Abstract
Species of Pyricularia (magnaporthe-like sexual morphs) are responsible for major diseases on grasses. Pyricularia oryzae (sexual morph Magnaporthe oryzae) is responsible for the major disease of rice called rice blast disease, and foliar diseases of wheat and millet, while Pyricularia grisea (sexual morph Magnaporthe grisea) is responsible for foliar diseases of Digitaria. Magnaporthe salvinii, M. poae and M. rhizophila produce asexual spores that differ from those of Pyricularia sensu stricto that has pyriform, 2-septate conidia produced on conidiophores with sympodial proliferation. Magnaporthe salvinii was recently allocated to Nakataea, while M. poae and M. rhizophila were placed in Magnaporthiopsis. To clarify the taxonomic relationships among species that are magnaporthe- or pyricularia-like in morphology, we analysed phylogenetic relationships among isolates representing a wide range of host plants by using partial DNA sequences of multiple genes such as LSU, ITS, RPB1, actin and calmodulin. Species of Pyricularia s. str. belong to a monophyletic clade that includes all P. oryzae/P. grisea isolates tested, defining the Pyriculariaceae, which is sister to the Ophioceraceae, representing two novel families. These clades are clearly distinct from species belonging to the Gaeumannomyces pro parte/Magnaporthiopsis/Nakataea generic complex that are monophyletic and define the Magnaporthaceae. A few magnaporthe- and pyricularia-like species are unrelated to Magnaporthaceae and Pyriculariaceae. Pyricularia oryzae/P. grisea isolates cluster into two related clades. Host plants such as Eleusine, Oryza, Setaria or Triticum were exclusively infected by isolates from P. oryzae, while some host plant such as Cenchrus, Echinochloa, Lolium, Pennisetum or Zingiber were infected by different Pyricularia species. This demonstrates that host range cannot be used as taxonomic criterion without extensive pathotyping. Our results also show that the typical pyriform, 2-septate conidium morphology of P. grisea/P. oryzae is restricted to Pyricularia and Neopyricularia, while most other genera have obclavate to more ellipsoid 2-septate conidia. Some related genera (Deightoniella, Macgarvieomyces) have evolved 1-septate conidia. Therefore, conidium morphology cannot be used as taxonomic criterion at generic level without phylogenetic data. We also identified 10 novel genera, and seven novel species. A re-evaluation of generic and species concepts within Pyriculariaceae is presented, and novelties are proposed based on morphological and phylogenetic data.
Key words: Magnaporthaceae, Magnaporthe, Pyricularia, Pyriculariaceae, Phylogeny, Systematics
Introduction
The Magnaporthaceae contains several genera that are important plant pathogens of Poaceae, most notably Magnaporthe (now Nakataea sensu Luo & Zhang 2013), Pyricularia, and Gaeumannomyces. The family was originally described with six genera and 20 species, and presently includes 13 genera and more than 100 species (Cannon 1994, Bussaban et al. 2005, Thongkantha et al. 2009, Zhang et al. 2011). The family also includes genera (Ophioceras, Pseudohalonectria, Ceratosphaeria) that occur in aquatic habitats, or on dead plant materials such as wood (Shearer et al. 1999, Réblová 2006, Huhndorf et al. 2008, Thongkantha et al. 2009). The Magnaporthaceae is currently defined by having perithecial ascomata immersed in host tissue, frequently with long necks, and cylindrical asci that stain positive in Meltzer's reagent. Ascospores are highly variable in their morphology. Genera with filiform ascospores (Gaeumannomyces) tend to have simple, pigmented conidiophores with flared collarettes, and curved, aseptate conidia (harpophora-like). Genera with fusiform ascospores tend to have pigmented median cells (Nakataea = Magnaporthe), simple, pigmented conidiophores, or septate, pyriform to obclavate, pigmented conidia (Pyricularia and related genera).
The present study does not aim to revise all genera in Magnaporthales (Hernandez-Restrepo et al. unpubl data), but focuses primarily on species that are pyricularia-like in morphology. The genus Pyricularia (in reference to the pyriform shape of its conidia; Bussaban et al. 2005, Murata et al. 2014) includes species that are pathogenic on a wide range of monocot plants. Of these, Pyricularia oryzae (sexual morph Magnaporthe oryzae), the causal agent of the rice blast disease of rice, is one of the most widely distributed diseases of this crop, and is highly destructive leading to up to 30 % yield loss worldwide (Skamnioti & Gurr 2009). Pyricularia oryzae isolates from rice are mostly host-specific and only infect few host plants beside rice (barley and Lolium) (Ou 1985, Kato et al. 2000, Couch et al. 2005, Tosa & Chuma 2014). Pyricularia oryzae isolates from other host plants such as Eleusine, Setaria and Triticum are also host-specific, and unable to infect rice (Kato et al. 2000, Couch et al. 2005, Murata et al. 2014, Tosa & Chuma 2014). A close relative species of P. oryzae is Pyricularia grisea, which is indistinguishable in conidium, perithecium and ascopore morphology. Pyricularia grisea isolates from Digitaria were shown to form a distinct clade by phylogenetic analysis (Kato et al. 2000, Couch & Kohn 2002, Hirata et al. 2007, Faivre-Rampant et al. 2008, Choi et al. 2013) and infect crabgrass (Digitaria), but not other hosts (Mackill & Bonham 1986, Kato et al. 2000, Tsurushima et al. 2005, Chen et al. 2006, Faivre-Rampant et al. 2008, Choi et al. 2013). However, some P. oryzae isolates from rice and other grasses and some P. grisea isolates from crabgrass showing cross-infectivity on crabgrass and rice, respectively have been described (Choi et al. 2013). Sexual morphs were reported for P. grisea and P. oryzae. However, the genus Pyricularia comprises several other species for which the sexual morph has not yet been discovered. Such Pyricularia species include P. higginsii pathogenic on Cyperus (Luttrell 1954, Hashioka 1973), P. zingiberi pathogenic on Zingiber (Kotani & Kurata 1992), P. zizaniaecola pathogenic on Zizania (Hashioka 1973) and P. commelinicola on Commelina (Park & Shin 2009). Other notable pathogens from the Magnaporthaceae include Nakataea oryzae, Gaeumannomyces graminis, Magnaporthiopsis poae and M. rhizophila.
The aims of the present study were to determine the phylogenetic relationships among species of Pyricularia compared to P. oryzae/P. grisea, as well as those taxa now accommodated in Magnaporthiopsis and Nakataea, using multilocus sequence analysis. This study allowed defining two novel families, Ophioceraceae and Pyriculariaceae, as well as novel genera and species.
Materials and methods
Isolates
A global collection of 153 isolates was included in this study (Table 1). Cultures for morphological observation were inoculated in a three-point position onto the following agar media: Cornmeal agar (CMA), oatmeal agar (OA), 2 % potato dextrose agar (PDA) and 2 % malt extract agar (Oxoid) (MEA). All media were prepared as described previously (Crous et al. 2009, Samson et al. 2010). Representative isolates were deposited in the CBS-KNAW Fungal Biodiversity Centre (CBS), Utrecht, The Netherlands.
Table 1.
Collection details and GenBank accession numbers of isolates included in this study (“–” = unknown).
| Species | Location | Substrate | Collector | Culture collection no1 | GenBank Accession no2 |
||||
|---|---|---|---|---|---|---|---|---|---|
| ITS | LSU | RPB1 | ACT | CAL | |||||
| Bambusicularia brunnea | Japan: Aichi | Sasa sp. | S. Koizumi | CBS 133599 = MAFF 240225 = INA-B-92-45(Ss-1J) (ex-type) | KM484830 | KM484948 | KM485043 | AB274449 | AB274482 |
| Japan: Aichi | Phyllostachys bambusoides | S. Koizumi | CBS 133600 = MAFF 240226 = INA-B-93-19(Ph-1J) | AB274436 | KM484949 | KM485044 | AB274450 | AB274483 | |
| Barretomyces calatheae | Brazil: Minas Gerais | Calathea longifolia | D.J. Soares | CBMAI 1060 (ex-type) | GU294490 | – | – | – | – |
| Brazil: Minas Gerais | Calathea longifolia | P.W. Crous | CBS 129274 = CPC 18464 | KM484831 | KM484950 | KM485045 | KM485162 | KM485231 | |
| Buergenerula spartinae | USA | Spartina alterniflora, leaves | R.V. Gessner | ATCC 22848 | JX134666 | DQ341492 | JX134720 | – | – |
| Bussabanomyces longisporus | Thailand: Chiang Mai | Amomum siamense, leaf endophyte | B. Bussaban | CBS 125232 (ex-type) | KM484832 | KM484951 | KM485046 | – | – |
| Cryphonectria parasitica | USA: Connecticut | Castanea dentata | N. DePalma | EP155 = ATCC 38755 | Genome | Genome | Genome | Genome | Genome |
| Deightoniella roumeguerei | Netherlands: Utrecht | Phragmites australis, leaves | W. Quaedvlieg | CBS 128780 = CPC 18916 (ex-type) | JF951153 | JF951176 | KM485047 | KM485163 | KM485232 |
| Gaeumannomyces graminis var. avenae | Netherlands: Flevoland | Avena sativa, root | – | CBS 187.65 | JX134668 | JX134680 | JX134722 | – | – |
| Australia: Western Australia | Avena sativa | – | CBS 870.73 = DAR 20999 | KM484833 | DQ341495 | KM485048 | – | – | |
| Gaeumannomyces graminis var. graminis | USA: Arkansas | Oryza sativa | – | CBS 235.32 | JX134669 | JX134681 | KM485049 | – | – |
| Netherlands: near Barendrecht | Ctenanthe sp., stem base | – | CBS 352.93 = PD 93/290 | KM484834 | DQ341496 | KM485050 | – | – | |
| UK: England | Deschampsia caespitosa, dead culm and sheath | M.B. & J.P. Ellis | CBS 387.81 | KM484835 | KM484952 | KM485051 | – | – | |
| Australia: New South Wales | Stenotaphrum secundatum | J. Kuiper | CBS 902.73 = DAR 17502 | KM484836 | KM484953 | KM485052 | – | – | |
| Australia: New South Wales | Pennisetum clandestinum | P. Wong | CBS 903.73 = DAR 23471 | KM484837 | KM484954 | KM485053 | – | – | |
| USA: Florida | Stenotaphrum secundatum | – | M33 | JF710374 | JF414896 | JF710442 | – | – | |
| Gaeumannomyces graminis var. tritici | Netherlands: Flevoland | Hordeum vulgare | – | CBS 186.65 | KM484838 | KM484955 | KM485054 | KM485164 | – |
| Netherlands | – | – | CBS 247.29 | KM484839 | KM484956 | KM485055 | – | – | |
| – | Triticum aestivum | – | CBS 249.29 = IMI 083849 | KM484840 | KM484957 | KM485056 | – | – | |
| Australia: Western Australia | Triticum aestivum | A. Parker | CBS 905.73 = DAR 23140 | KM484841 | KM484958 | KM485057 | – | – | |
| USA: Montana | Triticum sp. | – | M55 | JF414850 | JF414900 | JF710445 | – | – | |
| USA: Washington | Triticum aestivum | – | R3-111a-1 | Genome | Genome | Genome | Genome | Genome | |
| Gaeumannomyces sp. | Netherlands: Groningen | Soil in potato field | – | CBS 117.83 | KM484842 | KM484959 | KM485058 | – | – |
| UK: Wales | Carex rostrata | M.B. Ellis | CBS 388.81 | KM484843 | KM484960 | KM485059 | – | – | |
| Harpophora radicicola | South Africa | Zea mays | – | CBS 149.85 = PREM 45754 (isotype of Phialophora zeicola) | KM484844 | KM484961 | KM485060 | KM485165 | KM485233 |
| Canada: Ontario | Zea mays, root | R.F. Cain | CBS 296.53 = MUCL 28970 = TRTC 23660 (isotype of Phialophora radicicola) | KM484845 | KM484962 | KM485061 | – | KM485234 | |
| South Africa | Zea mays, root | – | CPC 18682 = Z 383 Y | KM484846 | KM484963 | KM485062 | KM485166 | KM485235 | |
| South Africa | Zea mays, root | – | CPC 18683 = Z 390 G | KM484847 | KM484964 | KM485063 | KM485167 | KM485236 | |
| South Africa | Zea mays, root | – | CPC 18685 = Z 397 L | KM484848 | KM484965 | KM485064 | KM485168 | KM485237 | |
| South Africa | Zea mays, root | – | CPC 18689 = Z 426 AJ | KM484849 | KM484966 | KM485065 | KM485169 | KM485238 | |
| Harpophora sp. | UK: England | Zea mays, root | – | CBS 350.77 = ATCC 28234 = IMI 187786 | KM484850 | KM484967 | KM485066 | – | – |
| Germany | Triticum aestivum, seedling | – | CBS 541.86 | KM484851 | DQ341497 | KM485067 | – | – | |
| Kohlmeyeriopsis medullaris | USA: North Carolina | Juncus roemerianus | – | CBS 117849 = JK5528S | KM484852 | KM484968 | KM485068 | – | – |
| USA: North Carolina | Juncus roemerianus | – | CBS 118210 = JK5522N = ATCC MYA-3560 | KM484853 | KM484969 | KM485069 | – | – | |
| Macgarvieomyces borealis | UK: Scotland | Juncus effusus, leaf spots | G.D. MacGarvie | CBS 461.65 (ex-type) | KM484854 | DQ341511 | KM485070 | KM485170 | KM485239 |
| Macgarvieomyces juncicola | Netherlands | Juncus effusus, stem base | G.S. de Hoog | CBS 610.82 | KM484855 | KM484970 | KM485071 | KM485171 | KM485240 |
| Magnaporthe griffinii | Australia: Queensland | Cynodon dactylon × Cynodon transvaalensis | A.M. Stirling | TS99 | JQ390311 | – | – | – | – |
| Australia: South Australia | Cynodon dactylon × Cynodon transvaalensis | P. Toy | TY2 | JQ390312 | – | – | – | – | |
| Magnaporthiopsis incrustans | – | – | – | M35 | JF414843 | JF414892 | JF710437 | – | – |
| USA: Kansas | Zoysia matrella | – | M51 | JF414846 | JF414895 | JF710440 | – | – | |
| Magnaporthiopsis maydis | Egypt | Zea mays | H.A. Elshafey | CBS 662.82A | KM484856 | KM484971 | KM485072 | – | – |
| India: Rajasthan, Jaipur | Zea mays | B.S. Siradhana | CBS 663.82A | KM484857 | KM484972 | KM485073 | – | – | |
| India: Rajasthan, Jaipur | Zea mays | B.S. Siradhana | CBS 663.82B | KM484858 | KM484973 | KM485074 | – | – | |
| India: Bihar, Messina | Zea mays hybrid “Ganga Safed 2” | M.M. Payak | CBS 664.82 | KM484859 | KM484974 | KM485075 | – | – | |
| Magnaporthiopsis poae | USA | Triticum aestivum | P.J. Landschoot | ATCC 64411 | Genome | Genome | Genome | Genome | Genome |
| USA: New Jersey | Poa pratensis | – | M47 | JF414836 | JF414885 | JF710433 | – | – | |
| Magnaporthiopsis rhizophila | – | Poa pratensis | – | M23 | JF414834 | JF414846 | JF710432 | – | – |
| Nakataea oryzae | Japan | Oryza sativa | – | ATCC 44754 = M21 = Roku-2 | JF414838 | JF414887 | JF710441 | – | – |
| Italy | – | – | CBS 202.47 | KM484860 | KM484975 | KM485076 | – | – | |
| Italy | Oryza sativa | – | CBS 243.76 | KM484861 | DQ341498 | KM485077 | – | – | |
| Burma | Oryza sativa, straw | – | CBS 252.34 | KM484862 | KM484976 | KM485078 | – | – | |
| – | – | – | CBS 253.34 | KM484863 | KM484977 | KM485079 | – | – | |
| Japan: Takada | Oryza sativa, stem | – | CBS 288.52 | KM484864 | KM484978 | KM485080 | – | – | |
| USA: California | Oryza sativa | R.K. Webster | CBS 726.74 | KM484865 | KM484979 | KM485081 | – | – | |
| USA: California | Oryza sativa | R.K. Webster | CBS 727.74 | KM484866 | KM484980 | KM485082 | – | – | |
| Nakataea sp. | USA: Arkansas | Oryza sativa | – | CBS 332.53 | KM484867 | KM484981 | KM485083 | – | – |
| Neopyricularia commelinicola | South Korea: Hongcheon | Commelina communis, leaves | H.D. Shin & M.J. Park | CBS 128303 = KACC 44637 | KM484868 | KM484982 | KM485084 | KM485172 | KM485241 |
| South Korea: Pocheon | Commelina communis | M.J. Park | CBS 128306 = KACC 43869 | FJ850123 | KM484983 | KM485085 | KM485173 | KM485242 | |
| South Korea: Hongcheon | Commelina communis | H.D. Shin & M.J. Park | CBS 128307 = KACC 44083 | FJ850125 | KM484984 | KM485086 | KM485174 | KM485243 | |
| South Korea: Hongcheon | Commelina communis, leaves | H.D. Shin & M.J. Park | CBS 128308 = KACC 43081 (ex-type) | FJ850122 | KM484985 | KM485087 | KM485175 | – | |
| Omnidemptus affinis | Australia: Queensland | Panicum effusum var. effusum, grass leaves | V.P. Cooper | ATCC 200212 (ex-type) | JX134674 | JX134686 | JX134728 | – | – |
| Ophioceras commune | China: Yunnan | Rotten wood | – | M91 | JX134675 | JX134687 | JX134729 | – | – |
| China: Yunnan | Rotten wood | – | M92 | JX134676 | JX134688 | JX134730 | – | – | |
| Ophioceras dolichostomum | Hong Kong | Wood | – | CBS 114926 = HKUCC 3936 = KM 8 | JX134677 | JX134689 | JX134731 | – | – |
