Abstract
Introduction
The aim of this review was to investigate whether there is a faster cognitive decline in dementia with Lewy bodies (DLB) than in Alzheimer’s disease (AD) over time.
Methods
PsycINFO and Medline were searched from 1946 to February 2013. A quality rating from 1 to 15 (best) was applied to the included studies. A quantitative meta-analysis was done on studies with mini mental state examination (MMSE) as the outcome measure.
Results
A total of 18 studies were included. Of these, six (36%) reported significant differences in the rate of cognitive decline. Three studies reported a faster cognitive decline on MMSE in patients with mixed DLB and AD compared to pure forms, whereas two studies reported a faster decline on delayed recall and recognition in AD and one in DLB on verbal fluency. Mean quality scores for studies that did or did not differ were not significantly different. Six studies reported MMSE scores and were included in the meta-analysis, which showed no significant difference in annual decline on MMSE between DLB (mean 3.4) and AD (mean 3.3).
Conclusions
Our findings do not support the hypothesis of a faster rate of cognitive decline in DLB compared to AD. Future studies should apply recent diagnostic criteria, as well as extensive diagnostic evaluation and ideally autopsy diagnosis. Studies with large enough samples, detailed cognitive tests, at least two years follow up and multivariate statistical analysis are also needed.
1 Introduction
Dementia with Lewy bodies (DLB) and Alzheimer’s disease (AD) are the two most common subtypes of neurodegenerative dementia, representing 15 to 20% and 65% of all dementia cases, respectively [1]. DLB is characterized clinically by symptoms such as visual hallucinations, Parkinsonism and fluctuating cognition in addition to cognitive impairment with typically more visuospatial and executive impairment relative to memory impairment [2]. There is some evidence that DLB patients have more rapidly progressing dementia compared to AD [3], and more recent studies also reported a more severe course with shorter survival [4], higher rate of nursing home admissions [5] and higher costs in DLB as compared to AD [6].
An overlap in neuropathology between AD and DLB has been noted [7]. Parkinson’s disease (PD) and DLB also share some clinical and pathological features [8]. Subgroups with different cognitive profiles have been described in patients with PD [9], and there is evidence that this differentiation is related to the rate of cognitive decline [10]. Similar neuropsychologically defined subgroups may exist also in DLB [8], which could also predict differences in the rate of progression to end-stage dementia. Data supports accelerated disease progression when AD and DLB pathologies are present together [11].
To our knowledge, no systematic review has compared rate of cognitive decline in DLB versus AD. We therefore systematically reviewed the literature to find studies assessing overall cognitive decline in DLB and AD. We specifically noted studies that had investigated the potential differences in cognitive decline in subgroups with DLB and the effect of employing different diagnostic criteria.
2 Methods
PsycINFO and Medline were searched in February 2013, using key words listed in Table 1. References from reviewed articles were also searched for relevant studies. The following inclusion criteria were used: a) paper published in a peer-reviewed journal; b) written in English; c) DLB or mixed AD/DLB compared with AD; d) application of at least one neuropsychological test, and e) at least 6 months follow up. The following exclusion criteria were used: a) drug trials, and b) survival studies with death as the only outcome.
Table 1.
Search history
|
Medline |
PsycINFO |
|
|
|---|---|---|---|
| (1946 to February 2013) | (1806 to February 2013) | ||
|
Key words |
Alzheimer’s disease and Lewy body disease, or Lewy bodies |
Alzheimer’s disease and dementia with Lewy bodies |
|
|
Key words |
Neuropsychology, or neuropsychological tests, or Cognition, or cognition disorders |
Neuropsychology, or neuropsychological assessment, or neuropsychological assessment, or Cognition, or cognitive impairment, or |
|
|
Key words |
Disease progression, or longitudinal studies |
Disease course, or disease prognosis, or longitudinal studies |
|
|
Search results |
70 |
97 |
|
| Included | 18 |
2.1 Quality assessment
Two independent raters rated all studies with a self-designed quality scale and arrived at the same result. The domains, a) number of patients included; b) follow-up time; c) clinical criteria; d) autopsy, and e) neuropsychological tests) were rated on a four-point scale adapted from Aarsland et al. (2005) [12]: 0 (none), 1 (poor), 2 (fair) and 3 (good). See Table 2. Studies could be assigned 1 to 15 points.
Table 2.
Quality assessment criteria
| |
Score |
|||
|---|---|---|---|---|
| 3 | 2 | 1 | 0 | |
|
Patients at baseline, number |
>151 |
101 to 150 |
51 to 100 |
<50 |
|
Follow-up time, years |
>3 or mean ≥3 |
3 |
2 |
≤1 |
|
Clinical criteria |
Established criteria for AD + DLB criteria from 2005 |
Established criteria for AD + DLB criteria from 1992 or 1996 |
Used criteria for one type of dementia |
No criteria used |
| Autopsy, % of participants | 100 | >50 | >25 | None |
AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; BNT, Boston naming test; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease evaluation; DRS, dementia rating scale; ESD, extended scale for dementia; HVLT-R Hopkins verbal learning test-revised; mMMS, modified mini-mental state examination; MMSE, mini mental state examination; MTS, 37 item mental test score.
2.2 Statistical analysis
For studies reporting mini mental state examination (MMSE) results, standardized mean difference in annual progression between DLB and AD was calculated as the difference between annual progression between the DLB and AD groups divided by the pooled standard deviation across groups in each included study. The standardized mean differences were combined in a random-effects model to obtain summary estimates of the effect in each study. The overall results from each trial were then combined using a random-effects model to obtain a pooled summary estimate of effect across all trials [13]. To assess heterogeneity, the I2 as proposed by Higgins and colleagues [14] was chosen, indicating the percentage of total variation across studies due to heterogeneity.
3 Results
Of the 18 studies included in this review (see Table 3), six (36%) reported a statistically significant difference in cognitive decline over time between AD and DLB (see Table 4). Three studies reported a faster cognitive decline on cognitive screening tests in the neuropathologically mixed AD/DLB group [3],[15],[16] compared to those with pure AD or DLB. One study reported a faster decline in DLB than in AD on verbal fluency [17], and two in AD compared to DLB on memory [18],[19]. For a full description of neuropsychological tests used in included studies, see Table 3.
Table 3.
