Abstract
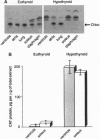
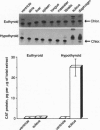
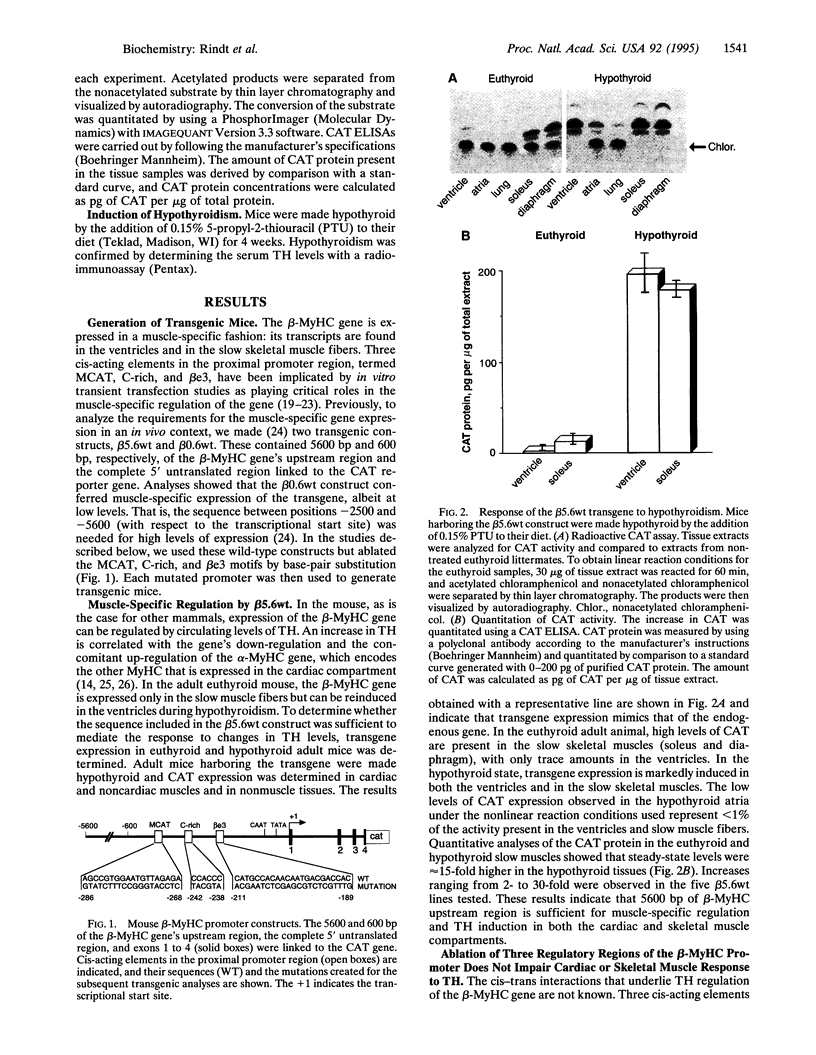
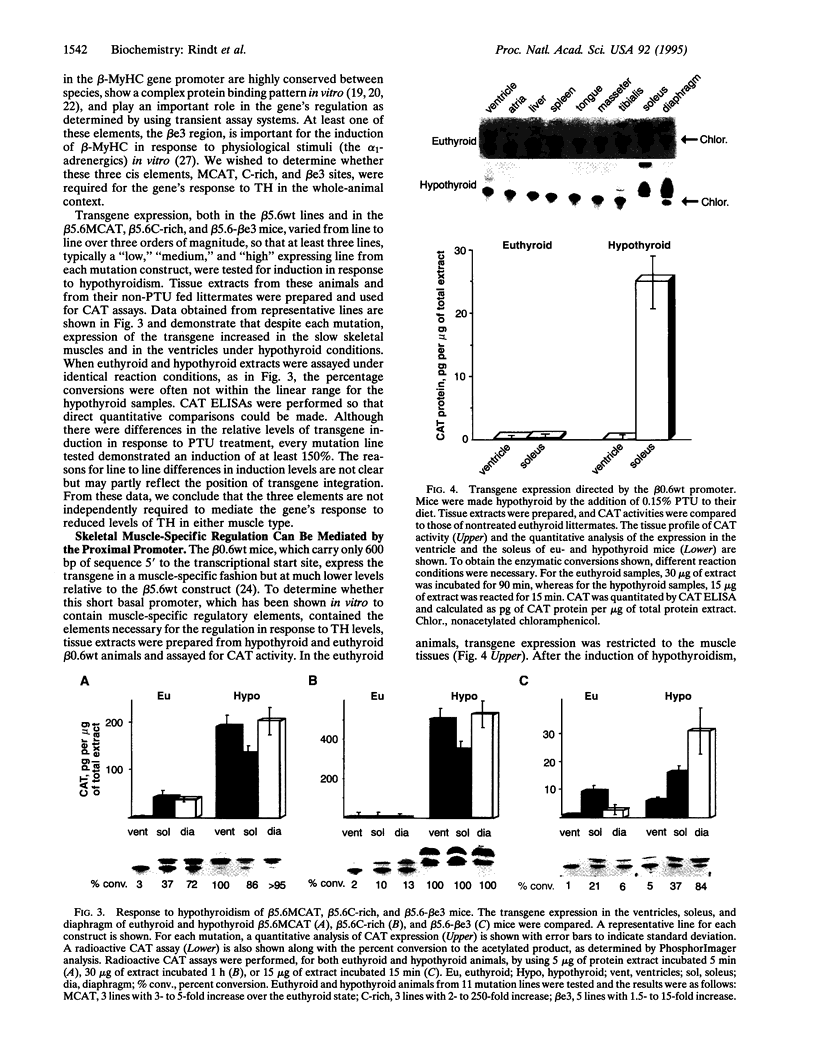
The beta-myosin heavy chain (beta-MyHC) gene is expressed in cardiac and slow skeletal muscles. To examine the regulatory sequences that are required for the gene's expression in the two compartments in vivo, we analyzed the expression pattern of a transgene consisting of the beta-MyHC gene 5' upstream region linked to the chloramphenicol acetyltransferase reporter gene. By using 5600 bp of 5' upstream region, the transgene was expressed at high levels in the slow skeletal muscles. Decreased levels of thyroid hormone led to the up-regulation of the transgene in both cardiac and skeletal muscles, mimicking the behavior of the endogenous beta-MyHC gene. After deleting the distal 5000 bp, the level of reporter gene expression was strongly reduced. However, decreased levels of thyroid hormone led to an 80-fold skeletal muscle-specific increase in transgene expression, even upon the ablation of a conserved cis-regulatory element termed MCAT, which under normal (euthyroid) conditions abolishes muscle-specific expression. In contrast, cardiac-specific induction was not detected with the deletion construct. These observations indicate that the cardiac and skeletal muscle regulatory elements can be functionally segregated on the beta-MyHC gene promoter.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boheler K. R., Chassagne C., Martin X., Wisnewsky C., Schwartz K. Cardiac expressions of alpha- and beta-myosin heavy chains and sarcomeric alpha-actins are regulated through transcriptional mechanisms. Results from nuclear run-on assays in isolated rat cardiac nuclei. J Biol Chem. 1992 Jun 25;267(18):12979–12985. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chizzonite R. A., Zak R. Regulation of myosin isoenzyme composition in fetal and neonatal rat ventricle by endogenous thyroid hormones. J Biol Chem. 1984 Oct 25;259(20):12628–12632. [PubMed] [Google Scholar]
- Christensen T. H., Prentice H., Gahlmann R., Kedes L. Regulation of the human cardiac/slow-twitch troponin C gene by multiple, cooperative, cell-type-specific, and MyoD-responsive elements. Mol Cell Biol. 1993 Nov;13(11):6752–6765. doi: 10.1128/mcb.13.11.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs L. L., Shimizu N., Yockey C. E., Levin J. E., Jakovcic S., Zak R., Umeda P. K. Muscle-specific regulation of a transfected rabbit myosin heavy chain beta gene promoter. J Biol Chem. 1989 Jun 25;264(18):10672–10678. [PubMed] [Google Scholar]
