Summary
L- Carnitine, a nutrient in red meat, was recently reported to accelerate atherosclerosis via a metaorganismal pathway involving gut microbial trimethylamine (TMA) formation and host hepatic conversion into trimethylamine-N-oxide (TMAO). Herein we show that following L-carnitine ingestion, γ-butyrobetaine (γBB) is produced as an intermediary metabolite by gut microbes at a site anatomically proximal to and at a rate ~1000-fold higher than the formation of TMA. Moreover, we show γBB is the major gut microbial metabolite formed from dietary L-carnitine in mice, and like dietary L-carnitine, in a gut microbiota-dependent manner is converted into TMA and TMAO, and accelerates atherosclerosis. Gut microbial composition and functional metabolic studies reveal distinct taxa are associated with the production of γBB versus TMA/TMAO from dietary L-carnitine. Moreover, despite their close structural similarity, chronic dietary exposure to L-carnitine versus γBB promotes development of functionally distinct microbial communities optimized for the metabolism of L-carnitine versus γBB, respectively.
Introduction
The past several years have witnessed a growing awareness that gut microbiota serve as active participants in the expression of complex metabolic phenotypes such as insulin resistance and obesity (Backhed et al., 2004; Karlsson et al., 2013; Ley et al., 2005; Sayin et al., 2013). Animal model and human clinical studies indicate gut microbial metabolism of certain dietary nutrients can also influence development of atherosclerosis (Bennett et al., 2013; Koeth et al., 2013; Tang et al., 2013; Wang et al., 2011). Specifically, gut microbial metabolism of phosphatidylcholine, the major dietary source of choline, was first shown to produce a proatherogenic metabolite, trimethylamine-N-oxide (TMAO), via initial formation of trimethylamine (TMA), followed by host hepatic flavin monooxygenase mediated conversion to TMAO (Wang et al., 2011) (Figure S1; Gut Pathway1). Subsequent studies identified an alternative trimethylamine nutrient found predominantly in red meat, L-carnitine, as another dietary source for gut microbe-dependent formation of TMAO and enhanced atherosclerosis (Koeth et al., 2013); (Figure S1; Gut Pathway2).
In recent human clinical studies plasma carnitine levels in subjects (n > 2,500) were independently associated with increased future risk for myocardial infarction, stroke or death, but only among subjects with concomitantly high TMAO levels, consistent with not carnitine, but the gut microbiota-dependent metabolite, TMAO, serving as the culprit promoting the pro-atherosclerotic phenotype (Koeth et al., 2013). TMAO formation from dietary L-carnitine was shown to significantly associate with omnivorous versus vegetarian or vegan eating habits, and together with microbiota composition, could discriminate chronic dietary patterns in humans and mice, suggesting gut microbial metabolism of L-carnitine into TMAO may in part help explain the commonly observed association between red meat consumption and cardiovascular risks (Bernstein et al., 2010; Koeth et al., 2013; Micha et al., 2010). Multiple mechanisms have been identified as contributing to TMAO-dependent enhancement in atherosclerosis, including inhibition of the reverse cholesterol transport pathway, alterations in hepatic and intestinal cholesterol and bile acid metabolism, and changes in macrophage phenotype in the artery wall (Bennett et al., 2013; Koeth et al., 2013; Wang et al., 2011). Importantly, in addition to the above mentioned relationship between carnitine levels and cardiac risks, human clinical studies also demonstrate plasma levels of choline independently predict both prevalent and incident CVD risks (Wang et al., 2011; Wang et al., 2014), and plasma TMAO levels are similarly independently associated with risk of both prevalent CVD (Wang et al., 2011) and prospective development of major adverse cardiovascular events including myocardial infarction, stroke and death (Koeth et al., 2013; Tang et al., 2013; Wang et al., 2014). Thus, a growing body of evidence suggests a mechanistic link between dietary trimethylamines such as choline, phosphatidylcholine or L-carnitine, and development of atherosclerosis via gut microbiome-dependent production of TMAO (for review see (Tang, 2014)).
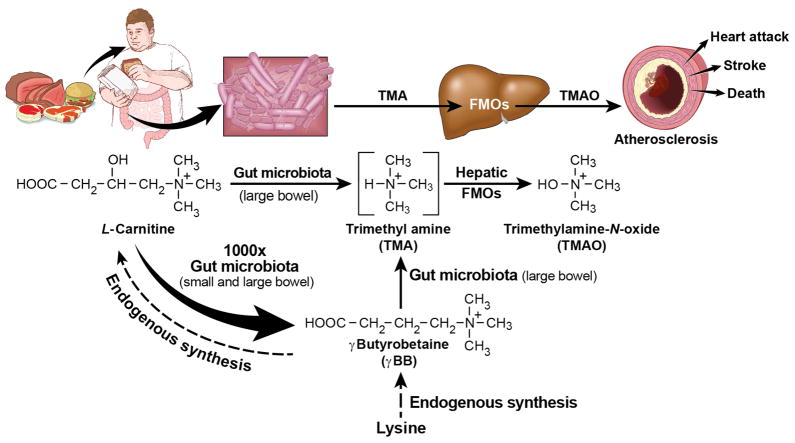
An obligatory role for gut microbes in the metabolism of dietary L-carnitine into TMA and TMAO has been established in both animal models and human clinical studies (Koeth et al., 2013); however, the pathways and enzyme(s) bacteria use to metabolize L-carnitine ultimately into TMA are poorly defined. Recently, Zhu and colleagues reported the cloning of a microbial enzyme capable of using L-carnitine as substrate to form TMA (Zhu et al., 2014), though its role in TMAO generation in vivo is unknown. Prior studies exploring the conversion of dietary L-carnitine into TMA/TMAO have not examined the possibility of sequential gut microbial reactions in the metabolism of L-carnitine into TMA. Interestingly, an early study in rats examining orally ingested radioactively labeled L-carnitine reported that γ-butyrobetaine (γBB), another trimethylamine, can be produced (Rebouche et al., 1984) (Figure S1; Gut Pathway 3, first reaction). γ-Butyrobetaine is used as a dietary supplement where it has also been called pre-carnitine (preCar), because of its known role as the immediate biosynthetic precursor to carnitine during endogenous carnitine synthesis. L-Carnitine is not required in the diet. It is synthesized by a multi-step pathway that begins with dietary lysine (Figure S1; Endogenous Pathway), an abundant amino acid in plant and animal proteins (Bremer, 1983; Rebouche and Seim, 1998).
Little is known regarding the relationship between γBB and the gut microbiome. Most studies consider the biochemical pathway of γBB → L-carnitine as unidirectional, catalyzed by the endogenous enzyme γBB hydroxylase (Rebouche and Engel, 1980), with γBB being a metabolite produced during L-carnitine biosynthesis, not catabolism (Figure S1; Endogenous Pathway). However, the production of γBB from L-carnitine by a gut microbial process has been suggested (Rebouche and Chenard, 1991; Rebouche et al., 1984; Seim et al., 1985). Herein we sought to test the hypothesis that γBB may serve as a pro-atherogenic intermediate in TMA/TMAO formation from dietary L-carnitine (Figure 1A, Figure S1, Gut Pathway 3). Such a finding would suggest additional potential bacterial pathways, and hence potential pharmacological targets, are involved in the mechanism(s) linking L-carnitine ingestion to enhanced atherosclerosis.
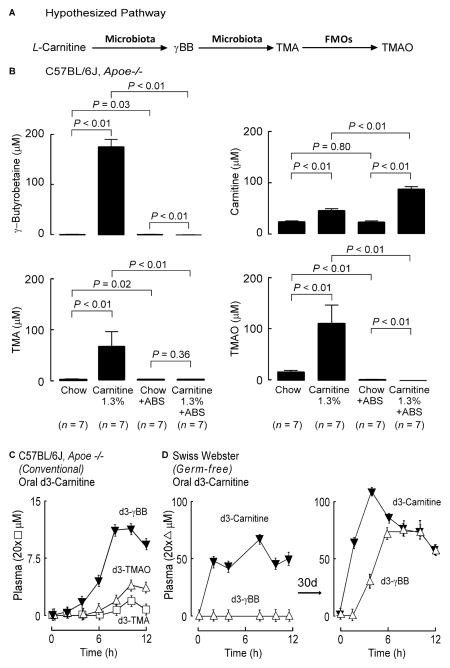
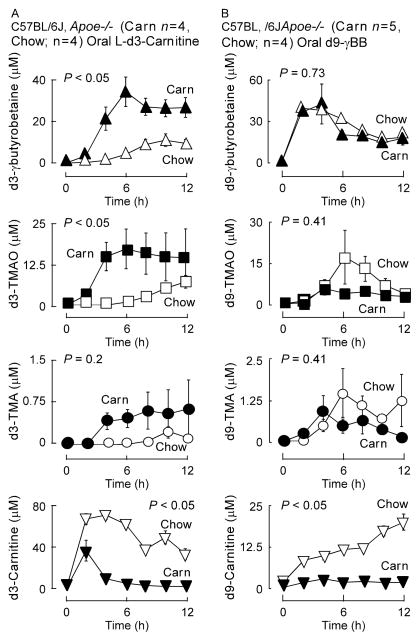
Figure 1. γBB is a quantitatively significant gut microbe generated metabolite of dietary L-carnitine.