| Ophioceras leptosporum | UK: England | Dead stem of dicot plant (probably Urtica dioica) | – | CBS 894.70 = ATCC 24161 = HME 2955 (ex-type of Gaeumannomyces leptosporus) | JX134678 | JX134690 | JX134732 | – | – |
| Proxipyricularia zingiberis | Japan: Hyogo | Zingiber mioga | I. Chuma | CBS 132195 = MAFF 240224 = HYZiM201-1-1-1(Z-4J) | KM484869 | KM484986 | KM485088 | AB274448 | KM485244 |
| Japan: Hyogo | Zingiber mioga | I. Chuma | CBS 132196 = MAFF 240223 = HYZiM202-1-2(Z-3J) | KM484870 | – | KM485089 | AB274447 | KM485245 | |
| Proxipyricularia zingiberis | Japan: Hyogo | Zingiber mioga | M. Ogawa | CBS 132355 = MAFF 240221 = HYZiM101-1-1-1(Z-1J) | AB274433 | KM484987 | KM485090 | KM485176 | AB274481 |
| Japan: Hyogo | Zingiber mioga | H. Kato | CBS 133594 = MAFF 240222 = HYZiM201-0-1(Z-2J) | AB274434 | KM484988 | KM485091 | AB274446 | KM485246 | |
| Japan | Zingiber officinale | Y. Nisikado | CBS 303.39 = MUCL 9449 | KM484871 | KM484989 | KM485092 | KM485177 | KM485247 | |
| Pseudopyricularia cyperi | Japan: Hyogo | Cyperus iria | H. Kato | CBS 133595 = MAFF 240229 = HYCI201-1-1(Ci-1J) (ex-type) | KM484872 | KM484990 | AB818013 | AB274453 | AB274485 |
| Israel | Cyperus rotundus | R. Kenneth | CBS 665.79 | KM484873 | DQ341512 | KM485093 | KM485178 | KM485248 | |
| Philippines: Sto Tomas, Batangas | Cyperus rotundus | IRRI | PH0053 = Cr88383 | KM484874 | – | KM485094 | KM485179 | KM485249 | |
| Pseudopyricularia higginsii | New Zealand: Auckland, Mount Albert | Typha orientalis, dead leaves | C.F. Hill | CBS 121934 = 09/2007/1470 | KM484875 | KM484991 | KM485095 | KM485180 | KM485250 |
| Pseudopyricularia kyllingae | Japan: Hyogo | Kyllinga brevifolia | I. Chuma | CBS 133597 = MAFF 240227 = HYKB202-1-2(K-1J) (ex-type) | KM484876 | KM484992 | KM485096 | AB274451 | AB274484 |
| Philippines: Los Banos | Cyperus brevifolius | IRRI | PH0054 = Cb8959 | KM484877 | KM484993 | KM485097 | KM485181 | KM485251 | |
| Pyricularia ctenantheicola | Greece: Almyros, imported from Brazil via Netherlands | Ctenanthe oppenheimiana | A.C. Pappas & E.J. Paplomatas | GR0001 = Ct-4 = ATCC 200218 | KM484878 | KM484994 | KM485098 | KM485182 | KM485252 |
| Greece: Almyros, imported from Brazil via Netherlands | Ctenanthe oppenheimiana | A.C. Pappas & E.J. Paplomatas | GR0002 (ex-type) | KM484879 | – | KM485099 | KM485183 | KM485253 | |
| Pyricularia grisea | Brazil: Goias, Goiana | Digitaria sanguinalis | J.-L. Nottéghem | BR0029 | KM484880 | KM484995 | KM485100 | DQ240874 | DQ240890 |
| Brazil | Digitaria horizontalis | – | Br33 | AB274430 | KM484996 | – | – | KM485254 | |
| Korea: Woanju | Echinochloa crus-galli var. frumentacea | H.K. Sim | CBS 128304 = KACC 41641 | KM484881 | – | KM485101 | KM485184 | KM485255 | |
| South Korea: Suwon | Lolium perenne | C.K. Kim | CR0024 | KM484882 | KM484997 | KM485102 | KM485185 | KM485256 | |
| Japan | Digitaria smutsii | – | JP0034 = NI980 | KM484883 | – | KM485103 | KM485186 | KM485257 | |
| Philippines: Sto Tomas, Batangas | Digitaria ciliaris | IRRI | PH0055 = Dc88420 | KM484884 | – | KM485104 | DQ240877 | DQ240893 | |
| USA: Delaware | Digitaria sp. | B. Valent | US0043 = G184 | KM484885 | – | KM485105 | KM485187 | KM485258 | |
| Pyricularia oryzae | Burkina Faso | Paspalum sp. | J.-L. Nottéghem | BF0028 | KM484886 | KM484998 | KM485106 | KM485188 | KM485259 |
| Brazil | Triticum sp. | J.-L. Nottéghem | BR0032 | KM484887 | – | KM485107 | DQ240884 | DQ240900 | |
| Brazil | Triticum sp. | J.-L. Nottéghem | BR0045 | KM484888 | – | KM485108 | KM485189 | KM485260 | |
| Romania | – | – | CBS 255.38 | KM484889 | KM484999 | KM485109 | KM485190 | KM485261 | |
| Pyricularia oryzae | Japan: Nagano | – | – | CBS 365.52 = MUCL 9451 | KM484890 | KM485000 | KM485110 | KM485191 | KM485262 |
| – | – | – | CBS 375.54 | KM484891 | KM485001 | KM485111 | KM485192 | KM485263 | |
| – | Oryza sativa, seed | – | CBS 433.70 | KM484892 | KM485002 | KM485112 | KM485193 | KM485264 | |
| Egypt | Oryza sativa | – | CBS 657.66 | KM484893 | KM485003 | KM485113 | KM485194 | KM485265 | |
| Israel | Echinochloa crus-galli | – | CBS 658.66 | KM484894 | KM485004 | KM485114 | KM485195 | KM485266 | |
| Israel | Stenotaphrum secundatum | – | CBS 659.66 | KM484895 | KM485005 | KM485115 | KM485196 | KM485267 | |
| Côte d'Ivoire: Bouaké | Leersia hexandra | J.-L. Nottéghem | CD0067 | KM484896 | KM485006 | KM485116 | KM485197 | KM485268 | |
| Côte d'Ivoire: Ferkessédougou | Eleusine indica | J.-L. Nottéghem | CD0156 | KM484897 | KM485007 | KM485117 | KM485198 | KM485269 | |
| South Korea: Suwon | Phleum pratense | C.K. Kim | CR0020 | KM484898 | KM485008 | KM485118 | KM485199 | KM485270 | |
| South Korea: Yongin | Panicum miliaceum | C.K. Kim | CR0021 | KM484899 | – | KM485119 | KM485200 | KM485271 | |
| South Korea: Suwon | Lolium hybridum | C.K. Kim | CR0026 | KM484900 | KM485009 | KM485120 | KM485201 | KM485272 | |
| South Korea: Suwon | Festuca elalior | C.K. Kim | CR0029 | KM484901 | KM485010 | KM485121 | KM485202 | KM485273 | |
| France: Camargue | Oryza sativa | J.-L. Nottéghem | FR0013 | KM484902 | KM485011 | KM485122 | DQ240885 | DQ240901 | |
| Gabon: Wey | Zea mays | J.-L. Nottéghem | GN0001 | KM484903 | KM485012 | KM485123 | DQ240882 | DQ240898 | |
| French Guiana | Oryza sativa | J.-L. Nottéghem | Guy11 = FGSC 9462 | KM484904 | KM485013 | KM485124 | KC167438 | AF396024 | |
| India: Uttar Pradesh | Setaria sp. | J. Kumar | IN0108 = VII-765-1 | KM484905 | KM485014 | KM485125 | KM485203 | KM485274 | |
| Japan | Eleusine indica | H. Yaegashi | JP0017 = C10 | AF074404 | KM485015 | – | AF395970 | AF396018 | |
| Japan | Eragrostis curvula | H. Yaegashi | JP0028 = K76-79 | KM484906 | KM485016 | KM485126 | AF395961 | KM485275 | |
| Japan | Eriochloa villosa | – | JP0033 = NI859 | KM484907 | KM485017 | KM485127 | KM485204 | KM485276 | |
| Japan | Eragrostis curvula | H. Kato | JP0038 = IN909 | KM484908 | – | KM485128 | AF395964 | KM485277 | |
| Japan | Anthoxanthum odoratum | – | JP0039 = NI904 | KM484909 | KM485018 | KM485129 | KM485205 | KM485278 | |
| Japan | Phalaris arundinacea | – | JP0040 = NI901 | KM484910 | KM485019 | KM485130 | KM485206 | KM485279 | |
| Philippines | Oryza sativa | IRRI | PH0014 = PO6-6 | KM484911 | – | KM485131 | DQ240888 | DQ240904 | |
| Philippines: Los Banos | Brachiaria mutica | IRRI | PH0035 = Bm8309 = PH0075 | KM484912 | – | KM485132 | KM485207 | KM485280 | |
| Philippines: Cabanatuan | Cynodon dactylon | IRRI | PH0051 = Cd88215 | KM484913 | KM485020 | KM485133 | KM485208 | KM485281 | |
| Philippines: Los Banos | Leptochloa chimensis | IRRI | PH0060 = LcA8401 | KM484914 | – | – | KM485209 | KM485282 | |
| Philippines: Cabanatuan | Paspalum distichum | IRRI | PH0062 = Pd8824 | KM484915 | KM485021 | KM485134 | KM485210 | KM485283 | |
| Philippines: Los Banos | Rottboellia exalta | IRRI | PH0063 = ReA8401 = ATCC 62619 | KM484916 | KM485022 | KM485135 | KM485211 | KM485284 | |
| Philippines | Echinochloa colona | IRRI | PH0077 = Ec8202 | KM484918 | KM485024 | KM485137 | KM485213 | KM485286 | |
| Philippines | Panicum repens | J. M. Bonman | PH0079 = GPr8212 | KM484919 | KM485025 | KM485138 | KM485214 | KM485287 | |
| Pyricularia oryzae | Portugal | Stenotaphrum secondatum | A. Lima | PR0067 | KM484920 | KM485026 | KM485139 | KM485215 | KM485288 |
| Portugal | Stenotaphrum secondatum | A. Lima | PR0104 | KM484921 | KM485027 | KM485140 | KM485216 | KM485289 | |
| Rwanda: Kunynya | Eleusine coracana | J.-L. Nottéghem | RW0012 | KM484922 | – | KM485141 | AF395959 | AF396014 | |
| USA: Kentucky | Setaria viridis | M. Farman | US0071 | KM484923 | KM485028 | KM485142 | KM485217 | – | |
| Vietnam: O Mon | Leersia hexandra | B. Couch | VT0032 | KM484924 | KM485029 | KM485143 | KM485218 | KM485290 | |
| – | Laboratory strain | – | 70-15 = ATCC MYA-4617 = FGSC 8958 | Genome | Genome | Genome | Genome | Genome | |
| “Pyricularia parasitica” | USA: Iowa | Phyllachora graminis | – | CBS 376.54 = ICMP 14696 = MUCL 9450 = QM 1092 | AY265340 | KM485030 | – | – | – |
| Pyricularia penniseticola | Burkina Faso: Kamboinse | Pennisetum typhoides | J.-L. Nottéghem | BF0017 | KM484925 | KM485031 | KM485144 | DQ240878 | DQ240894 |
| Côte d'Ivoire: Bouake | Pennisetum typhoides | J.-L. Nottéghem | CD0086 | KM484926 | – | KM485145 | DQ240879 | DQ240895 | |
| Côte d'Ivoire: Odienne | Digitaria exilis | J.-L. Nottéghem | CD0143 | KM484927 | – | KM485146 | KM485219 | – | |
| Côte d'Ivoire: Madiani | Pennisetum sp. | J.-L. Nottéghem | CD0180 | KM484928 | – | KM485147 | DQ240880 | DQ240896 | |
| Mali: Longorola Sikasso | Pennisetum typhoides | J.-L. Nottéghem | ML0031 (ex-type) | KM484929 | – | KM485148 | KM485220 | – | |
| Mali | Digitaria exilis | J.-L. Nottéghem | ML0048 | KM484930 | – | KM485149 | KM485221 | – | |
| Pyricularia pennisetigena | Brazil: Imperatriz | Cenchrus echinatus | – | BR0067 | KM484931 | KM485032 | KM485150 | KM485222 | KM485291 |
| Brazil: Primeiro de Maio | Echinochloa colona | H. Kato | BR0093 | KM484932 | – | KM485151 | KM485223 | KM485292 | |
| Brazil | Cenchrus echinatus | S. Igarashi | Br36 | KM484933 | KM485033 | – | – | KM485293 | |
| Japan: Kumamoto | Cenchrus ciliaris | N. Nishihara | CBS 133596 = MAFF 305501 = NI981(Cc-1J) | KM484934 | KM485034 | KM485152 | KM485224 | AB274475 | |
| Mali: Cinzana | Pennisetum sp. | J.-L. Nottéghem | ML0036 (ex-type) | KM484935 | – | KM485153 | KM485225 | KM485294 | |
| Philippines: Plaridel | Cenchrus echinatus | IRRI | PH0047 = Ce88454 | KM484936 | – | KM485154 | KM485226 | KM485295 | |
| USA: Tifton | Pennisetum glaucum | H. Wells | US0044 = 83P-25 | KM484937 | – | – | KM485227 | KM485296 | |
| USA: Tifton | Pennisetum glaucum | H. Wells | US0045 = 84P-19 | KM484938 | – | KM485155 | KM485228 | – | |
| Pyricularia sp. | Brazil | Setaria geniculate | S. Igarashi | Br37 | KM484939 | KM485035 | – | – | AB274474 |
| Pyricularia sp. | Japan: Chiba | Leersia oryzoides | N. Nishihara | CBS 133598 = MAFF 305509 = NI919(Leo-1J) = JP0036 | KM484940 | KM485036 | KM485156 | AB274440 | AB274473 |
| Pyricularia variabilis | Thailand | Amomum siamense, healthy leaves | – | CMUZE0229 = ICMP 14487 | AY265333 | – | – | – | – |
| Pyricularia zingibericola | Réunion | Zingiber officinale | J.-C. Girard | RN0001 | KM484941 | KM485037 | KM485157 | KM485229 | KM485297 |
| Pyriculariopsis parasitica | Hong Kong: Discovery Bay | Musa sp., leaves | K.D. Hyde | CBS 114973 = HKUCC 5562 = Maew HK 1 | – | DQ341514 | – | – | – |
| Rhexodenticula cylindrospora | Cuba: Pinar del Rio | Nectandra antillana, leaf litter | R.F. Castañeda & M. Saikawa | CBS 244.95 = INIFAT C94/182 | KM484942 | KM485038 | – | – | – |
| Cuba: Pinar del Rio | Nectandra antillana, leaf litter | R.F. Castañeda | CBS 318.95 = INIFAT C94/182 (ex-type) | KM484943 | KM485039 | – | – | – | |
| Slopeiomyces cylindrosporus | UK: England | Grass roots; associated with Phialophora graminicola | D. Hornby | CBS 609.75 (ex-type) | KM484944 | KM485040 | KM485158 | – | – |
| UK: England | Grass roots; associated with Phialophora graminicola | D. Hornby | CBS 610.75 (ex-type) | JX134667 | DQ341494 | JX134721 | – | – | |
| UK: England | Grass roots; associated with Phialophora graminicola | D. Hornby | CBS 611.75 (ex-type) | KM484945 | KM485041 | KM485159 | – | – | |
| Xenopyricularia zizaniicola | Japan: Kyoto | Zizania latifolia | K. Yoshida & K. Hirata | CBS 132356 = MAFF 240220 = KYZL201-1-1(Zz-2J) | KM484946 | KM485042 | KM485160 | AB274444 | AB274480 |
| Japan: Ibaraki | Zizania latifolia | N. Hayashi | CBS 133593 = MAFF 240219 = IBZL3-1-1(Zz-1J) (ex-neotype) | KM484947 | – | KM485161 | KM485230 | AB274479 | |
ATCC: American Type Culture Collection, Virginia, U.S.A.; BCC: BIOTEC Culture Collection, National Center for Genetic Engineering and Biotechnology (BIOTEC), Bangkok, Thailand; CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CPC: Culture collection of Pedro Crous, housed at CBS; DAR: Plant Pathology Herbarium, Orange Agricultural Institute, Forest Road, Orange. NSW 2800, Australia; FGSC: Fungal Genetics Stock Center, University of Kansas Medical Center, KS, U.S.A.; HKUCC: The University of Hong Kong Culture Collection, Hong Kong, China; ICMP: International Collection of Microorganisms from Plants, Landcare Research, Auckland, New Zealand; IMI: International Mycological Institute, CBI-Bioscience, Egham, Bakeham Lane, United Kingdom; INIFAT: Alexander Humboldt Institute for Basic Research in Tropical Agriculture, Ciudad de La Habana, Cuba; KACC: Korean Agricultural Culture Collection, National Institute of Agricultural Biotechnology, Rural Development Administration, Suwon, Republic of Korea; MAFF: Ministry of Agriculture, Forestry and Fisheries, Tsukuba, Ibaraki, Japan; MUCL: Université Catholique de Louvain, Louvain-la-Neuve, Belgium; PD: Plant Protection Service, nVWA, Division Plant, Wageningen, The Netherlands; PREM: South African National Collection of Fungi (NCF), Mycology Unit, Biosystematics Division, Plant Protection Institute, Agricultural Research Council, Roodeplaat, Pretoria, South Africa; QM: Quartermaster Research and Development Center, U.S. Army, Massachusetts, U.S.A.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: partial large subunit (28S) of the nrRNA gene operon; RPB1: partial RNA polymerase II largest subunit gene; ACT: partial actin gene; CAL: partial CAL gene. Genome sequences of C. parasitica strain EP155: JGI Genome Portal; Genome sequences of G. graminis var. tritici strain R3111a, P. oryzae strain 70-15 and M. poae strain ATCC 64411: Broad Institute.
DNA extraction, amplification and sequencing
Fungal cultures were grown on a cellophane disc on MEA to easily scrape off mycelium. Genomic DNA was extracted using the UltraClean Microbial DNA isolation kit (MoBio Laboratories, USA), according to the manufacturer’s instructions. Parts of the following loci were amplified and sequenced: RPB1, partial RNA polymerase II largest subunit gene; ITS, internal transcribed spacer regions and intervening 5.8S nuclear ribosomal RNA (nrRNA) gene; LSU, partial nrRNA gene large subunit (28S); ACT, partial actin gene and CAL, partial calmodulin gene.
The reactions were performed in 20 μL mixtures containing 1 μL of genomic DNA, 2 mM MgCl2 (Bioline, Germany), 4 μL 5× Colourless GoTaq® Flexi Buffer (Promega, USA), 80 μM dNTPs (Promega), 0.2 μM of each primer and 0.10 μL GoTaq® Flexi DNA Polymerase (Promega).
The primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990) were used to amplify the ITS + LSU region by using the following PCR programme: initial denaturation at 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 52 °C for 30 s, 72 °C for 2 min, and finally an additional 7 min at 72 °C. The primers ACT-512F and ACT-783R were used for actin and CAL-228F and CAL-737R for calmodulin (Carbone & Kohn 1999). The following PCR programme was used for actin/calmodulin: initial denaturation at 94 °C for 5 min, followed by 35 cycles of 95 °C for 15 s, 61/55 °C for 20 s, 72 °C for 40 s, and final extension at 72 °C for 5 min. For amplification of RPB1 the primers RPB1F and RPB1R (see Table 2) were designed for the Nakataea/Gaeumannomyces group from unpublished sequence data of eight P. oryzae strains and one P. grisea strain, as well as public genomes of P. oryzae 70-15, Magnaporthiopsis poae ATCC 64411 and Gaeumannomyces graminis var. tritici R3111a. The following PCR programme was used: initial denaturation at 94 °C for 5 min, followed by 12 cycles of 94 °C for 30 s, 57–51 °C (decreasing for 0.5° every cycle) for 20 s, 72 °C for 70 s; 25 cycles of 94 °C for 30 s, 51 °C for 20 s, 72 °C for 70 s; and finally an additional 5 min at 72 °C.
Table 2.
