Introduction
Initial attempts at obtaining a pregnancy by in vitro fertilization and embryo transfer (IVF-ET) were conducted in a natural cycle (Edwards et al., 1980). It soon became clear that ovarian stimulation using gonadotropins provided a useful tool to obtain more oocytes and increase the chance for success. This resulted in the need to monitor ovarian stimulation, based on serial sonographic assessments of follicular growth and to a lesser degree on serial measurements of serum estradiol (Golan et al., 1994; Lass, 2003). Estradiol measurements became less important and are now considered to be of value mainly in cases of threatening ovarian hyperstimulation syndrome (OHSS). Serial sonography as a sole method of monitoring follicular growth has become routine and cannot be dispensed with, both for optimal timing of HCG administration and for early detection of threatening OHSS.
Development of patient-friendly IVF includes self-administration of gonadotropins, milder stimulation protocols (Baart et al., 2007), reduction of multiple pregnancies by single embryo transfer (Gerris et al., 2002) and less ovarian hyperstimulation syndrome, more efficacious embryo freezing programs (Tiitinen et al., 2004) and less treatment-induced stress. Natural cycles or minimally modified natural cycles have been suggested (Pelinck et al., 2008) to further minimize the impact of IVF treatment on patients’ daily life. In vitro maturation (IVM) also minimizes gonadotropin use (Söderström-Anttila et al., 2006).
Still one major inconvenience persists i.e. the need for women to frequently visit the clinic to perform serial vaginal sonographies. Although no hard data exist, in our own centre, the last one hundred in-house attempts needed 440 follicular phase sonograms, i.e. a mean of 4,4 per fresh treatment cycle. The total annual number of IVF/ICSI cycles amounts to ~one million and is rising. Another one million of non-IVF/ICSI stimulations should be added. This implies eight to ten million annual sonographic examinations for pre-ovulatory monitoring only or, for ~300 working days per year, a daily trek to the IVF clinic for 90,000 women! In addition, huge distances in large countries where sonography is not available in reasonable distance from homes decreases access to treatment for many couples who need it. This is the case in large developed societies e.g. the USA, Australia, Canada, but also in many third-world and emerging countries where services used to be relatively scarce but are now quickly growing, e.g. the BRIC countries.
The possibility for patients to perform vaginal sonographies themselves at home includes theoretical advantages. Patients need not to come as often to the centre to have sonographies performed. They will save time and money spent on petrol, car usage, train or bus. They and their partners will avoid loss of income during working hours. Weekends with their important social and household functions will not be interrupted by trips to the centre. IVF centres will be less crowded during morning hours. Measurements will be standardized and reproducible and performed at ease, hence will be of a better quality. Communication with the patient will be smoother, more complete and explicitly contain all information. Images can be accompanied by questions that can be answered properly and not in a hurry by a stressed doctor, midwife or nurse. Sonograms can be made by the partner who is often the “cause” of the infertility and in many cases will be happy to participate in an active way. Time will become available for necessary interactions between doctors/nurses and patients who truly need to come physically to the centre, reducing excessively long waiting lists for consultations. Treatments will become possible in patients living far from the centre allowing them to show up just before egg retrieval from anywhere in the world. It is a development that fits in a flat world where telemedicine has already found a place in other fields of medicine e.g. radiology, cardiology, cardiotocography, etc. SOET could have a place in IVF treatment in the third world where several women in rural areas can have sonograms made through the use of a single instrument, data being to a central monitoring unit.
Even if applicable to a selected proportion of IVF patients, SOET could perfectly fit in the general tendency to make IVF more patient-friendly and to increase patient empowerment by supervised active participation to the treatment (Saltman, 1994).
It could fit in a politically desired trend to pay fixed amounts for professional efforts that can be organized and standardized using service level agreements. This would be in line with how other aspects of ART treatment are financed in Belgium (laboratory phase: 1,283€; gonadotrophins: 1,073€; oocyte pick-up: 591€; and embryo transfer: 155€).
