Abstract
Many key components of innate immunity to infection are shared between Drosophila and humans. However, the fly Toll ligand Spaetzle is not thought to have a vertebrate equivalent. We have found that the structurally related cystine-knot protein, Nerve Growth Factor β (NGFβ), plays an unexpected Spaetzle-like role in immunity to Staphylococcus aureus infection in chordates. Deleterious mutations of either human NGFβ or its high-affinity receptor TRKA were associated with severe S. aureus infections. NGFβ was released by macrophages in response to S. aureus exoproteins through activation of NLRP3 and NLRP4, and enhanced phagocytosis and superoxide-dependent killing, stimulated pro-inflammatory cytokine production, and promoted calcium-dependent neutrophil recruitment. TrkA knockdown in zebrafish increased susceptibility to S. aureus infection, confirming an evolutionarily conserved role for NGFβ-TRKA signaling in pathogen-specific host immunity.
Staphylococcus aureus causes a range of serious infections including skin ulceration, osteomyelitis, pneumonia and septicaemia (1, 2). Several evolutionarily conserved components of anti-staphylococcal immunity have been identified using Drosophila as a model organism (3,4). One of the key mediators of immunity to Gram-positive bacteria in Drosophila is the soluble protein Spaetzle which, when activated by Spaetzle processing enzyme (SPE) upon infection, triggers effector immunity in an autocrine and paracrine manner through Toll receptor activation (3, 5-8). To detect potential vertebrate equivalents of Spaetzle, we searched the human proteome using a relatively tolerant PROSITE pattern (C-X(35,45)-C-X(10,15)-C-X(25,32)-C-X-C; modified from ref. 9) to identify 166 soluble proteins potentially containing a >10 membered cystine knot domain (See Supplementary Material). We identified the neurotrophin Nerve Growth Factor β (NGFβ) as a possible vertebrate orthologue of Spaetzle (Figure 1A, B). NGFβ regulates the survival, differentiation and function of central and peripheral neurons (10, 11), predominantly through activation of its high affinity receptor, tropomyosin-related kinase receptor A (TRKA). Like Spaetzle, NGFβ is generated by enzymatic cleavage of a precursor pro-protein to form a biologically active cystine knot dimer (Ref. 11; Figure 1C). Because NGFβ is implicated in the modulation of inflammation in non-neuronal cells (12 – 14), we asked whether NGFβ could play a Spaetzle-like role in co-ordinating vertebrate immunity to S. aureus.
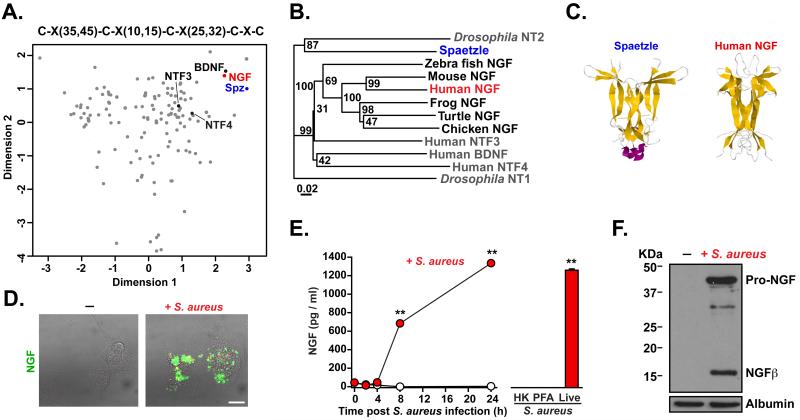
Figure 1. NGFβ is implicated in anti-staphylococcal immunity and is released from macrophages following S. aureus infection.
