Abstract
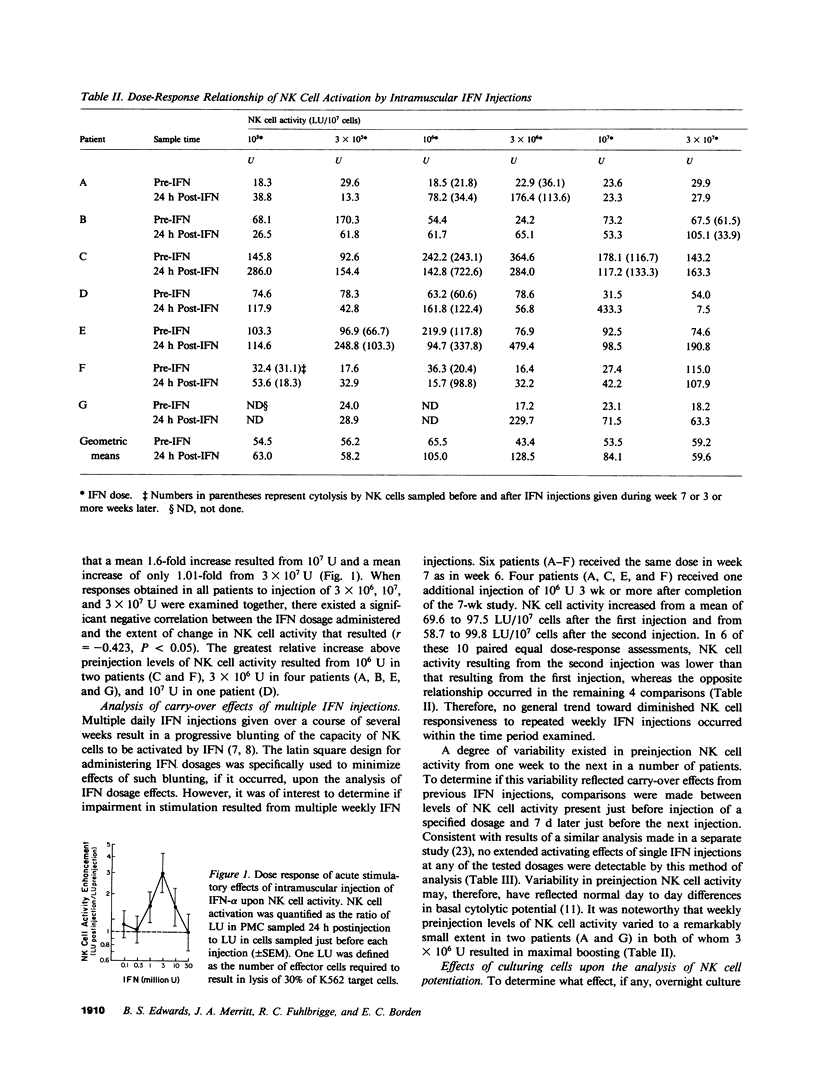
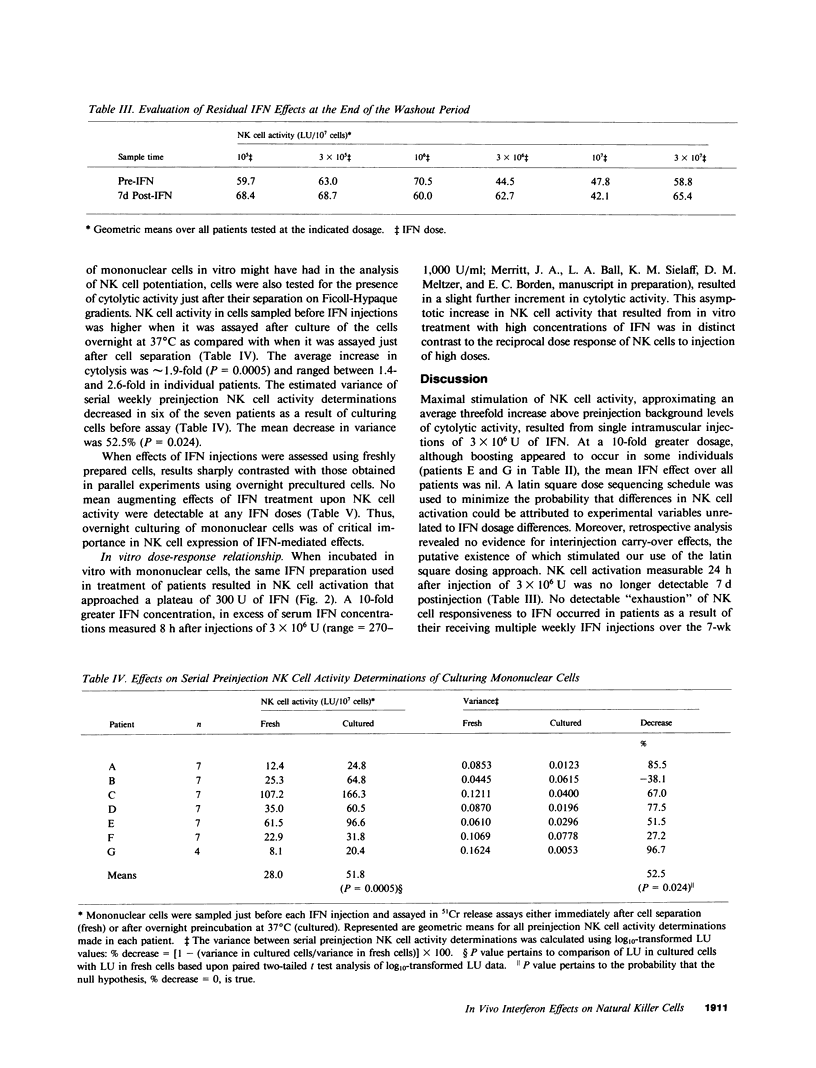
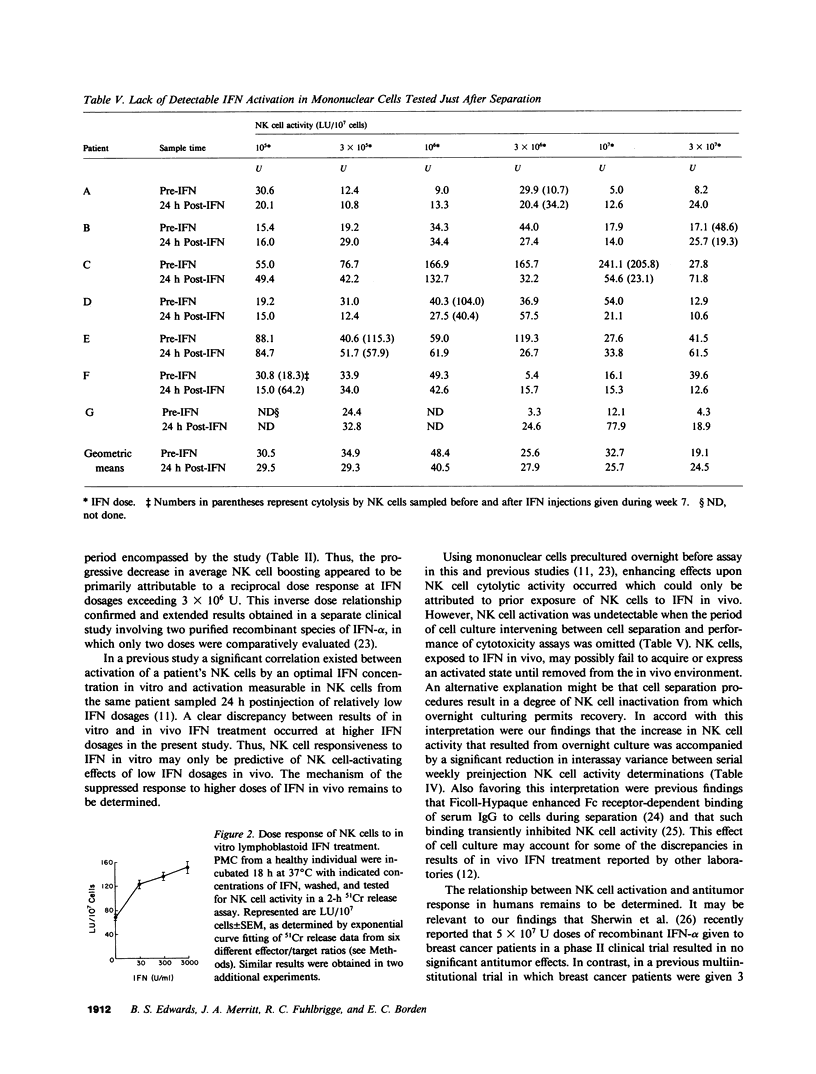
To define critical parameters concerning interferon (IFN) effects upon natural killer (NK) cells in vivo, we gave cancer patients serial weekly intramuscular injections of purified lymphoblastoid IFN in six doses ranging from 10(5) to 3 X 10(7) U. Dose sequences were determined by randomly allocating patients to one of six levels in a latin square ordering scheme. NK cell stimulation, a threefold peak increase above preinjection levels of cytolysis (P = 0.022), occurred in peripheral mononuclear cells (PMC) sampled 24 h postinjection, of 3 X 10(6) U, but was not detectable at any dose in PMC sampled 7 d postinjection. No blunting occurred in NK cell responsiveness to repeated injection of IFN dosages a second time at or several weeks after study completion. At IFN doses of 3 X 10(6), 10(7), and 3 X 10(7) U, a negative correlation existed between the amount of IFN injected and the average extent of NK cell activation (r = -0.423, P less than 0.05). This contrasted with the progressively increasing response of NK cells to in vitro incubation with increasing concentration of up to 3,000 U/ml of IFN. Overnight culturing of PMC sampled before IFN injections resulted in a mean 1.9-fold increase in cytolytic activity (P = 0.0005) and a mean 53% decrease in variance (P = 0.024) between serial preinjection NK cell activity determinations. Cell separation procedures may, therefore, have resulted in NK cell inactivation, from which overnight culturing permitted recovery. We found that maximal NK cell activation at a low IFN dose, decreasing NK cell responsiveness at higher doses, and the need to culture PMC to efficiently detect NK cell boosting may account for disparities in reported effects of IFN on NK cell function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abruzzo L. V., Rowley D. A. Homeostasis of the antibody response: immunoregulation by NK cells. Science. 