- Flink I. L., Edwards J. G., Bahl J. J., Liew C. C., Sole M., Morkin E. Characterization of a strong positive cis-acting element of the human beta-myosin heavy chain gene in fetal rat heart cells. J Biol Chem. 1992 May 15;267(14):9917–9924. [PubMed] [Google Scholar]
- Gulick J., Subramaniam A., Neumann J., Robbins J. Isolation and characterization of the mouse cardiac myosin heavy chain genes. J Biol Chem. 1991 May 15;266(14):9180–9185. [PubMed] [Google Scholar]
- Gunning P., Ponte P., Blau H., Kedes L. alpha-skeletal and alpha-cardiac actin genes are coexpressed in adult human skeletal muscle and heart. Mol Cell Biol. 1983 Nov;3(11):1985–1995. doi: 10.1128/mcb.3.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Johnson J. E., Wold B. J., Hauschka S. D. Muscle creatine kinase sequence elements regulating skeletal and cardiac muscle expression in transgenic mice. Mol Cell Biol. 1989 Aug;9(8):3393–3399. doi: 10.1128/mcb.9.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya K., Karns L. R., Simpson P. C. An enhancer core element mediates stimulation of the rat beta-myosin heavy chain promoter by an alpha 1-adrenergic agonist and activated beta-protein kinase C in hypertrophy of cardiac myocytes. J Biol Chem. 1994 Feb 4;269(5):3775–3782. [PubMed] [Google Scholar]
- Knotts S., Rindt H., Neumann J., Robbins J. In vivo regulation of the mouse beta myosin heavy chain gene. J Biol Chem. 1994 Dec 9;269(49):31275–31282. [PubMed] [Google Scholar]
- Litten R. Z., 3rd, Martin B. J., Low R. B., Alpert N. R. Altered myosin isozyme patterns from pressure-overloaded and thyrotoxic hypertrophied rabbit hearts. Circ Res. 1982 Jun;50(6):856–864. doi: 10.1161/01.res.50.6.856. [DOI] [PubMed] [Google Scholar]
- Lompre A. M., Schwartz K., d'Albis A., Lacombe G., Van Thiem N., Swynghedauw B. Myosin isoenzyme redistribution in chronic heart overload. Nature. 1979 Nov 1;282(5734):105–107. doi: 10.1038/282105a0. [DOI] [PubMed] [Google Scholar]
- Lompré A. M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984 May 25;259(10):6437–6446. [PubMed] [Google Scholar]
- Lyons G. E., Ontell M., Cox R., Sassoon D., Buckingham M. The expression of myosin genes in developing skeletal muscle in the mouse embryo. J Cell Biol. 1990 Oct;111(4):1465–1476. doi: 10.1083/jcb.111.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons G. E., Schiaffino S., Sassoon D., Barton P., Buckingham M. Developmental regulation of myosin gene expression in mouse cardiac muscle. J Cell Biol. 1990 Dec;111(6 Pt 1):2427–2436. doi: 10.1083/jcb.111.6.