(A) Hypothesized scheme of carnitine metabolism to TMA/TMAO through the intermediate production of γBB by gut microbes. (B) Stable isotope dilution LC/MS/MS analyses of plasma γBB, L-carnitine, TMA, and TMAO in plasma of C57BL/6J, Apoe−/− female mice on the indicated respective diets between the ages of weaning and 19 weeks of age. Error bars represent ± SE and P values represent Wilcoxon rank sums test. (C) C57BL/6J, Apoe−/− female mice challenged with d3-carnitine. Post challenge measurement of d3-metabolites was performed in serial venous blood draws by stable isotope dilution LC/MS/MS. (D) Female Swiss Webster Germ Free mice (n=4) challenged with d3-L-carnitine before and after conventionalization. Post challenge measurement of d3-L-carnitine and d3-γBB was performed in serial venous blood draws by stable isotope dilution LC/MS/MS. Data is expressed as means ± SE. (see also Figure S1).
Results
Gut microbial metabolism of L-carnitine produces γBB as a quantitatively significant product
To test whether gut microbial formation of γBB might represent a previously unrecognized intermediate in the pro-atherosclerotic effect of dietary L-carnitine and its conversion into TMA and TMAO (Koeth et al., 2013), we first evaluated plasma levels of γBB versus TMA, TMAO or carnitine in C57BL/6J, Apoe−/− mice placed on either a chemically defined diet (chow) versus the same diet supplemented with L-carnitine (+/− oral antibiotics cocktail to suppress gut microbes). Notably, plasma γBB levels in the L-carnitine supplemented mice (without antibiotics) demonstrated almost a 100-fold increase compared to chow-fed controls (Figure 1B). Further, suppression of gut microbes with an oral antibiotic cocktail virtually eliminated plasma levels of γBB, strongly indicating the majority of the analyte in L-carnitine supplemented mice was derived from gut microbial metabolism of dietary L-carnitine and not via the endogenous biosynthesis pathway. As was previously reported (Koeth et al., 2013), plasma TMA and TMAO levels were also suppressed in mice placed on the oral antibiotics, consistent with an obligatory role of gut microbes in the formation of these metabolites. Remarkably, plasma concentrations of γBB exceeded the concentration of carnitine, TMA or TMAO in the L-carnitine supplemented mice by approximately two-fold, suggesting production of γBB was a major gut microbial metabolite produced from L-carnitine (Figure 1B). Plasma clearance rates for trimethylamines were also determined, and observed to be within a factor of 2 of one another, with elimination of γBB being faster than that of either TMA or TMAO (Figure S2). Thus, in the carnitine supplemented mice, plasma γBB is not elevated relative to TMA or TMAO due to a reduced clearance rate.
To confirm a direct precursor → product relationship between dietary L-carnitine and γBB formation via a gut microbe-dependent process, conventional mice were provided L-d3(methyl)-carnitine orally by gastric gavage, and then time-dependent changes in plasma levels of the metabolites d3-γBB, d3-TMA and d3-TMAO were quantified (Figure 1C). Following ingestion, d3-γBB was observed, confirming a direct precursor → product relationship. Further, plasma levels of d3-γBB were significantly higher than that of either d3-TMA or d3-TMAO, again indicating formation of γBB was a major metabolite formed from dietary L-carnitine. To directly establish a role for gut microbes in γBB formation from dietary L-carnitine, synthetic L-d3(methyl)-carnitine was again provided by gastric gavage, but this time into germ-free mice. Serial plasma measurements following oral L-d3(methyl)-carnitine showed no detectable d3-γBB (Figure 1D). However, following housing in conventional cages to permit gut colonization with microbes, repeat oral L-d3(methyl)-carnitine challenge now readily showed d3-γBB in plasma (Figure 1D). Collectively, these results indicate that dietary L-carnitine serves as a precursor for gut microbe-dependent formation of γBB. Moreover, γBB is a quantitatively significant L-carnitine metabolite formed by the gut microbiome.
γBB produces TMA/TMAO in a gut microbe-dependent manner
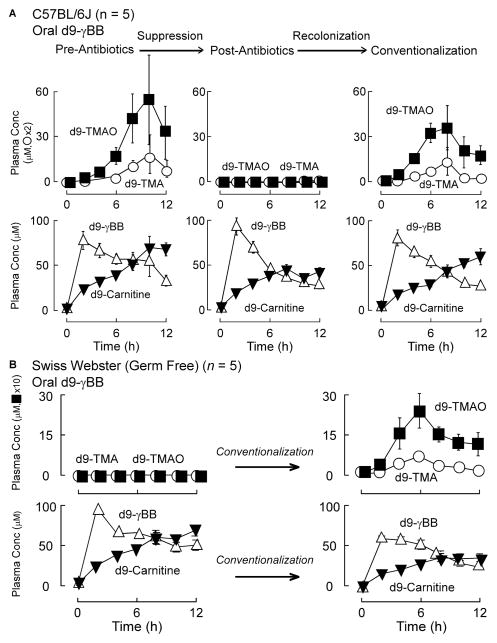
The demonstration of γBB production from dietary L-carnitine raises the possibility that this trimethylamine might contribute to TMA/TMAO formation by serving as an intermediate in gut microbial metabolism of L-carnitine into TMAO. To test this hypothesis, we first synthesized d9(trimethyl)-γBB (Methods) and then provided it by gastric gavage to conventionally housed C57BL/6J mice. Quantification of predicted d9-isotopologues of various trimethylamines (γBB, TMA, TMAO, carnitine, 3-dehydrocarnitine, choline, betaine, and trans crotonobetaine) in plasma was performed by LC/MS/MS. No d9-choline, d9-betaine or d9-3-dehydrocarnitine were detected, and only trace levels of d9-trans crotonobetaine were observed (over 2 orders of magnitude lower than d9-TMA or d9-TMAO, data not shown). Time dependent increases in both d9-TMA and d9-TMAO are shown in Figure 2A (top row, left). Interestingly, d9-carnitine was also produced (Fig. 2A, second row, left). While d9-carnitine formation from oral d9-γBB is consistent with γBB conversion into L-carnitine via the endogenous synthesis pathway, we could not rule out the possible contribution of gut microbes to the d9-carnitine generation (at this juncture). After marked reduction in gut microbe content with a period of oral poorly absorbed broad-spectrum antibiotics, mice were again challenged with d9-γBB by gastric gavage. Despite appearance of the d9-γBB and d9-carnitine in plasma (again consistent with the endogenous synthesis pathway), there was complete absence of any detectable d9-TMA or d9-TMAO in the circulation, indicating a gut microbe-dependence in TMA/TMAO formation from orally ingested γBB (Figure 2A, middle panels). After withdrawal of the oral antibiotics and a period of housing the mice in conventional cages to permit recolonization, there was reacquisition of the capacity to produce d9-TMA and d9-TMAO from oral d9-γBB, while production of d9-carnitine remained similar to that observed in the two previous challenges (Figure 2A, right panels). An obligatory role for gut microbes in formation of d9-TMA and d9-TMAO from d9-γBB was confirmed by performing a similar oral challenge using germ-free (Swiss Webster) mice (Figure 2B). As observed in the antibiotic suppressed C57BL/6J mice, no detectable d9-TMA or d9-TMAO were formed following gastric gavage of mice with d9-γBB, whereas d9-carnitine was produced, consistent with the endogenous (host) pathway. Following conventionalization, the prior germ-free mice acquired the capacity to produce d9-TMA and d9-TMAO from oral d9-γBB (Figure 2B).
Figure 2. Orally ingested γBB generates TMA and TMAO via a gut microbe dependent pathway.
