Abstract
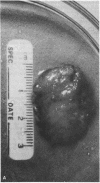
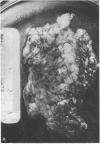
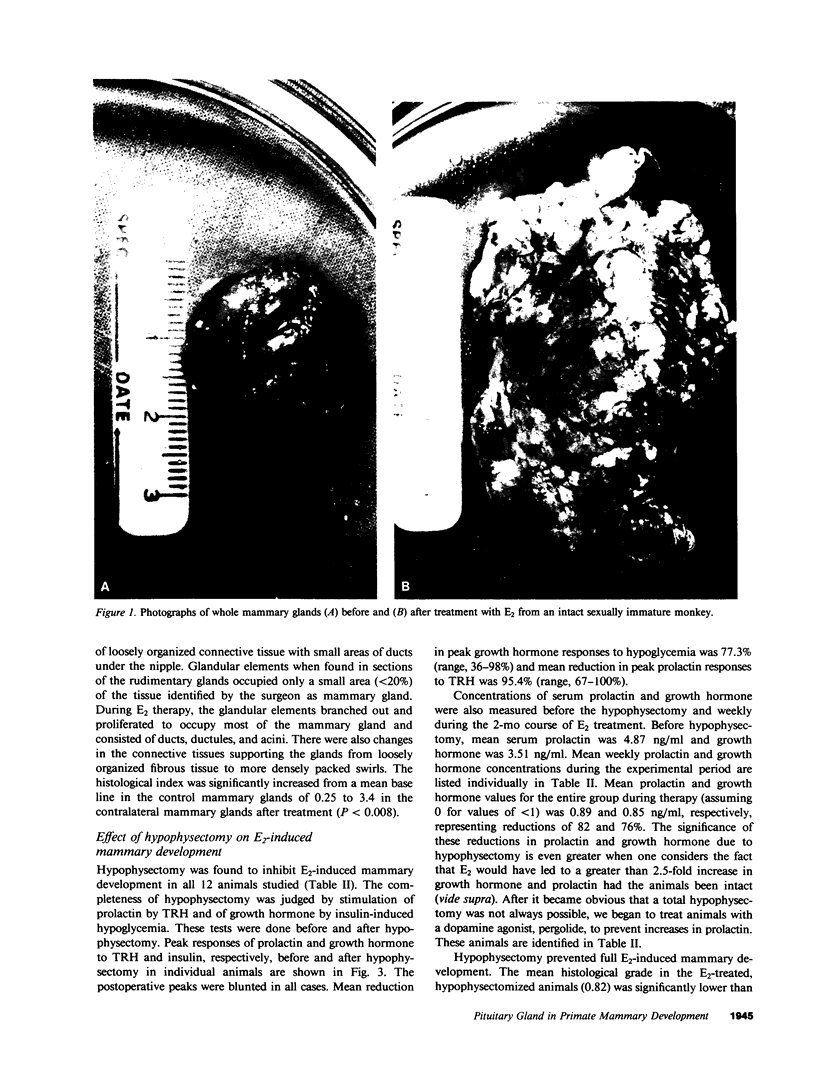
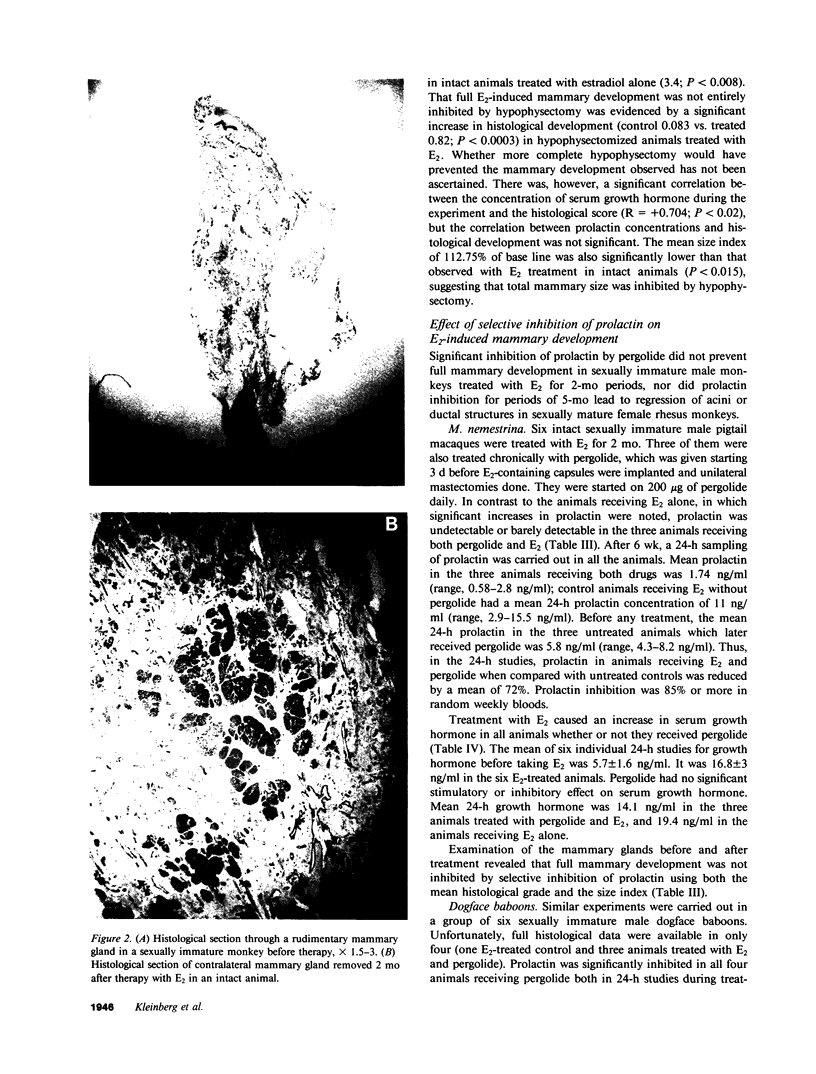
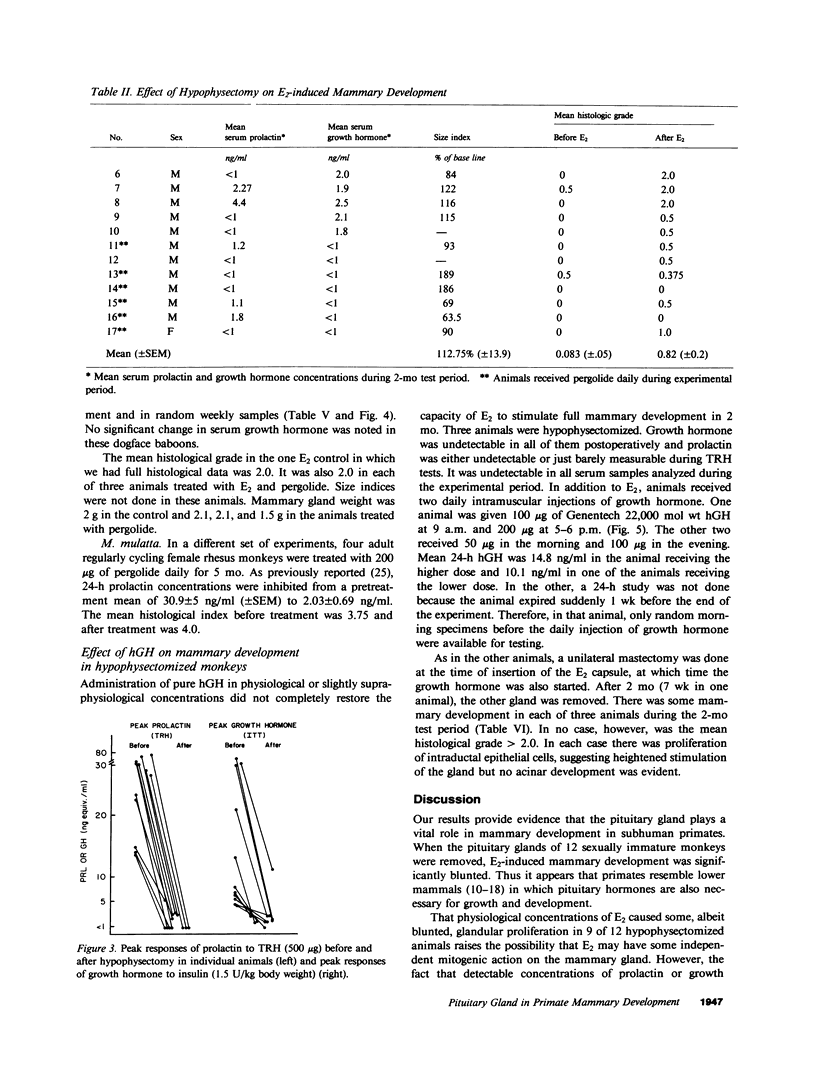
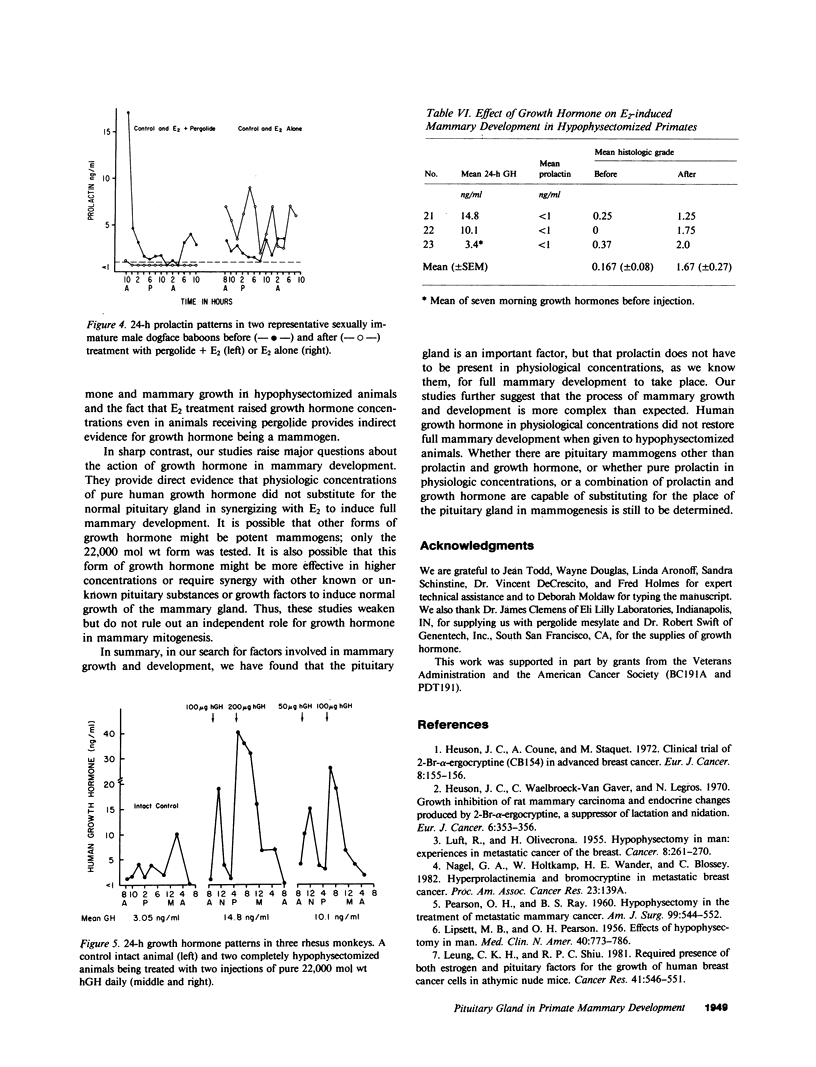
The pituitary gland has been found to be an important factor in mammary development in primates. Hypophysectomy in 12 sexually immature monkeys caused significant inhibition of estradiol (E2)-induced mammary growth and development. A histological index of mammary development in sexually immature hypophysectomized animals was lower (0.82) than in intact E2-treated controls (3.4; P less than 0.008). Hypophysectomy also inhibited growth of the mammary gland as judged by a size index. Despite the hypophysectomy, E2 stimulated some, albeit blunted, mammary growth and development, which may have been due to incomplete hypophysectomy. Selective inhibition of prolactin by ergot drugs in intact animals did not prevent full mammary development, suggesting that there may be pituitary mammogens other than prolactin, or that very low or unmeasurable concentrations of prolactin were sufficient to synergize with E2 to cause full acinar development. The mean histological index was 3.08 in E2-treated animals and 3.16 in animals treated with E2 plus pergolide. There was also no difference in the size of the glands. We evaluated the effect of growth hormone on mammary development by treating three hypophysectomized animals with pure 22,000 mol wt human growth hormone (hGH) (Genentech, Inc., South San Francisco, CA). We found that physiological or slightly supraphysiological concentrations