A. Bioinformatic identification of potential human orthologues of Spaetzle. The human proteome was search using a PROSITE pattern to find soluble proteins potentially containing a >10 membered cystine knot domain, which were then subjected to multifactorial analysis, incorporating structural prediction of disulphide bond formation with other structural and sequence parameters (See Supplementary Methods for details) to identify Nerve growth factor (NGF; red) as the closest human orthologue to Spaetzle (Spz; blue). The other human neurotrophins (brain-derived growth factor (BDNF), neurotrophic factor 3 (NTF3) and 4 (NTF4); black) are also highlighted. B. Phylogenetic alignment of vertebrate neurotrophic factors (NGF, BDNF, NTF 3 & 4), Drosophila neurotrophin (NT) 1 & 2 and the Drosophila immune regulator Spaetzle, with bootstrap values. C. Dimeric protein structures (from PDB) of Spaetzle and human NGFβ. D. Intracellular staining of NGFβ (green) in primary human macrophages uninfected (top) or infected with S. aureus (SH1000) (red; bottom) and then treated with monensin for 14h to prevent secretion. Scale: 5 μm. E. Time course of NGFβ release from primary human macrophages following infection with S. aureus. NGFβ secretion requires live bacteria since heat-killed (HK) or paraformaldehyde (PFA)-killed S. aureus do not trigger NGFβ release. F. Release of Pro-NGF and NGFβ from differentiated THP-1 cells upon infection with S. aureus for 12 h. * denotes p ≤ 0.05; ** denotes p ≤ 0.005. All experiments were carried out in at least triplicate and are representative of at least 3 independent repeats.
Deleterious biallelic mutations in the genes encoding NGFβ (NGF; Ref. 15, 16) or TRKA (NTRK1; Ref. 17) lead to a profound congenital sensory and autonomic neuropathy (termed Hereditary Sensory and Autonomic Neuropathy (HSAN) 4 and 5). We found that these individuals also had frequent severe S. aureus infections of skin, teeth, joints and bone (Figure S1) suggesting a pathogen-specific immune defect. To further explore the role of NGFβ in staphylococcal immunity, we measured its release from primary human macrophages obtained from healthy individuals. Infection of cells with live, but not killed, S. aureus stimulated de novo synthesis and secretion of both pro-NGF and mature NGFβ (Figure 1D-F). We found considerable variation in NGFβ stimulation by clinical isolates of S. aureus. Clones triggering lower levels of NGFβ were associated with increased all-cause patient mortality (Figure S1); again suggesting a protective role for NGFβ during S. aureus infection. The exact mechanisms generating mature NGFβ remain unclear but it is likely that endogenous and exogenous host proteases (such as furins (18), matrix metalloproteinase (MMP) 7 and plasmin (19)) as well as bacterial proteases (Figure S1) combine to cleave Pro-NGF during S. aureus infection, suggesting similarities with the regulation of Spaetzle processing (20).
We next examined whether other bacterial species were also able to stimulate NGFβ release from macrophages. Although a low level response was seen with some other bacteria (such as Enterococcus faecalis), only S. aureus effectively triggered NGFβ release (Figure S1). Indeed, the closely-related skin commensal Staphylococcus epidermidis was unable to stimulate significant NGFβ production, suggesting that macrophages can discriminate between pathogenic and non-pathogenic staphylococcal species. Furthermore macrophages only secreted NGFβ and not other neurotrophins (BDNF, NT3 and NT4) in response to infection (Figure S1). Thus, NGFβ may act as a specific and sensitive signal for S. aureus infection in man, potentially explaining the clinical phenotype of patients with HSAN 4 and 5 and suggesting a non-redundant and pathogen-specific role for NGFβ in innate immunity.
We then explored the cellular pathways triggering NGFβ generation. Rather than involving conventional surface pattern recognition receptors, S. aureus elicits NGFβ production through activation of NOD-like receptors (NLRs; Figure S2), a well-recognised consequence of infection with this bacteria (21), and suggests an additional potential role for NGFβ during tissue damage.