Details of primers used and/or developed for this study.
| Locus1 and primer name | Sequence (5′ – 3′) | Orientation | Reference |
|---|---|---|---|
| Actin | |||
| ACT-512F | ATG TGC AAG GCC GGT TTC GC | Forward | Carbone & Kohn (1999) |
| ACT-783R | TAC GAG TCC TTC TGG CCC AT | Reverse | Carbone & Kohn (1999) |
| Calmodulin | |||
| CAL-228F | GAG TTC AAG GAG GCC TTC TCC C | Forward | Carbone & Kohn (1999) |
| CAL-737R | CAT CTT TCT GGC CAT CAT GG | Reverse | Carbone & Kohn (1999) |
| ITS | |||
| ITS4 | TCC TCC GCT TAT TGA TAT GC | Reverse | White et al. (1990) |
| ITS5 | GGA AGT AAA AGT CGT AAC AAG G | Forward | White et al. (1990) |
| V9G | TTA CGT CCC TGC CCT TTG TA | Forward | de Hoog & Gerrits van den Ende (1998) |
| LSU | |||
| LR5 | TCC TGA GGG AAA CTT CG | Reverse | Vilgalys & Hester (1990) |
| NL1 | GCA TAT CAA TAA GCG GAG GAA AAG | Forward | O'Donnell (1993) |
| RPB1 | |||
| RPB1F | AGA CGA TYG AGG AGA TCC AGT T | Forward | This study |
| RPB1R | ART CCA CAC GCT TAC CCA TC | Reverse | This study |
ACT: partial actin gene; CAL: partial CAL gene; ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: partial large subunit (28S) of the nrRNA gene operon; RPB1: partial RNA polymerase II largest subunit gene.
Both strands of the PCR fragments were sequenced with the BigDye Terminator v. 3.1 Cycle Sequencing Kit (Applied Biosystems, USA) using the primers indicated in Table 2. The products were analysed on an ABI Prism 3730 XL DNA Sequencer (Applied Biosystems). Contigs were assembled by using the forward and reverse sequences with the programme SeqMan from the LaserGene v. 9 package (DNAstar, USA).
Genomic sequences of Cryphonectria parasitica strain EP155, Gaeumannomyces graminis var. tritici strain R3111a, P. oryzae strain 70-15 and M. poae strain ATCC 64411 were retrieved from Broad Institute (www.broadinstitute.org; G. graminis var. tritici, P. oryzae and M. poae) and JGI Genome Portal (http://genomeportal.jgi.doe.gov/; C. parasitica) databases (Dean et al. 2005).
Phylogenetic analyses
Megablast searches of the NCBI's GenBank nucleotide database were used to supplement the sequence data generated in this study, especially to populate the overview LSU phylogeny. Sequences were aligned using the online version of MAFFT (http://mafft.cbrc.jp/alignment/software/) and the alignments were manually adjusted using MEGA v. 5.2 (Tamura et al. 2011). Analyses were performed with the individual and combined datasets to test the robustness of each included locus. Phylogenetic trees were reconstructed by Bayesian Inference (BI) using MrBayes v. 3.2.2 ((Ronquist et al. 2012); LSU only) and maximum parsimony (MP) using PAUP v. 4.0b10 (Swofford 2003) for all datasets as described by Crous et al. (2006). To check the congruency of the individual datasets, a 70 % neighbour-joining (NJ) reciprocal bootstrap was performed (Mason-Gamer & Kellogg 1996, Lombard et al. 2010). Novel sequences derived in this study were lodged at GenBank, and the alignments and phylogenetic trees in TreeBASE (www.treebase.org/treebase/index.html).
Morphology
For morphological characterisation, cultures were grown on synthetic nutrient-poor agar (SNA; Nirenberg 1976), supplemented with autoclaved barley seeds, water agar supplemented with autoclaved barley seeds and leaves, as well as OA. Plates were inoculated with agar plugs from cultures growing on MEA, PDA or OA. Plates were incubated at 23–25 °C under a regime of 12 h dark/12 h near-ultaviolet light, and examined after 1–3 wk for sporulation. Observations were made with a Zeiss V20 Discovery stereo-microscope, and with a Zeiss Axio Imager 2 light microscope using differential interference contrast (DIC) illumination and an AxioCam MRc5 camera and software. Measurements and photographs were made from structures mounted in clear lactic acid. The 95 % confidence intervals were derived from 30 observations (×1 000 magnification), with the extremes given in parentheses. Ranges of the dimensions of other characters are given. Colony diameter and other macroscopic features were recorded after 1 wk of incubation at 25 °C in the dark. Colony colours were determined using the colour charts of Rayner (1970). Specimens were deposited in the fungarium at CBS (CBS H) in Utrecht, and taxonomic novelties in MycoBank (Crous et al. 2004).
Results
DNA phylogeny
We combined the LSU sequences obtained from our Pyricularia/Magnaporthe species (Table 1) with sequences from NCBI corresponding to other Pyricularia/Magnaporthe species. The LSU dataset consists of 99 aligned sequences, including the outgroup Peziza vesiculosa. It contains 772 characters, of which 336 constitute unique site patterns (BI analysis with the GTR model, dirichlet (1,1,1,1) state frequency distribution and inverse gamma-shaped rate variation across sites). 405 characters were constant, 62 were variable and parsimony-uninformative while 305 were parsimony informative (MP analysis). A maximum of 1 000 equally most parsimonious trees were retained from this analysis (Tree length = 1 362, CI = 0.438, RI = 0.785 and RC = 0.343, Fig. 1). The majority of strains clustered in the Magnaporthales (Thongkantha et al. 2009). However, “Pyricularia” parasitica, based on CBS 376.54, clusters in the Chaetothyriales (Eurotiomycetes) and Rhexodenticulata cylindrospora (=Pyricularia lauri, Nakataea cylindrospora) is placed incertae sedis in the Sordariomycetes, but in both the parsimony (69 % bootstrap support) and Bayesian analyses (posterior probability of 1.0), this clade is related to Boliniales and Sordariales.
Fig. 1.

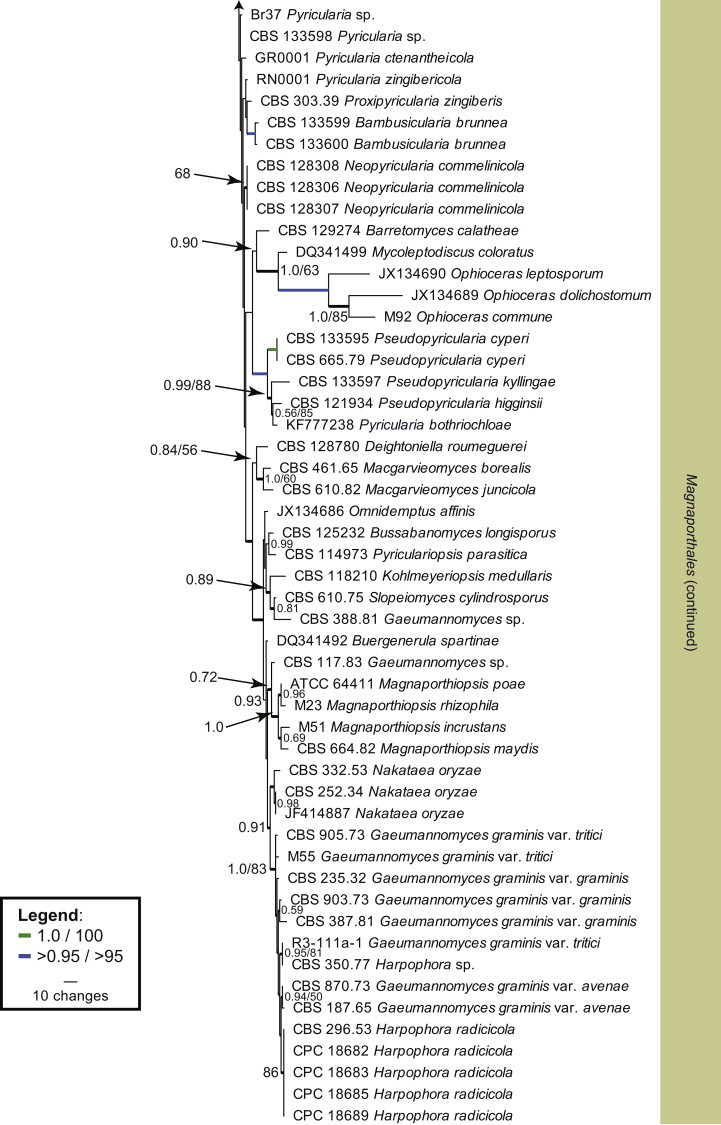
The first of 1000 equally most parsimonious trees (Tree length = 1362, CI = 0.438, RI = 0.785 and RC = 0.343) obtained from a maximum parsimony analysis of the LSU alignment. The bootstrap support values (integers) from 1000 replicates and the posterior probability values (values ≤1.0) are indicated as numbers at the nodes or as coloured branches (see legend) and the scale bar represents 10 changes. Thickened branches reflect those branches present in the strict consensus parsimony tree. Families are highlighted in the horizontal coloured boxes, orders in the vertical coloured boxes and classes are shown to the left of the tree. “Pyricularia” parasitica and Rhexodenticula cylindrospora are shown in bold text. The tree was rooted to Peziza vesiculosa (GenBank DQ470948).
Within Magnaporthales, the different clades were not well-resolved using LSU sequences (Fig. 1). Therefore, LSU was supplemented with RPB1 sequences to generate a novel phylogenetic tree restricted to species from Magnaporthales. The combined LSU/RPB1 dataset consists of 101 aligned sequences including Cryphonectria parasitica as outgroup. This dataset contains 1 391 characters, of which the LSU dataset contributed 748 characters and the RPB1 dataset contributed 643 characters; 772 characters were constant, while 131 were variable and parsimony-uninformative and 488 were parsimony informative (LSU: 539, 74, 135 characters respectively and RPB1: 233, 57, 353 characters respectively). Two equally most parsimonious trees were retained from this analysis (Tree length = 2 483, CI = 0.416, RI = 0.879 and RC = 0.365), the first of which is shown in Fig. 2. This phylogenetic tree delimited three families, of which two are described as new (Ophioceraceae, Pyriculariaceae), and 19 genus clades, ten of which represent novel genera, described in the Taxonomy Section. A further two lineages represent “Gaeumannomyces” spp., but these species defined clades distinct from other known species of the genus and are not treated further here.
Fig. 2.
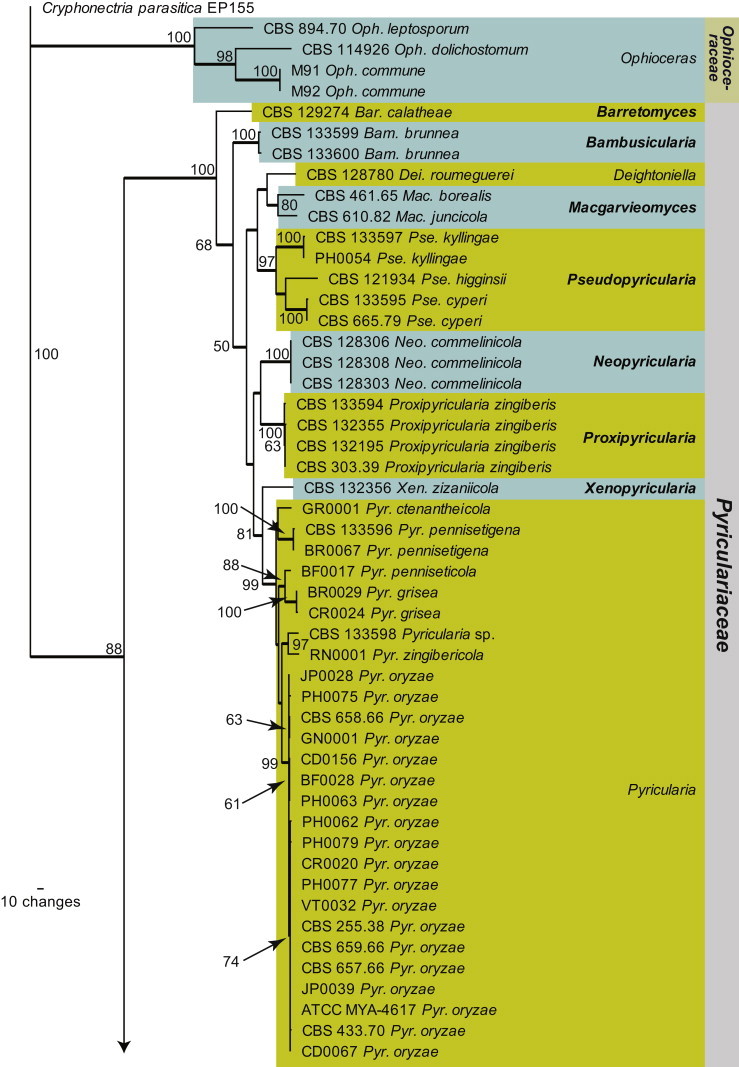
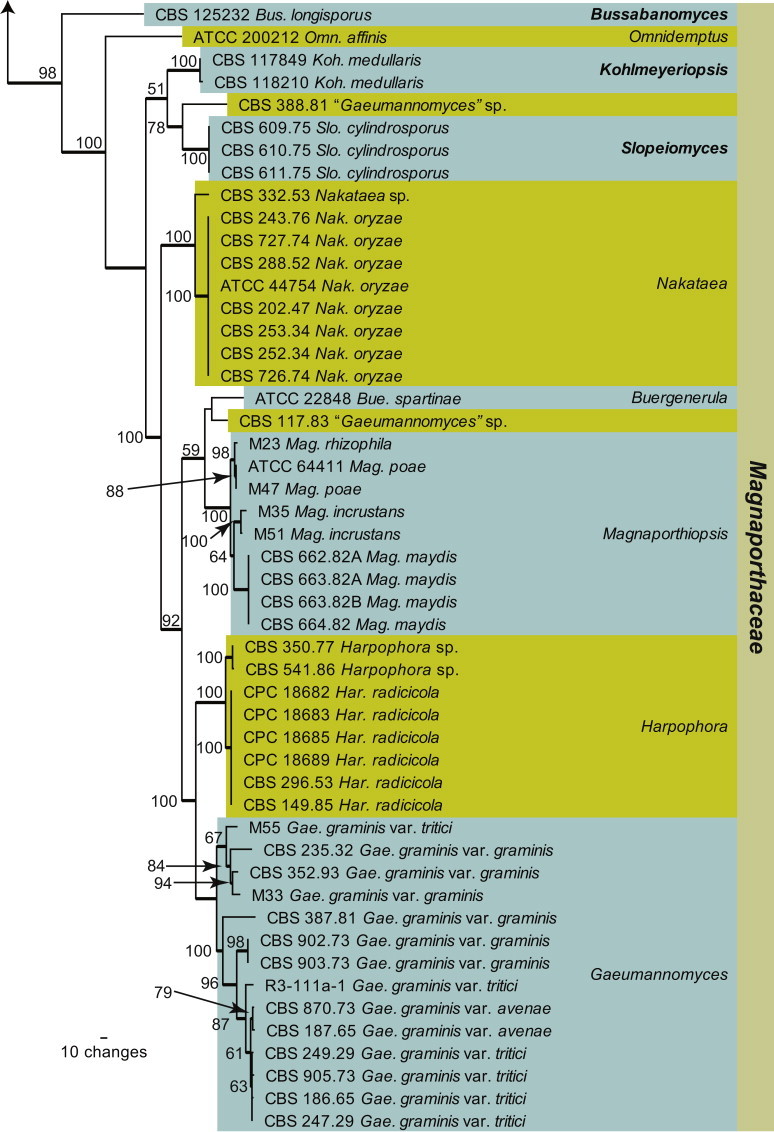
The first of two equally most parsimonious trees (Tree length = 2483, CI = 0.416, RI = 0.879 and RC = 0.365) obtained from a maximum parsimony analysis of the combined LSU/RPB1 alignment. The bootstrap support values from 1000 replicates are indicated at the nodes and the scale bar represents the number of changes. Thickened branches reflect those branches present in the strict consensus tree. Genera are highlighted in the horizontal coloured boxes, families in the vertical coloured boxes and novel species and families are shown in bold text. The tree was rooted to Cryphonectria parasitica strain EP155.
To improve the resolution of the clades within Pyriculariaceae, we combined ACT/ITS/RPB1 sequences. The combined dataset consists of 56 sequences including Barretomyces calatheae as outgroup, since it defines a clade basal to other species from this family (Fig. 2). This dataset contains 1 866 characters, of which the ACT dataset contributed 364 characters, the ITS dataset contributed 507 characters and the RPB1 dataset contributed 995 characters: 1 018 characters were constant, 118 were variable and parsimony-uninformative and 730 were parsimony informative (ACT: 94, 34, 236 characters respectively, ITS: 324, 27, 156 characters respectively, and RPB1: 600, 57, 338 characters respectively). A total of 192 equally most parsimonious trees were retained from this analysis (Tree length = 2 587, CI = 0.563, RI = 0.821 and RC = 0.462), the first of which is shown in Fig. 3. The phylogenetic tree delimited 17 species clades, seven of which represent novel species described in in the Taxonomy section.
Fig. 3.
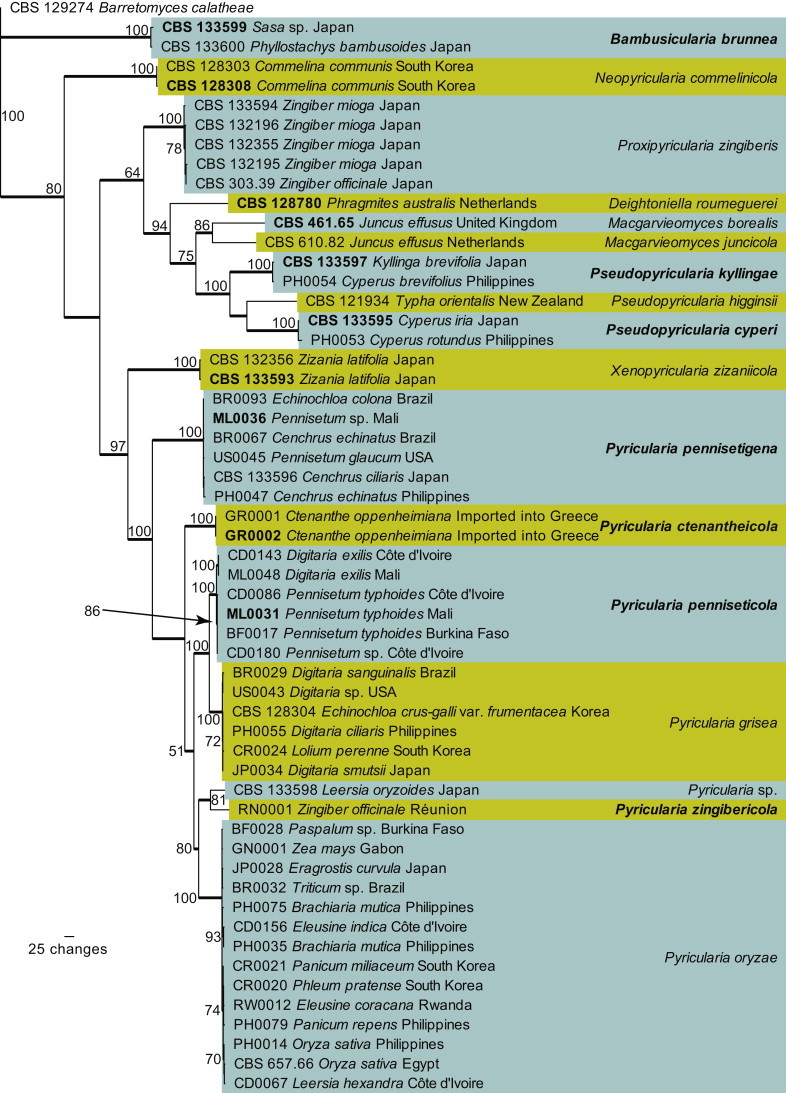
The first of 192 equally most parsimonious trees (Tree length = 2587, CI = 0.563, RI = 0.821 and RC = 0.462) obtained from a maximum parsimony analysis of the combined ACT/ITS/RPB1 alignment. The bootstrap support values from 1000 replicates are indicated at the nodes and the scale bar represents the number of changes. Thickened branches reflect those branches present in the strict consensus tree. Species are highlighted in the coloured boxes and ex-type strain numbers and novel species are shown in bold text. The tree was rooted to Barretomyces calatheae strain CBS 129274.