Study characteristics and main findings of included studies
| Study | Sample, male/female ratio (m/f), mean age (SD) | Follow-up period | Neuropsychological tests | AD versus DLB comparison | Test scores, mean (SD) |
|---|---|---|---|---|---|
|
McKeith
et al
., 1992 [[20]] |
AD 37 |
Baseline and late stage |
MTS |
No significant difference |
MTS baseline |
| m/f 13/24 |
AD 15.9 (1.8) |
||||
| y 74.7 (0.9) |
SDLT 24.5 (1.7) |
||||
| SDLT 21 |
MTS late stage |
||||
| m/f 12/9 |
AD 9.3 (2.1) |
||||
| y 73.3 (1.6) |
SDLT 18.2 (2.3) |
||||
|
Ballard
et al
., 1996 [[17]] |
AD 53 |
1 y |
CAMCOG |
SDLT faster decline of verbal fluency |
Scores for subtests n/a |
| m/f, n/a |
|
|
|
|
|
| Y, n/a |
CAMCOG total, baseline |
||||
| SDLT 7 |
AD 42.7 (17.9) |
||||
| |
SDLT 47.7 (18.0) |
||||
| m/f, n/a |
CAMCOG mean annual decline |
||||
| Y, n/a | |||||
| VaD 14 |
AD 13.2 (12.6) |
||||
| m/f, n/a |
SDLT 27.0 (19.8) |
||||
| Y, n/a | |||||
|
Ballard
et al
., 1998 [[21]] |
AD 30 |
1 y |
MMSE |
No significant difference |
MMSE baseline |
| m/f 9/21 |
AD 13.9 |
||||
| DLB 14.9 | |||||
| y 81.7 |
MMSE mean annual decline |
||||
| DLB 42 | |||||
| AD 4.1 | |||||
| m/f 19/24 |
DLB 3.9 |
||||
| y 73.6 | |||||
|
Olichney
et al
., 1998 [[3]] |
AD 148 |
Mean 3 y |
MMSE |
LBV faster decline |
MMSE baseline |
| m/f 80/68 | |||||
| y 74.0 (7.9) |
AD 17.8 (6.0) |
||||
| LBV 40 |
LBV 18.2 (5.5) |
||||
| m/f 25/15 |
MMSE 1 y (n = 136/35) |
||||
| y 72.4 (6.5) | |||||
| AD 14.3 (7.2) | |||||
| LBV 12.5 (7.5) | |||||
| MMSE 2 y (n = 93/17) | |||||
| AD 12.3 (7.9) | |||||
| LBV 8.1 (6.3) | |||||
| MMSE 3 y (n = 59/12) | |||||
| AD 10.1 (8.4) | |||||
| LBV 4.5 (6.5) | |||||
| MMSE 4 y (n = 35/4) | |||||
| AD 9.1 (7.9) | |||||
| LBV 2.5 (3.0) | |||||
| MMSE mean annual decline | |||||
| AD 4.1 (3.0) | |||||
| LBV 5.8 (4.5) | |||||
|
Heyman
et al
., 1999 [[18]] |
AD 74 |
Annual controls |
CERAD (including CDT, calculation test, serial subtraction, CDR, BNT, MMSE, 10-item word list memory, recall and recognition, constructional praxis, two of the six items of the orientation-memory-concentration test) |
AD faster decline in delayed recall |
32% of LBV versus 15% of AD remembered any item on word list recall at last evaluation |
| m/f 47/27 | |||||
| y 41% >74 y | |||||
| AD/LBV 27 | |||||
| m/f 14/13 | |||||
| y 37% >74 y | |||||
|
Lopez
et al
., 2000 [[22]] |
AD 98 |
Mean 59 months |
MMSE |
No significant difference |
MMSE baseline |
| m/f 50/48 | |||||
| y 70.8 (9.4) |
AD 16.0 (6.5) |
||||
| AD/DLB 44 |
AD/DLB 16.2 (5.1) |
||||
| m/f 20/24 | |||||
| y 72.3 (6.0) | |||||
|
Stern
et al
., 2001 [[23]] |
AD 32 |
Annual controls, longest 9.9 y |
mMMSE (including WAIS-R digit span forward, backward, attention, calculation, general knowledge, language, construction), CDR |
No significant difference |
mMMSE baseline |
| m/f 16/16 |
AD 36.7 (6.3) |
||||
| y 73.0 (9.0) |
LBV 37.3 (6.2) |
||||
| LBV 19 |
mMMSE mean annual decline 3.6 (both groups) |
||||
| m/f 17/2 | |||||
| y 73.6 (6.8) | |||||
|
Ballard
et al
., 2001 [[24]] |
AD 101 |
1y |
MMSE, CAMCOG |
No significant difference |
MMSE n = 203 |
| m/f 30/71 |
MMSE baseline |
||||
| probable AD 61 m/f 17/44 |
prob AD 17.7 (5.1) |
||||
| poss AD 17.2 (5.2) | |||||
| y 81.9 (4.8) | |||||
| DLB 15.6 (7.0) | |||||
| possible AD 40 | |||||
| MMSE mean annual decline | |||||
| m/f 13/27 | |||||
| y 79.0 (7.8) |
AD 4.9 (3.6) |
||||
| DLB 64 |
DLB 4.3 (4.2) |
||||
| m/f 26/38 |
CAMCOG n = 154 |
||||
| Baseline 57.5 (18.8) | |||||
| y 76.6 (7.7) | |||||
| VaD 38 |
CAMCOG mean annual decline |
||||
| m/f 22/16 | |||||
| y 76.8 (7.7) |
Probable AD 15.0 (10.1) |
||||
| Possible AD 14.4 (9.8) | |||||
| DLB 11.9 (12.2) | |||||
|
Helmes
et al
., 2003 [[25]] |
AD 15 |
50 months |
ESD |
No significant difference |
Scores n/a |
| m/f 9/6 | |||||
| y 70.3 (7.6) | |||||
| AD/DLB 8 | |||||
| m/f 5/3 | |||||
| y 69.3 (11.2) | |||||
| DLB 7 | |||||
| m/f 5/2 | |||||
| y 69.1 (4.1) | |||||
|
Johnson
et al
., 2005 [[26]] |
AD 66 |
Annual controls, |
WMS (digits forward, backward, logical memory and associate learning), BVRT, word fluency, BNT, WAIS (Digit Symbol and Block Design), TMT A, Crossing Off, CDR |
No significant difference |
Follow-up scores n/a. For baseline scores for all tests see article |
| m/f 39/27 |
1 to 20 assessments |
||||
| y 77.0 (8.1) | |||||
| AD/DLB 57 | |||||
| m/f 31/26 | |||||
| y 75.2 (9.7) | |||||
| DLB 9 | |||||
| m/f 8/1, age 72.6 (5.7) | |||||
|
Kraybill
et al
., 2005 [[15]] |
AD 48 |
Annual controls |
MMSE, DRS |
AD/LBP faster decline than AD and LBP |
MMSE baseline |
| |
m/f 18/30 |
|
|
|
AD 20.6 (3.9) |
| y at onset 77.5 |
AD/LBP 20.7 (3.7) |