To define the bacterial components responsible for NGFβ release from macrophages, we screened the Nebraska library of S. aureus transposon mutants (22) for their ability to stimulate NGFβ release from THP-1 cells. This identified a number of genes involved in bacterial cell wall synthesis, macromolecular transport, metabolism and cellular regulation (Figure S3; Additional Data Table S1) including the saeR/saeS 2 component gene system and autolysin, which regulate exoprotein and peptidoglycan release, respectively (23,24). As expected, a number of purified S. aureus-derived exoproducts (protein A, peptidoglycan and α-haemolysin) were able to stimulate NGFβ release in a proteinase K-dependent manner (Figure S3). Because most single exoprotein deletion mutants were still capable of stimulating NGFβ release suggesting redundancy (Figure S4), we turned to comparative mass spectroscopy of conditioned media from wild type and saeS- mutant S. aureus to define further bacterial components mediating NGFβ release (Figure S4) and identified alpha phenol soluble modulins (α-PSMs), a recently described family of secreted peptides capable of membrane rupture (25), as putative factors (Figure S4). Thus, multiple S. aureus exoproteins can stimulate NGFβ release from macrophages. We asked whether this regulatory mechanism might be evolutionarily conserved to control Spaetzle production in Drosophila. Intriguingly, although the regulation of Spaetzle activity has focused on its SPE-mediated activation (26), pro-Spaetzle levels in Drosophila phagocytes (S2 cells) were stimulated by wild type but not saeR- S. aureus, by conditioned media and by peptidoglycan (Figure S4), mirroring our results with NGFβ.
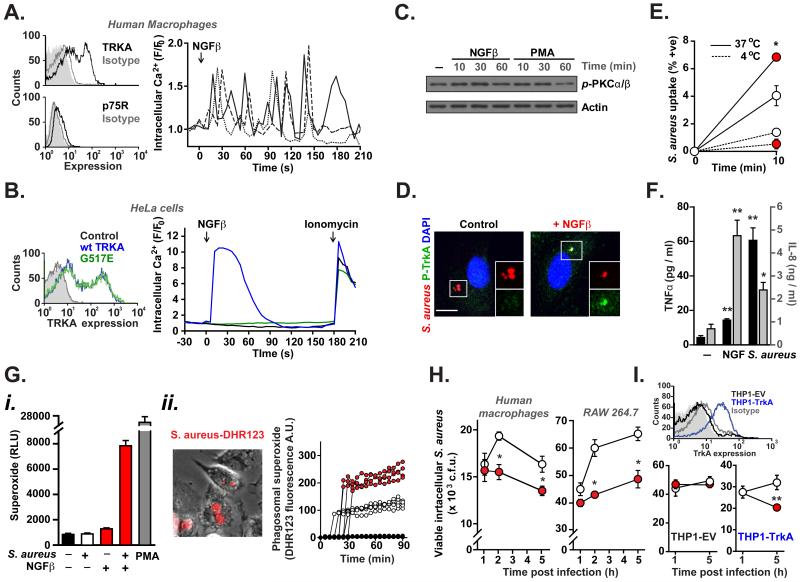
We then evaluated the effects of NGFβ on macrophage function. Primary human macrophages, which have constitutively high surface expression of TRKA but not the low affinity NGF receptor p75, responded to NGFβ with sustained calcium signaling (Figure 2A), which could be reconstituted in HeLa cells expressing wild type TRKA but not the HSAN5-associated mutation G517E (Figure 2B). TRKA signaling in macrophages also triggered rapid activation of calcium-dependent PKC isoforms (Figure 2C) as well as other recognised components of TRKA signaling observed in neuronal cells (Additional Data Table S2). Since TRKA is thought to continue signaling following internalisation, thereby permitting signal transmission along axons (27), we examined whether phagosomal TRKA activation might occur and found persistent tyrosine phosphorylation of TRKA within S. aureus-containing phagosomes (Figure 2D). Functionally, TRKA activation led to enhanced phagocytosis (Figure 2E), pro-inflammatory cytokine release from uninfected cells (Figure 2F) and increased S. aureus-induced phagosomal superoxide generation (Figure 2G). TRKA activation also enhanced intracellular killing of S. aureus in human and mouse macrophages (Figure 2H) and in TRKA-transfected, but not control, THP-1 cells (Figure 2I). This increased killing was dependent on intact receptor signaling (since it was not observed in cells from HSAN4 patients), and was principally mediated through enhanced superoxide generation (Figure S5) and autophagy (Figure S6). TRKA-dependent effector responses also depended on intact TLR signaling, because intracellular killing in S. aureus-infected cells and cytokine production in uninfected cells were abrogated in Myd88−/− and Trif−/− macrophages (Figure S7), suggesting an evolutionarily conserved interaction between cystine knot proteins and Toll family receptors.
Figure 2. Effects of NGFβ-TRKA signaling in human macrophages.