Taxonomy
Magnaporthales Thongk., Vijaykr. & K.D. Hyde, Fungal Diversity 34: 166. 2009.
Magnaporthaceae P.F. Cannon, Systema Ascomycetum 13: 26. 1994.
Ascomata perithecial, immersed, scattered to separate, globose to subglobose, black, with long unilateral, cylindrical, black, periphysate neck; wall of several layers of textura epidermoidea. Paraphyses hyaline, thin-walled, septate, intermingled among asci. Asci 8-spored, subcylindrical, unitunicate, short-stipitate or not, with a large apical ring staining in Meltzer’s iodine reagent. Ascospores curved to sigmoid, septate, filiform or fusoid, hyaline to olivaceous, with bluntly rounded ends, lacking sheath. Mycelium with simple to lobed brown appressoria. Asexual morphs hyphomycetous, at times formed from sclerotia, with simple, unbranched or branched conidiophores. Conidiogenous cells integrated, pigmented, phialidic with collarettes, or denticulate. Conidia hyaline to pale brown, septate to aseptate, variable in shape, straight or curved.
Type genus: Nakataea Hara (= Magnaporthe R.A. Krause & R.K. Webster)
Type species: Nakataea oryzae (Catt.) J. Luo & N. Zhang
Genera included: Buergenerula, Bussabanomyces, Endopyricularia, Gaeumannomyces, Harpophora, Kohlmeyeriopsis, Magnaporthiopsis, Nakataea, Omnidemptus, Pyriculariopsis and Slopeiomyces.
Notes: Other than being phylogenetically distinct, the Magnaporthaceae is distinguished from the Pyriculariaceae by their asexual morphs, which are either phialophora-like, or with falcate versicoloured conidia on brown, erect conidiophores.
Bussabanomyces Klaubauf, Lebrun & Crous, gen. nov. MycoBank MB810195.
Etymology: Named after Dr. B. Bussaban, who collected this fungus from Chiang Mai, Thailand.
Mycelium consisting of verruculose, pale brown, branched, septate hyphae. Conidiophores macronematous, rarely branched, straight, septate, pale brown near the base, subhyaline at the apex. Conidiogenous cells cylindrical, terminal, denticulate; denticles cylindrical, thin-walled, mostly cut off by a septum to form a separating cell. Conidia solitary, dry, obclavate, hyaline to pale brown, smooth, 4(–5)-septate.
Type species: Bussabanomyces longisporus (Bussaban) Klaubauf, Lebrun & Crous
Notes: Morphologically similar to Pyricularia, but distinct in that conidiophores are usually unbranched, with terminal conidiogenous cells that give rise to 4(–5)-septate, pale brown conidia.
Bussabanomyces longisporus (Bussaban) Klaubauf, Lebrun & Crous, comb. nov. MycoBank MB810196.
Basionym: Pyricularia longispora Bussaban, Mycologia 95: 520. 2003.
Illustrations: See Bussaban et al. (2003).
Mycelium consisting of verruculose, pale brown, branched, septate hyphae, 3–5 μm diam. Conidiophores macronematous, up to 400 μm long, 3–4.6 μm diam, rarely branched, straight, septate, pale brown near the base, subhyaline at the apex. Conidiogenous cells cylindrical, denticulate; each denticle cylindrical, thin-walled, mostly cut off by a septum to form a separating cell. Conidia 47–72 × 5.6–7.6 μm, solitary, dry, obclavate, hyaline to pale brown, smooth, 4(–5)-septate. (Description from Bussaban et al. 2003).
Culture characteristics: Colonies on MEA pale olivaceous-grey, irregularly raised with a hairy edge, velutinous, reaching 2.3–2.4 cm after 1 wk; reverse umber to chestnut. Similar appearance on CMA and OA with slightly bigger colony diameters, 2.6–3.1 cm. On PDA colonies were olivaceous, with central tufts. No sporulation was observed.
Material examined: Thailand, Chiang Mai, Doi Suthep-Pui National Park, isolated as an endophyte from leaves of Amomum siamense, Feb. 2000, B. Bussaban (holotype BCC11377, culture ex-type CBS 125232).
Harpophora W. Gams, Stud. Mycol. 45: 192. 2000.
Mycelium consisting of olivaceous-brown hyphae, with typical “runner hyphae” and narrower lateral hyphae. Conidiogenous cells phialidic, resembling those of Phialophora, solitary on hyphae or aggregated in clusters, faintly pigmented, with a conspicuous, divergent collarette. Conidia borne in slimy heads, cylindrical, but prominently curved, hyaline. (Description from Gams 2000).
Type species: Harpophora radicicola (Cain) W. Gams
Harpophora radicicola (Cain) W. Gams, Stud. Mycol. 45: 192. 2000.
Basionym: Phialophora radicicola Cain, Canad. J. Bot. 30: 340. 1952.
= Phialophora zeicola Deacon & D.B. Scott, Trans. Brit. mycol. Soc. 81: 256. 1983.
≡ Harpophora zeicola (Deacon & D.B. Scott) W. Gams, Stud. Mycol. 45: 192. 2000.
Materials examined: Canada, Ontario, Chatham, on Zea mays, 1950, R.F. Cain, isotypes of P. radicicola, specimens CBS H-7592, 7593, cultures ex-isotype CBS 296.53 = MUCL 28970 = TRTC 23660. South Africa, on Zea mays, isotype of P. zeicola, specimens PREM 45754, CBS H-7597, culture ex-isotype CBS 149.85.
Notes: When Gams (2000) introduced the genus Harpophora, it was assumed to be the asexual morph of Gaeumannomyces. The latter genus however, clusters apart in the Magnaporthaceae, and has harpophora-like asexual morphs. Furthermore, based on phylogenetic analyses of several isolates of H. zeicola from South Africa (Fig. 1), as well as the ex-type isolate of H. radicicola and H. zeicola, the latter must be reduced to synonymy under the older name H. radicicola.
Kohlmeyeriopsis Klaubauf, Lebrun & Crous, gen. nov. MycoBank MB810197.
Etymology: Named after Jan Kohlmeyer and Brigitte Volkmann-Kohlmeyer, who dedicated their careers to studying marine fungi, and collected this genus in the process.
Ascomata ellipsoid, immersed, ostiolate, dark brown, solitary, with long cylindrical periphysate necks, lateral or central; wall consisting of 3–4 layers of textura angularis. Paraphyses hyaline, septate, unbranched. Asci 8-spored, fusoid to cylindrical, short stipitate, unitunicate, with a large apical ring staining in Meltzer’s iodine reagent. Ascospores filamentous, tapering towards the base, indistinctly septate, hyaline, coiled in the ascus, producing appressoria at germination. Asexual morph trichocladium-like. Mycelium consisting of pale brown, septate, branched hyphae. Conidiophores reduced to conidiogenous cells, short, with lateral branches, giving rise to conidia. Conidia 2-celled, with a brown, large ellipsoidal, rarely with kidney-shaped apical cell, and 1–2 small, cylindrical or doliiform, pale brown basal cells.
Type species: Kohlmeyeriopsis medullaris (Kohlm., Volkm.-Kohlm. & O.E. Erikss.) Klaubauf, Lebrun & Crous
Notes: Gaeumannomyces medullaris was originally described from dead culms of Juncus roemerianus in North Carolina (Kohlmeyer et al. 1995). They described it as an aggressive cellulose decomposer, specific to the marine environment, commonly forming the trichocladium-like asexual morph in culture (Kohlmeyer & Volkmann-Kohlmeyer 1995). The genus Gaeumannomyces has harpophora-like asexual morphs, and the genus Trichocladium is heterogeneous (Seifert et al. 2011), and genetically unrelated to this fungus, for which a new genus is introduced.
Kohlmeyeriopsis medullaris (Kohlm., Volkm.-Kohlm. & O.E. Erikss.) Klaubauf, Lebrun & Crous, comb. nov. MycoBank MB810198.
Basionym: Gaeumannomyces medullaris Kohlm., Volkm.-Kohlm. & O.E. Erikss., Mycologia 87: 540. 1995.
= Trichocladium medullare Kohlm. & Volkm.-Kohlm., Mycotaxon 53: 349. 1995.
Illustrations: See Kohlmeyer et al. (1995).
Materials examined: USA, North Carolina, Broad Creek, Carteret County, on Juncus roemerianus, isol. Kohlmeyer JK5528S, deposited by C. Schoch, CBS 117849; North Carolina, Broad Creek, Carteret County, on Juncus roemerianus, isol. Kohlmeyer JK 5522N, deposited by C. Schoch, CBS 118210.
Magnaporthiopsis J. Luo & N. Zhang, Mycologia 105: 1021. 2013.
Plant pathogenic. Ascomata perithecial, solitary or gregarious, superficial or immersed, globose, with a cylindrical neck, black, smooth; wall consisting of two layers. Asci unitunicate, clavate, with a refractive ring. Ascospores fusoid, septate, hyaline or yellow-brown, smooth, biseriate. Paraphyses hyaline, septate, branched. Hyphopodia simple. Conidiophores solitary, branched or not. Conidiogenous cells phialidic, hyaline. Conidia subglobose to ovoid, aseptate, hyaline, smooth. (Description from Luo & Zhang 2013).
Type species: Magnaporthiopsis poae (Landsch. & N. Jacks.) J. Luo & N. Zhang
Notes: Luo & Zhang (2013) introduced Magnaporthiopsis to accommodate species with black, globose perithecia with long cylindrical necks, clavate asci with an apical ring, septate, fusoid ascospores, and a harpophora-like asexual morph.
Magnaporthiopsis maydis (Samra, Sabet & Hing.) Klaubauf, Lebrun & Crous, comb. nov. MycoBank MB810225.
Basionym: Cephalosporium maydis Samra, Sabet & Hing., Phytopathology 53: 404. 1963.
≡ Harpophora maydis (Samra, Sabet & Hing.) W. Gams, Stud. Mycol. 45: 192. 2000.
Materials examined: Bihar, Messina, on Zea mays hybrid “Ganga Safed 2”, Mar 1976, M.M. Payak, CBS 664.82. Egypt, on Zea mays, Dec. 1982, H.A. Elshafey, CBS 662.82A. India, Rajasthan, Jaipur, on Zea mays, Dec. 1982, B.S. Siradhana, CBS 663.82A, CBS 663.82B.
Notes: Gams (2000) introduced the genus Harpophora, based on H. radiciola for a group of species that are phialophora-like in morphology, with cylindrical, curved conidia. Harpophora is however heterogeneous (e.g. Gaeumannomyces has harpophora-like asexual morphs), and H. maydis clusters with species of Magnaporthiopsis (see Fig. 2), hence a new combination is introduced to accommodate it.
Nakataea Hara, The diseases of the rice-plant, 2nd ed.: 185. 1939.
= Nakataea Hara, Nippon-gaikingaku: 318. 1936. nom. nud.
Plant pathogenic. Sclerotia spherical to subspherical, black, formed on the host and in culture. Ascomata perithecial, globose, dark brown, immersed in leaf sheaths; wall consisting of 5–12 layers of thick-walled dark cells; neck frequently protruding from the leaf tissue. Asci 8-spored, subcylindrical, thin-walled, short-stipitate, deliquescing at maturity, spirally twisted, 3-septate, slightly constricted at septa, fusiform, curved, granular, with median cells turning yellowish brown. Conidiophores solitary, erect, brown, smooth, branched or not, septate, with integrated terminal conidiogenous cells forming a rachis with several denticles, each separated from the conidiogenous cell by a septum. Conidia solitary, falcate to sigmoid, smooth, 3-septate, widest in the middle, end cells hyaline, median cells medium brown.
Type species: Nakataea sigmoidea (Cavara) Hara
Nakataea oryzae (Catt.) J. Luo & N. Zhang, Mycologia 105: 1025. 2013.
Basionym: Sclerotium oryzae Catt., Arch. Triennale Lab. Bot. Crittog. 1: 10. 1877.
= Helminthosporium sigmoideum Cavara, Mat. Lomb.: 15. 1889.
≡ Nakataea sigmoidea (Cavara) Hara, as “sigmoideum”, Nippon-gaikingaku: 318. 1936. nom. nud.
≡ Nakataea sigmoidea (Cavara) Hara, as “sigmoideum”, The diseases of the rice-plant 2nd ed.: 185. 1939.
= Leptosphaeria salvinii Catt., Arch. Labor. Bot. Critt. Univ. Pavia 2, 3: 126. 1879.
≡ Magnaporthe salvinii (Catt.) R.A. Krause & R.K. Webster, Mycologia 64: 110. 1972.
Additional synonyms listed in MycoBank.
Materials examined: Burma, on straw of Oryza sativa, date and collector unknown, CBS 252.34. Italy, no collection details, CBS 202.47; on Oryza sativa, sent to CBS for identification by Centro di Ricerche sul Riso, Mortara, Italy, Nov 1975, collector unknown, specimen CBS H-14204, culture CBS 243.76. Japan, on Oryza sativa, date and collector unknown, ATCC 44754 = M21 = Roku-2; Takada, on stem of Oryza sativa, date and collector unknown, CBS 288.52. USA, Calivornia, Davis, on Oryza sativa, Dec. 1974, R.K. Webster, specimens CBS H-14203; CBS H-14205, cultures CBS 726.74, CBS 727.74. Unknown, CBS 253.34.
Notes: The genus Nakataea (based on N. sigmoidea, described from rice in Italy) has some similarity to Pyricularia in general morphology, but differs in having falcate conidia with darker median cells (Luo & Zhang 2013). Magnaporthe oryzae (=M. salvinii), the type of Magnaporthe, forms a Nakataea asexual morph, and hence Luo & Zhang (2013) introduced the combination N. oryzae for this fungus, as the name Nakataea (1939) is older than Magnaporthe (1972). This decision effectively reduced Magnaporthe to synonymy under Nakataea. The majority of species formerly treated as Magnaporthe, fall in the Pyricularia complex (Murata et al. 2014).
Pyriculariopsis M.B. Ellis, In: Ellis, Dematiaceous Hyphomycetes (Kew): 206. 1971.
Plant pathogenic. Mycelium consisting of smooth, hyaline to brown, branched, septate hyphae; hyphae developing chains of globose, swollen chlamydospores that give rise to black microsclerotia. Conidiophores forming from hyphae or microsclerotia, solitary, erect, straight or curved, unbranched, medium brown, thick-walled, smooth, subcylindrical, septate; base bulbous, lacking rhizoids. Conidiogenous cells integrated, terminal, medium brown, smooth, forming a rachis with several protruding denticles, and minute marginal frill due to rhexolytic secession. Conidia solitary, obclavate, smooth, guttulate, 3-septate, two median cells brown, apical and basal cell olivaceous to subhyaline; hilum truncate, slightly protruding, with marginal frill, unthickened, not darkened; apex tapering, subacutely rounded, with persistent mucoid cap.
Type species: Pyriculariopsis parasitica (Sacc. & Berl.) M.B. Ellis
Pyriculariopsis parasitica (Sacc. & Berl.) M.B. Ellis, Dematiaceous Hyphomycetes (Kew): 207. 1971. Fig. 4.
Fig. 4.
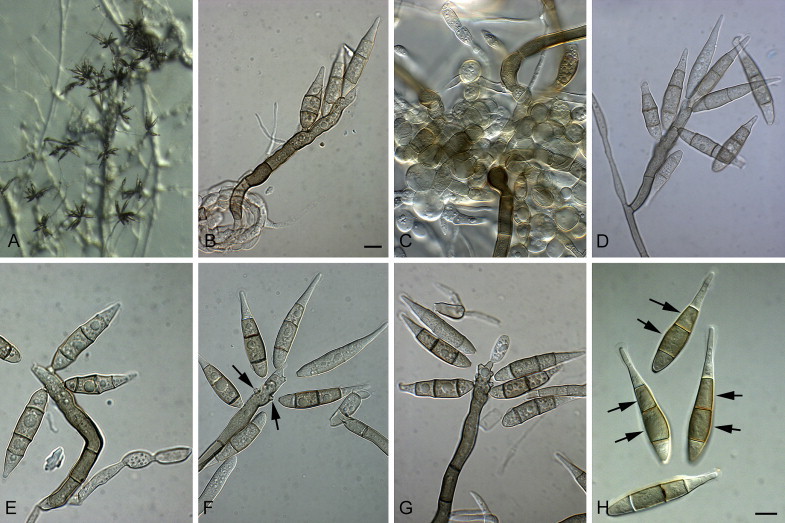
Pyriculariopsis parasitica (CBS 114973). A–G. Conidiophores sporulating on SNA, having a rachis with conidia. H. Arrows indicate conidial median cells with darker pigmentation. Scale bars = 10 μm.
Basionym: Helminthosporium parasiticum Sacc. & Berl., Revue mycol., Toulouse 11: 204. 1889.
On SNA on sterile barley seed. Mycelium consisting of smooth, hyaline to brown, branched, septate hyphae, 3–4 μm diam; hyphae developing chains of globose, swollen chlamydospores that give rise to black microsclerotia. Conidiophores forming from hyphae or microsclerotia, solitary, erect, straight or curved, unbranched, medium brown, thick-walled, smooth, subcylindrical, 60–180 × 6–8 μm, 3–10-septate; base bulbous, 10–16 μm diam, lacking rhizoids. Conidiogenous cells 10–50 × 7–8 μm, integrated, terminal, medium brown, smooth, forming a rachis with several protruding denticles, 2–4 μm long, 3–5 μm diam, and minute marginal frill due to rhexolytic secession. Conidia solitary, obclavate, smooth, guttulate, 3-septate, two median cells brown, apical and basal cell olivaceous to subhyaline, (30–)40–55(–60) × (7–)8–9(–12) μm; apical cell 18–22 μm long, basal cell 8–11 μm long; hilum truncate, slightly protruding, 2–3 μm diam with marginal frill, unthickened, not darkened; apex tapering, subacutely rounded, with persistent mucoid cap, 2–3 μm diam.
Culture characteristics: Colonies on MEA with white aerial mycelium, mouse-grey in centre, raised, cottony, round, reaching up to 5 cm diam after 1 wk; reverse with dark mouse-grey in centre. Colonies on CMA and OA transparent, with very thin, spreading mycelium with scattered dark spots of sporulation, covering full plate after 1 wk. Colonies on PDA transparent with dark mouse-grey areas, flat, covering plate after 1 wk; reverse with some dark spots.
Material examined: Hong Kong, Discovery Bay, Lantau Island, on leaves of Musa sp., 5 Oct. 1999, K.D. Hyde, CBS 114973 = HKUCC 5562 = Maew HK 1.
