5 | 43.3±3.3 | <0.001 |
| NEAP, mEq/d | 41.2±13.1 | 39.2±10.7 | 36.4±10.9 | 0.001 |
BMI, body mass index; NEAP, net endogenous acid production.
P value by chi-squared test and not by trend.
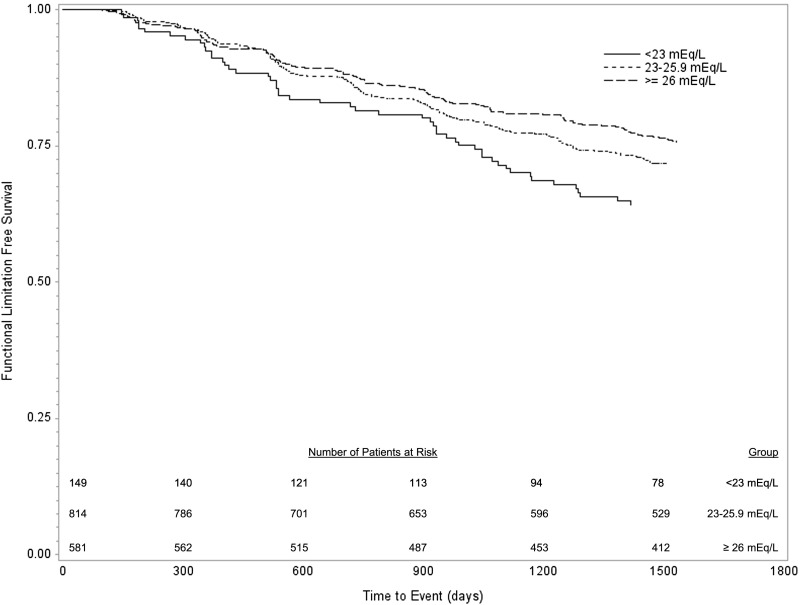
During follow-up, 412 individuals developed incident persistent lower extremity functional limitation. The risk of incident functional limitation increased with lower serum bicarbonate values (Figure 1). Table 2 shows the multivariable analyses for incident functional limitation. The risk was greatest with serum bicarbonate <23 mEq/L, with intermediate hazard ratio (HR) for bicarbonate from 23 to 25.9 mEq/L compared with a serum bicarbonate value ≥26 mEq/L. Adjustment for demographics, CKD, baseline gait speed, or other variables had minimal effect on the association of bicarbonate with functional limitation. Similar results were found for bicarbonate as a continuous variable (Table 2). Similar results were also found if eGFR rather than CKD as a continuous variable was included in the model. Because a low bicarbonate value could reflect respiratory alkalosis, we performed an analysis excluding individuals with Pco2<38 and pH>7.42. Excluding these individuals did not affect the results. There was a borderline association of low pH with functional impairment in unadjusted analyses, which remained nonsignificant with adjustment (Table 2).
Figure 1.
Development of persistent lower extremity functional limitation by bicarbonate category (P=0.01 by log rank test).
Table 2.
Association of serum bicarbonate level and low pH with incident lower extremity functional limitation
| Outcome | Hazard Ratio (95% Confidence Interval) | ||||
|---|---|---|---|---|---|
| Unadjusted | Adjusted for Age, Race, and Sex | Plus CKD | Plus DM, Smoking, BMI, and Diuretic Use | Plus Gait Speed | |
| Any persistent functional limitation | |||||
| Continuous (per 1 mEq/L lower) | 1.07 (1.02 to 1.12) | 1.08 (1.04 to 1.14) | 1.07 (1.02 to 1.12) | 1.08 (1.03 to 1.14) | 1.07 (1.02 to 1.12) |
| ≥26 mEq/L | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 23–25.9 mEq/L | 1.19 (0.96 to 1.47) | 1.26 (1.02 to 1.56) | 1.23 (1.00 to 1.53) | 1.32 (1.05 to 1.65) | 1.35 (1.08 to 1.68) |
| <23 mEq/L | 1.61 (1.17 to 2.21) | 1.69 (1.23 to 2.34) | 1.58 (1.14 to 2.18) | 1.68 (1.20 to 2.24) | 1.58 (1.12 to 2.22) |
| pH<7.40 | 1.23 (1.00 to 1.52) | 1.08 (0.87 to 1.34) | 1.03 (0.83 to 1.28) | 1.15 (0.92 to 1.44) | 1.10 (0.88 to 1.38) |
| Severe persistent functional limitation | |||||
| ≥26 mEq/L | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 23–25.9 mEq/L | 1.05 (0.71 to 1.56) | 1.09 (0.74 to 1.62) | 1.07 (0.72 to 1.59) | 1.12 (0.74 to 1.69) | 1.14 (0.75 to 1.71) |
| <23 mEq/L | 2.02 (1.19 to 3.45) | 2.10 (1.23 to 3.59) | 1.87 (1.09 to 3.23) | 1.85 (1.04 to 3.27) | 1.73 (0.98 to 3.08) |
| pH<7.40 | 1.46 (1.20 to 1.78) | 1.23(1.05 to 1.58) | 1.37 (1.00 to 1.51) | 1.32 (1.08 to 1.63) | 1.31 (1.06 to 1.61) |
DM, diabetes; BMI, body mass index.
