Abstract
Sequence learning and spatial alternation were examined in rats with anterior thalamic lesions or sham surgeries. There was a lesion-induced deficit in spatial alternation but not in sequence learning. During sequence learning, rats discriminated between six different sequentially presented compounds (e.g. reinforce A before B, but not B before A), composed of audio-visual elements. The solution required rats to learn both specific stimulus sequences and the reward contingencies associated with these specific temporal relationships. The failure of anterior thalamic lesions to affect the acquisition of this sequential configural task complements the recent finding that anterior thalamic lesions also spare the acquisition of a configural task involving specific stimulus pairings and their spatial relationships. These finding suggest that such ‘structural’ learning is more reliant on cortico-hippocampal than thalamo–hippocampal interactions.
Keywords: configural learning, hippocampus, rat, anterior thalamus, structural learning
The anterior thalamic nuclei may be important for the ability of rats to learn the temporal sequence in which stimuli occur. This evidence comes from a study (Wolff, Gibb & Dalrymple-Alford, 2006) that used a recency discrimination in which rats received a sequence of stimuli (e.g., five odours) before the presentation of two of those odours in a test in which appetitive reinforcement was obtained for choice of the older odour.Rats with anterior thalamic lesions showed impaired performance (Wolff et al., 2006).While sequence learning would support this odour task, a far simpler account is available: Performance could be governed by discriminations among stimulus traces that differ in strength at test, having had different amounts of time to decay.The assumption is that trace intensity would typically be greater for the newer odour than the older odour (cf., McLaren & Dickinson, 1990). The concern is, therefore, that brain regions implicated in stimulus sequence learning may instead be involved in discriminating among stimuli differing in intensity.Similar concerns apply to studies of odour sequence learning by rats with hippocampal lesions (Fortin, Agster & Eichenbaum, 2002; Kesner, Gilbert & Barua, 2002), studies of particular relevance given the close functional ties between the hippocampus and anterior thalamic nuclei (Aggleton & Brown, 1999).The present study, therefore, re-examined the impact of anterior thalamic nuclei lesions on sequence learning but used a task designed to eliminate the alternative strategy of trace discrimination.
Rats were presented with sequential compound-trials of the form: Reinforce A before B (A→B+) but do not reinforce B before A (i.e. B→ A−). Interest focussed on the rats’ responding during the second element of the compound. During the second element there are no differences in gross stimulus intensity between reinforced (A → B+) and non reinforced (B→ A−) pairs because both types of compound consist of the second (present) element along with any stimulus traces from the first element. Any ability to discriminate between the compounds’ sequences must reside elsewhere. A single pair of sequential compounds does not, however, provide a sufficient test because the task could, for example, be solved by simply attending to just the second element (if B then reward, if A then no reward).To avoid this problem, we required rats to attend to both elements of such trial types by including six trial types in a discrimination of the form: A → B+, C → A+, B → C+, B → A−, A → C−, C → B− (cf.,Murphy, Mondragón, Murphy & Fouquet, 2004). The elements used were two different auditory stimuli and a visual stimulus.Each element (A, B, C) now appears with equal frequency at the beginning or end of both reinforced (+) and non-reinforced (−) compound stimuli.Thus, to solve the discrimination the rats need to learn each element pairing and their sequence.
Method
Rats underwent surgery designed to create relatively selective lesions of the anterior thalamic nuclei. After recovery, all rats performed two behavioural procedures: (a) an appetitive, Pavlovian discrimination, requiring rats to learn the sequence of pairs of stimulus elements; (b) a spatial alternation task, which is a reliable index of effective anterior thalamic lesions in rats (e.g., Aggleton, Neave, Nagle & Hunt, 1995; Aggleton, Poirier, Aggleton, Vann & Pearce, 2009).
Subjects
Fourteen male, Lister Hooded rats (Harlan, Bicester, UK) were pair housed under diurnal conditions. During surgery rats weighed 226 - 265 g. One week before the beginning of the behavioural procedures, the rats’ weights were gradually reduced to between 80 % and 90 % of free-feeding weights.