Notes: The denticles of Pyriculariopsis are similar to those of Pyricularia. The main difference lies in the conidium pigmentation, septation, and the persistent apical mucoid cap. In Pyricularia conidia are 2-septate, uniformly olivaceous to medium brown, and the apical mucoid cap is not persistent, leaving the apex with what appears to be a marginal frill surrounding the apex (mucoid remnant?), from where the globoid mucoid cap extended.
Slopeiomyces Klaubauf, Lebrun & Crous, gen. nov. MycoBank MB810199.
Etymology: Named after D.B. Slope, who collected this fungus from cereal roots in Rothamsted Experimental Station, UK.
Perithecia superficial, globose, black, solitary, sometimes 2–3 aggregated, with cylindrical, black, periphysate neck bearing hyphae; wall consisting of several layers of textura prismatica to angularis. Paraphyses hyaline, septate, unbranched. Asci 8-spored, clavate, straight to curved, with a non-amyloid apical ring staining in Congo red. Ascospores hyaline, cylindrical to fusoid, septate, slightly curved, tapering somewhat to base, forming appressoria at germination. Asexual morph phialophora-like. Conidiogenous cells developing on hyphae, phialidic, subcylindrical to ampulliform with flared collarette, hyaline. Conidia hyaline, aseptate, apex rounded, pointed towards base, straight to curved or sigmoid.
Type species: Slopeiomyces cylindrosporus (D. Hornby, Slope, Gutter. & Sivan.) Klaubauf, Lebrun & Crous
Notes: Slopeiomyces is morphologically similar to Gaeumannomyces in the general morphology of its sexual and asexual morphs, the production of appressoria, and its ecology, being a root pathogen of Poaceae (Hornby et al. 1975). The only obvious morphological difference lies in its ascospores, which are much shorter and wider than observed in species of Gaeumannomyces. The link between S. cylindrosporus and the asexual morph originally used in inoculation experiments, Phialophora radiciola var. graminis, could not be confirmed. Phylogenetically, however, Slopeiomyces is clearly distinct from Gaeumannomyces (see Fig. 2).
Slopeiomyces cylindrosporus (D. Hornby, Slope, Gutter. & Sivan.) Klaubauf, Lebrun & Crous, comb. nov. MycoBank MB810200.
Basionym: Gaeumannomyces cylindrosporus D. Hornby, Slope, Gutter. & Sivan., Trans. Br. mycol. Soc. 69: 21 (1977).
Materials examined: UK, on grass roots, associated with Phialophora graminicola, Dec. 1975, D. Hornby, cultures ex-type CBS 609.75, CBS 610.75, CBS 611.75.
Ophioceraceae Klaubauf, Lebrun & Crous, fam. nov. MycoBank MB810201.
Ascomata perithecial, immersed to superficial, scattered to separate, globose to subglobose, black, with long cylindrical, black, periphysate neck, pale brown at apex; wall consisting of several layers of textura angularis. Paraphyses hyaline, thin-walled, septate, intermingled among asci. Asci 8-spored, subcylindrical to narrowly fusoid, unitunicate, short-stipitate or not, with a large apical ring staining in Meltzer’s iodine reagent. Ascospores curved to sigmoidal, septate, filiform, hyaline to olivaceous, with bluntly rounded ends, lacking sheath.
Type genus: Ophioceras Sacc., Syll. fung. (Abellini) 2: 358. 1883.
Type species: Ophioceras dolichostomum (Berk. & M.A. Curtis) Sacc., Syll. fung. (Abellini) 2: 358 (1883)
Genus included: Ophioceras.
Notes: Although Ophioceras is morphologically similar to Gaeumannomyces, the two genera can be distinguished by the aquatic habit of Ophioceras, occurring on wood and herbaceous material, versus the plant pathogenic nature of Gaeumannomyces, which has harpophora-like asexual morphs, mycelial appressoria, and a perithecial peridium of textura epidermoidea (Walker 1980, Chen et al. 1999). Although the family placement of Ophioceras was not resolved, the genus was temporarily added to the Magnaporthaceae (established for nectrotrophic and hemibiotrophic plant pathogens infecting root and shoots of Poaceae and Cyperaceae; Cannon 1994) awaiting further study (Shearer 1989, Shearer et al. 1999, Chen et al. 1999). As shown in the present analyses (Fig. 2) Ophioceras clearly clusters separate from the Magnaporthaceae in the Magnaporthales, and hence a separate family, the Ophioceraceae, is introduced to accommodate it.
Pyriculariaceae Klaubauf, Lebrun & Crous, fam. nov. MycoBank MB810202.
Ascomata perithecial, immersed, black, with long cylindrical necks covered in setae. Asci subcylindrical, unitunicate, short-stipitate, with a large apical ring staining in Meltzer's iodine reagent. Paraphyses hyaline, thin-walled, septate, intermingled among asci. Ascospores septate, fusiform, often with median cells pigmented, lacking sheath. Asexual morphs hyphomycetous, with simple, branched conidiophores. Conidiogenous cells integrated, pigmented, denticulate. Conidia hyaline to brown, transversely septate, apical mucoid appendage rarely present.
Type genus: Pyricularia Sacc.
Type species: Pyricularia grisea Sacc.
Genera included: Bambusicularia, Barretomyces, Deightoniella, Macgarvieomyces, Neopyricularia, Proxipyricularia, Pseudopyricularia, Pyricularia, Xenopyricularia.
Bambusicularia Klaubauf, Lebrun & Crous, gen. nov. MycoBank MB810203.
Etymology: Named after its occurrence on bamboo.
Plant pathogenic. Mycelium consisting of smooth, hyaline, branched, septate hyphae. Conidiophores solitary, erect, straight or curved, unbranched, flexuous to geniculate, dark brown, finely roughened, up to 500 μm long, multi-septate; base bulbous, lacking rhizoids. Conidiogenous cells integrated, terminal and intercalary, pale brown at apex, intercalary cells medium brown, finely roughened, with several protruding denticles. Conidia solitary, ellipsoid to obclavate, medium brown, finely roughened, granular to guttulate, 2-septate, hilum truncate, somewhat protruding.
Type species: Bambusicularia brunnea Klaubauf, Lebrun & Crous
Notes: The main distinguishing character between Bambusicularia and Pyricularia is in their conidiophore morphology. Conidiophores in Bambusicularia are flexuous, longer, wider and darker brown than seen in species of Pyricularia. Conidia are pale brown, but appear to have darker brown septa. The two genera are also phylogenetically distinct (Figs 2, 3).
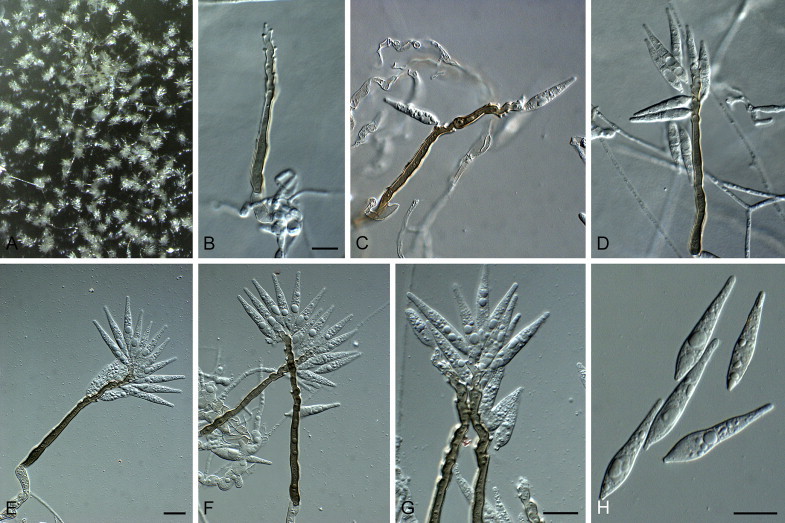
Bambusicularia brunnea Klaubauf, Lebrun & Crous, sp. nov. MycoBank MB810204. Fig. 5.
Fig. 5.

Bambusicularia brunnea (CBS 133599). A. Sporulation on sterile barley seed on SNA. B, C. Sporulation on sterile barley leaves. D–H. Conidiophores bearing conidia. I. Conidia. Scale bars = 10 μm.
Etymology: Named after its dark brown conidiophores.
On SNA on sterile barley seed. Mycelium consisting of smooth, hyaline, branched, septate hyphae, 2–3 μm diam. Conidiophores solitary, erect, straight or curved, unbranched, flexuous to geniculate, dark brown, finely roughened, 280–500 × 5–7 μm, 5–11-septate; base bulbous, lacking rhizoids, 7–10 μm diam. Conidiogenous cells 20–120 × 4–6 μm, integrated, terminal and intercalary, pale brown at apex, intercalary cells medium brown, finely roughened, with several protruding denticles, 1–2 μm long, 1.5–2 μm diam. Conidia solitary, ellipsoid to obclavate, medium brown, finely roughened, granular to guttulate, 2-septate, (20–)21–25(–27) × 10–11(–11.5) μm; apical cell 4–7 μm long, basal cell 6–9 μm long; hilum truncate, protruding, 0.5–1 μm long, 1.5–2 μm diam.
Culture characteristics: Colonies on MEA white, round, cottony, slightly raised, reaching 3.8 cm diam after 1 wk; reverse ochreous. Colonies on PDA transparent with white centre, flat, round, slightly cottony, reaching up to 3.7 cm after 1 wk, with diffuse, hairy margin. Colonies on CMA and OA transparent, smooth, flat, round, reaching up to 3.3 cm diam after 1 wk; colonies fertile.
Materials examined: Japan, Aichi, on Sasa sp. (Poaceae), 1992, S. Koizumi [holotype CBS H-21839, culture ex-type CBS 133599 = MAFF 240225 = INA-B-92-45(Ss-1J)]; Aichi, on Phyllostachys bambusoides (Poaceae), 1993, S. Koizumi, CBS 133600 = MAFF 240226 = INA-B-93-19(Ph-1J).
Note: Isolate CBS 133600 sporulated poorly, and had slightly larger conidia than CBS 133599, measuring (23–)25–30(–34) × (7–)8–9 μm; apical cell 7–11 μm long, basal cell 7–10 μm long.
Barretomyces Klaubauf, Lebrun & Crous, gen. nov. MycoBank MB810205.
Etymology: Named after Prof. dr. Robert W. Barreto, in acknowledgement of his contribution to mycology and plant pathology in Brazil.
Plant pathogenic. Mycelium consisting of verruculose, pale brown, branched, septate hyphae. Conidiophores macronematous, rarely branched, straight, septate, pale brown near the base, subhyaline at the apex. Conidiogenous cells cylindrical, terminal, denticulate; each denticle cylindrical, thin-walled, mostly cut off by a septum to form a separating cell. Conidia solitary, dry, obclavate, basal and terminal cell hyaline to pale brown, median cell darker brown, smooth, 4(–5)-septate.
Type species: Barretomyces calatheae (D.J. Soares, F.B. Rocha & R.W. Barreto) Klaubauf, Lebrun & Crous
Notes: Barretomyces calatheae, which is a foliar pathogen of Calathea longifolia in Brazil (Soares et al. 2011), was originally described in Pyriculariopsis based on its versicoloured conidia (with paler basal cell). Furthermore, they noted this species to have schizolytic secession, and Ellis (1971) defined Pyriculariopsis as having schizolytic secession, in contrast to the rhexolytic secession observed in Pyricularia. We have however found conidiogenesis to be variable, and not a good taxonomic criterion in distinguishing these genera.
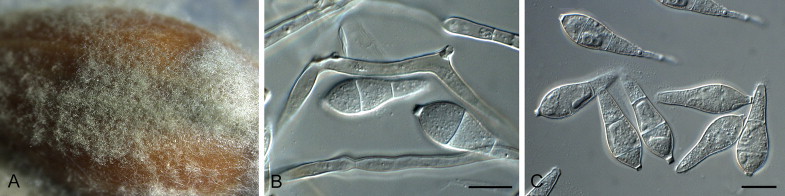
Barretomyces calatheae (D.J. Soares, F.B. Rocha & R.W. Barreto) Klaubauf, Lebrun & Crous, comb. nov. MycoBank MB810206. Fig. 6.
Fig. 6.

Barretomyces calatheae (CBS 129274). A. Leaf spot on Calathea longifolia in Brazil. B–G. Conidiophores bearing conidia. H. Conidia. Scale bars = 10 μm.
Basionym: Pyriculariopsis calatheae D.J. Soares, F.B. Rocha & R.W. Barreto, Mycol. Prog. 10: 317. 2011.
Leaf spots amphigenous, 0.5–11 cm diam, progressing from small yellow spots to large, circular to elliptic, grey-brown lesions, sometimes with a darker centre and with concentric circles, the outer region being dark-brown, surrounded by a large chlorotic border; sometimes coalescing, leading to leaf necrosis; disease symptoms also occurring on leaf petioles, as brown spots. On SNA medium. Mycelium consisting of smooth, hyaline, branched, septate hyphae, 2–3.5 μm diam. Conidiophores forming from hyphae, solitary, erect, straight or curved, unbranched, medium brown, smooth, 70–160 × 4–6 μm, 2–9-septate. Conidiogenous cells 20–70 × 5–6 μm, integrated, terminal and intercalary, pale to medium brown, smooth, forming a rachis with several protruding flat-tipped denticles, 1–3 μm long, 1–2 μm diam. Conidia solitary, obclavate, smooth, basal and terminal cell hyaline to pale brown, median cell darker brown, granular to guttulate, 2-septate, (19–)28–32(–35) × (5.5–)6–7(–8) μm; apical cell tapered, 9–12 μm long, basal cell 7–9 μm long; base tapering prominently to a truncate, protruding hilum, 1–1.5 μm diam.
Culture characteristics: Colonies on MEA white, round, raised, with a thick, furry texture, reaching 3 cm diam after 1 wk; reverse cinnamon. Colonies on OA white with a mouse grey centre, reaching 3.2 cm after 1 wk. Colonies on CMA white to pale mouse grey, round with entire edge, flat, felty, exuding droplets, reaching 3.3 cm after 1 wk, sporulating in centre. Colonies on PDA whitish, transparent with vinaceous-buff centre, irregular in shape, felty, reaching 2.8 cm after 1 wk.
Materials examined: Brazil, Minas Gerais, Viçosa, ‘Mata do Seu Nico’ on Calathea longifolia (Marantaceae), Dec. 2003, D.J. Soares (holotype VIC 30699, culture ex-type culture CBMAI 1060); Minas Gerais, Viçosa, on C. longifolia, Aug. 2010, P.W. Crous, CBS 129274 = CPC 18464.
Notes: A microconidial state was observed being similar in morphology to that reported for P. oryzae (Chuma et al. 2009, Zhang et al. 2014), and also observed in this study for P. grisea. The denticles of Barretomyces are different to those of Pyricularia, in that they are flat-tipped, but with a central pore.
Deightoniella S. Hughes, Mycol. Pap. 48: 27. 1952.
= Utrechtiana Crous & Quaedvl., Persoonia 26: 153. 2011.
Plant pathogenic. Conidiophores solitary, erect, aggregated, brown, smooth, becoming pale brown towards apex, base swollen, partly immersed in epidermis, but lacking rhizoids, with circular scar where base of conidiophore is attached to immersed hyphal network; conidiophore with swellings (twisted growth) along its axis, swellings coinciding with internal conidiophore proliferation (percurrently) through conidial scars; lacking transverse septa and reduced to conidiogenous cells (though some species have a basal septum). Conidiogenous cells integrated terminal, with truncate and flattened scar; sometimes thickened, not darkened, nor refractive. Conidia pale brown, ellipsoid to pyriform, guttulate to granular, finely verruculose, 1-septate slightly above the conidial median, thin-walled, apex bluntly to acutely rounded, base obtusely rounded with a flattened, darkened and thickened hilum that has a central pore, and minute marginal frill.
Type species: Deightoniella africana S. Hughes
Deightoniella roumeguerei (Cavara) Constant., Proc. K. Ned. Akad. Wet., Ser. C, Biol. Med. Sci. 86(2): 137. 1983. Fig. 7.
Fig. 7.

Deightoniella roumeguerei (CBS 128780). A. Leaf spot on Phragmites australis. B. Close-up of conidiophores on leaf surface. C–G. Conidiophores bearing conidia. H. Germinating conidium. I, J. Conidia. Scale bars = 10 μm.
Basionym: Scolicotrichum roumeguerei Cavara (as “roumegueri”), in Briosi & Cavara, Funghi Parass. Piante Colt. od Utili, Fasc. 5: no. 112. 1890.
= Utrechtiana cibiessia Crous & Quaedvl., Persoonia 26: 153. 2011.
Description and illustration: Constantinescu (1983), Crous et al. (2011).
Material examined: Netherlands, Utrecht, De Uithof University Campus, intersection of Harvardlaan with Uppsalalaan, on leaves of Phragmites australis growing along water canals, 14 Dec. 2010, W. Quaedvlieg (holotype of U. cibiessiae CBS H-20594, cultures ex-type CPC 18917, 18916 = CBS 128780).
Notes: Deightoniella as presently defined is heterogeneous. The genus Deightoniella (based on D. africana, occurring on leaves of Imperata cylindrica var. africana; Poaceae) has solitary conidiophores, with conidiogenous cells that rejuvenate percurrently. Deightoniella is distinct from Neodeightoniella, as the latter does not undergo percurrent rejuvenation, has conidiophores arranged in fascicles, well-developed apical and intercalary conidiogenous loci, and conidia with mucoid caps (Crous et al. 2013).
Macgarvieomyces Klaubauf, Lebrun & Crous, gen. nov. MycoBank MB810207.
Etymology: Named after Quentin D. MacGarvie, the Scottish plant pathologist that first named these species.
Plant pathogenic. Mycelium consisting of smooth, hyaline, branched, septate hyphae. Chlamydospores brown, ellipsoid, arranged in chains. Conidiophores solitary, erect, straight or curved, mostly unbranched, medium brown, smooth, septate. Conidiogenous cells integrated, terminal, rarely intercalary, medium brown, smooth, forming a rachis with several protruding denticles, appearing flat-tipped. Conidia solitary, narrowly obclavate, hyaline, smooth, granular and guttulate, medianly 1-septate; hilum somewhat thickened, not refractive, nor darkened.
Type species: Macgarvieomyces borealis (de Hoog & Oorschot) Klaubauf, Lebrun & Crous
Notes: MacGarvie described two species occurring on Juncus in the genus Diplorhinotrichum. de Hoog (1985) treated this genus as synonym of Dactylaria, but preferred to retain the plant pathogenic species in Pyricularia. As these taxa are clearly not congeneric with Pyricularia (Figs 2, 3), a new genus, Macgarvieomyces, is herewith introduced to accommodate them.
Macgarvieomyces borealis (de Hoog & Oorschot) Klaubauf, Lebrun & Crous, comb. nov. MycoBank MB810208.
Basionym: Pyricularia borealis de Hoog & Oorschot (as “boreale”), Stud. Mycol. 26: 114. 1985. (a nom. nov. for D. juncicola MacGarvie 1965).
≡ Diplorhinotrichum juncicola MacGarvie, Trans. Br. mycol. Soc. 48(2): 269. 1965.
≡ Dactylaria juncicola (MacGarvie) G.C. Bhatt & W.B. Kendr., Canad. J. Bot. 46: 1257. 1968.
Illustration: de Hoog (1985).