The idea is to have patients have their sonographies perform themselves, after proper instruction by a midwife or a nurse-practitioner, using a small, safe, cheap and easy-to-use device, allowing registration of real time images under direct visual inspection of the patient or her partner. Images can be sent with proper identification over the internet to the centre where specialized personnel and technology receives, stores, analyzes and interprets the images and takes swift appropriate action. Analysis and response could be semi-automated using specific new software applications. They comprise proper instructions regarding the dose of gonadotropins to be self-injected during the following day(s), the timing for a subsequent sonogram, the precise timing of HCG injection and of oocyte retrieval. If extended to intrauterine insemination or freeze/thaw cycles after IVF, even in a natural cycle or after oral ovulation induction, planning of insemination or embryo replacement can be communicated. If needed, these self-operated endovaginal sonographies (SOETs) can be performed with a backup of same-day (if needed) or next-day “live” sonography in the centre. Many secondary questions and considerations will need to be addressed, a.o. (re)organisational aspects, protection of privacy, legal and financial aspects, the impact of failure from the patients’ side to correctly apply the technology, etc.
It is not the idea to replace all vaginal sonography in the context of IVF, let alone all gynaecological sonography, by SOET. “Real” sonography will remain a very important tool in IVF, and even if implemented, SOET will not replace sonography in complicated cases, when follicular growth is abnormally slow or quick, when abnormal images (cysts, hydrosalpinges, unexpected tumours, endometriosis, etc.) are observed, or on simple request from anxious patients. It is conceived in the first place to alleviate an abnormal amount of routine and to allow patients living far from the centre to demand less sacrifices.
We wanted to find out whether patients would in general welcome the possibility to perform vaginal sonograms themselves, whether images recorded by one person would result in the same clinical decisions when analysed by another person and finally whether patients are also able to actually produce good quality video recordings of their internal genital organs.
Methods
First, patients should in general have a positive attitude towards the idea, see and value its advantages over traditional follow-up. Therefore, in a first step (SOET1), we interviewed twenty-five randomly chosen patients who approached our centre for IVF treatment.
Second, images made by one operator had to be be readable and interpreted with clinical efficacy by another operator. Therefore, in a second step (SOET2), we had serial sonographies from five patients analyzed by an assessor with sonographic experience in IVF to see whether she would take the same clinical decisions regarding dose adaptation and other decisions as those taken in reality by the physician who monitored the cycle in person in the traditional way.
Most importantly, patients must be able to make sonographies themselves after an instruction session by an experienced midwife. Therefore, in a third step (SOET3), twenty-one patients were instructed on how to make sonograms themselves and send them over an electronic channel.
SOET 1: Research question: Do patients and their partners see advantages in SOET over traditional monitoring?
The aim of the study was to assess the perception and acceptance of the concept by the patients and their partners. This opinion-finding SOET-1 study was approved by the ethical committee of the University of Ghent under project number EC/2006/330, Belgian registration number B6702006289.
Twenty-five couples, consulting our centre for IVF or ICSI were asked to fill out a questionnaire consisting of ten questions. Most patients lived at a considerable distance (50-250km) from the centre, either in Flanders itself, in The Netherlands or in Germany. Ten of them were first-ever IVF/ICSI patients, fifteen had undergone one or more previous attempts without obtaining an ongoing pregnancy. Questions regarding the idea of home sonography could be answered with a score ranging between 1 (do not agree at all) and 5 (fully agree) or by yes or no, when applicable.
I consider the frequent trafficking to and from the centre as one of the heaviest aspects of the whole IVF treatment (1-5).
I would welcome an evolution in which I would not have to visit the centre as often for monitoring stimulation of my ovaries provided the treatment is as efficacious and as safe as it is at present (1-5).
I have practical or other objections against introducing a vaginal probe into my vagina (1-5).