(A) C57BL/6J female mice (n=5) challenged with d9-γBB gastric gavage (left panels; upper (d9TMA/TMAO) and lower (d9-L-carnitine and d9-γBB)) followed with serial blood venous blood draws and quantification of deuterated plasma analytes by stable isotope dilution LC/MS/MS. Repeat gastric gavage with d9-γBB after 1 month gut suppression with a cocktail of broad-spectrum antibiotics as described in Experimental Procedures (middle panels). A final d9-γBB gastric challenge and sequential measurement of deuterated plasma compounds (right panels) was performed after a month long reconventionalization period. (B) Female Swiss Webster Germ Free mice (n=5) were challenged with d9-γBB before and after conventionalization. Post challenge measurement of d9-TMA, d9-TMAO, (upper panels), d9-L-carnitine, and d9-γBB (lower panels) was performed in serial venous blood draws by stable isotope dilution LC/MS/MS. Data is expressed as means ± SE. (see also Figure S2).
γBB formation begins in a location anatomically proximal to TMA formation and is quantitatively a major gut microbial metabolite of L-carnitine
To further assess the relative synthetic capacity and location(s) along the intestinal tract for γBB and TMA formation from both L-carnitine and γBB, we co-incubated molar equivalent amounts of synthetic L-d3(methyl)-carnitine and d9(trimethyl)-γBB with distinct anatomic segments of mouse intestines (including luminal contents) and then examined production of the various trimethylamines (carnitine, γBB, TMA). Use of the different isotopically labeled precursors (d3-carnitine and d9-γBB) within gas tight reaction vials allowed us to simultaneously monitor direct precursor → product relationships individually for both carnitine and γBB by quantifying the d3- and d9-isotopologue metabolites formed from each precursor. Several results were noted from this experiment. First, the main site of TMA production from both carnitine and γBB were mainly in the bacterial rich cecum, with formation of TMA from γBB occurring at a rate approximately 1.5 fold higher than that from an equimolar amount of L-carnitine (Figure 3A). Second, the small bowel (jejunum and especially ileum) served as a major initial site of production of γBB from L-carnitine, anatomically proximal to those for TMA production (Figure 3A, right panel). Moreover, a remarkable 1,000-fold increase in d3-γBB production (relative to d3-TMA) from d3-carnitine was noted. To further establish that γBB production from carnitine is a dominant product of gut microbial catabolism of carnitine we also performed studies to measure the potential production of alternative suspected or hypothetical products including trans crotonobetaine, glycine betaine (betaine), glycine, and 3-dehydrocarnitine (Kleber, 1997). We observed no detectable production of d3-isotopologues of betaine, glycine, or 3-dehydrocarnitine in either plasma (multiple time points between 0–24 hours) of C57BL/6J female mice challenged (gastric gavage) with d3-carnitine or in the ex vivo gut segment incubation experiments under either anaerobic or aerobic conditions (Figure S3, and data not shown). We speculated that one potential reason these d3-trimethylamines were not detected in plasma may be due to an accelerated rate of elimination. However, the rate of plasma clearance of synthetic deuterium labeled forms of these trimethylamines were not remarkably different compared to γBB (Figure S2). We did detect modest production of trans crotonobetaine in the ex vivo gut segment experiments, as well as following oral (gastric gavage) ingestion (Figure S3 and S4). However, the production of trans crotonobetaine was significantly (almost 2 orders of magnitude) less than the production of γBB or TMA/TMAO from carnitine. In addition, the plasma clearance rate of trans crotonobetaine was significantly slower, not faster, than that observed with γBB (Figure S2). Finally, in studies with germ-free mice we confirmed that the trace levels of trans crotonobetaine formed were generated by gut microbes since virtually none was formed in the germ-free mice (Fig. S4B).
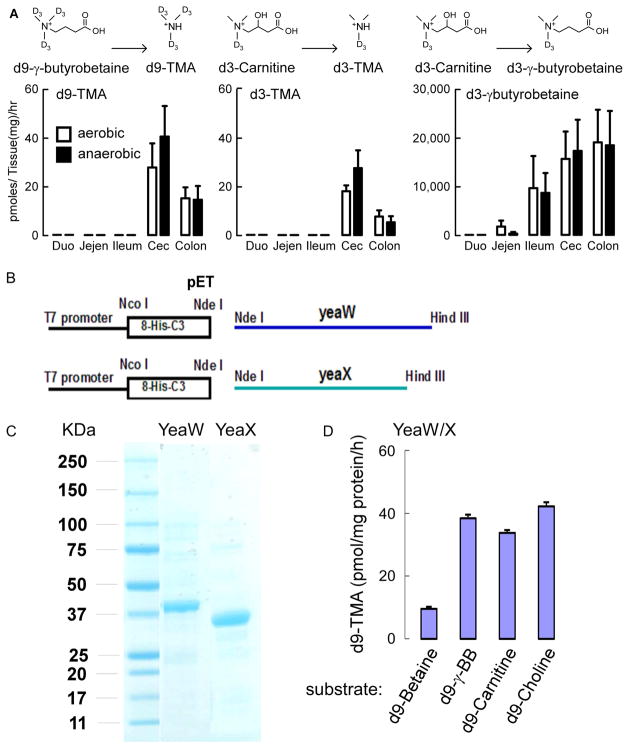
Figure 3. Gut microbes convert L-carnitine into γBB anatomically proximal to TMA, and the microbial enzyme yeaW/X shows TMA lyase activity with multiple trimethylamine nutrients.
(A) C57BL/6J Female mouse intestines (n=7) were sectioned into two complementary pieces for incubation at 37 °C for 18 hours with equimolar amounts of d3-L-carnitine (middle and right panels) or d9-γBB (left panel) under either aerobic (open bars) or anaerobic(closed bars)conditions, as indicated. Deuterated trimethylamine analytes were quantified by stable isotope dilution LC/MS/MS as described in Experimental Procedures. d3-γBB production from d3-L-carnitine is approximately 1,000-fold higher (lower panel) than d3-TMA production (middle panel). Duo =Duodenum, Jejen=Jejenum, Cec=Cecum (B) Cloning of YeaW/YeaX from E. coli DH10b into pET at NdeI and HindIII sites and transforming E. coli BL21. (C) SDS-PAGE confirmation of the purified yeaW and yeaX from E. coli BL21 lysate transformed with pET-yeaW and pET-yeaX, respectively. Both yeaW and yeaX contain 8xHis Tag. (D) YeaW/X catalyzes production of TMA from multiple TMA containing compounds. Data presented are mean ± SE for triplicate determinations from 2 independent replicates of purified proteins. (see also Figure S3–S7).
Collectively, our data thus suggested that sequential microbial reactions (L-carnitine → γBB, and then γBB → TMA) were a major pathway for orally ingested L-carnitine conversion into TMA and thus, TMAO. However, given our data opened the door to considering the concept of sequential reactions, we thought it prudent to perform additional studies to exclude a major contribution of alternative potential sequential pathways to either γBB or TMA production from ingested L-carnitine. Focusing first on generation of γBB from oral L-carnitine ingestion, we speculated that other trimethylamines produced during gut microbial dependent catabolism of L-carnitine, including TMA or TMAO themselves, might somehow serve as substrate to produce γBB via gut microbes. However, we could not experimentally demonstrate any d9-γBB formation from d9-TMA or d9-TMAO during either ex vivo gut segment incubations (Figure S5A,B) or following direct gastric gavage in mice (Figure S5C,D). We also sought to test whether gut microbe produced γBB (e.g. proximally in small bowel) might be converted back to carnitine, which could then be converted into TMA (i.e. γBB → L-carnitine → TMA). Indeed, the previous observation of the formation of d9-carnitine from oral d9-γBB in conventional mice (Fig. 2) raises the possibility that in addition to the endogenous synthesis pathway, gut microbes might have contributed to d9-carnitine formation. Comparison of the amount of d9-carnitine formed in conventional mice before vs. following antibiotic suppression of gut microbes (and following conventionalization) showed no difference in area under the curve (AUC) (i.e. the AUC of d9-carnitine in pre-antibiotics vs. post antibiotics (P=0.12); and AUC of d9-carnitine post-antibiotics vs. conventionalization (P=0.69) (Fig. 2A; Fig. S6)). Similarly, comparison of the AUC for d9-carnitine generation from oral d9-γBB in germ-free mice before vs. after conventionalization (Fig. 2B) revealed no significant differences (P=1.00). These results suggest no significant microbial production of d9-carnitine occurred following d9-γBB ingestion.