1983 Nov 11;222(4624):581–585. doi: 10.1126/science.6685343. [DOI] [PubMed] [Google Scholar]
- Alexander E. L., Titus J. A., Segal D. M. Quantitation of Fc receptors and surface immunoglobulin is affected by cell isolation procedures using plasmagel and ficoll-hypaque. J Immunol Methods. 1978;22(3-4):263–272. doi: 10.1016/0022-1759(78)90034-0. [DOI] [PubMed] [Google Scholar]
- Borden E. C., Holland J. F., Dao T. L., Gutterman J. U., Wiener L., Chang Y. C., Patel J. Leukocyte-derived interferon (alpha) in human breast carcinoma. The American Cancer Society phase II trial. Ann Intern Med. 1982 Jul;97(1):1–6. doi: 10.7326/0003-4819-97-1-1. [DOI] [PubMed] [Google Scholar]
- Brieva J. A., Targan S., Stevens R. H. NK and T cell subsets regulate antibody production by human in vivo antigen-induced lymphoblastoid B cells. J Immunol. 1984 Feb;132(2):611–615. [PubMed] [Google Scholar]
- Callewaert D. M., Smeekens S. P., Mahle N. H. Improved quantification of cellular cytotoxicity reactions: determination of kinetic parameters for natural cytotoxicity by a distribution-free procedure. J Immunol Methods. 1982;49(1):25–37. doi: 10.1016/0022-1759(82)90363-5. [DOI] [PubMed] [Google Scholar]
- Edwards B. S., Hawkins M. J., Borden E. C. Comparative in vivo and in vitro activation of human natural killer cells by two recombinant alpha-interferons differing in antiviral activity. Cancer Res. 1984 Jul;44(7):3135–3139. [PubMed] [Google Scholar]
- Edwards B. S., Hawkins M. J., Borden E. C. Correlation between in vitro and systemic effects of native and recombinant interferons-alpha on human natural killer cell cytotoxicity. J Biol Response Mod. 1983;2(5):409–417. [PubMed] [Google Scholar]