I am prepared to perform sonographies at home myself, after a teaching session by a specialist-midwife or specialist-nurse, if this could save me “echo-trafficking” (1-5).
I possess a computer and I or my partner can Email the recordings to the centre (yes-no).
I can technically handle a regular PC to send data via a USB-connection to the centre utilizing existing information channels (internet, e-mail) (yes-no).
I am prepared to learn what I need to learn in order to profit from this simplified IVF-treatment (1-5).
I can do this all days of the week, including weekend days (yes-no).
I would appreciate to receive clear instructions from the centre by return mail in view of injecting myself with the hormonal preparations needed or receive other practical instructions (1-5).
The benefit of this approach would be important for me with respect to (1-5): a: time; b: petrol use; c: morning stress; d: pure treatment comfort.
SOET 2: Research question: Can recorded images be interpreted by another operator and how good is the concordance between decisions taken using the “real” images versus the recorded ones?
We wanted to know whether there was concordance between clinical decisions made by the treating physician and another operator assessing the images at a later moment receiving only essential information concerning the patient (age, starting dose, rank of attempt, previous relevant outcome). This study was approved by the ethical committee of Ghent University Hospital under project number EC/2006/329, Belgian registration number B6702006288.
Five volunteers (two first-ever patients and three with previous attempts), all living at a long distance from the centre, were monitored in the traditional way. After performing each sonography and taking the appropriate decision, a second sonogram was made using an experimental set-up. The instrument used was a MyLab-30 ultrasound apparatus provided for the purpose by ESAOTE, PIE MEDICAL, Maastricht, The Netherlands. This is a hybrid between a sonograph and a regular computer. A standard sequence of scanning was followed: after sliding the probe into the vagina, it was panned from neutral to the right ovary, then the left ovary; then rocked to visualize the anterior and posterior segment of the pelvis; a scan was made lasting 30-60 seconds, followed by a second “sweep” lasting shorter. These images were recorded and provided with an anonymous code. All images were analyzed weeks later during one session by two blinded experienced assessors, who were given the following information: age of the patient, rank of the attempt, day of gonadotropin stimulation and starting dose. They were asked to analyze the data and formulate a clinical decision: maintain, increase or decrease the dose; for how many days and when to come back for a subsequent sonography (according to the principle: as much as needed but no more); administration of HCG and timing of oocyte retrieval; cancellation of the cycle in case of poor or exaggerated response.
Cycle preparation and standard stimulation consisted in all cases of the administration of a full strip of an oral contraceptive containing 20 µg of ethinylestradiol and 150 mg of desogestrel to improve cohort synchronization; after a basal sonogram to exclude cysts, the oral contraceptive was discontinued and triptorelin (Decapeptyl, Ipsen, Belgium), 0.1mg/day SC, was started on the fifth day after pill-stop for 10 days; gonadotropins were initiated on the seventh day; rec-FSH (follitropin β Gonal-F, Merck-Serono, Belgium), was used with a starting dose of 150 IU, except in two cases. One woman had suffered severe OHSS in a previous cycle and was started with 100 IU; another patient received 225 IU because previous stimulation elsewhere with a starting dose of 150 IU had resulted in only 3 oocytes. This information was also given to the blinded assessors. The rule of thumb was not to increase the dosage if sustained follicular growth was observed; any increase had to be by ≤ 75 IU if no growth was established, withholding FSH in case of threatening OHSS (coasting). The first sonography was planned in all cases on the seventh day of gonadotropin stimulation.
Further treatment regarding egg retrieval, fertilization, embryo culture and day-2 or day-3 transfer were conducted according to established methods published elsewhere (Gerris et al., 2004). Luteal phase support was given using 3 × 200 mg micronized progesterone (Utrogestan, Besins, Belgium) intravaginally. HCG was measured sixteen days after embryo transfer.
Patient data are shown in Table I.