In separate experiments we looked at the effect of mildronate, a reported γBB hydroxylase inhibitor, on d9-carnitine generation from oral d9-γBB, in hopes that this inhibitor (a carnitine hydrazine analogue) might serve as a tool to specifically inhibit the endogenous carnitine biosynthesis pathway. However, control studies showed that it was rather non-specific, inhibiting several microbial reactions (e.g. gut microbial production of TMA from γBB, data not shown). To more directly test for a potential microbial contribution to the observed d9-carnitine in plasma following d9-γBB ingestion, we quantified the production of d9-carnitine (and d9-betaine) from d9-γBB in freshly harvested gut segments incubated ex vivo under either aerobic or anaerobic conditions. No detectable d9-carnitine (or d9-betaine) production was observed (Figure S7), despite clear d9-TMA production (Figure 3). In a last set of studies we tested whether the circulating d9-carnitine observed (via endogenous pathway) following d9-γBB ingestion might reenter the intestinal lumen (via diffusion or entero-hepatic cycling) to ultimately produce d9-TMA and d9-TMAO. Two groups of mice were provided an equivalent amount of d9-carnitine via either oral (gastric gavage) or parenteral (intraperitoneal injection) route, and the time course of changes in plasma d9-carnitine and d9-TMAO monitored (Figure S7). While the plasma levels of d9-carnitine achieved in the two groups of mice were on the whole similar (i.e. within approximately a factor of two), the concentrations of plasma d9-TMAO observed were dramatically reduced (>100-fold less) in the mice that received parenteral d9-carnitine. Thus, carnitine synthesized via the endogenous pathway from γBB enters the circulation and essentially bypasses the gut lumen (and microbes), and does not significantly contribute to observed TMA/TMAO generation.
Identification of a microbial enzyme complex, yeaW/X, that directly produces TMA from γBB
It has long been known that some cultured bacterial isolates can use L-carnitine as major carbon source and form TMA (Seim et al., 1982a; Seim et al., 1982b; Unemoto et al., 1966). Moreover, very recently, using bioinformatics approaches with Acinetobacter baumannii as the model, a two-component Rieske-type oxygenase/reductase capable of using L-carnitine in culture and producing TMA was reported (Zhu et al., 2014). We too had used a similar bioinformatics search strategy to identify microbial carnitine TMA lyases. We searched for microbial enzymes of unknown function that were clustered with those known to synthesize or use either malate or succinate (potential products formed following carntine utilization) and a presumed betaine-carnitine-choline transporter. One potential candidate was the gene pair previously called yeaW/X in E. Coli DH10B (yeaW (dioxygenase), GeneID: 6060925 and yeaX (oxidoreductase), GeneID: 6060982). Using a modified pET20 plasmid we transformed E. coli BL21pLysS with each allele and subsequently individually purified recombinant yeaW and yeaX from bacterial lysates (Figure 3B,C). When the purified proteins are combined, the yeaW/X complex demonstrated carnitine TMA lyase activity (monitored by d9-TMA production from d9-carnitine). Interestingly, further characterization of the recombinant microbial yeaW/X complex revealed substrate promiscuity, catalyzing production of d9-TMA from multiple synthetic d9-trimethylamine precursors (γBB, L-carnitine, choline, and betaine; Figure 3D).
Gut microbial metabolism of dietary γBB accelerates atherosclerosis
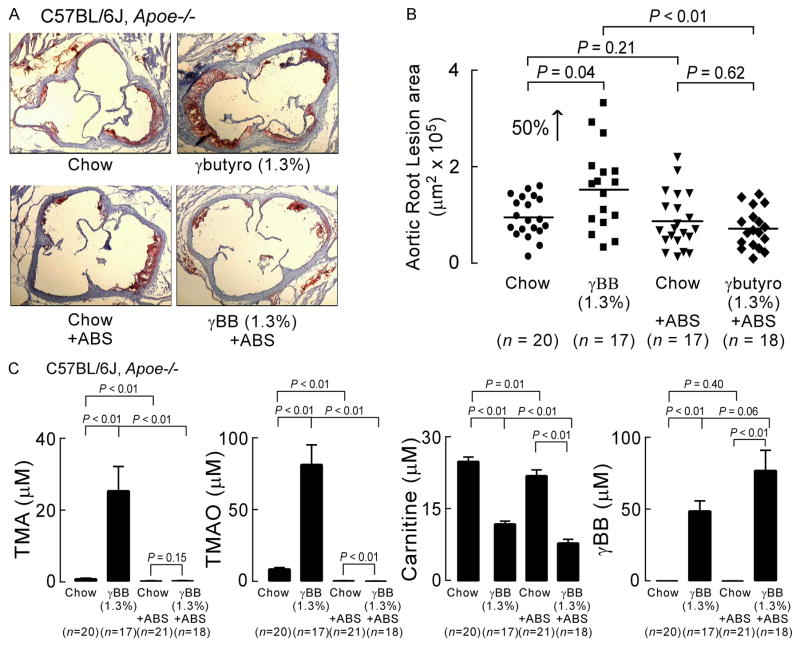
We next sought to test whether γBB could contribute to the enhanced development of atherosclerosis previously observed in mice placed on a diet supplemented with L-carnitine (Koeth et al., 2013). C57BL/6J, Apoe−/− littermates at time of weaning were placed in separate cages on a chemically defined diet equivalent to normal chow versus the same diet supplemented with γBB (1.3%, gm/gm). In parallel, in half the mice, gut microbes were suppressed by incorporating within the drinking water a cocktail of poorly absorbed antibiotics (Methods). At 19 weeks of age, mice were sacrificed and aortic root atherosclerotic plaque was quantified (Figure 4A,B). A significant 50% increase in total area of plaque was noted within the γBB diet supplemented mice compared to their littermate controls on chow diet (Figure 4A,B). Importantly, no increase in total plaque area was observed in mice on the γBB supplemented diet in which gut microbes was suppressed by the antibiotics cocktail (+ABS). Analyses of plasma levels of carnitine, γBB, TMA and TMAO showed neither carnitine nor γBB were likely the direct pro-atherogenic species since they remained high in the γBB-fed mice on antibiotics (Figure 4C). In fact, γBB-supplemented mice receiving antibiotics had the highest plasma γBB concentrations observed, yet showed no increase in atherosclerosis plaque (Figure 4A,B).
Figure 4. Gut microbes promote atherosclerosis in a gut microbe-dependent manner.
(A) Oil-red-O stained and hematoxylin counterstained representative aortic root slides of 19 week-old C57BL/6J, Apoe−/− female mice on the indicated diets in the presence versus absence of gut microbe suppression (± antibiotics (ABS)), as described under Experimental Procedures. (B) Quantification of mouse aortic root plaque lesion area of 19 week-old C57BL/6J, Apoe−/− female mice. Mice were started on the indicated diets at the time of weaning (4 weeks of age). Lesion area was quantified as described under Experimental Procedures. (C) Terminal plasma concentrations of γBB, L-carnitine, TMA, and TMAO were determined using stable isotope dilution LC/MS/MS analysis. Data is expressed as means ± SE. (see also Table S1).
In parallel studies we explored whether changes in known metabolic parameters that impact atherosclerosis might help explain the changes in aortic root plaque area observed between the groups of mice. Analyses of plasma cholesterol levels showed no differences amongst the mouse groups. A modest but statistically significant increase in plasma and liver triglyceride concentrations was observed within the γBB-supplemented mice (Table S1). A similar modest significant increase in plasma triglyceride concentrations, however, was also noted within antibiotic treated mice in the γBB-supplemented dietary arm versus controls (chow +ABS; Table S1). Further analyses of plasma showed no other pro-atherosclerotic alterations, with comparable HDL cholesterol and glucose levels observed among the different groups of mice.
Gut microbial production of γBB from L-carnitine is an inducible trait
We previously reported that gut microbe-dependent formation of TMA and TMAO from dietary L-carnitine is enhanced following chronic exposure, presumably due to compositional changes within the intestinal microbial community (Koeth et al., 2013). We therefore tested if gut microbial production of γBB from dietary L-carnitine was similarly inducible. L-carnitine challenges (gastric gavage with synthetic d3-L-carnitine) were performed in 10 week old C57BL/6J, Apoe−/− mice that had been maintained since weaning on either a chemically defined diet comparable to chow versus an otherwise identical diet supplemented with L-carnitine (1.3 gm%). Following oral d3-carnitine ingestion, production of d3-γBB was several-fold higher in mice that had been maintained on the L-carnitine-supplemented diet vs. chow (Figure 5A). As previously described (Koeth et al., 2013), production of both d3-TMA and d3-TMAO were also significantly induced in the L-carnitine supplemented mice, and plasma concentrations of d3-carnitine were significantly lower (consistent with a large portion of dietary L-carnitine being catabolized at greater efficiency by gut microbes following the chronic dietary exposure) (Figure 5A). When the mice on L-carnitine versus chow (control) diet were instead challenged with synthetic d9-γBB, not differences in d9-TMAO or d9-TMA production were noted. Moreover, post prandial (gastric gavage with d9-γBB) plasma levels of d9-γBB between L-carnitine supplemented versus chow mice were equivalent. These results indicate that there is a remarkable functional specificity induced following chronic L-carnitine exposure for gut microbe-dependent metabolism of the proximal dietary nutrient provided in abundance, L-carnitine, but not the more downstream metabolite, γBB (Figure 5B). Further, a significant increase in plasma d9-carnitine was noted in chow (control) mice compared to the L-carnitine supplemented mice. This latter data is consistent with oral d9-γBB being absorbed and converted into d9-carnitine in normal chow fed mice, and suppression of the endogenous L-carnitine biosynthesis pathway in mice supplemented with L-carnitine (Rebouche, 1983) (Figure 5B).