||||
| (7.34) |
LBP 20.7 (3.8) |
||||
| AD/LBP 65 |
MMSE mean annual decline |
||||
| m/f 24/41 |
AD 3.5 (0.4) |
||||
| y at onset 74.8 (6.6) |
AD/LBP 5.0 (0.5) |
||||
| LBP 3.4 (0.7) | |||||
| LBP 22 |
DRS baseline |
||||
| m/f 16/6 |
AD 114.7 (2.1) |
||||
| y at onset 76.5 (5.3) |
AD/LBP 114.2 (1.8) |
||||
| LBP 114.2 (2.7) | |||||
| DRS mean annual decline | |||||
| AD 9.6 (1.5) | |||||
| AD/LBP 15.3 (1.9) | |||||
| LBP 8.8 (1.7) | |||||
|
Stavitsky
et al
., 2006 [[19]] |
AD 55 |
Mean 3 y |
mMMSE (incl WAIS-R digit Span forward, backward, attention, calculation, general knowledge, language, construction), HVLT-R |
AD faster decline on recognition. |
mMMSE baseline |
| m/f 21/34 |
AD 39.0 (7.6) |
||||
| DLB 38.1 (8.3) | |||||
| y 73.1 (8.3) |
HVLT-R n/a |
||||
| DLB 28 | |||||
| m/f 19/9 | |||||
| y 73.5 (7.6) | |||||
|
Williams
et al
., 2006 [[27]] |
AD 252 |
< 5 y |
MMSE, CDR, WMS (mental control, logical memory, digit span forward and backward, associate learning), BVRT, WAIS (information, digit symbol, block design), word fluency, BNT, Crossing off, TMT A |
No significant difference. |
Scores n/a |
| m/f 95/157 | |||||
| y 77.8 (9.5) | |||||
| DLB 63 | |||||
| m/f 38/25 | |||||
| y 73.5 (8.7) | |||||
|
Hamilton
et al
., 2008 [[28]] |
AD 44 |
2 y |
DRS, WISC-R (block design), CDT copy, BNT |
Poor baseline visuospatial skills (block design <20, CDT copy <3) were strongly associated with faster decline in DLB, but not AD. |
DRS baseline |
| m/f 20/24 |
AD 114.4 (15.4) |
||||
| y 72.0 (5.6) |
DLB 109.5 (11.4) |
||||
| DLB 22 |
DRS 1 y mean decline |
||||
| m/f 14/8 | |||||
| y 73.4 (6.2) |
AD 7.9 (11.6) |
||||
| DLB 17 (24.2) | |||||
| DRS 2 y mean decline | |||||
| AD 23.9 (24.7) | |||||
| DLB 39.3 (35.1) | |||||
| Other scores n/a | |||||
|
Hanyu
et al
., 2009 [[29]] |
AD 111 |
5 y |
MMSE |
No significant difference |
MMSE |
| m/f 37/74 |
Baseline n = 111/56 |
||||
| y 77.5 (6.2) |
AD 20.3 (3.7) |
||||
| DLB 56 |
DLB 20.7 (3.8) |
||||
| m/f 30/26 |
1 y n = 111/56 |
||||
| y 78.1 (5.2) |
AD 19.4 (4.8) |
||||
| DLB 20.5 (4.2) | |||||
| 2 y n = 102/40 | |||||
| AD 17.7 (5.2) | |||||
| DLB 18.0 (4.8) | |||||
| 3 y n = 72/25 | |||||
| AD 16.2 (5.0) | |||||
| DLB 17.0 (5.3) | |||||
| 4 y n = 51/19 | |||||
| AD 14.2 (4.5) | |||||
| DLB 13.4 (4.0) | |||||
| 5 y n = 16/5 | |||||
| AD 11.4 (5.2) | |||||
| DLB 10.6 (4.0) | |||||
|
Nelson
et al
., 2009 [[16]] |
AD 107 |
Mean 4 y |
MMSE |
AD/DLB had a faster decline than DLB and AD. |
MMSE baseline n/a |
| m/f n/a |
MMSE final |
||||
| y n/a |
AD 10.7 (8.6) |
||||
| AD/DLB 27 |
AD/DLB 10.6 (8.6) |
||||
| m/f n/a |
DLB 15.6 (8.7) |
||||
| y n/a | |||||
| DLB 9 | |||||
| m/f n/a | |||||
| y n/a | |||||
|
Wood
et al
., 2012[30] |
AD 16 |
1 y |
MMSE, CAMCOG, NEVIP |
No significant difference. |
MMSE baseline |
| m/f 12/4 |
AD 21.3 (3.2) |
||||
| y 78.9 (6.1) |
DLB 24.5 (3.3) |
||||
| DLB 10 |
MMSE decline from baseline |
||||
| m/f 9/1 | |||||
| y 78.2 (7.4). |
AD 2.1 (3.6) |
||||
| Controls 28 |
DLB 1.8 (3.1) |
||||
| m/f 16/12 |
CAMCOG baseline |
||||
| y 79.5 |
AD 71.4 (9.7) |
||||
| DLB 79.1 (12.0) | |||||
| CAMCOG decline from baseline | |||||
| AD 7.4 (10.7) | |||||
| DLB 4.3 (7.3) | |||||
| Walker et al ., 2012[31] | AD 100 |
1 y | MMSE, CAMCOG-R, VOSP, CDR | No significant difference. | MMSE baseline |
| m/f 48/52 |
AD 21.5 (4.5) |
||||
| y 74,9 |
DLB 21.4 (3.9) |
||||
| DLB 58 |
MMSE follow up (n = 81/33) |
||||
| m/f 37/21 |
AD 19.0 (6.2) |
||||
| y 74,2 | |||||
| DLB 18.5 (6.0) | |||||
| CAMCOG-R baseline | |||||
| AD 66.3 (15.6) | |||||
| DLB 66.0 (13.5) | |||||
| CAMCOG-R follow up | |||||
| (n = 81/33) | |||||
| AD 59.6 (20.3) | |||||
| DLB 56.3 (19.7) |
AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; LBP, Lewy body pathology; LBV, Lewy body variant; n/a, not available; SDLT, senile dementia of Lewy body type; VaD, vascular dementia; y, years; BNT, Boston naming test; BVRT, Benton visual retention test; CAMCOG, Cambridge cognitive examination; CAMCOG-R, Cambridge cognitive examination-revised; CDR, clinical dementia rating; CDT, clock drawing test; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease evaluation; DRS, dementia rating scale; ESD, extended scale for dementia; HVLT-R, Hopkins verbal learning test-revised; MMSE, mini mental state examination; mMMS, modified mini-mental state examination; MTS, 37-item mental test score; NEVIP, Newcastle visual perception battery; TMT A, trail making test A; VOSP, visual object and space perception battery; WAIS, Wechsler adult intelligence scale; WISC-R, Wechsler intelligence scale for children-revised; WMS, Wechsler memory scale.
Table 4.