Table I. Patient data SOET2 trial.
| >Patient | >Age | >Rank of cycle | >Type of treatment | >Residence | >Remark |
|---|---|---|---|---|---|
| 1 | 38 | 1 | icsi | Neth | |
| 2 | 36 | 2 | icsi | Neth | |
| 3 | 30 | 4 | tese/icsi | Portugal | |
| 4 | 31 | 2 | ivf | Neth | Previous OHSS |
| 5 | 28 | 2 | icsi | Germany | Previous triplet lost |
SOET 3: Research question: Can we obtain proof of concept that women can make serial sonographies after instruction by an experienced midwife and send them over an electronic link?
We wanted to find out whether it was possible to obtain good-quality scanning images with patients performing their own vaginal sonographies. This study was approved by the ethical committee of Ghent University Hospital under project number EC/2007/2057, Belgian Registration Number B67020072057.
Twenty-one volunteers were followed in the centre from their basal sonography onwards by one physician, using the same protocols as described above. Immediately after taking each regular sonogram and communicating the appropriate clinical decision to the patient, the physician then left the room. All decicions were taken on the basis of these sonograms only, except in cases of threathening OHSS, where hormone levels were also considered and the patient had to call back in the afternoon (1 case). The self-operated sonogram was then repeated by the patient in the presence of an instructing midwife. The instrument used was again a MyLab-30 sonographic apparatus provided for the purpose by a company (ESAOTE, PIE MEDICAL, Maastricht, The Netherlands). This instrument still weighs 8 kg and although portable, can not be used in routine for home sonography. The instrument was connected by the ICT department of the hospital to the intranet system so that sonographic images could be sent to a receiving computer on a different location.
The patient was then instructed by a experienced midwife on how to slide in the vaginal probe, rock, pan and rotate it in the vagina and identify the images on the screen. Use was made of an instruction poster with typical images of a thin lined endometrium, a thickening endometrium, a triple-line endometrium, a quiescent ovary, incipient follicular growth, intermediate size and mature follicles, differences in follicles due to asynchrony in development, the bladder, large vessels and the bowel.
Results
SOET 1
Answers to these questions are reported in Table II and in Figure 1 (a-m). Patients’ and their partners’ positive attitude towards a self-operated vaginal sonographic technology was high. Perceived advantages were avoidance of frequent hospital visits, time loss, petrol cost, organizational stress and ease of treatment. There were no objections to introduce a device into the vagina. Most partners seemed to be willing to help in this regard, potentially replacing a strenuous patient-sonographist encounter by an intimate bonding contact.
Table II. Results from SOET1 trial.
| Q1 | Q2 | Q3 | Q4 | Q7 | Q9 | Q10a | Q10b | Q10c | Q10d | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients mean score | 3,24 | 4,32 | 1,36 | 4,00 | 4,32 | 4,52 | 4,44 | 2,84 | 3,20 | 3,88 |
| Partners mean score | 3,12 | 4,24 | 1,68 | 4,08 | 4,44 | 4,56 | 4,40 | 2,88 | 2,92 | 3,96 |
Fig. 1 (a-m). 25 patients’ and their partners’ responses to questions regarding the desirability of self-operated endovaginal telemonitoring (SOET).
SOET 2
For each of the five patients the concordance between the real time assessor and the blinded assessors was excellent. Although the image quality was not considered optimal in all cases so that all follicles could not be measured individually, the overall concordance between the clinical decisions takes by the real-time assessor and the two blinded assessors was excellent.
SOET 3
All patients completed their attempt treatment cycle and all of them produced good-quality sonograms in each instance. Images were sent over the hospital intranet as a proxy for the worldwide internet, stored at the receiving end and can be endlessly replayed. All consented on a very positive experience, as did the midwives involved in the study.
In general, the first, basal, sonography was the most difficult to clarify, because of lack of visible follicular structures. A visible bladder may help during this first-ever self-operated sonography to orient the woman in recognizing her uterus. Further sonographies proved easier and took less time.