of hGH in animals with unmeasurable prolactin were incapable of restoring the capacity of E2 to induce full mammary growth. These findings suggest that, if growth hormone is a mammary mitogen, that physiological concentrations are insufficient to synergize with E2 to induce full mammary growth or that other forms of hGH are mammogenic. Our studies suggest that the role of the pituitary gland in mammary mitogenesis in primates is more complicated than previously thought. They also raise the possibility that heretofore unidentified pituitary substances may be mammogenic.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buttle H. L., Cowie A. T., Jones E. A., Turvey A. Mammary growth during pregnancy in hypophysectomized or bromocriptine-treated goats. J Endocrinol. 1979 Mar;80(3):343–351. doi: 10.1677/joe.0.0800343. [DOI] [PubMed] [Google Scholar]
- Carmel P. W., Antunes J. L., Ferin M. Collection of blood from the pituitary stalk and portal veins in monkeys, and from the pituitary sinusoidal system of monkey and man. J Neurosurg. 1979 Jan;50(1):75–80. doi: 10.3171/jns.1979.50.1.0075. [DOI] [PubMed] [Google Scholar]
- Clinical trial of 2-Br- -ergocryptine (CB154) in advanced breast cancer. Eur J Cancer. 1972 Apr;8(2):155–156. doi: 10.1016/0014-2964(72)90037-0. [DOI] [PubMed] [Google Scholar]
- Dziuk P. J., Cook B. Passage of steroids through silicone rubber. Endocrinology. 1966 Jan;78(1):208–211. doi: 10.1210/endo-78-1-208. [DOI] [PubMed] [Google Scholar]
- FLUX D. S. Mammary gland growth in male mice of the CHI strain after hypophysectomy and castration. J Endocrinol. 1958 Sep;17(3):300–306. doi: 10.1677/joe.0.0170300. [DOI] [PubMed] [Google Scholar]