CKD and bicarbonate were independent risk factors for the development of functional limitation. The addition of bicarbonate only mildly attenuated the relationship of CKD with functional limitation. In the model with other covariates but without bicarbonate, the HR for CKD was 1.50 (95% confidence interval [95% CI], 1.17 to 1.93); with bicarbonate in the model, the HR for CKD was 1.42 (95% CI, 1.10 to 1.83). There was a trend toward a statistical interaction of CKD with bicarbonate on the association with functional limitation in unadjusted analysis (P=0.06), but this lessened with additional adjustment (P=0.15). Stratified analysis by CKD is shown in Supplemental Table 1.
Severe functional limitation showed more of a threshold relationship. There were 122 individuals without any functional limitation at baseline who developed severe functional limitation during follow-up. In unadjusted analyses, compared with a bicarbonate value ≥26 mEq/L, the risk associated with severe functional limitation was two times higher for a bicarbonate level <23 mEq/L; the risk associated with a bicarbonate level from 23 to 25.9 mEq/L was similar to that with a bicarbonate level ≥26 mEq/L. Full adjustment attenuated the relationship. With the addition of baseline gait speed, there was a modest attenuation of the HR, but the relationship was no longer statistically significant. CKD predicted severe functional impairment, but there was only modest attenuation with the addition of bicarbonate in the model (HR, 1.50; 95% CI, 1.19 to 1.89) compared with without bicarbonate in model (HR, 1.39; 95% CI, 1.09 to 1.76). There was no interaction of serum bicarbonate and CKD with severe functional limitation (P=0.96). In contrast to the functional limitation results, there was a significant association of lower pH with severe functional limitation in unadjusted and adjusted analyses.
In mixed models, a serum bicarbonate level <23 mEq/L was associated with lower gait speed in the main effect, but the interaction with time was not significant. This means that the average value was lower for those with lower bicarbonate but that bicarbonate did not predict change in gait speed over time. After controlling for time and other covariates (same as model 4 in the Cox models), the adjusted mean values for gait speed were 1.11 (95% CI, 1.09 to 1.13) for bicarbonate≥23 mEq/L versus 1.07 (95% CI, 1.04 to 1.10) for bicarbonate<23 mEq/L (P=0.003).
Discussion
We found that, among community-dwelling older adults without functional limitation at baseline, lower serum bicarbonate levels were associated with the subsequent development of persistent lower extremity functional limitation. The relationship seemed to be linear for the outcome of any functional limitation, whereas for severe functional limitation, there seemed to be a threshold relationship evident at serum levels <23 mEq/L. We did not find a statistically significant interaction of CKD and bicarbonate, although there was a nonsignificant trend, suggesting a stronger relationship in individuals with CKD. However, there may have been limited power to see an interaction given the relatively small proportion of individuals with CKD. Although individuals with CKD were more likely to have low serum bicarbonate, low bicarbonate values did not seem to explain the higher risk of functional limitation in CKD. Low bicarbonate and CKD were independent predictors, and the inclusion of both variables in the model did not appreciably attenuate the relationship of either variable. The independence of bicarbonate and CKD as risk factors for functional limitation suggests that there are other mediators or confounders of the risk.
Our findings extend the results by Abramowitz et al. (7). In a cross-sectional analysis, Abramowitz et al. (7) analyzed the association of lower serum bicarbonate with gait speed in individuals over the age of 50 years participating in the NHANES. Individuals with a serum bicarbonate level <23 mEq/L had a 43% higher odds of having a gait speed in the lowest sex-specific quartile. Similar results were seen in individuals with and without CKD (also mainly stage 3). In the adjusted analyses, we also found that individuals with lower bicarbonate had lower gait speed, but we did not find that bicarbonate predicted change in gait speed over time. This could relate partly to power with small changes over time or the development of physical limitation and other factors, such as fatigue.