We therefore conclude that patients’ acceptance of self-performed sonography and performance were high. The possibility of the women to visualise their own internal genital organs, recognize the structures, in particular their thickening endometrium and the growing follicles, elicited great interest and cooperation. Relevant images were easily recognized as soon as early follicular development became visible. The need for a didactic reference document exhibiting the most relevant images was noted and opens a line for midwife research to standardize and optimize such a teaching tool as an adjunct to “sono-anatomy” of the female pelvis.
Discussion
We have delivered proof of concept that performing high-quality sonographic images of the female pelvis by the patients themselves is possible and acceptable both for the patient and her partner. Patients are able to send the images over an electronic link.
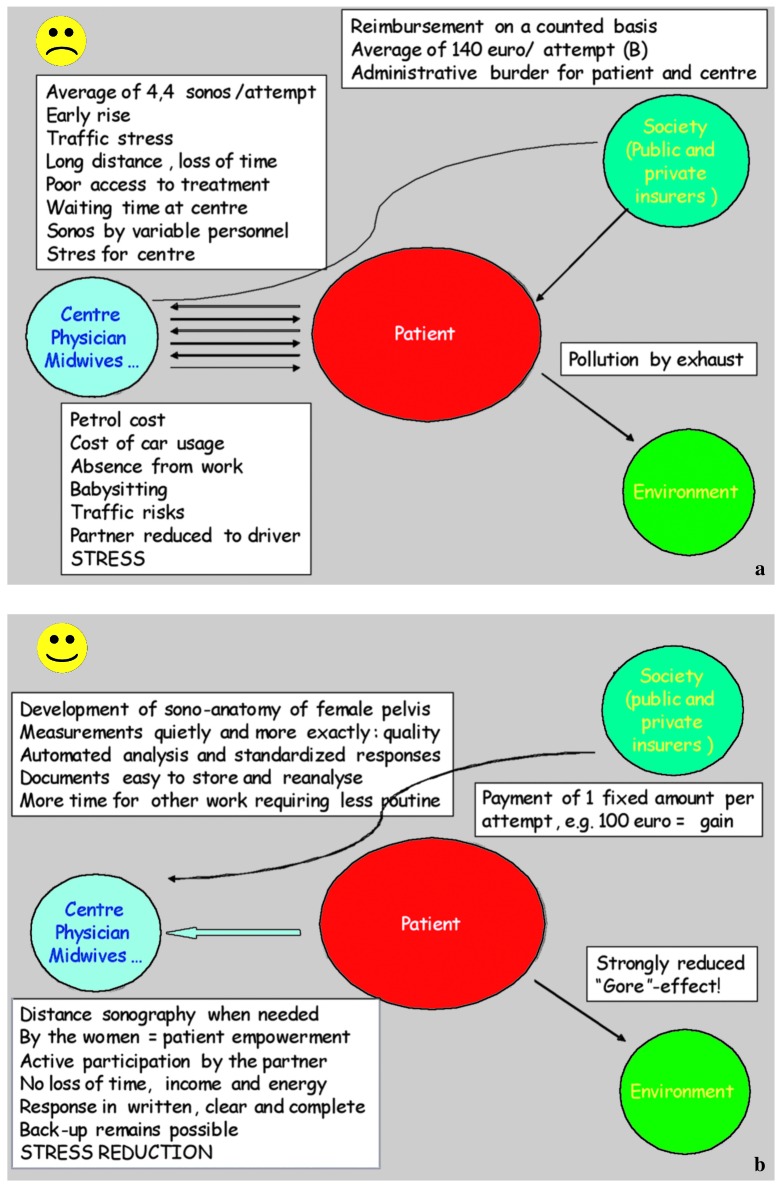
Comparison between the disadvantages of the present way of monitoring ovarian stimulation for ART and the advantages of self-operated home sonography is pictured in Figure 2a and Figure 2b. These a priori considerations were used as a starting point to explore in a systematic way the potential value of a home based approach.