- FRANTZ A. G., RABKIN M. T. HUMAN GROWTH HORMONE. CLINICAL MEASUREMENT, RESPONSE TO HYPOGLYCEMIA AND SUPPRESSION BY CORTICOSTEROIDS. N Engl J Med. 1964 Dec 31;271:1375–1381. doi: 10.1056/NEJM196412312712701. [DOI] [PubMed] [Google Scholar]
- Freeman C. S., Topper Y. J. Progesterone is not essential to the differentiative potential of mammary epithelium in the male mouse. Endocrinology. 1978 Jul;103(1):186–192. doi: 10.1210/endo-103-1-186. [DOI] [PubMed] [Google Scholar]
- Gout P. W., Beer C. T., Noble R. L. Prolactin-stimulated growth of cell cultures established from malignant Nb rat lymphomas. Cancer Res. 1980 Jul;40(7):2433–2436. [PubMed] [Google Scholar]
- Hart I. C., Morant S. V. Roles of prolactin, growth hormone, insulin and thyroxine in steroid-induced lactation in goats. J Endocrinol. 1980 Mar;84(3):343–351. doi: 10.1677/joe.0.0840343. [DOI] [PubMed] [Google Scholar]
- Heuson J. C., Waelbroeck-van Gaver C., Legros N. Growth inhibition of rat mammary carcinoma and endocrine changes produced by 2-Br-alpha-ergocryptine, a suppressor of lactation and nidation. Eur J Cancer. 1970 Oct;6(5):353–356. doi: 10.1016/0014-2964(70)90031-9. [DOI] [PubMed] [Google Scholar]
- Karsch F. J., Dierschke D. K., Weick R. F., Yamaji T., Hotchkiss J., Knobil E. Positive and negative feedback control by estrogen of luteinizing hormone secretion in the rhesus monkey. Endocrinology. 1973 Mar;92(3):799–804. doi: 10.1210/endo-92-3-799. [DOI] [PubMed] [Google Scholar]
- Kleinberg D. L. Human alpha-lactalbumin: measurement in serum and in breast cancer organ cultures by radioimmunoassay. Science. 1975 Oct 17;190(4211):276–278. doi: 10.1126/science.1179206. [DOI] [PubMed] [Google Scholar]