It is notable that, in the study by Abramowitz et al. (7), 22.7% of participants had a serum bicarbonate <23 mEq/L; in contrast, in our analysis, 9.6% of participants had a bicarbonate level <23 mEq/L. In the NHANES, serum bicarbonate was measured from venous chemistry samples, whereas in our study, it was arterialized venous blood gas. In addition to differences in technique, the bicarbonate values in our study were locally measured rather than measured at a central laboratory. A delay in measuring bicarbonate, which can be seen with measurement at a central laboratory, can produce lower bicarbonate values because of loss of CO2 (12). An alternative explanation is that individuals with lower serum bicarbonate levels are more likely to have functional limitation at baseline and were excluded from enrollment in the Health ABC Study.
The mechanism of the low serum bicarbonate association with higher risk of functional limitation may relate to the effect of acidosis on muscle. Acidosis leads to impaired insulin/IGF signaling; this leads to an upregulation of proteolytic pathways in muscle, which produces muscle wasting (5,13). The individuals in this study were not severely acidemic, with mean pH values in the normal range. There was not an association of low pH with functional impairment, although there was a relationship for severe functional impairment. This weaker relationship compared with bicarbonate could be because of the relatively narrow range of pH values in this population or the bicarbonate values reflecting increases of acid load or acid retention without frank acidosis.
With aging, there is a decreased ability of the kidneys to excrete an acid load (14,15). Low bicarbonate levels might reflect occult tubular dysfunction, leading to hydrogen ion retention (16), or an increased acid load that is greater than the aging kidney’s ability to excrete the load. One potential source of acid load is dietary acid intake. Diets that are higher in fruits and vegetables are more alkalogenic, whereas diets higher in animal protein and phosphorus are more acidogenic. Amodu and Abramowitz (17) analyzed the association of dietary acid intake and serum bicarbonate levels in the NHANES. Higher dietary acid was associated with lower serum bicarbonate levels, and this association was magnified in older individuals. Other studies have found that higher dietary acid load is associated with lower muscle mass (18), which could be a mediator of the association of acidosis with physical function. Although the food frequency questionnaire information was collected 1 year earlier than venous blood gas, it is notable that individuals with low bicarbonate had a higher estimated dietary acid load (NEAP).
Whether the findings of a more alkalogenic diet with better muscle reflect a healthier lifestyle with confounding factors that explain the relationship or whether acid load is contributing to muscle loss is not clear. In a recent pilot study, Abramowitz et al. (19) gave 20 individuals with CKD and lower serum bicarbonate (20–24 mEq/L) sodium bicarbonate. The study started with a placebo run in and then ramped up doses of bicarbonate every 2 weeks to 1 mEq/kg ideal body wt per day. At 6 weeks, there was improvement in the sit-to-stand time, a measure of physical performance (from 11.7 to 11.0 seconds for a five-repetition sit-to-stand test). Longer time to complete the five-repetition sit-to-stand test predicts the development of disability (20). This pilot study needs to be confirmed in a randomized study but suggests that the relationship of low bicarbonate with functional limitation might be causal. This would be important, because alkali supplementation or dietary modification would be easy and relatively inexpensive approaches to decreasing disability.
This study has a number of strengths. The Health ABC Study excluded individuals with functional limitation at baseline, and therefore, the development of functional limitation was incident. Furthermore, we were able to use persistent functional limitation so that transient declines in function related to an acute illness could be excluded. Another strength is the use of arterialized blood gas, which allowed measurement of pH and bicarbonate in triplicate, minimizing bias caused by measurement error. This study also has a number of limitations. The majority of individuals with CKD had stage 3a CKD, and the results may not be generalizable to more severe CKD. In addition, many older individuals with CKD have decreased physical function and were excluded from the study, affecting the ability to generalize to all older patients with CKD. We used reported functional limitation rather than a performance test-based definition, although this has been validated in other studies (21).
In conclusion, low serum bicarbonate was associated with the development of impaired physical function in previously well functioning older individuals. This association was independent of CKD and did not explain the association of CKD with functional limitation. Future studies are needed to determine whether supplementation with alkali or a diet lower in acid generation decreases the risk of functional limitation in individuals with and without CKD.
Disclosures
None.