Fig. 2a and b. Comparing the disadvantages of the present way of monitoring ovarian stimulation for ART with the advantages of home sonography.
The SOET-1 study in twenty-five couples indicated an almost unanimous enthusiasm in all patients and their partners for the development of SOET as a tool to ease the burden and stress of monitoring follicular growth. It should be noted that the participants to this opinion-seeking phase of the project were mostly patients living at a considerable distance from the centre. In a western European perspective, this means 50-250km at most. Informal enquiries in patients living nearer to our centre showed that many would still consider using home sonography because of the obstructed traffic in our highly urbanized society.
Recorded images in the SOET-2 study from five women that were analyzed some time after their cycle had elapsed were analyzed by blinded assessors who took exactly the same clinical decisions as the real-time assessor.
The SOET-3 study examined the acceptance and feasibility in twenty-one women who along their regular treatment, performed all their sonographies in duplo themselves. It is possible to obtain usable images and this gave a feeling of empowerment to the women. Transmission of recordings within the hospital was unproblematic.
The decision on whether to continue the technological development of a specific low-end device needed for home sonography is dependent on a positive experience in these three initial steps.
It is important to realize that SOET is not a simple and straightforward application of existing technology in a particular clinical setting. Specific research is needed with respect to the three following aspects:
1. Detailed specifications of the instrument must be explored and described.
The low end instrument to be developed for this very specific application must not necessarily yield the same high-quality images as existing sonographic instruments. It must yield images that induce the responsible clinician to take the same clinical decisions as if made using high end devices. This needs adapting existing technology. The crux is to produce an instrument at a substantially lower cost than existing “portable” sonographic machinery, resulting in the same clinical efficacy, while reducing the disadvantages and side-effects of static in situ sonography and not decreasing safety of treatment. The instrument must be light, strong, easy to transport and to handle for the woman and her partner.
2. Development of specific communication software
Several issues must be addressed that belong to the soft-ware engineering domain:
Simplify and customize the lay-out, usage, ergonomics, recording and image-sending instructions of the instrument to be used by users not usually familiar with electronic equipment; ideally, the probe should be made “intelligent”, i.e. directed towards recognizing ovarian tissue, follicular (= cystic) sonographic patterns, or “movable”, i.e. make angular movements on command after introduction into the vagina, thereby limiting hand- and wrist movements users would have to make themselves in order to visualize the ovaries; however, production cost considerations should be preponderant over gadget value or design;
Develop and implement software for automated measurements of follicular diameter growth (Raine-Fenning et al., 2008, 2009).
Study the modalities of sending short motion images over the internet in an encrypted format to guarantee correct data attribution as well as patient confidentiality;
Develop a semi-automated image analysis system: although a follicle is a three-dimensional structure, routine sonography relies on two-dimensional measurements of a multitude of follicles, that need not necessarily to be measured all; we need a measuring algorithm that takes into account the total number of follicles > 10 × 10 mm (reflecting the risk for OHSS) and the main dimensions of the 2 or 3 leading follicles (reflecting the criterion for clinical decisions).
Ultimately, a standardized reply software should be developed that integrates patient initials, measurements, treatment details (type of treatment, day of stimulation, drugs used, risk factors) and sends out a structured answer to the patient comprising dosage adaptation requirements, next-step instructions.
Integrate the concept into the future telemedicine that is about to emerge world-wide and find applications everywhere in the globe.
Develop business models adapted to specific societal circumstances.
Health-economists will have to explore the limits and possibilities of self-operated endovaginal telemonitoring.