- Edwards B. S., Merritt J. A., Jelen P. A., Borden E. C. Effects of diethyldithiocarbamate, an inhibitor of interferon antiviral activity, upon human natural killer cells. J Immunol. 1984 Jun;132(6):2868–2875. [PubMed] [Google Scholar]
- Einhorn S., Blomgren H., Strander H. Interferon and spontaneous cytotoxicity in man. V. Enhancement of spontaneous cytotoxicity in patients receiving human leukocyte interferon. Int J Cancer. 1980 Oct 15;26(4):419–428. doi: 10.1002/ijc.2910260406. [DOI] [PubMed] [Google Scholar]
- Fantes K. H., Allen G. Specific activity of pure human interferons and a non-biological method for estimating the purity of highly purified interferon preparations. J Interferon Res. 1981;1(4):465–471. doi: 10.1089/jir.1981.1.465. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Huddlestone J. R., Merigan T. C., Jr, Oldstone M. B. Induction and kinetics of natural killer cells in humans following interferon therapy. Nature. 1979 Nov 22;282(5737):417–419. doi: 10.1038/282417a0. [DOI] [PubMed] [Google Scholar]
- Kariniemi A. L., Timonen T., Kousa M. Effect of leucocyte interferon on natural killer cells in healthy volunteers. Scand J Immunol. 1980;12(5):371–374. doi: 10.1111/j.1365-3083.1980.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Lotzová E., Savary C. A., Gutterman J. U., Hersh E. M. Modulation of natural killer cell-mediated cytotoxicity by partially purified and cloned interferon-alpha. Cancer Res. 1982 Jun;42(6):2480–2488. [PubMed] [Google Scholar]
- Lucero M. A., Magdelenat H., Fridman W. H., Pouillart P., Billardon C., Billiau A., Cantell K., Falcoff E. Comparison of effects of leukocyte and fibroblast interferon on immunological parameters in cancer patients. Eur J Cancer Clin Oncol. 1982 Mar;18(3):243–251. doi: 10.1016/0277-5379(82)90043-8. [DOI] [PubMed] [Google Scholar]