A. Addition of NGFβ (250 ng/ml; 9.25 μM) triggers sustained calcium oscillations in Fluo3-loaded primary human macrophages (detected by single cell confocal imaging). Three representative recordings normalised for starting fluorescence (F/F0) are shown. Inset: Surface expression of TRKA and p75R (black) compared to isotype control (dark grey) or unstained cells (grey fill) on primary human macrophages. B. Single cell calcium signaling in GCamp3-expressing HeLa cells transfected with wild type TRKA (blue), HSAN4-associated TRKA mutation (G517E; green) or empty vector (black) in response to NGFβ (250 ng/ml; 9.25 μM). Inset: Surface expression of TRKA in transfected HeLa cells. C. TRKA signaling in macrophages triggered rapid activation of calcium-dependent PKC isoforms. D. Co-localisation of intracellular phospho-TRKA (green) with RFP-labelled S. aureus (SH1000; red) in primary human macrophages treated for 30 min with 100 ng/ml (3.7 μM) NGFβ. Σχαλε βαρ 10μμ. E,F. Addition of NGFβ to primary human macrophages increased (F) phagocytosis of RFP-labelled S. aureus and (G) release of TNFα and IL-8 (measured after 24h). G. (i) Luminol-based detection of superoxide in response to S. aureus, NGFβ or PMA. (ii) The generation of phagosomal superoxide, monitored by DHR123-labelled heat-killed S. aureus, is increased in cells treated with the TRKA-specific agonist gambogic amide (250 nM; red) compared to vehicle (white; p < 0.005) or bacteria without cells (black). Four representative fluorescence traces from individual cells are shown for each group. H,I. TRKA activation (by gambogic amide; 250 nM) enhanced intracellular killing of S. aureus in (H) primary human macrophages (left) and the mouse macrophage cell line RAW 264.7 (right) and in (I) TRKA-transfected (blue), but not control (black) THP-1 cells. Inset: surface TRKA expression in THP-1 cells transfected with TRKA (blue) or empty vector (black) compared to isotype control (grey) and unstained cells (grey fill). All experiments were carried out in at least triplicate and are representative of at least 3 independent repeats.
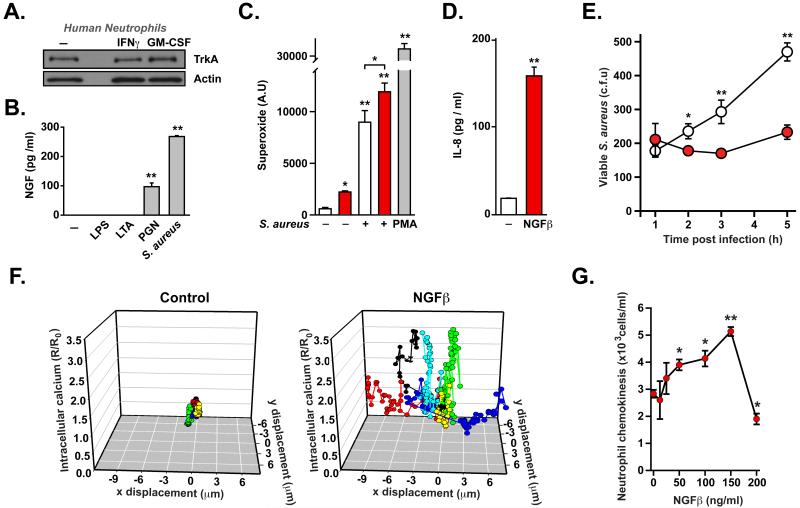
We next determined the role of NGFβ-TRKA in human neutrophils, which are critical components of the host response to S. aureus infection (28). Neutrophils constitutively expressed TRKA (Figure 3A) and released NGFβ in response to live S. aureus and peptidoglycan (Figure 3B). As seen in macrophages, NGFβ stimulated neutrophils to generate superoxide (Figure 3C), secrete pro-inflammatory cytokines (Figure 3D) and enhanced intracellular killing of S. aureus (Figure 3E). NGFβ also stimulated chemokinesis and chemotaxis in a TRKA- and calcium-dependent manner (Figure 3F,G; Movie S1; Figure S8) suggesting that NGFβ may be an important chemotactic signal for neutrophil recruitment to sites of S. aureus infection.