Surgeries
Rats were divided into two groups (ns = 7) receiving anterior thalamic lesions (group ATx) and surgical controls (group Sham). All surgeries were performed under aseptic conditions. Animals were first anesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg/kg), before placement in a stereotaxic frame (David Kopf Instruments, Tujunga, CA), the incisor bar +5.0 mm to horizontal. An incision was made in the scalp, and the skin retracted to expose the skull. A dorsal craniotomy was made directly above the target region and the dura cut exposing the cortex. After, the skin was sutured over the skull and antibiotic powder was applied to the wound (Acramide, Dales Pharmaceuticals, UK). Rats received 5 mL of glucose saline solution, subcutaneously. Paracetamol and sucrose were dissolved in the rats’ drinking water.
The thalamic lesions were made by injecting 0.20 μL of 0.12 MN-methyl D-aspartate (NMDA, Sigma Chemicals, Poole, UK) in phosphate buffered saline at pH 7.2 into two sites per hemisphere using a 1-μL syringe (Hamilton, Switzerland) at: anterio-posterior −0.5 from bregma;medio-lateral 1.0 and 1.7 from the midline; dorso-ventral −6.3 and −5.7 from the top of the dura for the medial and lateral injections, respectively. Group Sham received surgical procedures identical to those described above except that the needle was filled with vehicle and injected into the thalamic sites.
Sequence Learning
Apparatus
Experimental procedures were performed in eight identically specified operant boxes. Boxes were individually housed in light- and sound-attenuated chambers that were equipped withan exhaust fan serving to ventilate the boxes and to provide background noise. Each box had three aluminium walls and an aluminium ceiling; a transparent plastic door served as the fourth wall. The floor was made from stainless steel rods. Each box’s internal dimensions measured: 24.5 cm wide; 23.0 cm deep; 21.0 cm high. The boxes were equipped with a concave, recessed tray to which 45-mg food pellets could be delivered (Formula P, P. J. Noyes, Lancaster, NH). A sprung, transparent plastic flap (6 cm high; 5 cm wide) covered the tray. Pushing this flap interrupted an infrared beam, which was recorded as a response. Each box had four, 2.8 –W lamps covered by 1.5-cm-diameter plastic discs. One lamp was located centrally in the ceiling and three on the wall that housed the food tray. Only two of the wall lamps were used, which were located 15.0 cm above floor level and 12.5 apart (centre to centre). Simultaneous and uninterrupted operation of the two lamps constituted the stimulus referred to as L. A loudspeaker located in the ceiling could be used to present a 2-kHz tone and a 10-Hz click train, referred to, respectively, as T and C. Auditory stimuli were approximately 10 dB (A) above the background noise.
Preliminary training
Rats received pellets in the food tray that were delivered every minute for two, thirty-minute sessions. Throughout this experiment the operant boxes were not illuminated,except during the presentation of the stimulus L.
Sequence learning
The procedure used an appetitive, Pavlovian discrimination.Food tray responding (see Apparatus) was measured. Rats received trials in which elements T, C and L were arranged in six, two-element sequential compounds. The first element was immediately followed by the second; both were 10 s in duration. Half of the trials preceded delivery of a single, response-independent, food pellet (+); the remainder did not (−). For 3 Sham rats and 4 ATx rats, trials were arranged: L→ T+, C → L+, T → C+, T → L−, L → C−, C → T−; for the remainder, the same compounds were presented but with reinforcement schedules reversed. Trials were separated by an inter-trial interval (ITI) that averaged 1 minute and were randomly scheduled with the constraints that: (a) the six trial types occurred 10 times in each session; (b) the same type of trial occurs only once in each block of six trials. Twelve such sessions were given with no more than one session per day.
Spatial Alternation
Training began approximately six weeks after the beginning of sequence learning.
Apparatus
Testing was in a three-arm maze that formed a T-shape when viewed from above.Each arm was 70 cm long and 10 cm wide, with clear Perspex side-walls (16.5 cm high). The floor was wooden and painted white. At the end of each arm was a circular, sunken food well, 3.0 cm diameter, 0.75 cm deep. The base of the maze was 100 cm above the floor. The maze was located in a room 3.0 m wide, 3.0 m long. Four distinctly different pictures were hung on the four walls of the laboratory and items of furniture were positioned on the floor. These items provided extra-maze spatial cues.