Figure 5. Mice chronically exposed to dietary L-carnitine demonstrate selective enhancement in gut microbial functional capacity to produce γBB and TMA/TMAO from L-carnitine, but not from γBB.
(A) d3-L-carnitine challenge of mice on an L-carnitine supplemented diet (1.3%) from weanining until 10 weeks of age or age-matched normal chow controls. Plasma concentrations of d3-γBB, d3-L-carnitine, d3-TMA, and d3-TMAO were measured in sequential venous blood draws at the indicated times post d3-L-carnitine challenge using stable isotope dilution LC/MS/MS. Data points represents mean ± SE of 4 replicates per group. (B) The same C57Bl/6J, Apoe−/− mice on L-carnitine and normal chow were challenged with d9-γBB oral gavage followed by venous blood draws at the indicated times over 12 hours. d9-γBB, d9-L-carnitine, d9-TMA, and d9-TMAO levels were analyzed using stable isotope dilution LC/MS/MS. Error bars represent ± SE and P values represent Wilcoxon rank sum test of the mean of the area under the curve for each replicate mouse.
Distinct microbial taxa are associated with production of γBB versus TMA or TMAO from dietary L-carnitine
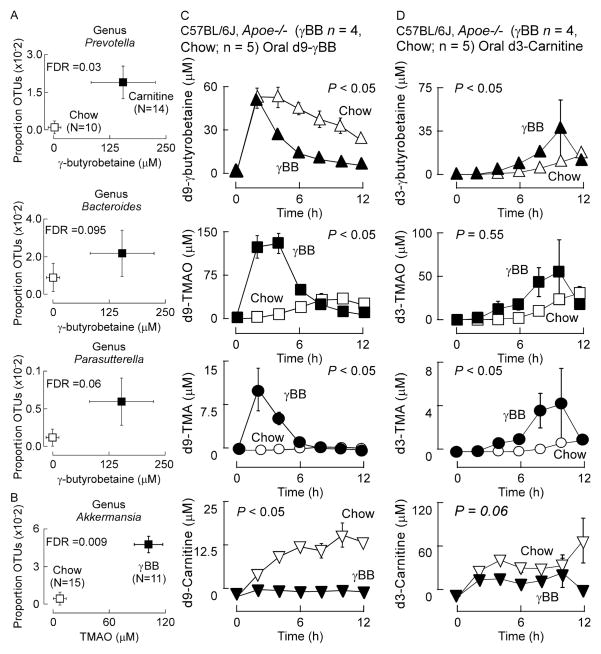
We next sought to test whether the enhancement of γBB versus TMA/TMAO production observed from L-carnitine in the chronic L-carnitine supplemented mice indicated enrichment of distinct taxa better suited for either γBB versus TMA/TMAO production. Microbial compositional analyses of intestinal contents recovered from L-carnitine versus chow supplemented mice were performed by sequencing 16S rRNA gene amplicons from cecum of mice. We initially characterized the gut microbiota of the cecum, a major site of carnitine conversion into both γBB and TMA. In parallel, stable isotope dilution LC/MS/MS analyses were performed to quantify plasma levels of gut microbe-dependent metabolites of L-carnitine (γBB, TMA and TMAO) within mice. Global analyses revealed multiple microbial genera showed increased proportions coincident with increased plasma levels of either γBB or TMAO after testing for multiple comparisons (Figure 6A,B). In separate analyses we examined 16S rRNA gene amplicons recovered from not only cecum, but also sections of small bowel shown to possess γBB production activity (ilium, and jejunum) from mice initially cohoused until time of weaning, whereupon they were separated and individually housed and maintained chronically on the indicated diets (γBB vs. carnitine) (Table S2). Interestingly, multiple similar taxa in the cecum were observed to have proportions that were significantly associated with levels of γBB (e.g. genera of Parasutterella, Prevotella, and Bacteroides), but they were distinct from taxa associated with TMAO levels. Further, the only microbial taxa in the small bowel (jejunum) that was significantly (FDR adjusted) associated with γBB levels was staphylococcus, which in cecum was not significantly associated with γBB levels (Table S2). Also of note, the genre Akkermansia was significantly associated with TMAO production in γBB supplemented mice in both the cecum and ilium, but was not significantly associated (all gut segment examined) with TMAO levels in carnitine supplemented mice.
Figure 6. Chronic dietary exposure to L-carnitine or γBB influences global function of the gut microbiome.
Plasma (A) γBB or (B) TMAO concentrations were determined by stable isotope dilution LC/MS/MS (plotted on x axes) and the proportion of taxonomic operational units (OTUs, plotted on Y axes) were determined as described in Experimental Procedures. The FDR (for multiple comparisons) P value shown is for comparisons between L-carnitine and normal chow dietary groups. (C) Mice on a 1.3% γBB supplemented diet from weaning until 10 weeks of age (n=4) and age-matched mice on a normal chow diet (n=5) were challenged with d9-γBB oral gavage. Plasma concentrations of d9-γBB, d9-L-carnitine, d9-TMA, and d9-TMAO recovered by sequential venous blood draws were measured using stable isotope dilution LC/MS/MS. P values represent Wilcoxon rank sum test of the mean of the area under the curve for each replicate mouse. (D) Mice on a 1.3% γBB-supplemented diet at 10 weeks of age (n=4) and age-matched mice on a normal chow diet (n=5) were challenged with d3-carnitine oral gavage. Plasma concentrations of d3-γBB, d3-L-carnitine, d3-TMA, and d3-TMAO recovered by sequential venous blood draws were measured using stable isotope dilution LC/MS/MS. P values represent Wilcoxon rank sum test of the mean of the area under the curve for each replicate mouse. (see also Table S2). All Error bars represent ± SE.
Gut microbial production of TMAO from dietary γBB is also an inducible trait with distinct microbial taxa associated with TMA and TMAO
The production of γBB from L-carnitine in the gut suggested γBB may also be involved in shaping the composition and function of gut microbial communities. At the time we performed the studies examining mice on L-carnitine vs. chow diets (described above), we in parallel examined cohoused C57BL/6J, Apoe−/− littermates that were separated at time of weaning and maintained on either a chemically defined diet comparable to chow versus an otherwise identical diet supplemented with γBB (1.3 gm%) for a similar period of time (weaning until 10 weeks of age), and then performed both gut microbial compositional analyses and quantification of plasma TMA and TMAO levels. Plasma TMAO levels were significantly associated with the relative abundances a distinct microbial genera, Akkermansia from the family Verrucomicrobia, within the cecum recovered from the γBB supplemented mice (Figures 6B). Further analyses were performed on mice placed on either L-carnitine vs. γBB diets (1.3 gm%, each), as described above, and 16S rRNA gene amplicons analyzed from different segments of the intestines (jejunum, ilium and cecum), and in parallel, plasma TMA, TMAO and γBB levels were determined. Plasma TMAO levels were significantly associated with the relative abundance of Akkermansia within the jejunum and cecum recovered from γBB supplemented mice (Table S2). Interestingly, TMA and TMAO production in the γBB-supplemented mice showed no association with bacteria from the Bacteroides or Proteobacteria phyla, taxa that co-associated with plasma γBB levels in L-carnitine fed mice (Table S2). Additionally, further global analyses of plasma concentrations of TMA and TMAO from mice on either carnitine-supplemented versus γBB-supplemented diets demonstrate virtually no overlap among proportions of gut microbiota genera that associated with plasma TMA/TMAO levels, suggesting that distinct genera may contribute to the metabolism of γBB into TMA/TMAO, versus gut microbial metabolism of dietary L-carnitine into TMA/TMAO (Table S2).