Studies reporting differences in cognitive decline
| Study | Cognitive function | Impairment | Contrast group | Test |
|---|---|---|---|---|
|
Olichney
et al
., 1998 [[3]] |
Total score |
AD/DLB |
AD |
MMSE |
|
Kraybill
et al
l., 2005 [[15]] |
Total score |
AD/DLB |
AD and DLB |
MMSE, DRS |
|
Nelson
et al
., 2009 [[16]] |
Total score |
AD/DLB |
AD and DLB |
MMSE |
|
Heyman
et al
., 1999 [[18]] |
Delayed recall |
AD |
AD/DLB |
CERAD |
|
Stavitsky
et al
., 2006 [[19]] |
Recognition |
AD |
DLB |
HVLT-R |
| Ballard et al ., 1996 [[17]] | Verbal fluency | DLB | AD | CAMCOG |
AD, Alzheimer’s disease; AD/DLB, mixed pathology; DLB, dementia with Lewy bodies;
CAMCOG, Cambridge cognitive examination; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease evaluation; DRS, dementia rating scale; HVLT-R, Hopkins verbal learning test-revised; MMSE, mini mental state examination.
Six studies either reported annual decline in MMSE scores, or included data enabling calculation of annual decline based on reported scores. In AD, mean annual decline was 3.3 (SD 1.7, range 1.8 to 4.9), and in DLB 3.4 (SD 1.4, range 1.8 to 5.8). One study also reported annual decline of 5.0 in AD/DLB (see Figure 1). The random-effects meta-analysis revealed an overall effect-size of −0.035 (negative sign indicates faster progression in DLB) (P = 0.764; 95% CI = 0.261, 0.192). I2 was 50.3, which is considered to represent moderate heterogeneity [14].
Figure 1.
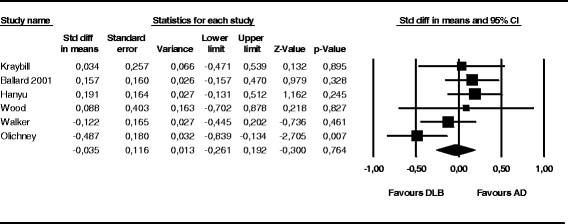
Forrest plot of annual progression of mini-mental state examination scores. The random-effects meta-analysis revealed an overall effect-size of −0.035 (negative sign indicates faster progression in dementia with Lewy bodies (DLB) (P = 0.764; 95% CI = 0.261, 0.192). AD, Alzheimer's disease.
3.1 Cognitive domains
Six studies measured memory, and two reported differences in memory over time, both a faster decline in AD. Delayed recall was found to have a faster decline in AD compared to AD/DLB when measured with the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) evaluation, with 15% of patients with AD versus 32% of patients with AD/DLB remembering any item at the last evaluation [17]. Recognition was found to have a faster decline in AD compared to DLB as measured with Hopkins verbal learning test- revised (HVLT-R) (scores not available) [19]. Eight studies measuring language and ten studies measuring visuospatial ability reported no differences in rate of decline. Seven studies measured explicit executive functions, and one reported differences over time. In that study, verbal fluency was found to have a more rapid decline in DLB compared to AD, measured with the Cambride cognitive examination (CAMCOG) (subscores not available) [17].
3.2 Subgroups
Two studies [28],[30] divided patients into two groups according to high or low visuospatial functioning. In the first study, DLB patients with a low baseline score (<20) on the Wechsler intelligence scale for children-revised, block design (WISC-R) and impaired clock drawing test (CDT) had a faster decline on the dementia rating scale (DRS), compared to DLB patients with a high baseline score. In the latter study, DLB patients with a low baseline score on the Newcastle visual perception battery (NEVIP) had a faster decline in activities of daily living (ADL) than those with higher score, but no difference on any of the cognitive tests. There were no differences in the AD groups.
3.3 Quality assessment
The mean quality score for all the included studies was 9.4 points (SD 2.5, range 5 to 14) (see Table 5). Only two studies were rated fair or good on all quality measures [26],[27]. Three studies were rated poor on one variable, but fair and good on the others [15],[16],[22]. Mean quality scores for studies that found any differences in cognitive decline was 9.8 points (SD 2.4, range 5 to 11) compared to 9.3 points (SD 2.6, range 5 to 14) in the group with no differences (P = 0.335).
Table 5.
Quality assessment results
| Study | Sum | Patients | Neuropsychological tests | Time | Autopsy | Clinical criteria |
|---|---|---|---|---|---|---|
|
Williams
et al
., 2006 [[27]] |
14 |
3 |
3 |
3 |
3 |
2 |
|
Johnson
et al
., 2005 [[26]] |
13 |
2 |
3 |
3 |
3 |
2 |
|
Heyman
et al
., 1999 [[18]] |
11 |
1 |
3 |
3 |
3 |
1 |
|
Lopez
et al
., 2000 [[22]] |
11 |
2 |
1 |
3 |
3 |
2 |
|
Kraybill
et al
., 2005 [[15]] |
11 |
2 |
2 |
3 |
3 |
1 |
|
Olichney
et al
., 1998 [[3]] |
11 |
3 |
1 |
3 |
3 |
1 |
|
Nelson
et al
., 2009 [[16]] |
11 |
2 |
1 |
3 |
3 |
2 |
|
Stern
et al
., 2001 [[23]] |
10 |
1 |
2 |
3 |
3 |
1 |
|
Stavitsky
et al
., 2006 [[19]] |
10 |
1 |
3 |
3 |
1 |
2 |
|
Hamilton
et al
., 2008 [[28]] |
10 |
1 |
3 |
1 |
3 |
2 |
|
Helmes
et al
., 2003 [[25]] |
9 |
0 |
2 |
3 |
3 |
1 |
|
Hanyu
et al
., 2009 [[29]] |
9 |
3 |
1 |
3 |
0 |
2 |
|
McKeith
et al
., 1992 [[20]] |
8 |
1 |
1 |
3 |
3 |
0 |
|
Ballard
et a
l., 2001 [[24]] |
8 |
3 |
2 |
0 |
1 |
2 |
|
Walker
et al
., 2012 [[31]] |
8 |
3 |
3 |
0 |
0 |
2 |
|
Wood
et al
., 2012 [[30]] |
6 |
0 |
3 |
0 |
0 |
3 |
|
Ballard
et al
., 1998 [[21]] |
5 |
1 |
1 |
0 |
1 |
2 |
| Ballard et al ., 1996 [[17]] | 5 | 1 | 2 | 0 | 0 | 2 |
3.4 Clinical and neuropathological diagnostic criteria
There were no systematical differences in clinical or neuropathological criteria between studies that found differences in cognitive decline and those who did not (see Table 6). Of 18 included studies, 16 (89%) used National Institute of Neurological and Communication Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) or CERAD clinical criteria for AD and 12 (67%) used DLB consensus criteria, only one of them used the revised criteria from 2005. To diagnose AD neuropathologically, mainly CERAD neuropathological criteria for the diagnosis of AD and neuropathological DLB consensus criteria from 1996 were used. A diagnosis of mixed AD/DLB was made, if in addition to the Alzheimer’s pathology the characteristic Lewy bodies were found in subcortical and cortical areas. Eleven studies (61%) used autopsy-confirmed diagnosis on all patients. In three studies (17%), some of the diagnoses were autopsy-confirmed. In four studies (22%) autopsy was not performed. One of the studies used 123I-FP-CIT-SPECT only as a method of verifying of clinical diagnosis [31].