Alternation learning
Rats were first trained to eat sucrose reward pellets (45 mg, Noyes, Reward Pellets, Lancaster, NH) placed in the food wells. All rats then received four sessions, each of 10 trials. A trial consisted of a “forced-sample” followed by a “free choice”. During the forced-sample, rats were released from the central arm and forced to enter one of the two side arms by blocking the alternative side arm with metal barrier at the junction of the maze. After reaching the end of the forced-sample arm, the rat ate two sucrose pellets from the food well. Immediately after eating the pellets, the rat was returned to the start of the central arm and, after 10 s, was given a free choice in which the rat received two pellets if it visited the arm that had not been visited during the forced-sample.No reinforcement was given for revisiting the forced-sample arm. The ITI ranged from 3-4 minutes. The left and right side arms were designated as forced-samples in a random sequence with the constraint that each occurred five times in each session.
Results
Histological
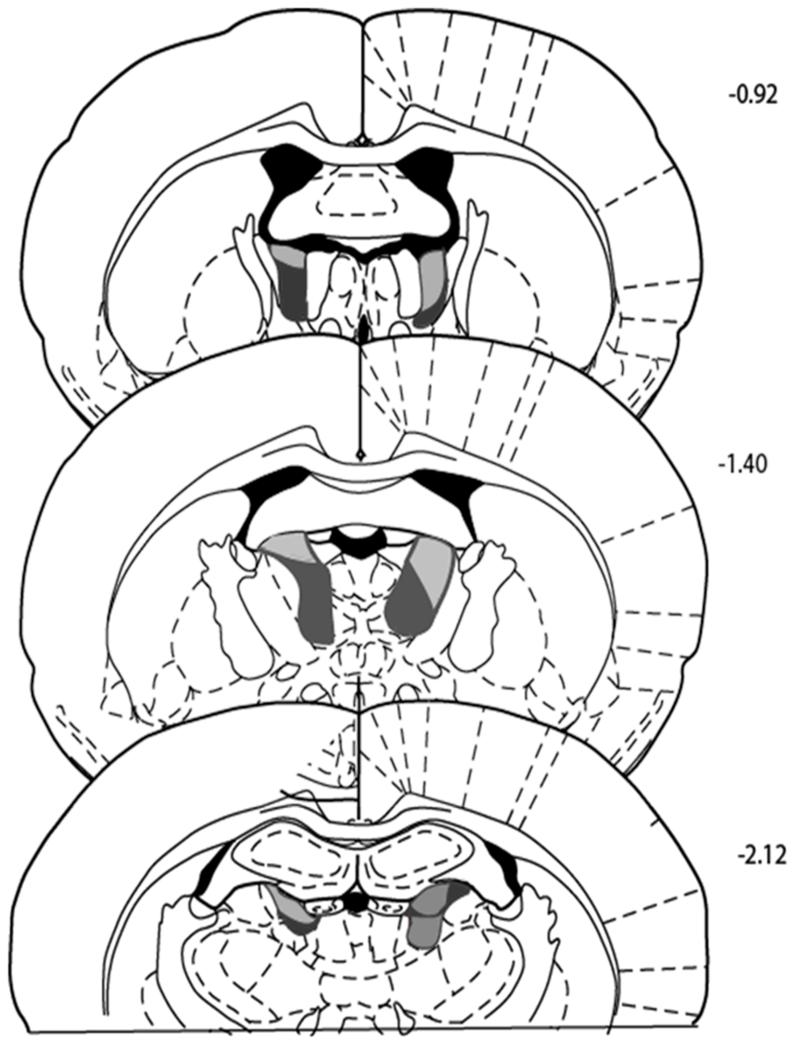
Two ATx cases were excluded because one had additional fornix damage and in the other the lesions were unusually small. Group ATx, therefore, comprised five cases; group Sham, seven.The thalamic lesions were discrete and essentially confined to the target nuclei (Figure 1).In all cases the anterior dorsal thalamic nucleus was almost completed removed, though there was consistent sparing of the most medial parts of the anterior medial thalamic nucleus. Additional, unilateral damage was found in that part of the ventral blade of dentate gyrus immediately above the anterior thalamic nuclei (n = 2), the parataenial nucleus (n = 1), and the rostral limit of the lateral dorsal thalamic nucleus (n =3).