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer Y., Czosnek H., Zeelon P. E., Yaffe D., Nudel U. Expression of the genes coding for the skeletal muscle and cardiac actions in the heart. Nucleic Acids Res. 1984 Jan 25;12(2):1087–1100. doi: 10.1093/nar/12.2.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Alonso S., Caravatti M., Buckingham M. E. A fetal skeletal muscle actin mRNA in the mouse and its identity with cardiac actin mRNA. Cell. 1982 Aug;30(1):185–192. doi: 10.1016/0092-8674(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Ng W. A., Grupp I. L., Subramaniam A., Robbins J. Cardiac myosin heavy chain mRNA expression and myocardial function in the mouse heart. Circ Res. 1991 Jun;68(6):1742–1750. doi: 10.1161/01.res.68.6.1742. [DOI] [PubMed] [Google Scholar]
- Parmacek M. S., Ip H. S., Jung F., Shen T., Martin J. F., Vora A. J., Olson E. N., Leiden J. M. A novel myogenic regulatory circuit controls slow/cardiac troponin C gene transcription in skeletal muscle. Mol Cell Biol. 1994 Mar;14(3):1870–1885. doi: 10.1128/mcb.14.3.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmacek M. S., Leiden J. M. Structure and expression of the murine slow/cardiac troponin C gene. J Biol Chem. 1989 Aug 5;264(22):13217–13225. [PubMed] [Google Scholar]
- Rindt H., Gulick J., Knotts S., Neumann J., Robbins J. In vivo analysis of the murine beta-myosin heavy chain gene promoter. J Biol Chem. 1993 Mar 5;268(7):5332–5338. [PubMed] [Google Scholar]
- Shimizu N., Dizon E., Zak R. Both muscle-specific and ubiquitous nuclear factors are required for muscle-specific expression of the myosin heavy-chain beta gene in cultured cells. Mol Cell Biol. 1992 Feb;12(2):619–630. doi: 10.1128/mcb.12.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N., Prior G., Umeda P. K., Zak R. cis-acting elements responsible for muscle-specific expression of the myosin heavy chain beta gene. Nucleic Acids Res. 1992 Apr 11;20(7):1793–1799. doi: 10.1093/nar/20.7.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A. M., Friedman D. J., Nigro J. M., Jakovcic S., Rabinowitz M., Umeda P. K. Expression of rabbit ventricular alpha-myosin heavy chain messenger RNA sequences in atrial muscle. J Biol Chem. 1984 May 25;259(10):6674–6680. [PubMed] [Google Scholar]
- Thompson W. R., Nadal-Ginard B., Mahdavi V. A MyoD1-independent muscle-specific enhancer controls the expression of the beta-myosin heavy chain gene in skeletal and cardiac muscle cells. J Biol Chem. 1991 Nov 25;266(33):22678–22688. [PubMed] [Google Scholar]
- Walter C. A., Nasr-Schirf D., Luna V. J. Identification of transgenic mice carrying the CAT gene with PCR amplification. Biotechniques. 1989 Nov-Dec;7(10):1065–1070. [PubMed] [Google Scholar]