Table 6.
Clinical and neuropathological criteria
| Study | Sample | Database | Neuropathological criteria | Autopsy | Dementia criteria |
|---|---|---|---|---|---|
|
McKeith
et al
., 1992 [[20]] |
AD 37 |
Newcastle, UK |
AD: plaque/tangle quantification, H + E, CFV, Loyez, Palmgren. |
All |
DLB: proposed consensus (1992) |
| SDLT 21 | |||||
| LB: H + E, pholxine, erythrosin | |||||
|
Ballard
et al
., 1996 [[17]] |
AD 53 |
West Midlands and Bristol, UK |
|
0 |
AD: NINCDS/ADRDA (1984) |
| SDLT 7 |
DLB: McKeith, operational criteria for senile dementia of Lewy body type (1992) |
||||
| VaD 14 | |||||
|
Ballard et al., 1998 [[21]] |
AD 30 |
Newcastle General Hospital, UK |
AD: CERAD, plaque – Braunmuhl stain, tangle – modified Palmgren |
19 |
AD: NINCDS/ADRDA (1984) |
| DLB 42 |
DLB: McKeith, operational criteria for senile dementia of Lewy body type (1992) |
||||
| LB: consensus criteria (1996), ubiquitin, anti-tau2, anti-Alz50, anti-AT8 to detect and distinguish cortical LB | |||||
|
Olichney
et a
l., 1998 [[3]] |
AD 148 |
Cohort from: |
AD: CERAD, ADRC |
All |
AD: NINCDS/ADRDA (1984), |
| LBV 40 |
Univeristy of California, San Diego Alzheimer’s Disease Research Center, USA; |
LB: ubiquitin, H + E (brainstem, cerebral cortex) |
DSM-III for dementia |
||
| CERAD centers, multinational | |||||
|
Heyman
et al
., 1999 [[18]] |
AD 74 |
Subjects with premortem diagnosis of probable and possible AD from 24 centers participating in CERAD, 1986 to 1995, USA |
AD: CERAD |
All |
AD: NINCDS/ADRDA (1984) |
| AD/LBV 27 |
LB: consensus criteria (1996), modified (brainstem, limbic/transitional and noecortical). |
||||
|
Lopez
et al
., 2000 [[22]] |
AD 98 |
University of Pittsburg 1983 to 1998, USA |
AD: CERAD, NIA-RI |
All |
AD: NINCDS/ADRDA (1984) |
| AD/DLB 44 |
LB: H + E, ubiqutin (SN, neocortex, limbic areas) |
DLB: consensus criteria (1996) |
|||
|
Stern
et al
. 2001 [[23]] |
AD 32 |
From cohort of 236 patients with probable AD |
AD: CERAD |
All |
AD: NINCDS/ADRDA (1984) |
| LBV 19 |
LB: semi quantitative ubiquitin (SN, hippocampus, cingulate gyrus, insula cortex) |
||||
| Recruited: | |||||
| Columbia University College, New York, USA | |||||
| Johns Hopkins University, Baltimore, USA | |||||
| Massachusetts General Hospital, Boston, USA | |||||
|
Ballard
et al
., 2001 [[24]] |
AD 101 |
Cohort of 227 patients |
AD: CERAD, plaque - Braunmuhl stain, tangle - modified Palmgren |
50 |
AD: NINCDS/ADRDA (1984) |
| DLB 64 |
Institute of the Health of the Elderly (IHE), Newcastle, UK |
DLB: consensus criteria (1996) |
|||
| VaD 38 |
LB: consensus criteria (1996), ubiquitin, anti-tau2, anti-Alz50, anti-AT8 to detect and distinguish cortical LB |
||||
|
Helmes
et al
., 2003 [[25]] |
AD 15 |
University of Western Ontario Dementia Study, Canada |
No criteria are referred to. Only referred to LB staining methods (Bielschovsky, anti-ubiquitin, anti-synuclein). |
All |
Not specified. |
| AD/DLB 8 | |||||
| DLB 7 | |||||
|
Johnson
et al
., 2005 [[26]] |
AD 66 |
Washington University, from 1979, USA |
AD: NIA-RI quantification of diffuse and neuritic depositions in 10 cortical regions |
All |
AD: NINCDS/ADRDA (1984) |
| AD/DLB 57 |
DLB: consensus criteria (1996) or McKeith, operational criteria for senile dementia of Lewy body type (1992) |
||||
| DLB 9 |
LB: synuclein |
||||
|
Kraybill
et al
., 2005 [[15]] |
AD 48 |
Cohort from University of Washington/Group Health Cooperative Alzheimer’s Disease Patient Registry, USA |
AD: CERAD, Braak stages > IV |
All |
AD: NINCDS/ADRDA (1984) |
| AD/LBP 65 |
LB/AD: AD + synuclein (amygdala, SN) |
DLB: missing criteria because study was started before the consensus criteria for DLB was established. |
|||
| LBP 22 | |||||
| LB: Braak stages < III, synuclein (amygdala, SN) | |||||
|
Stavitsky
et a
l., 2006 [[19]] |
AD 55 |
Cohort of the Predictors Study, 1997: |
AD: CERAD |
12 |
AD: NINCDS/ADRDA (1984) |
| DLB 28 |
LB: semi quantitative ubiquitin (hippocampus, cingulate gyrus, insula cortex) |
DLB: consensus criteria (1996) |
|||
| Columbia University | |||||
| Johns Hopkins University, | |||||
| Massachusetts General Hospital, USA | |||||
|
Williams
et al
., 2006 [[27]] |
AD 252 |
Cohort from Washington University, USA |
AD: NIA-RI quantification of diffuse and neuritic depositions in 10 cortical regions |
All |
AD: NINCDS/ADRDA (1984) |
| DLB 63 |
DLB: consensus criteria (1996) |
||||
| LB: synuclein | |||||
|
Hamilton
et al
., 2008 [[28]] |
AD 44 |
University of California, Alzheimer’s disease center San Diego, 1985 to 2002, USA |
AD: modified Braak staging, NIA-RI (1997) and CERAD (1991) |
All |
AD: NIA-RI and CERAD (1988) |
| DLB 22 |
DLB: consensus criteria (1996) |
||||
| LB: H + E, ubiquitin (1996) synuclein (2005) | |||||
|
Hanyu
et al
., 2009 [[29]] |
AD 111 |
Memory Clinic of Tokyo Medical University, 2000 to 2006, Japan |
|
0 |
AD: NINCDS/ADRDA (1984) |
| DLB 56 |
DLB: consensus criteria (1996) |
||||
|
Nelson
et al
., 2009 [[16]] |
AD 107 |
National Alzheimer’s Coordinating Center (NACC) Registry - 31 AD centers in USA, |
AD: NIA-RI |
All |
AD: CERAD (1988) |
| AD/DLB 27 |
University of Kentucky Alzheimer’s Disease Center, USA |
LB: Braak staging and CERAD |
DLB: consensus criteria (1996) |
||
| DLB 9 | |||||
|
Wood
et a
l., 2012 [[30]] |
AD 16 |
Newcastle University, UK |
|
0 |
AD: NINCDS/ADRDA (1984) |
| DLB 12 |
DLB: consensus criteria (2005) or (1996) |
||||
| Walker et al . 2012 [[31]] | AD 100 |
40 European sites | 123I-FTP-SPECT as verifying method | 0 | AD: NINCDS/ADRDA (1984) |
| DLB 58 | DLB: consensus criteria (1996) |
AD, Alzheimer’s disease; ADRC, Alzheimer’s Disease Research Center; CERAD, The Consortium to Establish a Registry for Alzheimer's Disease; CFV, creasyl fast violet; DLB, dementia with Lewy bodies; H + E, hematoxylin and eosin staining; I-FTP-SPECT, ioflupane single-photon emission computed tomography; LB Lewy body; LBV, Lewy body variant; LBP, Lewy body pathology; NIA-RI, National Institute on Aging-Reagan; NINCDS/ADRDA, National Institute of Neurological and Communication Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association; SDLT, senile dementia of Lewy body type; SN, substantia nigra.