Figure 1.
Coronal sections depicting the cases with the smallest (gray) and largest (black) of the anterior thalamic nuclei lesions. The numbers refer to the approximate distance of the section in mm caudal to bregma. Sections are based on The Rat Brain in Stereotaxic Coordinates, by G. Paxinos and C. Watson, San Diego, CA: Academic Press. (2005). Reproduced with permission from Elsevier.
Spatial Alternation
Over the course of the 40 spatial alternation trials, group Sham’s performance was superior to group ATx’s with, respective, mean scores of 33.4 (SEM [standard error of the mean]: 0.48) and 28.4 (SEM: 2.02).These scores differed using a between-subject comparison t(10) = 2.85,p = 0.009 (one tailed).
Sequence Learning
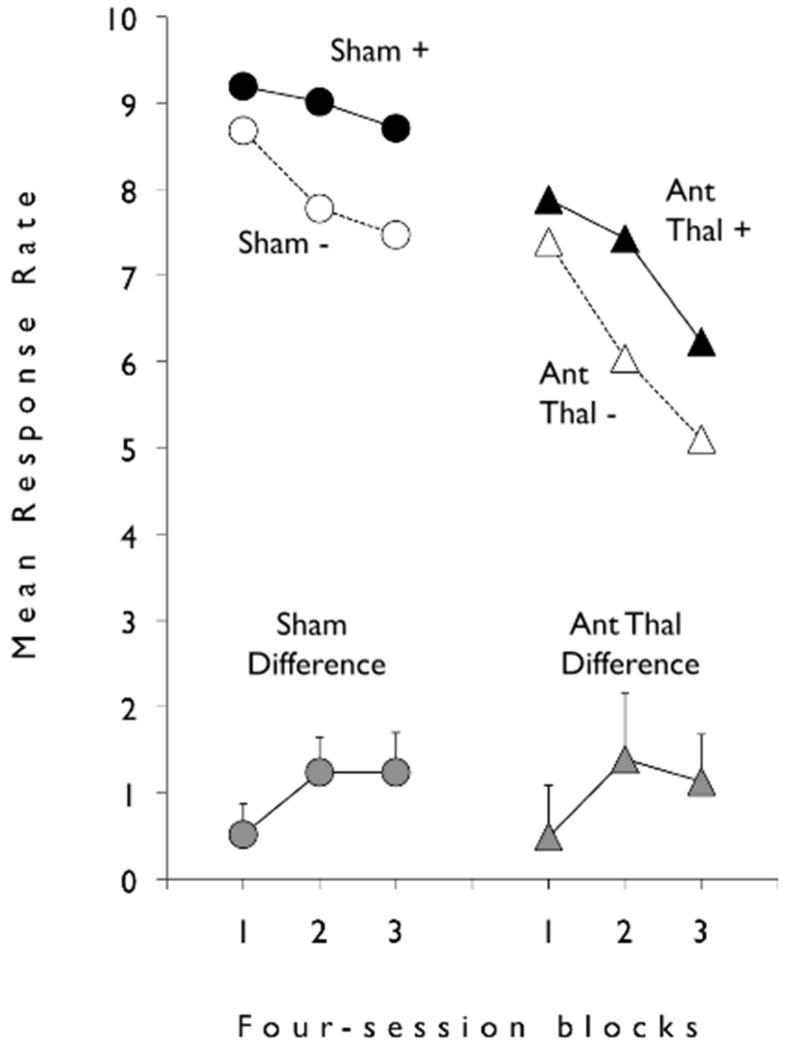
Because the stimuli T, C and L served equally often as the first and as the second elements of the trial, data are collapsed over the stimulus factor in our summary and analyses. Tray responses during first element of the compound were, for group Sham and group ATx, respectively, 7.47 rpm (responses per minute; SEM: 0.846) and 6.81 rpm (SEM: 0.592), which did not differ, t(10) < 1. The data of central importance are those of the second element of the compound, which are summarized in Figure 2. It is apparent that rats tended to emit tray responses at a reasonably high rate across the twelve sessions but that the discrimination was gradually solved with higher response rates on reinforced trials (+) than on non-reinforced trials (−). It is also apparent that the groups solved the discrimination at similar rates, and to a similar asymptote.