- Maluish A. E., Ortaldo J. R., Conlon J. C., Sherwin S. A., Leavitt R., Strong D. M., Weirnik P., Oldham R. K., Herberman R. B. Depression of natural killer cytotoxicity after in vivo administration of recombinant leukocyte interferon. J Immunol. 1983 Jul;131(1):503–507. [PubMed] [Google Scholar]
- Perussia B., Fanning V., Trinchieri G. A human NK and K cell subset shares with cytotoxic T cells expression of the antigen recognized by antibody OKT8. J Immunol. 1983 Jul;131(1):223–231. [PubMed] [Google Scholar]
- Perussia B., Starr S., Abraham S., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983 May;130(5):2133–2141. [PubMed] [Google Scholar]
- Pross H. F., Baines M. G., Rubin P., Shragge P., Patterson M. S. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J Clin Immunol. 1981 Jan;1(1):51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- Pross H. F., Baines M. G. Studies of human natural killer cells. I. In vivo parameters affecting normal cytotoxic function. Int J Cancer. 1982 Apr 15;29(4):383–390. doi: 10.1002/ijc.2910290404. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Pross H. F. The biology of the human natural killer cell. J Clin Immunol. 1982 Oct;2(4):249–263. doi: 10.1007/BF00915064. [DOI] [PubMed] [Google Scholar]
- Sherwin S. A., Mayer D., Ochs J. J., Abrams P. G., Knost J. A., Foon K. A., Fein S., Oldham R. K. Recombinant leukocyte A interferon in advanced breast cancer. Results of a phase II efficacy trial. Ann Intern Med. 1983 May;98(5 Pt 1):598–602. doi: 10.7326/0003-4819-98-5-598. [DOI] [PubMed] [Google Scholar]
- Sulica A., Gherman M., Galatiuc C., Manciulea M., Herberman R. B. Inhibition of human natural killer cell activity by cytophilic immunoglobulin G. J Immunol. 1982 Mar;128(3):1031–1036. [PubMed] [Google Scholar]