Figure 3. NGFβ-TRKA signaling stimulates functional activation of neutrophils.
A. TRKA expression on untreated, IFN-γ(10 ng/ml) or GM-CSF (100 ng/ml) -primed primary human neutrophils. B. Neutrophils secrete NGFβ in response to live S. aureus, peptidoglycan (PGN) but not lipopolysaccharide (LPS; 100 ng/ml) or lipoteichoic acid (LTA; 5 μg/ml). C, D. Neutrophils generate superoxide (C) and release IL-8 (D) in response to S. aureus, PMA and/or NGFβ (red). E. Killing of S. aureus by human neutrophils is enhanced by treatment with NGFβ (100 ng/ml; red) compared to control (white). F. Representative plots of x-y displacement and calcium levels in individual neutrophils following addition of vehicle (control) or NGFβ<G. Chemokinesis of human neutrophils assess using a transwell assay in response to increasing concentrations of NGFβ. * denotes p ≤ 0.05; ** denotes p ≤ 0.005. All experiments were carried out in at least triplicate and are representative of at least 3 independent repeats.
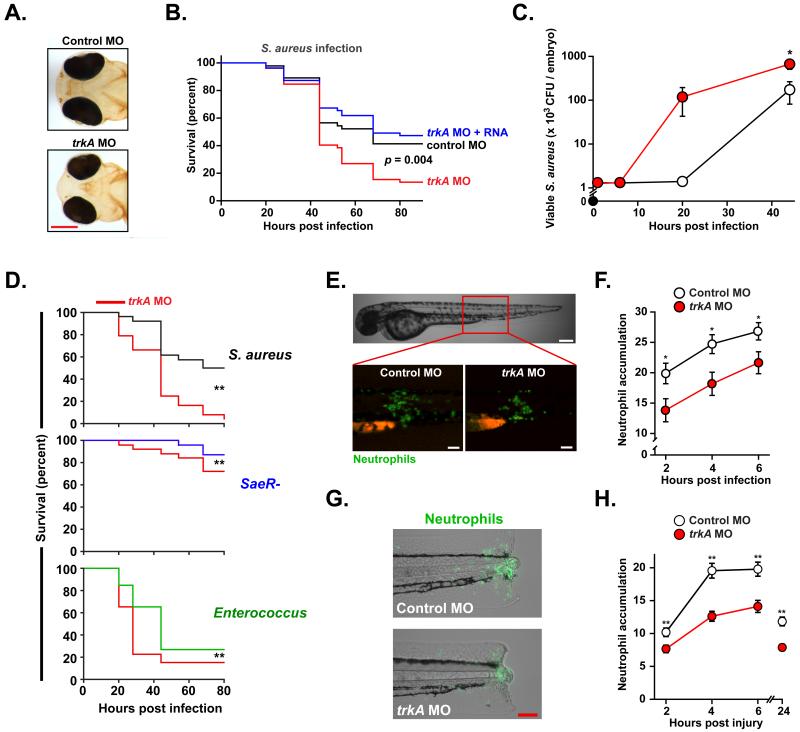
To establish whether NGFβ-TRKA signaling represents a critical, evolutionarily conserved component of vertebrate immunity to S. aureus infection, we examined its role during in vivo infection of zebrafish. Effective morpholino knockdown of trkA was confirmed by immunohistochemistry, where we observed the expected loss of trkA protein in the forebrain and nose of zebrafish larvae (Figure 4A). Knockdown of trkA had a major effect on the host response to S. aureus: trkA morphants were more susceptible to S. aureus infection than controls; a phenotype that could be rescued by concomitant injection of morpholino-resistant trkA RNA (Figure 4B) and was only partially rescued in a transgenic line expressing trkA specifically in macrophages (Figure S9), suggesting the critical importance of trkA signaling in other cells (such as neutrophils). Bacterial counts in trkA-deficient fish rose faster and remained significantly higher than in controls (Figure 4C). We then explored the relationship between the ability of bacteria to stimulate NGFβ release from macrophages and the in vivo impact of silencing trkA expression during infection (Figure 4D). We observed a greater effect of trkA knockdown in fish infected with wild type (SH1000) S. aureus compared to animals infected with bacteria less able to trigger NGFβ release from macrophages: the saeR- S. aureus mutant (causing a mild infection) and Enterococcus (causing a severe infection). Furthermore trkA knockdown compromised neutrophil migration to sites of S. aureus infection (Figure 4E,F) as well as sterile inflammation (Figure 4G,H) supporting a role for NGFβ as an ‘alarmin’ for both S. aureus infection and for non-specific tissue damage.