Figure 2.
Black and white filled symbols summarise (mean) response rates (responses per minute) during the second element of the food reinforced (+) and non-reinforced (−) sequential compound stimuli.Grey filled symbols summarise (mean and one standard error of the mean) each rat’s differences score (food reinforced minus non-reinforced response rates) during the second element. Circles summarise group Sham’s data; triangles summarise group Ant Thal’s data. The twelve sessions of discrimination training are grouped into three 4-session blocks. The experimental design appears in the bottom right side of the figure.
This description of the data was confirmed using ANOVA (analysis of variance) with the data blocked into three sets of four sessions (Figure 2).The main effect of Reinforcement, F(1, 10) = 8.23,p < 0.018 reflected the higher rates of responding during reinforced than non-reinforced trials.In addition, the Reinforcement × Block interaction was close to significance, F(2, 20) = 3.32, 0.057 <p< 0.056, and its source was examined using simple main effects analysis with separate error-terms. This analysis revealed that while responding on S+ and S− trials did not differ on Block 1, p> 0.167, significantly higherresponding was found for reinforced than non-reinforced trials on Blocks 2 and 3[smaller F(1, 11) = 9.64,p< 0.011].No other main effect, nor interaction, was significant (smallest p > 0.236), that is there were no differences between the ATx and Sham groups.
Discussion
Rats with discrete anterior thalamic nuclei lesions were impaired on spatial alternation (see also, Aggleton et al., 1995, 2009) but were seemingly unimpaired in their sequence learning using a task reported by Murphy et al. (2004).Interest in examining the effects of anterior thalamic nuclei lesions on sequence learning stemmed from a study showing that such lesions attenuate rats’ sequence learning when assessed using a recency discrimination (Wolff, et al., 2006).A concern is that rats might succeed on a recency discrimination by comparing, during test, solely the different stimulus intensities, based on trace decay – with no necessity to appreciate the actual sequence of the odours. For example, an intense (recent) odour may signal one outcome (e.g., the absence of food) whereas the same odour in its less intense (older) form may indicate a different outcome (e.g., food); notice that there is no necessity in such a scheme to encode the fact that the less intense odour accompanied a more intense odour as part of a sequence.The present task (cf., Murphy et al., 2004) eliminated solutions based solely upon gross differences in stimulus intensity: The situation during the second element of both reinforced and non-reinforced compounds was identical in aggregate stimulus intensity, being composed of the currently presented stimulus and any stimulus traces from the first element. Our finding, therefore, conflicts with that of Wolff et al. (2006), with the implication that the anterior thalamic nuclei might be unimportant for true sequence learning but are important for odourintensity discriminations.
Previous studies of stimulus order have recognised the potential problem that rats might solve that task by comparing trace strength;that is, select the odour that seems least familiar.For this reason the study by Wolff et al. (2006) also tested odour recognition (odours novel for that session versus familiar odours from within that session).The reasoning is that a rat with intact recognition is less likely to show a trace strength discrimination deficit, so any deficit presumably arises from a problem with true sequence learning.Unfortunately, the rats with anterior thalamic lesions were impaired on the additional recognition task (Wolff et al.), although they did improve such that they did not differ from the control rats by the end of training.An anterior thalamic lesion deficit was, however, reinstated by a subsequent odour recognition reversal task (Wolff et al.).These recognition deficits highlight the difficulty of excluding a trace-strength discrimination deficit in that study (Wolff et al.).Other differences between the present study and that of Wolff et al. include the fact that the present study involved multiple trials and sessions on the same problem, while learning on the odour-recency task (Wolff et al.) involved single trials within each session.Furthermore, the anterior thalamic lesions in the present study appear to be slightly smaller, although they were sufficient to impair T-maze alternation. It is, therefore, possible that several factors account for the apparent difference in findings across these two studies.