Supplementary Material
Acknowledgments
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA Grants R01-AG028050 and R01-AG029364; and National Institute of Nursing Research (NINR) Grant R01-NR012459. This research was supported, in part, by the Intramural Research Program of the National Institutes of Health, National Institute on Aging. R.Y. was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant T3-2DK061296.
The results were presented, in part, at the American Society of Nephrology Meeting, San Diego, CA, in November 2012.
The contents do not represent the views of the Department of Veterans Affairs or the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05480614/-/DCSupplemental.
See related editorial, “Acid-Base Balance and Physical Function,” on pages 2030–2032.
References
- 1.Fried LF, Lee JS, Shlipak M, Chertow GM, Green C, Ding J, Harris T, Newman AB: Chronic kidney disease and functional limitation in older people: Health, aging and body composition study. J Am Geriatr Soc 54: 750–756, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Gopinath B, Harris DC, Burlutsky G, Mitchell P: Use of community support services and activity limitations among older adults with chronic kidney disease. J Gerontol A Biol Sci Med Sci 68: 741–747, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Liu CK, Lyass A, Massaro JM, D’Agostino RB, Sr., Fox CS, Murabito JM: Chronic kidney disease defined by cystatin C predicts mobility disability and changes in gait speed: The Framingham Offspring Study. J Gerontol A Biol Sci Med Sci 69: 301–307, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey JL: Metabolic acidosis: An unrecognized cause of morbidity in the patient with chronic kidney disease. Kidney Int Suppl 96: S15–S23, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Franch HA, Mitch WE: Catabolism in uremia: The impact of metabolic acidosis. J Am Soc Nephrol 9[Suppl]: S78–S81, 1998 [PubMed] [Google Scholar]
- 6.Mitch WE: Metabolic and clinical consequences of metabolic acidosis. J Nephrol 19[Suppl 9]: S70–S75, 2006 [PubMed] [Google Scholar]
- 7.Abramowitz MK, Hostetter TH, Melamed ML: Association of serum bicarbonate levels with gait speed and quadriceps strength in older adults. Am J Kidney Dis 58: 29–38, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermeulen J, Neyens JC, van Rossum E, Spreeuwenberg MD, de Witte LP: Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: A systematic review. BMC Geriatr 11: 33, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 10.Ryder KM, Shorr RI, Bush AJ, Kritchevsky SB, Harris T, Stone K, Cauley J, Tylavsky FA: Magnesium intake from food and supplements is associated with bone mineral density in healthy older white subjects. J Am Geriatr Soc 53: 1875–1880, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Frassetto LA, Todd KM, Morris RC, Jr., Sebastian A: Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr 68: 576–583, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Kirschbaum B: Spurious metabolic acidosis in hemodialysis patients. Am J Kidney Dis 35: 1068–1071, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Workeneh BT, Mitch WE: Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr 91: 1128S–1132S, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Berkemeyer S, Vormann J, Günther AL, Rylander R, Frassetto LA, Remer T: Renal net acid excretion capacity is comparable in prepubescence, adolescence, and young adulthood but falls with aging. J Am Geriatr Soc 56: 1442–1448, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Schlanger LE, Bailey JL, Sands JM: Electrolytes in the aging. Adv Chronic Kidney Dis 17: 308–319, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wesson DE, Simoni J, Broglio K, Sheather S: Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol 300: F830–F837, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Amodu A, Abramowitz MK: Dietary acid, age, and serum bicarbonate levels among adults in the United States. Clin J Am Soc Nephrol 8: 2034–2042, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sellmeyer DE, Stone KL, Sebastian A, Cummings SR, Study of Osteoporotic Fractures Research Group : A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Am J Clin Nutr 73: 118–122, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Abramowitz MK, Melamed ML, Bauer C, Raff AC, Hostetter TH: Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol 8: 714–720, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang F, Ferrucci L, Culham E, Metter EJ, Guralnik J, Deshpande N: Performance on five times sit-to-stand task as a predictor of subsequent falls and disability in older persons. J Aging Health 25: 478–492, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Simonsick EM, Kasper JD, Guralnik JM, Bandeen-Roche K, Ferrucci L, Hirsch R, Leveille S, Rantanen T, Fried LP: Severity of upper and lower extremity functional limitation: Scale development and validation with self-report and performance-based measures of physical function. WHAS Research Group. Women’s Health and Aging Study. J Gerontol B Psychol Sci Soc Sci 56: S10–S19, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.