To further explore potential functional metabolic differences between the microbes that contribute to dietary γBB versus dietary L-carnitine metabolism into TMA/TMAO, we took initially cohoused C57BL/6J, Apoe−/− mice and at time of weaning placed them into individual cages with either chemically defined (chow) or γBB (1.3%) supplemented diet. After chronic exposure to the diets (10 weeks old), we challenged the mice with gastric gavage of equimolar amounts of d9-γBB and d3-carnitine (Figure 6C). Production of plasma d9-TMA and d9-TMAO from the d9-γBB was markedly higher in the mice that had been on the γBB supplemented diet, consistent with functional induction of microbes optimized to use γBB as fuel source following long-term dietary exposure (Figure 6B). Correspondingly, the post prandial plasma levels of d9-γBB observed were lower in the γBB supplemented mice, consistent with the previous dietary exposure inducing enhanced catabolic capacity of specific taxa better suited to utilize γBB as a fuel source (and TMA/TMAO formation) (Figure 6B). Notably, the γBB-supplemented mice failed to show enhanced d3-TMAO production following gavage with d3-carnitine (P=0.55; Figure 6D), but did show modest increases in the production of d3-TMA and d3-γBB (Figure 6D). The small increases in d3-TMA production paralleled the kinetics of production of d3- γBB, and may thus be the result of microbial metabolism of d3-γBB formed within the gut (luminal) (i.e. oral d3-carnitine → gut (microbial) d3-γBB → d3-TMA → d3-TMAO). Thus, chronic exposure to diets equivalent in chemical composition except for the addition of either L-carnitine or γBB, despite their remarkable similarity in structure, resulted in development of functionally distinct microbial communities optimized for the catabolism of L-carnitine versus γBB.
Discussion
The present studies unambiguously showing that gut microbial catabolism of L-carnitine into TMA/TMAO in mice can occur via at least two distinct routes involving functionally distinct genera of microbiota (Figure 7). The first pathway represents the direct scission of the C-N bond in L-carnitine liberating TMA within the cecum and proximal large bowel, which is then converted into TMAO by host FMO3, as previously described (Koeth et al., 2013). The second pathway involves the catabolism of L-carnitine through two sequential microbial reactions, first into the intermediate γBB, and then into TMA, which is then converted into TMAO by host hepatic FMO3 (Figure 7). Both functionally and compositionally distinct genera of microbes associate with this latter pathway, which appears to quantitatively account for a majority of L-carnitine catabolism in the mouse gut microbiome. Further, the anatomical distribution of gut microbial production of γBB from L-carnitine is distinct from that observed for direct TMA production. In the small bowel, where the bacterial load is considerably reduced compared to cecum and colon, L-carnitine catabolism to γBB begins. Moreover, biochemical assays along the intestinal tract indicate γBB formation in this proximal site occurs at a rate kinetically favored ~1,000-fold compared to direct cleavage of L-carnitine into TMA (Figure 7). There have been previous studies demonstrating that gut microbes can convert carnitine into γBB (Rebouche et al., 1984; Seim et al., 1985). Additionally, there is a study linking γBB to TMA production in culture with the bacterial isolate Acinetobacter calcoaceticu (Kleber et al., 1977). However, no studies have either linked γBB to TMA generation in vivo, or the sequential catabolism of L-carnitine into TMA and TMAO via the intermediate γBB. Further, the present studies are the first to show a pro-atherosclerotic effect of orally ingested γBB in the murine model (Figure 7).
Figure 7. Overall scheme showing gut microbe-dependent pathways for conversion of dietary L-carnitine into the pro-atherosclerotic metabolite TMAO.
γBB is endogenously produced as part of the L-carnitine biosynthetic pathway from lysine, but can also be produced by the metabolism of L-carnitine by commensal gut microbes. L-carnitine and γBB both serve as sources of TMA production via gut microbes.
Through a variety of studies we sought to rule out a quantitatively significant contribution of other alternative sequential microbe-catalyzed pathways within the intestinal lumen besides the proposed L-carnitine → γBB → TMA/TMAO pathway (Fig. 7). We first investigated whether or not L-carnitine → TMA → γBB, or TMA (or TMAO) → γBB were plausible. Neither oral ingestion nor gut segment incubations under a variety of conditions with d9-TMA or d9-TMAO resulted in detectable generation of d9-γBB or d9-carnitine, arguing these more terminal trimethylamine catabolites do not significantly participate as an intermediate in the formation of γBB. Similarly, no d9-carnitine could be detected following gut segment incubations with d9-γBB, d9-TMA or d9-TMAO. These latter studies strongly argue against microbe generated γBB formed in the proximal small bowel being subsequently converted by microbes into carnitine further down the intestinal tract (i.e. γBB → (via microbes) L-carnitine → TMA in the cecum and more distal colon as a potential pathway does not occur). Orally ingested d9-γBB does result in detection of d9-carnitine within plasma in both germ free and conventional mice, consistent with the endogenous carnitine synthesis pathway (Figure 7). However, no significant conversion of parenterally administered d3-carnitine into d3-TMA or d3-TMAO was observed in comparison to oral route, despite comparable plasma levels of carnitine achieved. Thus, once in the circulation (a separate compartment from gut microbes), carnitine does not significantly contribute quantitatively to TMA and TMAO formation. Additionally, quantification of other trimethylamines like betaine, 3-dehydrocarnitine, choline, and the related metabolite glycine similarly failed to indicate any significant metabolic production by the gut microbial community following isotope labeled carnitine ingestion. Only a minimal (2 orders of magnitude less) level of d3-trans crotonobetaine was detected following ingestion of d3-carnitine, and our studies with gut segments show similarly modest trans crotonobetaine production. Thus, while some contribution of trans crotonobetaine in TMA production may occur (e.g. L-carnitine → trans crotonobetaine → TMA; or L-carnitine → trans crotonobetaine → γBB → TMA; or L-carnitine → γBB → trans crotonobetaine → TMA), our data collectively argue that the overall contribution of trans crotonobetaine to TMA and TMAO formation in vivo will be modest. Also notable is our identification and cloning of yeaW/X, a promiscuous microbial TMA lyase complex capable of catalyzing the C-N bond cleavage of multiple trimethylamine substrates including γBB, thus affirming that gut microbial enzyme machinery exists for generating TMA directly from γBB. Finally, by synthesizing heavy isotope labeled standards of the various trimethylamines we were able to examine their rates of plasma clearance. These studies showed that the high plasma levels of γBB were not the result of slower elimination. Our studies thus collectively indicate that the sequential conversion of L-carnitine → γBB → TMA serves as a major gut microbial pathway for conversion of L-carnitine into TMA in the rodent model (Fig. 7).
Another interesting finding is that the production of γBB from L-carnitine in the gut is much more proximally distributed than TMA generation, beginning in the mid and distal small bowel (latter part of jejunum and ileum). This contrasts with the site of gut microbial production of TMA from either L-carnitine or γBB, which is localized to the cecum and proximal large bowel. L-carnitine is mostly absorbed through active transport in the upper gastrointestinal tract and only when the active transport system is saturated will appreciable levels of L-carnitine reach the more distal segments of the gastrointestinal tract to be catabolized by the gut micobiota (Rebouche and Seim, 1998). The more proximal anatomical location of γBB formation thus further argues for its physiological relevance. We acknowledge, however, that the ex vivo gut segment incubations have the inherent limitation of not being subject to neuro-endocrine inputs, nor the dynamic metabolism of the intact GI tract within a host. As such, conclusions from these experiments should be considered in this context. Despite these limitations, it is noteworthy that the distinct cecal microbial taxa identified whose proportions are associated with TMA or TMAO production from dietary L-carnitine in the past (Koeth et al., 2013) (e.g. Prevotella, Mucispirillium, Prevotellaceae-Unclassified, Anaeroplasma), or present studies (Lachnospiraceae or other Ruminococcaceae), or from dietary γBB in the present studies (e.g. Bacteroides, Prevotella, Parasutterella and several taxa from the phyla Proteobacteria) are known to be predominantly populated with anaerobic species. The discovery that gut microbial metabolism of L-carnitine involves a second quantitatively significant sequential pathway for TMA formation from distinct microbial taxa suggests numerous targets are at hand if one wishes to pharmacologically manipulate the catabolism of carnitine into TMA/TMAO for potential treatment or prevention of CVD, such as through shifting of gut bacterial composition (probiotic, prebiotic) or inhibition of bacterial enzymatic activities.
Early studies demonstrated that mammals completely lack the capacity to catabolize L-carnitine (Bremer, 1983; Kleber, 1997; Rebouche and Chenard, 1991). The importance of γBB in mammalian physiology has thus traditionally centered on its role in L-carnitine endogenous biosynthesis where γBB serves as the proximal biosynthetic intermediate in endogenous L-carnitine production that begins with dietary lysine and also utilizes methionine (Figure 7) (Bremer, 1983; Rebouche, 1983; Rebouche and Chenard, 1991). Our results reveal the quantitative importance of γBB as an unrecognized intermediate in the multi-step catabolism of L-carnitine by the gut microbiome into TMA and TMAO formation in mice. The results herein also allude to the complexity of the ‘meta-metabolome’. In the present case, sequential symbiotic microbial community-host interactions participate in an overall metaorganismal pathway that impacts the development of a complex phenotypic trait, atherosclerosis. The complexity of the global metabolome that reflects both host and symbiont inputs is even more evident when one considers that host genetic factors influence gut microbial communities, while diet shapes gut inhabitant composition (Wu et al., 2011). Thus, the interaction between host genetic factors, dietary and other environmental exposures, and microbial factors collectively contribute to the overall metabolic network or ‘meta-metabolome’ that impacts atherosclerosis expression in the present example.