4 Discussion
In the 18 studies included in this review, no consistent faster rate of decline in DLB as compared to AD on cognitive screening tests was found. When combining studies that used MMSE, the most frequently used scale, a meta-analysis revealed no difference in the annual rate of cognitive decline. There were mixed findings on decline in specific cognitive domains. Two of six studies of memory found a more rapid decline in AD. Only one of seven studies of executive function found a more rapid decline in DLB, and differences in visuospatial or language tests were not found. The hypothesis of a more rapid cognitive decline in autopsied patients with both AD and DLB pathology was supported in three studies. However, findings were inconsistent and other studies did not find differences.
Differences in methods such as selection criteria, design, neuropsychological tests, dementia severity, diagnostic procedures and criteria can explain the diverse findings and lack of firm conclusions. However, quality assessment did not reveal any systematic differences between studies with high or low quality scores. There were large differences in sample sizes (n = 28 to 315), and the studies that could not be included in the meta-analysis or used other tests than MMSE, thus, may have had varying statistical power to detect significant differences between groups. To be able to compare the overall results and draw some general conclusions it would have been ideal that uniform diagnostic criteria had been used in all the studies. Some of the studies initially included patients with a clinical diagnosis of AD only, where analyses were based on autopsy diagnosis which included both AD and DLB.
A common weakness in the included studies was the choice of neuropsychological measures. When studying cognitive decline over time, cognitive tests that are designed for a specific cognitive domain are required. Screening tests or batteries that use a total score only, often designed for purposes other than research are less suitable. In this review, the MMSE was the most used test, either alone, or in combination with others. The MMSE may not be an optimal measure, especially when using only the total score and not separate subscores for different cognitive domains, as AD and DLB have different cognitive profiles at onset [32]. This difference in cognitive profile leads to difficulties in choosing an optimal cognitive screening instrument to compare AD and DLB. The MMSE is heavily based on memory and language and is thus more sensitive to changes in AD than in DLB [33]. DLB is associated with a more severe visuospatial deficit than AD [32],[34], but only 1 of 30 points on the MMSE comes from a measure of visuospatial functioning. MMSE may also be less than optimal because of the ceiling and floor effect [35], which refers to a test being too easy or too difficult to discriminate below or above a certain point, which is a common problem when testing people with dementia. In one of the reviewed studies the children’s version of the Wechsler intelligence scale was used to avoid this. The test then lacks age adjusted norms, but it gains a wider range in scores, and therefore can monitor the cognitive decline over a longer period of time. Studies differed also with regard to the time period of observation, from 1 to 20 years. In studies with short follow-up periods, the MMSE may not be a reliable measure, as Clark, Sheppard, Fillenbaum et al. (1999) [36] have argued that MMSE registrations need to be separated by at least three years in order to be a reliable measure of cognitive decline in AD.
Only few studies investigated, or reported, subgroups with different cognitive profiles in DLB. It could be due to a low number of cases in several studies, and subsequent low statistical power. People die from dementia or reach an endpoint where they are not capable of performing cognitive tests, and therefore in several studies there was a lower number of patients towards the end of the study. This is challenging when performing statistical analysis. Our search did not cover the issue of subgroups with different cognitive profiles thoroughly, as we only included studies comparing DLB with AD, and not studies describing cognitive decline in DLB and potential subgroups alone. However, there are some data that support the hypothesis that there are subgroups in DLB with different cognitive profiles, and subgroups with poor initial visuospatial function may have a more rapid decline than DLB with good visuospatial function [28].
Due to overlapping symptoms, it can be difficult to determine the correct diagnosis ante mortem between the pure form of AD, mixed AD/DLB and the pure form of DLB. Because clinical criteria cannot distinguish with certainty the individual pathology, the gold standard for validating the clinical assessment is neuropathological diagnosis. Clinical criteria may have a low sensitivity in particular for DLB, which could have been a source of bias in studies that did not include a neuropathological validation of the diagnosis. However, dementia is a clinical diagnosis and both AD and DLB pathology can be found also in cognitively normal elderly subjects. In one study with autopsy, 50% of cases with widespread α-synucleinopathy did not show any clinical signs of dementia [37].
In most studies with autopsy, consensus neuropathological criteria were used. Even though not all included studies used consistent and the same neuropathological methods and criteria, and many also used varying combinations, use of post-mortem verification at least increases the validity of the clinical diagnosis.
It is also important to mention that the sensitivity for detecting Lewy bodies has increased with anti-ubiquitin immunostaining, where tau-positive samples indicate Alzheimer’s pathology. Anti-α-synuclein immunostaining has been incorporated in the assessment, which is most sensitive for Lewy body pathology [2]. Thus, the neuropathological identification of cases may have been less accurate before the new methods were established, and more reliable staging strategies have been developed [38].
A complicating issue is the frequent occurrence of mixed pathology [39], and to underline the complexity of dementia and its pathology, at least four distinct pathological phenotypes have been identified between AD and DLB [40]. According to Schneider et al. (2012) [7], the locus of neuropathology is associated with a faster decline in cognition. A neocortical type of Lewy body pathology is associated with increased odds of dementia and a faster decline in episodic, semantic and working memory. The limbic-type is more associated with more rapid decline in visuospatial function. Olichney et al. (1998) [3], concluded that patients with Lewy body variant decline faster than patients with Alzheimer’s disease. This statement has often been used with reference to rapid progression in DLB, but it actually refers to an AD variant with Lewy body pathology, not to pure DLB. It should be emphasized that it is still uncertain whether AD and DLB are two independent pathologies that may coexist, or the pathologies are related, or one of them is a consequence of the other.