Figure 4. Disruption of NGFβ-TrkA signaling compromises S. aureus immunity in vivo.
A. Reduced TRKA protein expression (assessed by immunohistochemistry) in the forebrain and nose of 72 hpf zebrafish larvae injected with trkA-targeted (bottom) but not control (top) morpholinos. B. Kaplan-Meier survival curves of fish infected with S. aureus. TrkA morphants (red) were more susceptible to S. aureus infection than controls (black) and could be rescued by concomitant injection of morpholino-resistant trkA RNA (blue). N at least 45 per group, performed as three independent experiments. C. Numbers of viable S. aureus were significantly greater in trkA morphant (red) than control (black) fish assessed as colony forming units (c.f.u.) per embryo. D. Morpholino trkA knockdown (red) caused a greater effect on mortality in fish infected with wild type (SH1000) S. aureus (black) compared to animals infected with bacteria less able to trigger NGFβ release from macrophages: the saeR- S. aureus mutant (causing a mild infection; blue) and Enterococcus faecalis (causing a severe infection; green).E-H. Reduced migration of GFP-tagged neutrophils to sites of S. aureus infection (E,F) or sterile inflammation (G,H) in trkA morphants (red) compared to controls (white). Representative images at 4h post infection (E; scale bar: brightfield 200 μm, fluorescence 100 μm) or tail injury (G; scale bar 100 μm). N at least 32 per group, performed as 3 independent experiments. * denotes p ≤ 0.05; ** p ≤ 0.005 and *** p ≤ 0.0005. Unless otherwise stated, data shown is representative of at least 3 independent experiments.
In summary, our results indicate a critical role for NGFβ-TRKA signaling in controlling vertebrate innate immunity during S. aureus infection. It is also conceivable that other vertebrate cystine-knot proteins might play similar roles to NGFβ for other bacterial pathogens. The recent finding that Spaetzle also functions as a neurotrophin in Drosophila (29) suggests an evolutionarily conserved dual function for cystine knot proteins in both nerve development and anti-staphylococcal immunity and may explain stimulation of aberrant nerve growth by soft tissue infection by S. aureus (30). Our findings reveal pleotropic effects of the NGFβ-TRKA pathway that may particularly influence innate immunity to S. aureus infection suggesting that, potentially, person-to-person variability in phagocyte secretion of, or response to, NGFβ may influence vulnerability to S. aureus infection and may provide opportunities for therapeutic intervention, particularly in multi-drug resistant disease.
Supplementary Material
Acknowledgements
We would like to thank S. Clegg and E. Henderson for help with patient samples, R. Mifsud and D. Cusens for initial phylogenetic and functional analysis, A. Segal for provision of Nod2−/− mouse bone marrow and the aquarium staff of the Bateson Centre, University of Sheffield for zebrafish husbandry. This work was supported by The Wellcome Trust (Senior Clinical Research Fellowship to RAF (084953), project grant to SJF/SAR (089981), The Medical Research Council, UK (Research centre grant (G0700091), Senior Clinical Fellowship to SAR (G0701932)), Papworth Hospital and NIHR Cambridge Biomedical Research Centre, the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), U.S. National Institutes of Health (NIH).