On OA. Conidiophores scattered, pale olivaceous-brown, thick-walled near the base, 7–9 μm diam, tapering towards the apex, 30–70 μm long, 1–3-septate. Conidiogenous cells apical, with flat-tipped denticles, 2 μm diam, unthickened, not pigmented. Conidia solitary, 1–4 per conidiogenous cell, subhyaline, ellipsoid with obtuse apex, tapering in basal cell towards obconically truncate base, slightly constricted at median septum, 16–17(–40) × 6–9 μm. (Description from de Hoog 1985).
Culture characteristics: Colonies on MEA buff to rosy buff with entire edge, umbonate to conical colony with somewhat velvety texture, reaching up to 3.3 cm diam after 2 wk; reverse ochreous and buff towards the edge. Colonies on CMA and OA transparent with smooth surface, reaching up to 3.5 cm diam after 2 wk. On PDA whitish to buff colony with honey centre, irregular outline, slightly furrowed in centre, reaching up to 3 cm diam after 2 wk; colony reverse whitish to buff with honey centre. No sporulation was observed.
Material examined: UK, Scotland, Moorland near Carnwat in Lanarkshire, 275 m alt. and near East Graigs, Edinburgh, 33 m alt., associated with leaf spots on Juncus effusus, Apr 1964, G.D. MacGarvie, culture ex-type CBS 461.65.
Macgarvieomyces juncicola (MacGarvie) Klaubauf, Lebrun & Crous, comb. nov. MycoBank MB810209. Fig. 8.
Fig. 8.
Macgarvieomyces juncicola (CBS 610.82). A. Colony sporulating on OA. B–G. Conidiophores and conidia forming on SNA. H. Conidia. Scale bars = 10 μm.
Basionym: Pyricularia juncicola MacGarvie, Scientific Proc. R. Dublin Soc., Ser. B 2(no. 16): 155. 1968.
On SNA on sterile barley seed. Mycelium consisting of smooth, hyaline, branched, septate hyphae, 1.5–2 μm diam. Chlamydospores arranged in intercalary chains, ellipsoid, hyaline to pale brown, smooth, 5–7 μm diam, frequently giving rise to conidiophores. Conidiophores solitary, erect, straight or curved, mostly unbranched, medium brown, smooth, 50–200 × 3–5 μm, with basal septum, developing additional septum if branched. Conidiogenous cells 50–180 × 3–5 μm, integrated, terminal, rarely intercalary, medium brown, smooth, forming a rachis with several protruding denticles, 1.5–2 μm long, 1–1.5 μm diam. Conidia solitary, narrowly obclavate, hyaline, smooth, granular and guttulate, medianly 1-septate, (17–)25–30(–32) × (4–)5 μm; hilum somewhat thickened, 1–1.5 μm diam.
Culture characteristics: Colonies on MEA isabelline with pale olivaceous grey central mycelium, slightly raised wool-like texture, round and hairy edge, reaching up to 2.6 cm after 1 wk; reverse iron grey. On CMA and OA olivaceous to grey olivaceous, flat, smooth and velutinous surface, undulate edge. Colonies fertile on MEA, CMA and OA. Colonies on PDA white with buff centre, round, flat, fringed edge, reverse white with buff centre.
Material examined: Netherlands, on stem base of Juncus effusus, 3 Nov. 1982, G.S. de Hoog, specimens CBS H-11668; CBS H-1764; CBS H-17648, culture CBS 610.82.
Note: Macgarvieomyces borealis and M. juncicola can be distinguished based on conidial dimensions, because conidia of M. juncicola are on average longer and narrower.
Neopyricularia Klaubauf, Lebrun & Crous, gen. nov. MycoBank MB810210.
Etymology: Named after its morphological similarity to Pyricularia.
Plant pathogenic. Conidiophores solitary or in fascicles, subcylindrical, erect, olivaceous, smooth, rarely branched, septate, with sympodial growth. Conidiogenous cells terminal and intercalary, olivaceous, with denticulate conidiogenous loci, slightly darkened, and rhexolitic secession. Conidia solitary, formed sympodially, pyriform to obclavate, narrowed toward tip, rounded at the base, 2-septate, subhyaline to pale brown, with a distinct protruding basal hilum, and minute marginal frill.
Type species: Neopyricularia commelinicola (M.J. Park & H.D. Shin) Klaubauf, Lebrun & Crous
Neopyricularia commelinicola (M.J. Park & H.D. Shin) Klaubauf, Lebrun & Crous, comb. nov. MycoBank MB810507. Fig. 9.
Fig. 9.
Neopyricularia commelinicola (CBS 128308). A. Sporulation on sterile barley seed on SNA. B. Conidiophores and conidia. C. Conidia. Scale bars = 10 μm.
Basionym: Pyricularia commelinicola M.J. Park & H.D. Shin, Mycotaxon 108: 452. 2009.
Description: Park & Shin (2009).
Materials examined: South Korea, Hongcheon, Bukbang-ri, 37°48′1″ N, 127°51′9″ E, on leaves of Commelina communis, 9 Sep. 2007, H.D. Shin & M.J. Park (holotype KUS (F) 22838, culture ex-type CBS 128308 = KACC 43081); Hongcheon, on C. communis, 30 June 2009, H.D. Shin & M.J. Park, CBS 128303 = KACC 44637; Pocheon, on C. communis, 29 July 2008, M.J. Park, CBS 128306 = KACC 43869; Hongcheon, on C. communis, 27 Oct. 2008, H.D. Shin & M.J. Park, CBS 128307 = KACC 44083.
Notes: Characteristic for this species is its long, flexuous, branched, pale brown, smooth conidiophores, with a terminal rachis, with terminal and intercalary conidiogenous cells with denticle-like loci that are 2–3 μm long and wide, not thickened, but trapping air (also in conidial hila), so appearing thickened. Conidia are pyriform to obclavate, subhyaline to pale brown, 2-septate, (27–)30–38(–40) × (9–)10–11(–13) μm (on SNA). Phylogenetically P. commelinicola does not cluster within clades corresponding to species of Pyricularia s. str. (Figs 2, 3), and hence a new genus is introduced to accommodate it.
Proxipyricularia Klaubauf, Lebrun & Crous, gen. nov. MycoBank MB810211.
Etymology: Named after the fact that it is morphologically similar to the genus Pyricularia.
Plant pathogenic. Conidiophores solitary or in fascicles, subcylindrical, erect, olivaceous to medium brown, smooth, septate. Conidiogenous cells terminal and intercalary, pale brown, with denticulate conidiogenous loci and rhexolitic secession. Conidia solitary, formed sympodially, pyriform to obclavate, narrowed toward tip, rounded at the base, 2-septate, subhyaline to pale brown, with a distinct protruding basal hilum, frequently with minute marginal frill.
Type species: Proxipyricularia zingiberis (Y. Nisik.) Klaubauf, Lebrun & Crous
Note: Proxipyricularia is morphologically similar to Pyricularia, but phylogenetically distinct (Figs 2, 3).
Proxipyricularia zingiberis (Y. Nishik.) Klaubauf, Lebrun & Crous, comb. nov. MycoBank MB810212. Fig. 10.
Fig. 10.
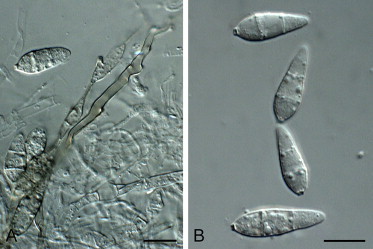
Proxipyricularia zingiberis (CBS 133594). A. Conidiophore forming on SNA. B. Conidia. Scale bars = 10 μm.
Basionym: Pyricularia zingiberis Y. Nishik. (as “Piricularia zingiberi”), Ber. Ohara Inst. Landwirt. Forsch. 1(2): 216. 1917.
On SNA on sterile barley seed. Conidiophores solitary or in fascicles, subcylindrical, erect, olivaceous to medium brown, smooth, 2–4-septate, 50–180 × 1.5–4 μm. Conidiogenous cells terminal and intercalary, pale brown, with denticulate conidiogenous loci and rhexolitic secession. Conidia 14–20(–24) × (5–)6–8(–9.5) μm, apical cell 5–8 μm long, basal cell 5–7 μm long, solitary, pyriform to obclavate, narrowed toward tip, rounded at the base, 2-septate, subhyaline to pale brown, with a distinct protruding basal hilum and marginal frill.
Materials examined: Japan, Hyogo, on Zingiber mioga, 2002, H. Kato, CBS 133594 = MAFF 240222 = HYZiM201-0-1(Z-2J); location unknown, on Zingiber officinale, Jan 1939, Y. Nisikado, CBS 303.39 = MUCL 9449; Hyogo, on Zingiber mioga, 2003, I. Chuma, CBS 132195 = MAFF 240224 = HYZiM201-1-1-1(Z-4J); Hyogo, on Zingiber mioga, 2003, I. Chuma, CBS 132196 = MAFF 240223 = HYZiM202-1-2(Z-3J); Hyogo, on Zingiber mioga, 1990, M. Ogawa, CBS 132355 = MAFF 240221 = HYZiM 101-1-1-1(Z-1J).
Notes: Proxipyricularia zingiberis is phylogenetically distant (Figs 2, 3) from Pyricularia s. str., although morphologically, it appears similar, with medium brown conidiophores and a terminal and intercalary denticulate rachis, and subhyaline, 2-septate, obclavate conidia. Isolates of P. zingiberis from Zingiber mioga and Z. officinale are able to infect both plants, but not Oryza, Setaria or Panicum spp. (Nishikado 1917, Kato et al. 2000). Nishikado (1917) regarded the fungus from Zingiber as genetically distant from Pyricularia species isolated from rice or other Poaceae, as well as (Kato et al. 2000) using RFLP patterns and (Hirata et al. 2007) using multilocus sequence analysis.
Pseudopyricularia Klaubauf, Lebrun & Crous, gen. nov. MycoBank MB810213.
Etymology: Named after its morphological similarity to Pyricularia.
Plant pathogenic. Mycelium consisting of smooth, hyaline, branched, septate hyphae. Conidiophores solitary, erect, straight or curved, branched or not, medium brown, finely roughened, septate. Conidiogenous cells integrated, terminal, rarely intercalary, medium brown, finely roughened, forming a rachis with several protruding, flat-tipped denticles. Conidia solitary, obclavate, pale to medium brown, finely roughened, guttulate, 2-septate; hilum truncate, slightly protruding, unthickened, not darkened.
Type species: Pseudopyricularia kyllingae Klaubauf, Lebrun & Crous
Notes: Several isolates previously identified as representative of P. higginsii were found to belong to a complex of three related species (Fig. 3) classified into Pseudopyricularia (P. cyperi, P. kyllingae and P. higginsii). Taxa in this complex are primarily distinguished from Pyricularia s. str. by having short, determinate, brown conidiophores with an apical rachis with flat-tipped denticles. It was also based on this character, that Ellis (1976) originally suspected P. higginsii to represent a species of Dactylaria.
Pseudopyricularia cyperi Klaubauf, Lebrun & Crous, sp. nov. MycoBank MB810214. Fig. 11.
Fig. 11.
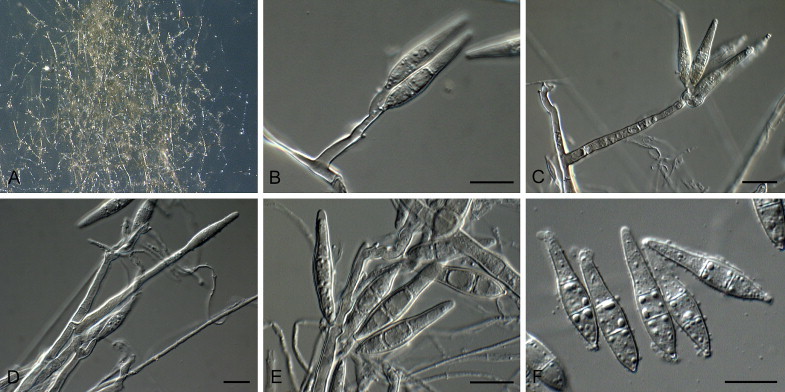
Pseudopyricularia cyperi (CBS 133595). A. Sporulation on SNA. B–E. Conidiophores. F. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Cyperus.
On SNA on sterile barley seed. Mycelium consisting of smooth, hyaline, branched, septate hyphae, 1–2 μm diam. Conidiophores solitary, erect, straight or curved to geniculate, branched, medium brown, smooth, 40–100 × 3–4 μm, 1–5-septate. Conidiogenous cells 35–70 × 3–4 μm, integrated, terminal and intercalary, pale brown, smooth, forming a rachis with several protruding, flat-tipped denticles, 2–3 μm long, 1.5–2 μm diam. Conidia solitary, obclavate, medium brown, smooth to finely roughened, granular and guttulate, 2-septate, (22–)25–28(–35) × (4–)5(–6) μm; apical cell 12–17 μm long, basal cell 7–9 μm long; hilum truncate, slightly protruding, 1.5–2 μm diam, unthickened, not darkened.
Culture characteristics: Colonies on MEA buff, round, raised, cottony, reaching up to 1.8 cm diam after 1 wk; reverse ochreous. On CMA and OA transparent, round to undulate colonies with smooth surface. Colonies on PDA white, round, diffuse edge, cottony, reaching up to 2.2 cm diam after 1 wk; reverse buff.
Materials examined: Israel, on Cyperus rotundus, date unknown, R. Kenneth, specimen CBS H-17647, culture CBS 665.79. Japan, Hyogo, on Cyperus iria, 2002, H. Kato (holotype CBS H-21840, culture ex-type CBS 133595). Philippines, Sto Tomas, Batangas, on Cyperus rotundus, 1983, IRRI collector unknown, CR88383 (Borromeo et al. 1993) = PH0053.
Notes: The distinguishing character of this species is its conidiophores that are commonly branched, forming a rachis with flat-tipped denticles. Morphologically it is similar to P. higginsii, except that conidia are longer and narrower in culture (26.1–28.6 × 6–6.1 μm; av. 26.1 × 6.1 μm) (Luttrell 1954).
Pseudopyricularia higginsii (Luttr.) Klaubauf, Lebrun & Crous, comb. nov. MycoBank MB810215.
Basionym: Pyricularia higginsii Luttr., Mycologia 46: 810. 1954.
≡ Dactylaria higginsii (Luttr.) M.B. Ellis, Dematiaceous Hyphomycetes (Kew): 173. 1976.
Material examined: New Zealand, Auckland, Mount Albert, Carrington Road, UNITEC Technical Institute, on dead leaves of Typha orientalis, 30 Apr. 2007, C.F. Hill, specimen in PDD, culture CBS 121934.
Notes: Pyricularia higginsii was originally described from Cyperus sp. in Georgia (Luttrell 1954). Conidiophores were described as being 3-septate, up to 76 um long, while conidia were 2-septate, 17.5–36.5 × 5.3–6.5 μm (av. 28 × 6 μm), in culture 26.1–28.6 × 6–6.1 μm (av. 26.1 × 6.1 μm) (Luttrell 1954). Species in the Pseudopyricularia higginsii complex are all very similar based on their conidial dimensions, and fresh collections from Georgia would be required to resolve the phylogeny of P. higginsii.
Pseudopyricularia kyllingae Klaubauf, Lebrun & Crous, sp. nov. MycoBank MB810218. Fig. 12.
Fig. 12.
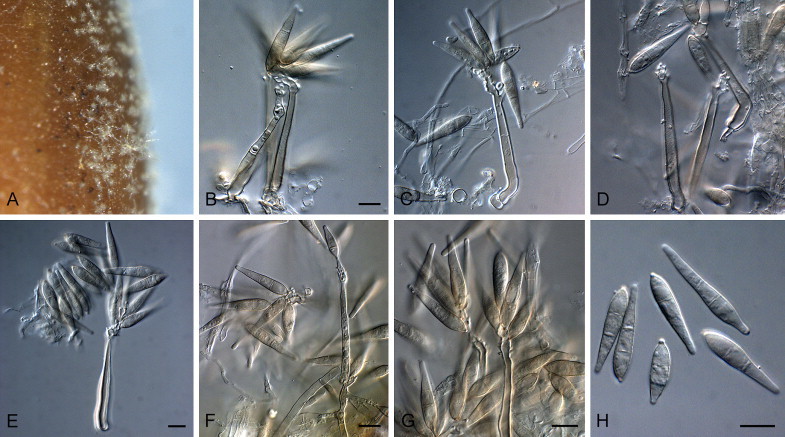
Pseudopyricularia kyllingae (CBS 133597). A. Sporulation on sterile barley seed on SNA. B–G. Conidiophores and conidia. H. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Kyllinga.
On SNA on sterile barley seed. Mycelium consisting of smooth, hyaline, branched, septate hyphae, 1.5–2 μm diam. Conidiophores solitary or in fascicles of 2–3, erect, straight or curved, branched or not, medium brown, finely roughened, 50–80 × 4–6 μm, 1–3-septate. Conidiogenous cells 15–60 × 3–4 μm, integrated, terminal, rarely intercalary, medium brown, finely roughened, forming a rachis with several protruding, flat-tipped denticles, 1–2 μm long, 1–1.5 μm diam. Conidia solitary, obclavate, pale to medium brown, finely roughened, guttulate, 2-septate, (23–)27–30(–35) × (5–)6(–7) μm; apical cell 12–20 μm long, basal cell 9–10 μm long; hilum truncate, slightly protruding, 1–1.5 μm diam, unthickened, not darkened.
Culture characteristics: Colonies on MEA transparent, funiculate, reaching up to 6.5 cm diam after 1 wk; reverse ochreous. On CMA transparent smooth colony, reaching up to 5 cm diam after 1 wk. On PDA transparent colony, plate covering after 1 wk; transparent reverse.
Materials examined: Japan, Hyogo, on Kyllinga brevifolia, 2003, I. Chuma (holotype CBS H-21841, culture ex-type CBS 133597). Philippines, Los Banos, Laguna, on Cyperus brevifolius, 1989, IRRI collector unknown, CB8959 (Borromeo et al. 1993) = PH0054.
Note: Morphologically similar to P. higginsii (26.1–28.6 × 6–6.1 μm; av. 26.1 × 6.1 μm sensu Luttrell 1954), except that conidia of P. kyllingae (23–35 × 5–7 μm; av. 29 × 6 μm) are longer in culture.
Pyricularia Sacc., Michelia 2(no. 6): 20. 1880.
Plant pathogenic. Conidiophores solitary or in fascicles, subcylindrical, erect, brown, smooth, rarely branched, with sympodial proliferation. Conidiogenous cells terminal and intercalary, pale brown, with denticulate conidiogenous loci and rhexolytic secession. Conidia solitary, pyriform to obclavate, narrowed toward tip, rounded at the base, 2-septate, hyaline to pale brown, with a distinct basal hilum, sometimes with marginal frill. Ascomata perithecial, solitary to gregarious, subspherical, brown to black, base immersed in host tissue, with long neck protruding above plant tissue; wall consisting of several layers of brown textura angularis. Asci 8-spored, hyaline, subcylindrical to clavate, unitunicate, short-stipitate, with prominent apical ring. Paraphyses intermingled among asci, unbranched, septate. Ascospores bi- to multiseriate in asci, hyaline, guttulate, smooth-walled, fusiform, curved with rounded ends, transversely 3-septate, slightly constricted at septa.
Type species: Pyricularia grisea Sacc., Michelia 2(no. 6): 20. 1880.