5 Conclusion
Only 6 of the 18 included studies in this review found some differences in cognitive decline between DLB and AD over time, and only one of them found a faster decline in DLB. It is difficult to draw firm conclusions based on available studies, since the results are contradictory. Future studies will need to apply recent diagnostic criteria, as well as extensive diagnostic evaluation and autopsy to confirm the diagnosis. Studies with large enough samples, adapted cognitive tests, more than one year of follow up and multivariate statistical analysis are also needed. Inclusion of mild cognitive impairment patients, with subclinical manifestations and an increased risk of developing DLB (for example, who present rapid eye-movement (REM) sleep behavior disorder) could also strengthen the studies. Our final conclusion is that the studies in this review support neither the hypothesis of a faster cognitive decline in DLB, nor in AD.
Abbreviations
AD: Alzheimer’s disease
ADL: activities of daily living
CAMCOG: Cambride cognitive examination
CDT: clock drawing test
CERAD: Consortium to Establish a Registry for Alzheimer’s Disease evaluation
DLB: dementia with Lewy bodies
DRS: dementia rating scale
HVLT-R: Hopkins verbal learning test-revised
MMSE: mini mental state examination
NEVIP: Newcastle visual perception battery
NINCDS/ADRDA: National Institute of Neurological and Communication Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association
SPECT: ioflupane single-photon emission computed tomotgraphy
WISC-R: Wechsler intelligence scale for children-revised
Competing interests
Dag Aarsland has received research support and honoraria from H Lundbeck, Novartis Pharmaceuticals and GE Health. None of the other authors have competing interests.
Authors’ contributions
MHB and LJC have made the conception and design, data acquisition, analysis and interpretation of data, and drafted the manuscript. AR, MJH, CJ, KB and DA have contributed to the analysis and interpretation of data, and revised the manuscript critically for important intellectual content. KB also performed the meta-analysis. All authors have read and approved the final version of the manuscript.
Contributor Information
Monica H Breitve, Email: monica.breitve@helse-fonna.no.
Luiza J Chwiszczuk, Email: luiza.chwiszczuk@helse-fonna.no.
Minna J Hynninen, Email: minna.hynninen@psykp.uib.no.
Arvid Rongve, Email: arvid.rongve@helse-fonna.no.
Kolbjørn Brønnick, Email: bronnick@gmail.com.
Carmen Janvin, Email: carmen.janvin@gmail.com.
Dag Aarsland, Email: daarsland@gmail.com.
Acknowledgement
We want to thank the librarian in Helse Fonna, Tonje Velde, for helping us with the systematic literature search.
References
- Aarsland D, Rongve A, Piepenstock Nore S, Skogseth R, Skulstad S, Ehrt U, Hoprekstad D, Ballard C. Frequency and Case Identification of Dementia with Lewy Bodies Using the Revised Consensus Criteria. Dement Geriatr Cogn Disord. 2008;26:445–452. doi: 10.1159/000165917. [DOI] [PubMed] [Google Scholar]
- McKeith IGI, Dickson DWD, Lowe JJ, Emre MM, O. apos Brien JTJ, Feldman HH, Cummings JJ, Duda JEJ, Lippa CC, Perry EKE, Aarsland DD, Arai HH, Ballard CGC, Boeve BB, Burn DJD, Costa DD, Del Ser T, Dubois BB, Galasko DD, Gauthier SS, Goetz CGC, Gomez-Tortosa EE, Halliday GG, Hansen LAL, Hardy JJ, Iwatsubo TT, Kalaria RNR, Kaufer DD, Kenny RAR, Korczyn AA. et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Galasko D, Salmon DP, Hofstetter CR, Hansen LA, Katzman R, Thal LJ. Cognitive decline is faster in Lewy body variant than in Alzheimer’s disease. Neurology. 1998;51:351–357. doi: 10.1212/WNL.51.2.351. [DOI] [PubMed] [Google Scholar]
- Oesterhus R, Soennesyn H, Rongve A, Ballard C, Aarsland D, Vossius C. Long-term mortality in a cohort of home-dwelling elderly with mild Alzheimer’s disease and Lewy body dementia. Dement Geriatr Cogn Disord. 2014;8:161–169. doi: 10.1159/000358051. [DOI] [PubMed] [Google Scholar]
- Rongve A, Vossius C, Nore S, Testad I, Aarsland D. Time until nursing home admission in people with mild dementia: comparison of dementia with Lewy bodies and Alzheimer’s dementia. Int J Geriat Psychiatry. 2014;29:392–398. doi: 10.1002/gps.4015. [DOI] [PubMed] [Google Scholar]
- Vossius C, Rongve A, Testad I, Wimo A, Aarsland D. The use and costs of formal care in newly diagnosed dementia: a three-year prospective follow-up study. Am J Geriatr Psychiatry. 2014;22:381–388. doi: 10.1016/j.jagp.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain. 2012;135:3005–3014. doi: 10.1093/brain/aws234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa CF, Duda JE, Grossman M, Hurtig HI, Aarsland D, Boeve BF, Brooks DJ, Dickson DW, Dubois B, Emre M, Fahn S, Farmer JM, Galasko D, Galvin JE, Goetz CG, Growdon JH, Gwinn-Hardy KA, Hardy J, Heutink P, Iwatsubo T, Kosaka K, Lee VM-Y, Leverenz JB, Masliah E, McKeith IG, Nussbaum RL, Olanow CW, Ravina BM, Singleton AB, Tanner CM. et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68:812–819. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord. 2006;21:1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Foltynie T, Brayne CEG, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130:1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and α-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30:7281–7289. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord. 2005;20:1255–1263. doi: 10.1002/mds.20527. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Wiley, New Jersey; 2011. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraybill ML, Larson EB, Tsuang DW, Teri L, McCormick WC, Bowen JD, Kukull WA, Leverenz JB, Cherrier MM. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64:2069–2073. doi: 10.1212/01.WNL.0000165987.89198.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Kryscio RJ, Jicha GA, Abner EL, Schmitt FA, Xu LO, Cooper G, Smith CD, Markesbery WR. Relative preservation of MMSE scores in autopsy-proven dementia with Lewy bodies. Neurology. 2009;73:1127–1133. doi: 10.1212/WNL.0b013e3181bacf9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Patel A, Oyebode F, Wilcock G. Cognitive decline in patients with Alzheimer’s disease, vascular dementia and senile dementia of Lewy body type. Age Ageing. 1996;25:209–213. doi: 10.1093/ageing/25.3.209. [DOI] [PubMed] [Google Scholar]