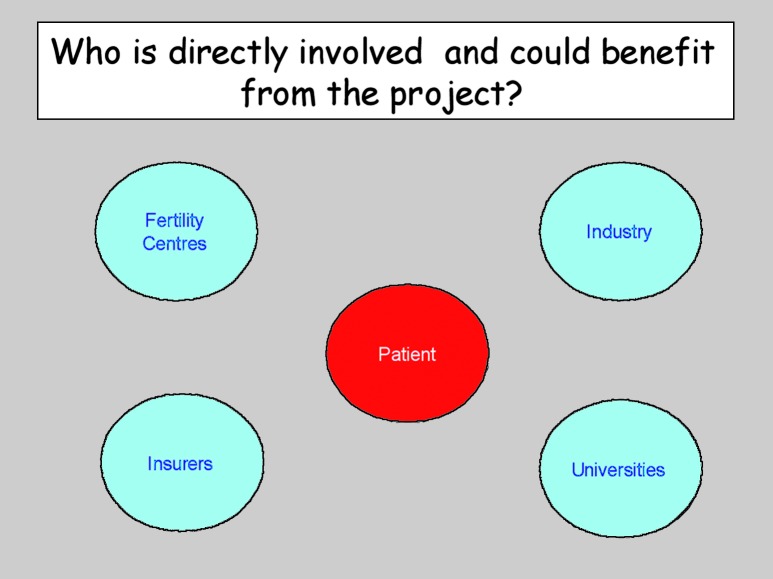
In Belgium, the situation with respect to ART funding is quite different from most countries in the world, where the patient pays it all. The names of the players and potential beneficiaries of this new approach are depicted in Figure 3.
Fig. 3. Who are possibly involved in the development or implementation of home sonography?
Parallel to the technical and clinical development of SOET, we will a new reflection on the payment of sonographies. This will be influenced by developments on a political level. Reasons to choose for SOET will be quite different between countries. Where patients pay, the instrument should be marketed in such a way as to reduce the (high) total cost for the patient. Where public insurance pays, the challenge will be to create savings for the government without increasing the (already limited) cost for the patient to reach an equilibrium that makes for a win(patient)-win(centre)-win(government). In Belgium, at present, the laboratory costs of IVF/ICSI are paid for by the government with a fixed amount, whereas medical acts are also subject to third-party payment using negotiated fixed “prices”. Even gonadotropins used for IVF/ICSI are now funded by fixed amount payments per attempt leading to an oocyte recovery. It seems logical to expect that before long the monitoring of the follicular phase and, to a lesser extent, the luteal phase, will also be paid for with agreed fixed amounts. At that moment, one all-comprising fixed amount will be paid for the totality of an IVF/ICSI cycle.
The most important long term objective advantage of SOET in our view is the tremendous gain in secondary profits (reduction of indirect costs and stress plus environmental considerations).
A question that needs answering is: SOET for whom?
In a first phase, only motivated ART patients are likely to be interested, especially those who have had at least one attempt already. Full instruction by a midwife to get acquainted with the sono-anatomy of the female pelvis is mandatory. Back-up using traditional sonography must remain available. Only if wanted by the patient should the method be considered. Later extensions possible to other infertility treatments or early pregnancy could be possible but initially it should be used exclusively for regular ART stimulations.
Is there a “market” for SOET?
In Belgium, the annual number of IVF/ICSI attempts has stabilized around ~15.000/year, of which an educated guess allows to state that ~50% could be candidates for SOET. World wide, there are ~1.000.000 attempts/year of which half take place in Europe. A further increase of this number is likely but hampered by accessibility for which SOET could be a solution. This is especially the case in large countries of which a number are at the same time third-world-countries where there is quickly emerging market for reproductive technologies.
In conclusion, self-operated endovaginal telemonitoring (SOET) appears to be a welcome idea and the concept is proven. The next phase will consist in designing and implementing a specific portable instrument via a home-to-centre internet connection and further document the clinical validity of the concept.
Some of its prerequisites are: ease and safety of introduction into the vagina, ease of handling in recording and in sending the recordings, connection modalities to most commercially available home computers, possibility of repeated usage within the centre, low cost to fit in any realistic health-economic perspective (from the point of view of the patient, the manufacturer, the centre and the health-insurers).