- Kleinberg D. L., Lieberman A., Todd J., Greising J., Neophytides A., Kupersmith M. Pergolide mesylate: a potent day-long inhibitor of prolactin in rhesus monkeys and patients with Parkinson's disease. J Clin Endocrinol Metab. 1980 Jul;51(1):152–154. doi: 10.1210/jcem-51-1-152. [DOI] [PubMed] [Google Scholar]
- Kleinberg D. L., Noel G. L., Frantz A. G. Galactorrhea: a study of 235 cases, including 48 with pituitary tumors. N Engl J Med. 1977 Mar 17;296(11):589–600. doi: 10.1056/NEJM197703172961103. [DOI] [PubMed] [Google Scholar]
- Kleinberg D. L., Todd J. Evidence that human growth hormone is a potent lactogen in primates. J Clin Endocrinol Metab. 1980 Nov;51(5):1009–1013. doi: 10.1210/jcem-51-5-1009. [DOI] [PubMed] [Google Scholar]
- Kleinberg D. L., Todd J., Groves M. L. Studies on human alpha-lactalbumin: radioimmunoassay measurements in normal human breast and breast cancer. J Clin Endocrinol Metab. 1977 Dec;45(6):1238–1250. doi: 10.1210/jcem-45-6-1238. [DOI] [PubMed] [Google Scholar]
- Kleinberg D. L., Todd J., Niemann W. Prolactin stimulation of alpha-lactalbumin in normal primate mammary gland. J Clin Endocrinol Metab. 1978 Aug;47(2):435–441. doi: 10.1210/jcem-47-2-435. [DOI] [PubMed] [Google Scholar]
- LIPSETT M. B., PEARSON O. H. Effects of hypophysectomy in man. Med Clin North Am. 1956 May;40(3):773–786. doi: 10.1016/s0025-7125(16)34565-5. [DOI] [PubMed] [Google Scholar]