The present sequence-learning task is an example of a more general class of structural discriminations (e.g., George et al., 2001). Unlike most configural discriminations (e.g. biconditional discriminations: AB+, CD+, AD−, CB−), structural tasks cannot be solved by learning solely the specific combinations of elements. Rather, this subset of configural discriminations require learning the relationships between specific elements, be they sequential (as in the present study), spatial (e.g., George et al., 2001) or when the relationship is with a second stimulus property (George & Pearce, 2003). We have recently reported that anterior thalamic lesions in rats leave unaffected the acquisition of a spatial, structural discrimination (Aggleton, et al. 2009).In that task, rats swam toward an escape platform in a water tank. The location of the platform was indicated by a compound visual stimulus (e.g., black to the left of white) while the mirror-image version (white to the left of black – i.e., the same elements but with the alternative spatial structure) was not reinforced. Thus, these findings appear to indicate that the anterior thalamic nuclei are generally unimportant in the solution of structural discriminations (be they based on spatial or sequential relationships). The failure to detect effects on spatial (Aggleton et al., 2009) or temporal on structural discrimination learning after anterior thalamic lesions may be contrasted with the attenuation on spatial structural discrimination in rats following lesions of the hippocampus (Sanderson, Pearce, Kyd & Aggleton, 2006). Thus, in total, these results suggest an important role for cortico-hippocampal, rather than thalamo-hippocampal interactions for structural learning.
Footnotes
All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act (1986) and complying with ethical standards for the treatment and care of animals specified by the Experimental Psychology Society.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia and the hippocampal - anterior thalamic axis. Behavioral and Brain Sciences. 1999;22:425–444. [PubMed] [Google Scholar]
- Aggleton JP, Neave NJ, Nagle S, Hunt PR. A comparison of the effects of anterior thalamic, mamillary body and fornix lesions on reinforced spatial alternation. Behavioural Brain Research. 1995;68:91–101. doi: 10.1016/0166-4328(94)00163-a. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Poirier GL, Aggleton HS, Vann SD, Pearce JM. Lesions of the fornix and anterior thalamic nuclei dissociate different aspects of hippocampal-dependent spatial learning: implications for the neural basis of scene learning. Behavioral Neuroscience. 2009;123:504–519. doi: 10.1037/a0015404. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DN, Pearce JM. Discrimination of structure: II. Feature binding. Journal of Experimental Psychology: Animal Behavioral Processes. 2003;29:107–117. doi: 10.1037/0097-7403.29.2.107. [DOI] [PubMed] [Google Scholar]
- George DN, Ward-Robinson J, Pearce JM. Discrimination of structure: I. Implications for connectionist theories of discrimination learning. Journal of Experimental Psychology: Animal Behavioral Processes. 2001;27:206–219. doi: 10.1037//0097-7403.27.3.206. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behavioral Neuroscience. 2002;116:286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- McLaren IPL, Dickinson A. The conditioning connection. Philosophical Transactions of the Royal Society (London) 1990;329:179–186. doi: 10.1098/rstb.1990.0163. [DOI] [PubMed] [Google Scholar]
- Murphy RA, Mondragón E, Murphy VA, Fouquet N. Serial order of conditional stimuli as a discriminative cue for Pavlovian conditioning. Behavioural Processes. 2004;67:303–311. doi: 10.1016/j.beproc.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Pearce JM, George DN, Haselgrove M, Erichsen JT, Good MA. Influence of Hippocampal Lesions on the Discrimination of Structure and on Spatial Memory in Pigeons (Columba livia) Behavioral Neuroscience. 2005;119:1316–1330. doi: 10.1037/0735-7044.119.5.1316. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. Academic Press; New York: 2005. [Google Scholar]
- Sanderson D, Pearce JM, Kyd R, Aggleton JP. The importance of the rat hippocampus for learning the structure of visual arrays. European Journal of Neuroscience. 2006;24:1781–1788. doi: 10.1111/j.1460-9568.2006.05035.x. [DOI] [PubMed] [Google Scholar]
- Wolff M, Gibb SJ, Dalrymple-Alford JC. Beyond spatial memory: The anterior thalamus and memory for the temporal order of a sequence of odor cues. Journal of Neuroscience. 2006;26:2907–2913. doi: 10.1523/JNEUROSCI.5481-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]