Who is afraid of SOET?
References
- Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NGM, Verhoeff A, Macklon NS, Fauser BCJM. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22:980–988. doi: 10.1093/humrep/del484. [DOI] [PubMed] [Google Scholar]
- Edwards RG, Steptoe PC, Purdy JM. Establishing full-term human pregnancies using cleaving embryo grown in vitro. Br J Obstet Gynaecol. 1980;87:737–755. doi: 10.1111/j.1471-0528.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- Gerris J, De Neubourg D, Mangelschots K, Van Royen E, Vercruyssen M, Vasquez J, Valkenburg M, Ryckaert G. Elective single day 3 embryo transfer halves the twinning rate without decrease in the ongoing pregnancy rate of an IVF/ICSI programme. Hum Reprod. 2002;17:2626–2631. doi: 10.1093/humrep/17.10.2626. [DOI] [PubMed] [Google Scholar]
- Gerris J, De Sutter P, De Neubourg D, Van Royen E, Van Der Elst J, Mangelschots K, Vercruyssen M, Annemans L, Elseviers M, Kok P, Pauwels P, Valkenburg M, Dhont M. A prospective health economic study of elective single embryo versus double embryo transfer in first IVF/ICSI cycles. Hum Reprod. 2004;19:917–923. doi: 10.1093/humrep/deh188. [DOI] [PubMed] [Google Scholar]
- Golan A, Herman A, Soffer Y, Bukovsky I, El R. Ultrasonic control without hormone determination for ovulation induction in in-vitro fertilization/embryo transfer with gonadotropin-releasing hormone analogue and human menopausal gonadotrophin. Hum Reprod. 1994;9:1631–1633. doi: 10.1093/oxfordjournals.humrep.a138764. [DOI] [PubMed] [Google Scholar]
- Lass A. Fertil Steril. Vol. 80. UK Timing of HCG Group; 2003. pp. 80–85. [DOI] [PubMed] [Google Scholar]
- Pelinck MJ, Knol HM, Vogel NEA, Arts EGJM, Simons AHM, Heineman MJ, Hoek A. Cumulative pregnancy rates after sequential treatment with modified natural cycle IVF followed by IVF with controlled ovarian stimulation. Hum Reprod. 2008;23:1808–1814. doi: 10.1093/humrep/den155. [DOI] [PubMed] [Google Scholar]
- Fenning NL, Jayaprakasan K, Clewes JS, Joergner I, Bonaki SD, Chamberlain S et. SonoAVC: a novel method of automated volume calculation. Ultrasound Obstet Gynecol. 2008;31:691–696. doi: 10.1002/uog.5359. [DOI] [PubMed] [Google Scholar]
- Fenning N, Jayaprakasan K, Chamberlain S, Devlin L, Priddle H, Johnson I. Automated measurements of follicular diameter: a chance to standardize? Fertil Steril. 2009;91:1469–1472. doi: 10.1016/j.fertnstert.2008.07.1719. [DOI] [PubMed] [Google Scholar]
- Saltman RB. Patient choice and patient empowerment in northern European health systems: a conceptual framework. Int J Health Serv. 1994;24:201–229. doi: 10.2190/8WMP-RR2K-ABM7-NVNH. [DOI] [PubMed] [Google Scholar]
- Anttila V, Salokorpi T, Pihlaja M, Sirve S, Suikkari A. Obstetric and perinatal outcome and preliminary results of development of children born after in vitro maturation of oocytes. Hum Reprod. 2006;21:1508–1513. doi: 10.1093/humrep/dei503. [DOI] [PubMed] [Google Scholar]
- Tiitinen A, Granskog C, Gissler M. What is the most relevant standard of success in assisted reproduction? The value of cryopreservation on cumulative pregnancy rates per single oocyte retrieval should not be forgotten. Hum Reprod. 2004;19:2439–2441. doi: 10.1093/humrep/deh446. [DOI] [PubMed] [Google Scholar]