- LUFT R., OLIVECRONA H. Hypophysectomy in man; experiences in metastatic cancer of the breast. Cancer. 1955 Mar-Apr;8(2):261–270. doi: 10.1002/1097-0142(1955)8:2<261::aid-cncr2820080205>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- LYONS W. R., LI C. H., JOHNSON R. E. The hormonal control of mammary growth and lactation. Recent Prog Horm Res. 1958;14:219–254. [PubMed] [Google Scholar]
- Leung C. K., Shiu R. P. Required presence of both estrogen and pituitary factors for the growth of human breast cancer cells in athymic nude mice. Cancer Res. 1981 Feb;41(2):546–551. [PubMed] [Google Scholar]
- Mittra I. A novel "cleaved prolactin" in the rat pituitary: Part II. In vivo mammary mitogenic activity of its N-terminal 16K moiety. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1760–1767. doi: 10.1016/s0006-291x(80)80102-1. [DOI] [PubMed] [Google Scholar]
- Mittra I. A novel "cleaved prolactin" in the rat pituitary: part I. Biosynthesis, characterization and regulatory control. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1750–1759. doi: 10.1016/s0006-291x(80)80101-x. [DOI] [PubMed] [Google Scholar]
- NANDI S. Endocrine control of mammarygland development and function in the C3H/ He Crgl mouse. J Natl Cancer Inst. 1958 Dec;21(6):1039–1063. [PubMed] [Google Scholar]
- PEARSON O. H., RAY B. S. Hypophysectomy in the treatment of metastatic mammary cancer. Am J Surg. 1960 Apr;99:544–552. doi: 10.1016/0002-9610(60)90149-5. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Shiu R. P., Gout P. W., Beer C. T., Noble R. L., Friesen H. G. A new sensitive and specific bioassay for lactogenic hormones: measurement of prolactin and growth hormone in human serum. J Clin Endocrinol Metab. 1980 Nov;51(5):1058–1063. doi: 10.1210/jcem-51-5-1058. [DOI] [PubMed] [Google Scholar]