Pyricularia ctenantheicola Klaubauf, Lebrun & Crous, sp. nov. MycoBank MB810219. Fig. 13.
Fig. 13.
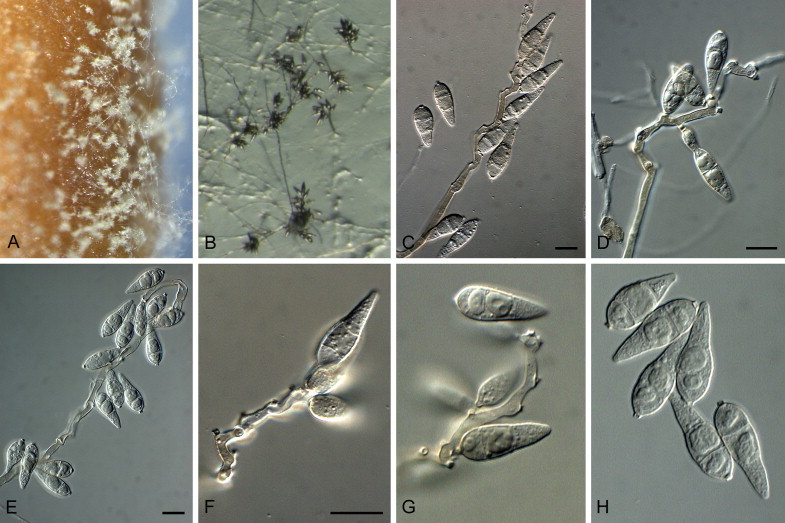
Pyricularia ctenantheicola (GR0002). A. Sporulation on sterile barley seed on SNA. B. Sporulation on SNA. C–G. Conidiophores and conidia. H. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Ctenanthe.
On SNA on sterile barley seed. Mycelium consisting of smooth, hyaline, branched, septate hyphae, 1.5–2 μm diam. Conidiophores solitary, erect, straight or curved, branched or not, medium brown, smooth, 70–200 × 3–5 μm, 1–6-septate; base bulbous, lacking rhizoids, 7–10 μm diam. Conidiogenous cells 40–110 × 3–5 μm, integrated, terminal and intercalary, pale brown, smooth, with several protruding denticles, 1–2 μm long, 1–1.5 μm diam. Conidia solitary, pyriform to obclavate, pale brown, finely roughened, granular to guttulate, 2-septate, (19–)20–24(–33) × (6–)7(–8) μm; apical cell 7–10 μm long, basal cell 5–7 μm long; hilum truncate, 0.5–1.5 μm long, 1.5–2 μm diam, unthickened, not darkened.
Culture characteristics: Colonies on MEA white to vinaceous buff, cottony, with undulating margin, reaching up to 2.7 cm diam after 1 wk; reverse ochreous to umber. Colonies on CMA pale luteous, with hazel centre, reaching up to 2.5 cm diam after 1 wk. Colonies on PDA hazel, with smoke grey tufts, reaching up to 3.5 cm diam after 1 wk; reverse hazel. Colonies on OA reaching up to 3.5 cm after 1 wk, sporulating abundantly after 1 wk in the dark.
Materials examined: Greece, Almyros, on Ctenanthe oppenheimiana imported from Brazil via Netherlands, 1998, A.C. Pappas & E.J. Paplomatas (holotype CBS H-21842, culture ex-type CBS 138601 = GR0002); ibid., GR0001 = Ct-4 = ATCC 200218.
Note: Although the leaf spot disease of Ctenanthe has previously been reported (Pappas & Paplomatas 1998), the fungus was never officially named.
Pyricularia grisea Sacc., Michelia 2(no. 6): 20. 1880. Fig. 14.
Fig. 14.
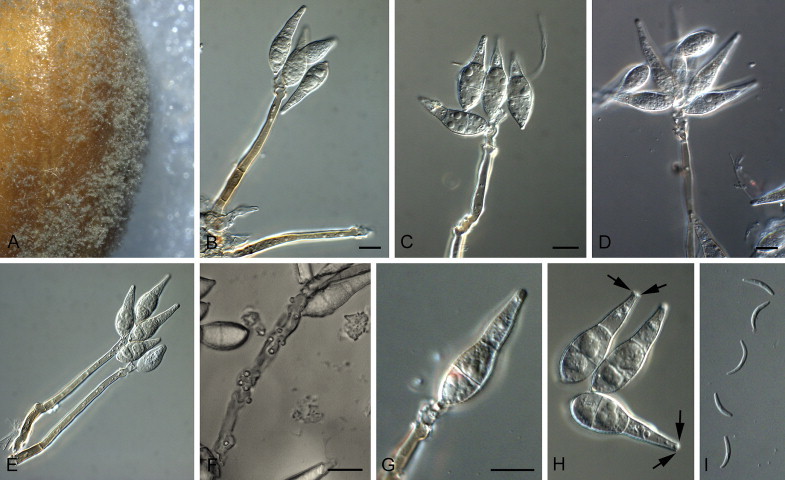
Pyricularia grisea (BR0029). A. Sporulation on sterile barley seed on SNA. B–G. Conidiophores and conidia. H. Macroconidia (arrows indicate apical marginal frill, which is a remnant of the apical mucoid cap). I. Microconidia. Scale bars = 10 μm.
Basionym: Ceratosphaeria grisea T.T. Hebert, Phytopathology 61(1): 86. 1971.
= Magnaporthe grisea (T.T. Hebert) M.E. Barr, Mycologia 69(5): 954. 1977.
Materials examined: Brazil, on Digitaria horizontalis, date and collector unknown, Br33; Goias, Goiana, on Digitaria sanguinalis, 1989, J.-L. Nottéghem, BR0029. Japan, on Digitaria smutsii, date and collector unknown, JP0034 = NI980. Korea, Woanju, on Echinochloa crus-galli var. frumentacea, date unknown, H.K. Sim, CBS 128304 = KACC 41641. Philippines, Sto Tomas, Batangas, on Digitaria ciliaris, 1988, IRRI collector unknown, Dc88420 (Borromeo et al. 1993) = PH0055. South Korea, Suwon, on Lolium perenne, 1991, C.K. Kim, CR0024. USA, Delaware, on Digitaria sp., 1991, B. Valent, US0043 = G-184.
Note: Isolates of P. grisea were observed to form apical mucilaginous droplets on their macroconidia in culture, as well as produce microconidia on SNA, as observed previously in P. oryzae (Chuma et al. 2009, Zhang et al. 2014).
Pyricularia oryzae Cavara, Fung. Long. Exsicc. 1: no. 49. 1892. Fig. 15.
Fig. 15.
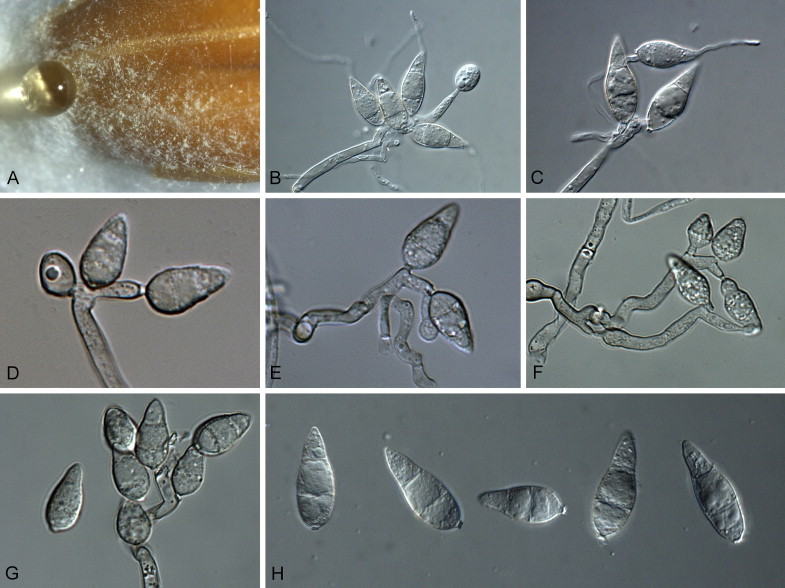
Pyricularia oryzae (BF0028). A. Sporulation on sterile barley seed on SNA. B–G. Conidiophores and conidia. H. Conidia. Scale bars = 10 μm.
= Magnaporthe oryzae B.C. Couch, Mycologia 94(4): 692. 2002.
Materials examined: Brazil, on Triticum aestivum, 1989, J.-L. Nottéghem, BR0032, BR0045. Burkina Faso, on Paspalum sp., 1990, collector unknown, BF0028 = CBS 138602. Côte d'Ivoire, Bouaké, on Leersia hexandra, 1983, J.-L. Nottéghem, CD0067; Ferkessédougou, on Eleusine indica, 1989, J.-L. Nottéghem, CD0156. Egypt, on Oryza sativa, date and collector unknown, CBS 657.66. France, Camargue, on Oryza sativa, 1988, J.-L. Nottéghem, FR0013. French Guyana, on Oryza sativa, 1978, J.-L. Nottéghem, Guy11 = FGSC 9462. Gabon, Wey, on Zea mays, 1985, J.-L. Nottéghem, GN0001. India, Uttar Pradesh, on Setaria sp., date unknown, J. Kumar, IN0108. Israel, Masmiah, on Echinochloa crus-galli, date and collector unknown, CBS 658.66; Rishon-le-Zien, on Stenotaphrum secundatum, date and collector unknown, CBS 659.66. Japan, on Eragrostis curvula, 1983, H. Kato, JP0038; on Eriochloa villosa, date and collector unknown, JP0033; on Phalaris arundinacea, date and collector unknown, JP0040; on Anthoxanthum odoratum, date and collector unknown, JP0039; on Eleusine indica, 1974, H. Yaegashi, JP0017; on Eragrostis curvula, 1976, H. Yaegashi, JP0028; Nagano, host, date and collector unknown, CBS 365.52 = MUCL 9451. Philippines, Los Banos, Laguna, on Brachiaria mutica, 1983 IRRI collector unknown, BmA8309 (Borromeo et al. 1993) = PH0035 = PH0075; Cabanatuan, Nueva Ecija, on Cynodon dactylon, 1988, IRRI collector unknown, Cd88215 (Borromeo et al. 1993) = PH0051; on Echinochloa colona, 1982, IRRI collector unknown, PH0077 = Ec8202; Los Banos, Laguna, on Leptochloa chimensis, 1984, IRRI collector unknown, Lc8401 (Borromeo et al. 1993) = PH0060; on Oryza sativa, 1980, IRRI collector unknown, PO6-6 (Wang et al. 1994) = PH0014; on Panicum repens, 1982, J. M. Bonmam, Pr8212 = PH0079; Cabanatuan, Nueva Ecija, on Paspalum distichum, 1988, IRRI collector unknown, Pd8824 (Borromeo et al. 1993) = PH0062; Los Banos, Laguna, on Rottboellia exalta, 1984, IRRI collector unknown, ReA8401(Borromeo et al. 1993) = PH0063 = ATCC 62619. Portugal, on Stenotaphrum secondatum, 1992, A. Lima, PR0067, PR0104. Romania, no further details, CBS 255.38. Rwanda, Kunynya, on Eleusine coracana, 1990, J.-L. Nottéghem, RW0012. South Korea, Suwon, on Festuca elalior, date unknown, C.K. Kim, CR0029; Suwon, on Lolium hybridum, 1991, C.K. Kim, CR0026; Suwon, on Phleum pratense, 1991, C.K. Kim, CR0020; Yongin, on Panicum miliaceum, date unknown, C.K. Kim, CR0021. USA, Kentucky, on Setaria viridis, 1998, M. Farman, US0071. Vietnam, Ô Môn, on Leersia hexandra, 2002, B. Couch, VT0032. Unknown, no collection details, CBS 375.54; on Oryza sativa, date and collector unknown, 70-15 = ATCC MYA-4617 = FGSC 8958; laboratory strain, progeny from a cross between strains with different host specificity, CBS 433.70.
Pyricularia penniseticola Klaubauf, Lebrun & Crous, sp. nov. MycoBank MB810220. Fig. 16.
Fig. 16.

Pyricularia penniseticola (ML0031). A. Sporulation on SNA. B–G. Conidiophores and conidia. H. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Pennisetum.
On SNA on sterile barley seed. Mycelium consisting of smooth, hyaline, branched, septate hyphae, 1.5–2 μm diam. Conidiophores solitary, erect, straight or curved, frequently branched, medium brown, smooth, 100–350 × 4–6 μm, multi-septate; base bulbous, lacking rhizoids. Conidiogenous cells 40–130 × 3–4 μm, integrated, terminal and intercalary, pale brown, smooth, forming a rachis with several protruding denticles, 1–2 μm long, 1–1.5 μm diam, with rhexolytic secession. Conidia solitary, pyriform to obclavate, pale brown, finely roughened, granular to guttulate, 2-septate, (23–)25–30(–35) × (8–)9(–10) μm; apical cell 9–13 μm long, basal cell 7–10 μm long; attenuated towards a truncate hilum, 0.5–1 μm long, 1.5–2 μm diam, with minte marginal frill.
Culture characteristics: Colonies on MEA pale olivaceous grey, cottony, reaching up to 3 cm diam after 1 wk; reverse olivaceous-black. Colonies on CMA reaching up to 3 cm diam after 1 wk. Colonies on PDA iron-grey, reaching up to 4.5 cm diam after 1 wk, reverse olivaceous-black. Colonies on OA up to 3.6 cm diam after 1 wk, surface sectoring.
Materials examined: Burkina Faso, Kamboinse (Guaga), Pennisetum typhoides, 27 Sept. 1990, J.-L. Nottéghem, BF0017. Côte d'Ivoire, Bouake, P. typhoides, 1 Dec. 1983, J.-L. Nottéghem, CD0086; Odienne, Digitaria exilis, 1 Oct. 1989, J.-L. Nottéghem, CD0143; Madiani, Pennisetum sp., 17 Oct. 1991, J.-L. Nottéghem, CD0180. Mali, Segou field 2, D. exilis, 17 Oct. 1993, J.-L. Nottéghem, ML048; Longorola Sikasso, on P. typhoides, 14 Sept. 1990, J.-L. Nottéghem (holotype CBS H-21843, culture ex-type ML0031 = CBS 138603).
Pyricularia pennisetigena Klaubauf, Lebrun & Crous, sp. nov. MycoBank MB810221. Fig. 17.
Fig. 17.

Pyricularia pennisetigena (ML0036). A. Sporulation on sterile barley leaf on SNA. B–G. Conidiophores and conidia. H. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Pennisetum.
On SNA on sterile barley seeds. Mycelium consisting of smooth, hyaline, branched, septate hyphae, 1.5–2 μm diam. Conidiophores solitary, erect, straight or curved, unbranched, medium brown, smooth, 60–150 × 4–6 μm, 2–3-septate; base arising from hyphae, not swollen, lacking rhizoids. Conidiogenous cells 40–95 × 3–5 μm, integrated, terminal and intercalary, pale brown, smooth, forming a rachis with several protruding denticles, 0.5–1 μm long, 1.5–2 μm diam. Conidia solitary, pyriform to obclavate, pale brown, smooth, granular to guttulate, 2-septate, (25–)27–29(–32) × (8–)9(–10) μm; apical cell 10–13 μm long, basal cell 6–9 μm long; hilum truncate, protruding, 1–1.5 μm long, 1.5–2 μm diam, unthickened, not darkened.
Culture characteristics: Colonies on MEA cottony to velvety, buff, smoke grey, with broad white rim, reaching up to 4.8 cm diam after 1 wk; reverse iron grey with pale margin. Colonies on CMA buff with grey dots, reaching up to 5.0 cm diam after 1 wk. Colonies on OA buff, reaching up to 5.0 cm diam after 1 wk, sporulating after 4 d in the dark. Colonies on PDA fuscous black with grey centre, and broad white rim, flat, erose, reaching up to 5.0 cm diam after 1 wk; reverse brown.
Materials examined: Brazil, on Cenchrus echinatus, date unknown, S. Igarashi, Br36; Imperatriz, on C. echinatus, 28 Feb. 1990, collector n.a., BR0067; Primeiro de Maio, on Echinochloa colona, 1 Apr. 1990, H. Kato, BR0093. Japan, Kumamoto, on Cenchrus ciliaris, 1975, N. Nishihara, CBS 133596 = MAFF 305501 = NI981(Cc-1J). Mali, Cinzana, on Pennisetum sp., 19 Sept. 1990, J.-L. Nottéghem (holotype CBS H-21844, culture ex-type ML0036 = CBS 138604). Philippines, Plaridel, Bucalan, on Cenchrus echinatus, 1988, IRRI collector unknown, Ce88454 (Borromeo et al. 1993) = PH0047. USA, Tifton, Pennisetum glaucum, 1983, H. Wells, US0044 = 83P-25, Tifton, Pennisetum glaucum, 1984, H Wells, US0045 = 84P-19 (Kang et al. 1995).
Notes: Another forgotten species on this host is P. penniseti (Prasada & Goyal 1970). Pyricularia penniseti was described as having conidia that are pyriform and 2-septate, 18.4–36.7 × 7.4–11 μm. In spite of differences in conidial dimensions to P. penniseticola and P. pennisetigena, no cultures are presently available to determine if it would also be distinct on a phylogenetic basis.
Pyricularia zingibericola Klaubauf, Lebrun & Crous, sp. nov. MycoBank MB810222. Fig. 18.
Fig. 18.
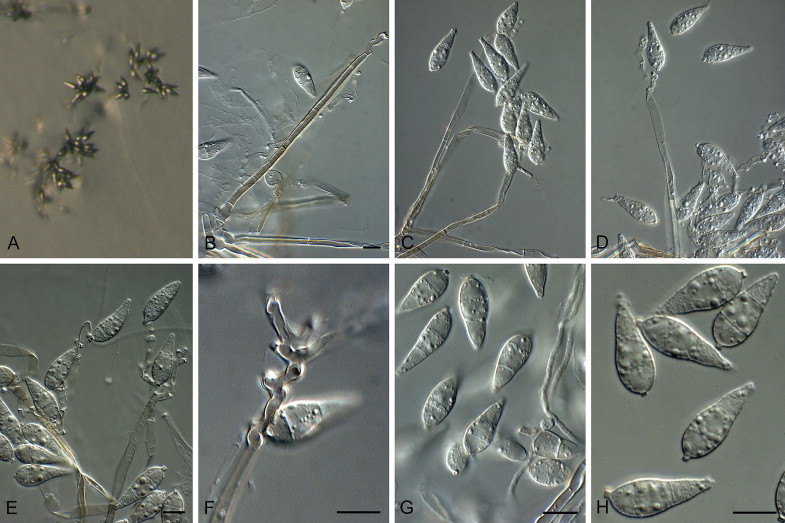
Pyricularia zingibericola (RN0001). A. Sporulation on SNA. B–F. Conidiophores and conidia. G, H. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Zingiber.
On SNA on sterile barley seed. Mycelium consisting of smooth, hyaline, branched, septate hyphae, 1.5–2 μm diam. Conidiophores solitary, erect, straight or curved, branched or not, medium brown, smooth, 100–200 × 4–6 μm, 3–8-septate; base bulbous, lacking rhizoids, 5–7 μm diam. Conidiogenous cells 45–70 × 3–4 μm, integrated, terminal and integrated, pale brown, smooth, with several protruding apical denticles, 1–1.5 μm long, 1–2 μm diam. Conidia solitary, pyriform to obclavate, pale brown, smooth to finely roughened, guttulate, 2-septate, (18–)20–23(–25) × (7–)8(–10) μm; apical cell 8–10 μm long, basal cell 5–7 μm long; hilum truncate, protruding, 0.5–1 μm long, 1.5–2 μm diam, unthickened, not darkened.