References and Notes
- 1.Lowy FD. Staphylococcus aureus infections. N. Engl. J. Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Thwaites GE, et al. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect. Dis. 2011;11:208. doi: 10.1016/S1473-3099(10)70285-1. [DOI] [PubMed] [Google Scholar]
- 3.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 4.Stuart LM, Ezekowitz RA. Phagocytosis and comparative innate immunity: learning on the fly. Nat. Rev. Immunol. 2008;8:131–141. doi: 10.1038/nri2240. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- 6.Valanne S, Wang J-H, Rämet M. The Drosophila Toll signaling pathway. J. Immunol. 2011;186:649–656. doi: 10.4049/jimmunol.1002302. [DOI] [PubMed] [Google Scholar]
- 7.Belvin MP, Anderson KV. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Annu. Rev. Cell. Dev. Biol. 1994;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- 8.Weber N, et al. Binding of the Drosophila cytokine Spätzle to Toll is direct and establishes signalling. Nat. Immunol. 2003;4:794–800. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- 9.Vitt UA, Hsu SY, Hsueh AJ. Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol. Endocrinol. 2001;15:681–694. doi: 10.1210/mend.15.5.0639. [DOI] [PubMed] [Google Scholar]
- 10.Cohen S, Levi-Montalcini R. A nerve growth-stimulating factor isolated from snake venom. Proc. Natl. Acad. Sci. U.S.A. 1956;42:571. doi: 10.1073/pnas.42.9.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 2001;24:1217. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 12.Aloe F, Levi-Montalcini R. Mast cells increase in tissues of neonatal rats injected with the nerve growth factor. Brain Res. 1977;133:358–366. doi: 10.1016/0006-8993(77)90772-7. [DOI] [PubMed] [Google Scholar]
- 13.Otten U, Ehrhard P, Peck R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 1989;86:10059. doi: 10.1073/pnas.86.24.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bischoff SC, Dahinden SA. Effect of nerve growth factor on the release of inflammatory mediators by mature human basophils. Blood. 1992;79:2662. [PubMed] [Google Scholar]
- 15.Einarsdottir E, et al. A mutation in the nerve growth factor beta gene (NGFB) causes loss of pain perception. Hum. Mol. Genet. 2004;13:799. doi: 10.1093/hmg/ddh096. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho OP, et al. A novel NGF mutation clarifies the molecular mechanism and extends the phenotypic spectrum of the HSAN5 neuropathy. J. Med. Genet. 2011;48:131. doi: 10.1136/jmg.2010.081455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotthier A, Baets J, Timmerman V, Janssens K. Mechanisms of disease in hereditary sensory and autonomic neuropathies. Nat. Rev. Neurol. 2012;8:73. doi: 10.1038/nrneurol.2011.227. [DOI] [PubMed] [Google Scholar]
- 18.Seidah NG, Benjannet S, Pareek S, Chrétien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Letts. 1996;379:247. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- 19.Bruno MA, Cuello AC. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and it’s degradation by a protease cascade. PNAS. 2006;103:6735–6740. doi: 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Chamy L, Leclerc V, Calderari I, Reichhart JM. Sensing of danger signals and pathogen-associated molecular patterns defines binary signaling pathways ‘upstream’ of Toll. Nat. Immunol. 9:1165–70. doi: 10.1038/ni.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muñoz-Planillo R, Franchi L, Miller LS, Núñez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J. Immunol. 2009;183:3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fey PD, et al. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. Mbio. 2013;12:e00537–12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benson MA, Lilo S, Nygaard T, Voyich JM, Torres VJ. Rot and SaeRS cooperate to activate expression of the staphylococcal superantigen-like exoproteins. J Bacteriol. 2012;194:4355–4365. doi: 10.1128/JB.00706-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vollmer W, Joris B, Charlier P, Foster S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 2008;32:259–286. doi: 10.1111/j.1574-6976.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- 25.Peschel A, Otto M. Phenol-soluble modulins and staphylococcal infection. Nat. Rev. Microbiol. 2013;11:667–673. doi: 10.1038/nrmicro3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang IH, et al. A Spätzle-processing enzyme required for toll signaling activation in Drosophila innate immunity. Dev Cell. 2006;10:45–55. doi: 10.1016/j.devcel.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Howe CL, Valletta JS, Rusnak AS, Mobley WC. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron. 2001;32:801–814. doi: 10.1016/s0896-6273(01)00526-8. [DOI] [PubMed] [Google Scholar]
- 28.Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin. Immunopathol. 2012;34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu B, et al. Drosophila Neurotrophins Reveal a Common Mechanism for Nervous System Formation. PLoS Biol. 2008;6:2476. doi: 10.1371/journal.pbio.0060284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiu IM, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.