- Heyman A, Fillenbaum GG, Gearing M, Mirra SS, Welsh-Bohmer KA, Peterson B, Pieper C. Comparison of Lewy body variant of Alzheimer’s disease with pure Alzheimer’s disease: Consortium to Establish a Registry for Alzheimer’s Disease, Part XIX. Neurology. 1999;52:1839–1844. doi: 10.1212/WNL.52.9.1839. [DOI] [PubMed] [Google Scholar]
- Stavitsky K, Brickman AM, Scarmeas N, Torgan RL, Tang M-X, Albert M, Brandt J, Blacker D, Stern Y. The progression of cognition, psychiatric symptoms, and functional abilities in dementia with Lewy bodies and Alzheimer disease. Arch Neurol. 2006;63:1450–1456. doi: 10.1001/archneur.63.10.1450. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Perry RH, Fairbarin AF, Jabeen S, Perry EK. Operational criteria for senile dementia for Lewy body type (SDLT) Psychol Med. 1992;22:911–922. doi: 10.1017/S0033291700038484. [DOI] [PubMed] [Google Scholar]
- Ballard CG, O’Brien J, Lowery K, Ayre GA, Harrison R, Perry R, Ince P, Neill D, McKeith IG. A prospective study of dementia with Lewy Bodies. Age Ageing. 1998;27:631–636. doi: 10.1093/ageing/27.5.631. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Wisniewski S, Hamilton RL, Becker JT, Kaufer DI, DeKosky ST. Predictors of progression in patients with AD and Lewy bodies. Neurology. 2000;54:1774–1779. doi: 10.1212/WNL.54.9.1774. [DOI] [PubMed] [Google Scholar]
- Stern Y, Jacobs D, Goldman J, Gomez-Tortosa E, Hyman BT, Liu Y, Troncoso J, Marder K, Tang MX, Brandt J, Albert M. An investigation of clinical correlates of Lewy bodies in autopsy-proven Alzheimer disease. Arch Neurol. 2001;58:460–465. doi: 10.1001/archneur.58.3.460. [DOI] [PubMed] [Google Scholar]
- Ballard C, O’Brien J, Morris CM, Barber R, Swann A, Neill D, McKeith I. The progression of cognitive impairment in dementia with Lewy bodies, vascular dementia and Alzheimer’s disease. Int J Geriatri Psychiatry. 2001;16:499–503. doi: 10.1002/gps.381. [DOI] [PubMed] [Google Scholar]
- Helmes E, Bowler J, Merskey H, Munoz DG, Hachinski VC. Rates of cognitive decline in Alzheimer’s disease and dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2003;15:67–71. doi: 10.1159/000067969. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Morris JC, Galvin JE. Verbal and visuospatial deficits in dementia with Lewy bodies. Neurology. 2005;65:1232–1238. doi: 10.1212/01.wnl.0000180964.60708.c2. [DOI] [PubMed] [Google Scholar]
- Williams MM, Xiong C, Morris JC, Galvin JE. Survival and mortality differences between dementia with Lewy bodies vs Alzheimer disease. Neurology. 2006;67:1935–1941. doi: 10.1212/01.wnl.0000247041.63081.98. [DOI] [PubMed] [Google Scholar]
- Hamilton JM, Salmon DP, Galasko D, Raman R, Emond J, Hansen LA, Masliah E, Thal LJ. Visuospatial deficits predict rate of cognitive decline in autopsy-verified dementia with Lewy bodies. Neuropsychology. 2008;22:729–737. doi: 10.1037/a0012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyu H, Sato T, Hiraro K, Kanetaka H, Sakurai H, Iwamoto T. Differences in clinical course between dementia with Lewy bodies and Alzheimer’s disease. Eur J Neurol. 2009;16:212–217. doi: 10.1111/j.1468-1331.2008.02388.x. [DOI] [PubMed] [Google Scholar]
- Wood JS, Watson R, Firbank MJ, Mosimann UP, Barber R, Blamire AM, O’Brien JT. Longitudinal testing of visual perception in dementia with Lewy bodies and Alzheimer’s disease. Int J Geriat Psychiatry. 2013;28:567–572. doi: 10.1002/gps.3860. [DOI] [PubMed] [Google Scholar]
- Walker Z, McKeith I, Rodda J, Qassem T, Tatsch K, Booij J, Darcourt J, O’Brien J. Comparison of cognitive decline between dementia with Lewy bodies and Alzheimer’s disease: a cohort study. BMJ Open. 2012;2:e000380. doi: 10.1136/bmjopen-2011-000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C. A review of cognitive impairments in dementia with Lewy bodies relative to Alzheimer’s disease and Parkinson’s disease with dementia. Cortex. 2007;43:583–600. doi: 10.1016/S0010-9452(08)70489-1. [DOI] [PubMed] [Google Scholar]
- Brown J, Pengas G, Dawson K, Brown LA, Clatworthy P. Self administered cognitive screening test (TYM) for detection of Alzheimer’s disease: cross sectional study. BMJ. 2009;338:b2030. doi: 10.1136/bmj.b2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa H, Vonsattel JPG, Honig LS. Early neuropsychological discriminants for Lewy body disease: an autopsy series. J Neurol Neurosurg Psychiatr. 2013;84:1326–1330. doi: 10.1136/jnnp-2012-304381. [DOI] [PubMed] [Google Scholar]
- Galasko DR, Gould RL, Abramson IS, Salmon DP. Measuring cognitive change in a cohort of patients with Alzheimer’s disease. Stat Med. 2000;19:1421–1432. doi: 10.1002/(SICI)1097-0258(20000615/30)19:11/12<1421::AID-SIM434>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Clark CM, Sheppard L, Fillenbaum GG, Galasko D, Morris JC, Koss E, Mohs R, Heyman A. Variability in annual Mini-Mental State Examination score in patients with probable Alzheimer disease: a clinical perspective of data from the Consortium to Establish a Registry for Alzheimer’s Disease. Arch Neurol. 1999;56:857–862. doi: 10.1001/archneur.56.7.857. [DOI] [PubMed] [Google Scholar]
- Parkkinen L, Pirttilä T, Alafuzoff I. Applicability of current staging/categorization of α-synuclein pathology and their clinical relevance. Acta Neuropathol. 2008;115:399–407. doi: 10.1007/s00401-008-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alafuzoff I, Ince PG, Arzberger T, Al-Sarraj S, Bell J, Bodi I, Bogdanovic N, Bugiani O, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Ironside JW, Kavantzas N, King A, Korkolopoulou P, Kovács GG, Meyronet D, Monoranu C, Parchi P, Parkkinen L, Patsouris E, Roggendorf W, Rozemuller A, Stadelmann-Nessler C, Streichenberger N, Thal DR, Kretzschmar H. Staging/typing of Lewy body related α-synuclein pathology: a study of the BrainNet Europe Consortium. Acta Neuropathol. 2009;117:635–652. doi: 10.1007/s00401-009-0523-2. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- McCann H, Stevens CH, Cartwright H, Halliday GM. α-Synucleinopathy phenotypes. Parkinsonism Relat Disord. 2014;20:62–67. doi: 10.1016/S1353-8020(13)70017-8. [DOI] [PubMed] [Google Scholar]