It is significant to note that similar to prior observed results involving dietary supplementation with choline (Wang et al., 2011) and L-carnitine (Koeth et al., 2013), supplementation of γBB also increased atherosclerotic plaque area in a gut microbe-dependent manner. From a mechanistic standpoint, the present data clarify that gut microbial metabolism of γBB to TMA/TMAO, and not γBB itself directly, is associated with enhanced atherosclerosis. This conclusion is supported by the observation that mice supplemented with γBB and concomitantly treated with oral antibiotics to suppress gut microbes (and TMA/TMAO production) did not have an increase in aortic root atherosclerotic plaque area, despite having the highest plasma concentrations of γBB. Rather, mice with the highest plasma concentrations of TMAO had the most plaque at the aortic root, whereas those with suppressed gut microbes and lacking TMA/TMAO showed no increase in atherosclerosis. While multiple human studies with distinct clinical populations have now confirmed a strong association between plasma TMAO levels and cardiovascular disease risks (Koeth et al., 2013; Tang et al., 2013; Tang WHW, (2014); Wang et al., 2011; Wang et al., 2014), a causal role between TMAO and atherosclerosis and its adverse events in humans remains to be proven. Despite these limitations, taken together with prior studies demonstrating direct pro-atherosclerotic effects of dietary TMAO in the murine model (Koeth et al., 2013; Wang et al., 2011), the present studies add to the growing data indicating that enhanced microbial production of TMA and subsequently, TMAO, fosters a pro-atherogenic phenotype.
One of the more notable findings of the present study is the extreme functional plasticity of the gut microbial community in response to relatively small dietary changes. Previous studies from our laboratory (Koeth et al., 2013) and others (Muegge et al., 2011; Wu et al., 2011; Zimmer et al., 2012) have demonstrated that preceding dietary habits can greatly influence the gut microbial composition. In general, these studies have been observational, exploring the influence of long standing eating habits with large changes in complex dietary nutrients (e.g. meat eaters (omnivores) versus high fiber vegetarian or vegan diets) (Cotillard et al., 2013; David et al., 2014; Faith et al., 2013; Koeth et al., 2013; Muegge et al., 2011; Wang et al., 2011; Wu et al., 2011; Zimmer et al., 2012). In the present studies, however, it is noteworthy that the dietary differences between animal groups were quite modest. The only structural difference between γBB and L-carnitine is the presence of a hydroxyl group at carbon 3 in carnitine (Fig. 7). Yet despite the close structural similarity, distinct gut (cecal, jejunal and ileal) microbial compositions with discernibly distinct biochemical functionality were observed in mice chronically fed diets identical in composition except for modest supplementation with equivalent amounts of either L-carnitine or γBB. Remarkably, chronic L-carnitine supplemented mice demonstrated marked induced synthetic capacity to produce TMAO from L-carnitine but not γBB, whereas mice chronically supplemented with γBB demonstrated enhanced TMAO formation from oral γBB but not L-carnitine. Furthermore, gut microbial taxa that are associated with the metabolism of L-carnitine to γBB, the first step in the pathway, were also mostly different from the taxa associated with L-carnitine metabolism into TMA /TMAO (Table S2). It remains unclear whether diet-induced differences in microbial composition, or other mechanisms such as transcriptional or post-transcriptional regulation of microbial enzymes (or more likely a combination), are responsible for the functional differences observed. Indeed, there are data to show that bacterial isolates grown on L-carnitine can induce enzymes involved in carnitine catabolism (Aurich, 1966; Seim et al., 1982b). Regardless, even the subtle dietary change employed in the present studies between L-carnitine versus γBB supplementation was sufficient to substantially alter the gut microbial community biochemical output and function.
In summary, the present studies reveal that γBB serves as a gut microbial intermediate in the metabolism of L-carnitine to TMA/TMAO and provides an important advance in further understanding the connection between gut microbe-dependent metabolism of L-carnitine and atherosclerosis. Over the counter supplementation with γBB (PreCar) is marketed as a direct to consumer product. Indeed, over the counter nutritional supplements in general is a rapidly growing commercial enterprise, yet fall outside of the mandate of the Food and Drug Administration, and only need to demonstrate minimal safety data. Although there are no current studies directly linking γBB to human atherosclerosis, the present studies suggest additional investigation into γBB and atherosclerosis pathogenesis are warranted, as are long term safety studies with supplemental γBB, as well as choline and carnitine, particularly within otherwise healthy individuals where no clinical benefit with supplementation is established.
Experimental Procedures
Materials and general procedures
All animal studies were performed under approval of the Animal Research Committee of the Cleveland Clinic. Mouse plasma total cholesterol and triglycerides were measured using the Abbott ARCHITECT platform model ci8200 (Abbott Diagnostics, Abbott Park, IL). HDL cholesterol concentration, liver triglyceride and cholesterol contents were quantified as previously described (Wang et al., 2011). Gut microbial suppression studies were performed by dissolving antibiotics in drinking water (Wang et al., 2011). D3(methyl)-betaine and d9(trimethyl)-betaine, were purchased from C/D/N Isotopes Inc. (Pointe-Claire, Quebec, Canada), and d9-TMA and d9-TMAO were purchased from Cambridge Isotope Laboratories (Andover, MA).
Mouse challenge and atherosclerosis studies
An oral γBB or L-carnitine challenge in mice consisted of a gastric gavage of 150 ul of 150 mM of d9-γBB or d3-L-carnitine dissolved in water, respectively and as further described in Supplemental Methods. C57BL/6J, Apoe−/− female mice used in atherosclerosis studies were placed on a standard chow control diet (Teklad 2018) or γBB-supplemented diet (mouse drinking water with 1.3% γBB; BOC Sciences; Shirley, NY) in the presence of absence of antibiotics at time of weaning as further described in Supplemental Methods.
Mouse microbiota and statistical analyses
Microbial community composition was assessed by pyrosequencing 16S rRNA genes derived from the mice cecum, jejunum or ilium of mice on the indicated diets as described in detail within Supplementary Methods. Briefly, DNA was isolated using the MoBio PowerSoil DNA isolation kit according to the manufacturer’s instructions and sequenced using 454 GS FLX titanium chemistry at the GenoSeq Facility at the University of California, Los Angeles. Sequences that passed quality control were analyzed and assigned to operational taxonomic units (OTUs) using UCLUST, and the taxonomic composition assigned using Ribosomal Database Project (RDP) Classifier 2.0.1 (Wang et al., 2007). All data was analyzed using R software version 2.15, JMP (SAS Inc, Cary NC). False discovery rates (FDR) of the multiple comparisons were estimated for each taxon based on the p-values resulted from Spearman correlation estimates. The Wilcoxon Rank-Sum test was used for two-group comparison.
Deuterium labeled compound syntheses and LC/MS/MS quantification of trimethylamines
Detailed descriptions of syntheses, isolation and characterization of all compounds are described under Supplemental Methods. Briefly, d9-γBB was prepared as the chloride salt (3-carboxypropyl)trimethyl(d9)ammonium chloride; d9-γBB) from γ-aminobutyric acid in methanol with potassium hydrogen carbonate and d3-methyl iodide (all from Sigma-Aldrich, St. Louis, MO) (Morano et al., 2008). D9-(trimethyl)-trans crotonobetaine was prepared by acid treatment of L-carnitine using a modification of a previously described method (Heinz and Hermann, 1996). D3(methyl)-L-carnitine was synthesized by methylating L-norcarnitine with CD3I (Koeth et al., 2013). Racemic d9-(trimethyl)carnitine was prepared in an analogous manner to d3-L-carnitine, but starting with 4-amino-3-hydroxybutyric acid (Chem Impex International; Wood Dale, IL). The purity (>98%) and identity of all compounds were confirmed by NMR, mass spectrometry, and thin layer chromatography. Stable isotope dilution LC/MS/MS (Koeth et al., 2013; Wang et al., 2011; Wang et al., 2014) was used to quantify trimethylamine compounds from mouse plasma (and diets) in positive ion MRM mode. Precursor -> product ion transitions specific for each trimethylamine isotopologue are further described in Supplemental Methods.
Plasma clearance studies
C57BL/6J female mice were injected i.v. with an equimolar isotonic neutral pH cocktail of synthetic d9-γBB, d3-carnitine, d9-TMAO, d9-TMA, d9-trans crotonobetaine, and d9-betaine. Multiple sequential venous blood draws were performed over a one hour period and the concentration of the respective isotopologues of the various labeled trimethylamines was determined by LC/MS/MS. Initial plasma clearance rates were determined using the equation Ct=Co*e-k(t) where Ct is the final concentration (defined at 60 minutes) Co is the initial (peak) concentration, k is the calculated rate constant, and t is time (one hour).
Ex vivo mouse gut segment incubations
C57BL/6J female mouse (n=7) intestines were harvested, sectioned into the indicated anatomic parts, and then each piece was longitudinally cut into 2 halves, and placed into gas tight reaction vials containing 50mM HEPES buffer pH 7.4, and 150 μM each of d9-γBB and/or d3-L-carnitine, respectively, depending upon study design. Reaction vials were incubated under anaerobic (Argon) conditions. Following 16 hour incubation at 37°C, reactions were halted by addition of 0.1% (final) formic acid, d4-choline added as internal standard, and then analyzed by LC/MS/MS.
Cloning and expression of yeaW/X
Bioinformatic searching of the E. Coli genome for close physically localized ORFs encoding a potential carnitine transporter, oxygenase/dioxygenase, a reductase and malic acid/succinate dehydrogenases consistent with the known published biochemical pathways identified for carnitine utilization in Acinetobacter (Ditullio et al., 1994; Englard et al., 1983; Kleber et al., 1977; Seim et al., 1982a) was used to identify potential candidate E. Coli K-12 DH10B genes as further described in Supplemental Methods.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health and Office of Dietary Supplements grants R01 HL103866 (S.L.H.), P20 HL113452 (S.L.H.), PO1 HL30568 (A.J.L.), PO1 H28481 (A.J.L.), R01 HL-094322 (A.J.L.), R01 HL098193 (J.D.S.), and the Leducq Fondation (S.L.H. and A.J.L.). SLH is also partially supported by a gift from the Leonard Krieger Fund. E.O. was supported in part by a MOBILITAS Postdoctoral Research Grant (MJD252). R.A.K. was supported in part by US NIH grant T32 GM007250.
Footnotes
Competing Financial Interest Disclosure
Drs. Wang and Levison are named as co-inventors on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Dr. Tang received research grant support from Abbott Laboratories, and served as consultants for Medtronic Inc and St. Jude Medical. Drs. Hazen and Smith are named as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Smith reports he has been paid as a consultant by Esperion, and has the right to recieve royalty payments for inventions from Cleveland Heart Lab and Esperion. Dr. Hazen reports he has been paid as a consultant or speaker by the following companies: Cleveland Heart Lab, Inc., Esperion, Liposciences Inc., Merck & Co., Inc., Pfizer Inc., and Proctor & Gamble. Dr. Hazen reports he has received research funds from Abbott, Cleveland Heart Lab, Liposciences, Inc., Proctor & Gamble, and Takeda. Dr. Hazen has the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics and therapeutics from Cleveland Heart Lab, Inc., Esperion, Frantz Biomarkers, and Liposciences, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aurich H. Composition of the amino acid pool of Neurospora in deficiency of growth substance. Acta biologica et medica Germanica. 1966;16:123–134. [PubMed] [Google Scholar]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell metabolism. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876–883. doi: 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J. Carnitine--metabolism and functions. Physiological Reviews. 1983;63:1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto JM, Renault P, Dore J, Zucker JD, Clement K, Ehrlich SD. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditullio D, Anderson D, Chen CS, Sih CJ. L-carnitine via enzyme-catalyzed oxidative kinetic resolution. Bioorganic & medicinal chemistry. 1994;2:415–420. doi: 10.1016/0968-0896(94)80009-x. [DOI] [PubMed] [Google Scholar]
- Englard S, Blanchard JS, Miurafraboni J. Production of Trimethylamine from Structurally Related Trimethylammonium Compounds by Resting Cell-Suspensions of Gamma-Butyrobetaine-Grown and D,L-Carnitine-Grown Acinetobacter-Calcoaceticus and Pseudomonas-Putida. Archives of microbiology. 1983;135:305–310. [Google Scholar]
- Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz L, Hermann S. SYNTHESIS OF [methyl-14C1CROTONOBETAlNE FROM DL-[methyl-14CICARNITINE. Journal of Labelled Compounds and Radiopharmaceuricals. 1996;38:179–186. [Google Scholar]
- Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, Nielsen J, Backhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- Kleber HP. Bacterial carnitine metabolism. FEMS microbiology letters. 1997;147:1–9. doi: 10.1111/j.1574-6968.1997.tb10212.x. [DOI] [PubMed] [Google Scholar]
- Kleber HP, Seim H, Aurich H, Strack E. Utilization of Trimethylammonium-Compounds by Acinetobacter-Calcoaceticus. Archives of microbiology. 1977;112:201–206. doi: 10.1007/BF00429336. [DOI] [PubMed] [Google Scholar]
- Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature medicine. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121:2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano C, Zhang X, Fricker LD. Multiple isotopic labels for quantitative mass spectrometry. Analytical chemistry. 2008;80:9298–9309. doi: 10.1021/ac801654h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muegge BD, Kuczynski J, Knights D, Clemente JC, Gonzalez A, Fontana L, Henrissat B, Knight R, Gordon JI. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–974. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebouche CJ. Effect of dietary carnitine isomers and gamma-butyrobetaine on L-carnitine biosynthesis and metabolism in the rat. J Nutr. 1983;113:1906–1913. doi: 10.1093/jn/113.10.1906. [DOI] [PubMed] [Google Scholar]
- Rebouche CJ, Chenard CA. Metabolic fate of dietary carnitine in human adults: identification and quantification of urinary and fecal metabolites. The Journal of nutrition. 1991;121:539–546. doi: 10.1093/jn/121.4.539. [DOI] [PubMed] [Google Scholar]
- Rebouche CJ, Engel AG. Significance of renal gamma-butyrobetaine hydroxylase for carnitine biosynthesis in man. J Biol Chem. 1980;255:8700–8705. [PubMed] [Google Scholar]
- Rebouche CJ, Mack DL, Edmonson PF. L-Carnitine dissimilation in the gastrointestinal tract of the rat. biochemistry. 1984;23:6422–6426. doi: 10.1021/bi00321a022. [DOI] [PubMed] [Google Scholar]
- Rebouche CJ, Seim H. Carnitine metabolism and its regulation in microorganisms and mammals. Annual Review of Nutrition. 1998;18:39–61. doi: 10.1146/annurev.nutr.18.1.39. [DOI] [PubMed] [Google Scholar]
- Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell metabolism. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Seim H, Loster H, Claus R, Kleber HP, Strack E. Splitting of the C-N Bond in Carnitine by an Enzyme (Trimethylamine Forming) from Membranes of Acinetobacter-Calcoaceticus. FEMS microbiology letters. 1982a;15:165–167. [Google Scholar]
- Seim H, Loster H, Kleber HP. Reductive metabolism of L-carnitine and structure-related trimethylammonium compounds in Escherichia coli. Acta biologica et medica Germanica. 1982b;41:1009–1018. [PubMed] [Google Scholar]
- Seim H, Schulze J, Strack E. Catabolic pathways for high-dosed L(−)- or D(+)-carnitine in germ-free rats? Biological chemistry Hoppe-Seyler. 1985;366:1017–1021. doi: 10.1515/bchm3.1985.366.2.1017. [DOI] [PubMed] [Google Scholar]
- Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. Journal of Clinical Investigation. 2014 doi: 10.1172/JCI72331. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. The New England journal of medicine. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WHW, WZ, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognostic Value of Elevated Levels of Intestinal Microbe-Generated Metabolite, Trimethylamine-N-oxide, in Patients with Heart Failure: Refining the Gut Hypothesis. Journal of the American College of Cardiology. 2014 doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemoto T, Hayashi M, Miyaki K. Formation of trimethylamine from DL-carnitine by Serratia marcescens. Biochimica et biophysica acta. 1966;121:220–222. doi: 10.1016/0304-4165(66)90382-5. [DOI] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. European heart journal. 2014;35:904–910. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science (New York, NY) 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Jameson E, Crosatti M, Schafer H, Rajakumar K, Bugg TD, Chen Y. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:4268–4273. doi: 10.1073/pnas.1316569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer J, Lange B, Frick JS, Sauer H, Zimmermann K, Schwiertz A, Rusch K, Klosterhalfen S, Enck P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. European journal of clinical nutrition. 2012;66:53–60. doi: 10.1038/ejcn.2011.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.