Culture characteristics: Colonies on MEA transparent to white with leaden grey centre, sulcate colony with entire edge, some irregular tufts, sporulating in centre, reaching up to 4 cm diam after 1 wk; reverse pale with olivaceous grey centre. Colonies on OA white with some dark spots, greenish olivaceous in centre, flat, smooth, cotton-like surface, reaching up to 4.5 cm diam after 1 wk. Colonies on CMA grey olivaceous to olivaceous black with olivaceous grey centre, entire edge, flat colony, slightly wool-like surface, reaching up to 4 cm diam after 1 wk. Colonies on PDA transparent with some greenish olivaceous parts, white centre, umbonate, powdery surface in centre, reaching up to 4.5 cm diam after 1 wk; reverse greenish olivaceous.
Material examined: Réunion, on Zingiber officinale, J.-C. Girard (holotype CBS H-21845, culture ex-type RN0001 = CBS 138605).
Notes: Pyricularia zingibericola, which appears to be unique on Zingiber, has smaller conidia than P. leersiae (20–)27(–35) × (7–)8.6(–10) μm, which is also known to occur on Leersia (Hashioka 1973). Presently no cultures of P. leersiae are available to facilitate a molecular comparison.
Xenopyricularia Klaubauf, Lebrun & Crous, gen. nov. MycoBank MB810223.
Etymology: Named after its morphological similarity to Pyricularia.
Plant pathogenic. Conidiophores solitary or in fascicles, subcylindrical, erect, medium brown, smooth, flexuous, branched, with sympodial growth. Conidiogenous cells terminal and intercalary, pale brown, denticulate conidiogenous loci. Conidia solitary, formed sympodially, obovoid, narrowed toward tip, rounded at the base, 2-septate, pale brown, with central cell appearing slightly darker brown, with a distinct protruding basal hilum.
Type species: Xenopyricularia zizaniicola (Hashioka) Klaubauf, Lebrun & Crous
Xenopyricularia zizaniicola (Hashioka) Klaubauf, Lebrun & Crous, comb. nov. MycoBank MB810224. Fig. 19.
Fig. 19.
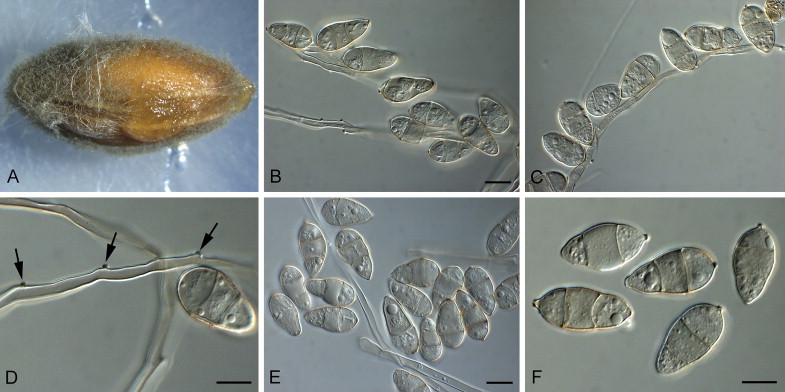
Xenopyricularia zizaniicola (CBS 133593). A. Sporulation on sterile barley seed on SNA. B–D. Conidiophores and conidia (arrows indicate conidiogenous loci in D). E, F. Conidia. Scale bars = 10 μm.
Basionym: Pyricularia zizaniicola Hashioka (as “zizaniaecola”), Trans. Mycol. Soc. Japan 14(3): 264. 1973.
≡ Pyricularia zizaniicola Hashioka (as “zizaniaecola”), Res. Bull. Fac. Agr. Gifu Univ. 29: 21. 1970. (nom. nud.)
Description and illustration: Hashioka (1973).
Materials examined: Japan, Gifu, on Zizania latifolia, 15 Sep. 1967, Y. Hashioka (holotype presumably lost); Ibaraki, on Zizania latifolia, 1985, N. Hayashi, (neotype designated here CBS H-21846, culture ex-neotype CBS 133593 = MAFF 240219 = IBZL3-1-1(Zz-1J)); Kyoto, on Zizania latifolia, 2003, K. Yoshida & K. Hirata, CBS 132356 = MAFF 240220 = KYZL201-1-1(Zz-2J).
Notes: Xenopyricularia zizaniicola has long, flexuous, pale brown, branched conidiophores. Conidia are brown, 2-septate, obovoid, (22–)25–28(–35) × (12–)13(–14) μm (on SNA), with a small protruding hilum, 0.5–1 μm long, 1 μm diam. Morphologically Xenopyricularia resembles Pyricularia, except that its conidia are very wide and more obovoid than are typical Pyricularia conidia, and some appear to be irregularly pigmented. The present culture corresponds very well with the original description and illustrations provided by Hashioka (1973), who cited conidia as being (24–)27.7(–33) × (10.5–)13.5(–15.5) μm, and is therefore designated as neotype.
Another forgotten species on this host is Pyricularia zizaniae Hara, (as “Piricularia”) Trans. Shizuoka Agric. Soc. 336: 29. 1925. Translated from Japanese: “Leaf spots small, circular, later elongate, brown, ellipsoid to fusiform, finaly greyish brown with brown border, 2–8 × 2–6 mm. Caespituli mainly hypophyllous, sooty-coloured. Conidiophores linear, 60–130 × 2.5–4 μm, rarely branched, solitary or densely fasciculate, dark brown and swollen at the base, paler and attenuate toward the apex, geniculate at the apex. Conidia pyriform to clavate, rounded at base, attenuate at apex, 1–2-septate, not constricted at septa, protruding at base, hyaline to pale smoky in colour. Notes: When it was inoculated onto rice, it was not pathogenic. This disease was observed in shaded area”. Pyricularia zizaniae has conidia that are described as being 1–2-septate, (18–)22(–28) × 7(–10) μm. No cultures are available, however, to determine if it could represent a second species of Xenopyricularia.
Sordariales, incertae sedis
Rhexodenticula W.A. Baker & Morgan-Jones, Mycotaxon 79: 363. 2001.
Mycelium immersed and superficial, consisting of branched, septate, pale brown to brown, smooth hyphae that become verruculose. Conidiophores solitary, erect, subcylindrical, straight or curved, unbranched, medium brown, finely verruculose, septate. Conidiogenous cells integrated, terminal, subclavate, pale brown, finely verruculose, forming a rachis with several protruding denticles, and rhexolytic secession. Conidia solitary, fusoid-ellipsoidal, finely verruculose, medium brown, guttulate, 3-septate; base rounded, hilum truncate, slightly protruding, with minute marginal frill.
Type species: Rhexodenticula cylindrospora (R.F. Castañeda, Saikawa & Hennebert) W.A. Baker & Morgan-Jones
Notes: An isolate deposited at CBS as Pyricularia lauri (CBS 244.95, on leaf litter of Nectandra antillana, Cuba) was morphologically identical to the ex-type isolate of Rhexodenticula cylindrospora (CBS 318.95, also isolated from leaf litter of Nectandra antillana, Cuba). Although the phylogenetic position of the genus is still unclear, it does not belong to the Magnaporthaceae, but appears to be sister to Boliniales and Sordariales (Fig. 1).
Rhexodenticula cylindrospora (R.F. Castañeda, Saikawa & Hennebert) W.A. Baker & Morgan-Jones, Mycotaxon 79: 363. 2001. Fig. 20.
Fig. 20.

Rhexodenticula cylindrospora (CBS 318.95). A. Sporulation on SNA. B–G. Conidiophores and conidia. H, I. Conidia. Scale bars = 10 μm.
Basionym: Nakataea cylindrospora R.F. Castañeda, Saikawa & Hennebert, Mycotaxon 59: 457. 1996.
On SNA on sterile barley seed. Mycelium consisting of finely verruculose, hyaline, branched, septate hyphae, becoming brown and verruculose, 2.5–3 μm diam. Conidiophores solitary, erect, subcylindrical, straight or curved, unbranched, medium brown, finely verruculose, 40–90 × 4–5 μm, 1–6-septate. Conidiogenous cells 10–20 × 3–5 μm, integrated, terminal, subclavate, pale brown, finely verruculose, forming a rachis with several protruding denticles, 1 μm long and in diam, with rhexolytic secession. Conidia solitary, fusoid-ellipsoidal, finely verruculose, medium brown, guttulate, 3-septate, (15–)17–19(–20) × (4–)5(–6) μm; base rounded, hilum truncate, slightly protruding, 1 μm long and diam, with minute marginal frill.
Culture characteristics: Colonies on MEA mouse-grey, vinaceous buff at the margin, sulcate, velutinous, reaching up to 1.7 cm diam after 15 d; reverse isabelline with sepia centre. Colonies on OA dark mouse-grey with greenish black rim, undulate, funiculose, reaching up to 2.1 cm diam after 15 d. Colonies on PDA buff to honey, isabelline in centre, undulate, sulcate, reaching up to 1.5 cm diam after 15 d; reverse buff to honey, isabelline in centre.
Materials examined: Cuba, Pinar del Rio, leaf litter of Nectandra antillana, 9 Aug. 1994, R.F. Castañeda, culture ex-type CBS 318.95 = INIFAT C94/182; on leaf litter of N. antillana, 9 Aug. 1994, R.F. Castañeda & M. Saikawa, CBS 244.95 = INIFAT C94/182.
Discussion
Prior to this study, the Magnaporthales contained a single family, the Magnaporthaceae (Thongkantha et al. 2009). However, the elucidation of Nakataea as older name for Magnaporthe (Luo & Zhang 2013) justified a reevaluation of the genera included in this order, as many are quite extreme in their morphology and ecology. Based on the results of our phylogenetic analyses (Fig. 2), three clear clades could be distinguished, one corresponding to Magnaporthaceae (based on Nakataea), and two other clades corresponding to new families, Pyriculariaceae (based on Pyricularia), and Ophioceraceae (based on Ophioceras). The genus Pseudohalonectria, which clusters basal to these three families (Fig. 1) is polyphyletic (Thongkantha et al. 2009) and is closely related to species of Ceratosphaeria (Réblová 2006, Huhndorf et al. 2008, Thongkantha et al. 2009), but could not be treated due to a lack of cultures. These families have different ecological characteristics. Magnaporthaceae and Pyriculariaceae are mainly composed of plant pathogenic species, some of which are of major importance in plant pathology (Gaeumannomyces, Nakataea and Pyricularia). Ophioceraceae and Pseudohalonectria (incertae sedis) are mainly composed of aquatic or wood-associated saprobic species. Magnaporthaceae is distinguished from the Pyriculariaceae by their asexual morphs, which are phialophora- or harpophora-like, or with falcate versicoloured conidia on brown, erect conidiophores in the case of Magnaporthaceae, and Pyricularia or pyricularia-like, characterised by pyriform 2-septate conidia and rhexolytic secession, in the case of Pyriculariaceae. Although Ophioceras is morphologically similar to Gaeumannomyces, the two genera can be distinguished by the aquatic habit of Ophioceras, occurring on wood and herbaceous material, versus the plant pathogenic nature of Gaeumannomyces, which has harpophora-like asexual morphs, mycelial appressoria, and a perithecial peridium of textura epidermoidea (Walker 1980, Chen et al. 1999). The allocation of Ophioceras to the Magnaporthaceae has always been seen as a temporary measure, awaiting further study (Shearer 1989, Shearer et al. 1999). As shown in the present analyses (Fig. 2), Ophioceras clusters separate from the Magnaporthaceae and Pyriculariaceae in the Magnaporthales, and hence a separate family, the Ophioceraceae, had to be defined for these taxa. Several genera were distinguished in the Magnaporthaceae in the present study, namely Buergenerula, Bussabanomyces, Gaeumannomyces, Harpophora, Kohlmeyeriopsis, Magnaporthiopsis, Nakataea, Omnidemptus, Pyriculariopsis and Slopeiomyces. The Pyriculariaceae includes eight additional genera, namely Bambusicularia, Barretomyces, Deightoniella, Macgarvieomyces, Neopyricularia, Proxipyricularia, Pseudopyricularia and Xenopyricularia and four novel Pyricularia species.
Some previously published and rather broadly defined species of Pyricularia and Magnaporthe clustered outside these families. These include isolate CBS 244.95, which was originally identified as Pyricularia lauri, and is shown here to represent Rhexodenticula cylindrospora (incertae sedis) (Fig. 1). In addition, an isolate deposited at CBS as Pyricularia parasitica (CBS 376.54, sterile on SNA) clustered in the Chaetothyriales (Fig. 1), and sequences of Magnaporthe griffinii (ITS GenBank JQ390311, JQ390312) proved to be distant to the Sordariomycetes (not included).
The Magnaporthaceae phylogeny (Fig. 2) provided good support (BS = 100 %) for several genera that were included in the analysis, namely Magnaporthiopsis, Nakataea, and two new genera, Kohlmeyeriopsis (for Gaeumannomyces medullaris), and Slopeiomyces (for Gaeumannomyces cylindrosporus) except Gaeumannomyces pro parte. The genus Pyriculariopsis was omitted from the final analysis however, due to the lack of a RPB1 sequence.
The Pyriculariaceae phylogenies (Figs 2, 3) delineated Pyricularia from Deightoniella, as well as novel genera such as Bambusicularia (based on Bambusicularia brunnea), Barretomyces (based on Barretomyces calatheae = Pyriculariopsis calatheae), Macgarvieomyces (based on Macgarvieomyces borealis = Pyricularia borealis), Neopyricularia (based on Neopyricularia commelinicola = Pyricularia commelinicola), Proxi-pyricularia (based on Proxipyricularia zingiberis = Pyricularia zingiberis), Pseudopyricularia (based on Pseudopyricularia kyllingae), and Xenopyricularia (based on Xenopyricularia zizaniicola = Pyricularia zizaniicola).
Several new species were introduced in Pyricularia, namely P. ctenantheicola (occurring on Ctenanthe oppenheimiana in Greece), P. penniseticola (occurring on Digitaria exilis and Pennisetum typhoides in West African countries such as Burkina Faso, Ivory Coast, and Mali), P. pennisetigena (occurring on Cenchrus ciliaris, Cenchrus echinatus, Echinochloa colona and Pennisetum glaucum in Brazil, Japan, Mali, Philippines and the USA), and P. zingibericola (occurring on Zingiber officinale in Réunion Island). The surprising high number of undescribed Pyricularia species encountered in this study suggests that Pyricularia is actually a species-rich genus, and that sampling leaf spot diseases of different members of Poaceae could reveal many more novel taxa.
What started out as an investigation into the systematics of Pyricularia, not only delineated four novel species, but also several novel pyricularia-like genera. The genus Pyricularia is defined by having pale brown conidiophores and a terminal and intercalary denticulate rachis, and subhyaline, 2-septate, pyriform conidia (Yaegashi & Nihihara 1978, Murata et al. 2014). Surprisingly, the pyriform 2-septate conidial shape was also found for isolates from Neopyricularia (Fig. 3), whereas other Pyriculariaceae genera had conidia that varied in shape from obclavate to more ellipsoid. Other than conidial shape, it appears that conidial septation also varies among Pyriculariaceae species. Indeed, three species from two related genera (Deightoniella, Macgarviennomyces, Fig. 3) have 1-septate conidia. Since other related genera (Neopyricularia, Proxypyricularia, Pseudopyricularia) that are basal to Deightoniella and Macgarviennomyces (Fig. 3), have 2-septate conidia, it is likely that a common ancestor of these related genera had 2-septate conidia.
Our phylogenetic study showed that the host plant from which Pyricularia isolates were sampled could not be used as a taxonomic criterion, since the host range varied depending on the fungal species. For example, Pyricularia isolates sampled from infected leaves of Eleusine, Oryza, Setaria and Triticum were exclusively clustering in the P. oryzae clade (Table 1, Fig. 3). These isolates are known to be strictly host-specific, and to have a shared evolutionary origin (Tosa & Chuma 2014). The genetic groups (sub-species) underlying these host-specific forms could not be differentiated by the multilocus sequences used in this study, but were clearly delineated using additional genetic markers (Borromeo et al. 1993, Kato et al. 2000, Couch et al. 2005, Hirata et al. 2007, Choi et al. 2013, Saleh et al. 2014). On the contrary, isolates from host plants such as Cenchrus, Echinocloa, Lolium, Pennisetum and Zingiber belong to different Pyricularia clades corresponding to unrelated species. For example, isolates sampled from infected Pennisetum leaves in West Africa belong to two unrelated fungal species, P. pennisetigena and P. penniseticola (Fig. 3). Similarly, isolates sampled from infected Echinochloa leaves belong to three fungal species, P. oryzae, P. grisea and P. pennisetigena (Fig. 3). This could reflect that Echinochloa is infected by different Pyricularia species, as some P. oryzae isolates from rice are pathogenic to Echinochloa (Mackill & Bonham 1986, Serghat et al. 2005). It is therefore clear from this study that some host plants can be infected by more than one species of Pyricularia.
It would not be fitting to round off a paper on Pyricularia and Magnaporthe without commenting on the ongoing debate about generic names. The decision to allocate the rice pathogen M. salvinii to Nakataea, has reduced Magnaporthe to synonymy under Nakataea, rendering the family Magnaportaceae without the genus Magnaporthe. Although the genus Magnaporthe has proven to be polyphyletic, we would have advocated a different approach in view of stability for the application of this name in literature. Likewise, the same can be said for Pyricularia, which also turned out to be polyphyletic, forming a generic complex. Although we introduce several genera to address this heterogeneity, Pyricularia can fortunately be retained as a well-defined genus in the Pyriculariaceae.
Acknowledgements
We thank Prof. Yukio Tosa and Prof. Yong-Hwan Lee for providing cultures or DNA for phylogenetic analysis. We thank the technical staff, Arien van Iperen (cultures), Marjan Vermaas (photographic plates), and Mieke Starink-Willemse (DNA isolation, amplification and sequencing), as well as Federico Santoro and Alessandro Trotta (cultures, DNA isolation, amplification and sequencing) for their invaluable assistance.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
Contributor Information
P.W. Crous, Email: p.crous@cbs.knaw.nl.
M.-H. Lebrun, Email: marc-henri.lebrun@versailles.inra.fr.
References
- Borromeo E.S., Nelson R.J., Bonman J.M. Genetic differentiation among isolates of Pyricularia infecting rice and weed hosts. Phytopathology. 1993;83:393–399. [Google Scholar]
- Bussaban B., Lumyong S., Lumyong P. Three new species of Pyricularia are isolated as zingiberaceous endophytes from Thailand. Mycologia. 2003;95:519–524. [PubMed] [Google Scholar]
- Bussaban B., Lumyong S., Lumyong P. Molecular and morphological characterization of Pyricularia and allied genera. Mycologia. 2005;97:1002–1011. doi: 10.3852/mycologia.97.5.1002. [DOI] [PubMed] [Google Scholar]
- Cannon P.F. The newly recognized family Magnaporthaceae and its interrelationships. Systema Ascomycetum. 1994;13:25–42. [Google Scholar]
- Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Chen Q.H., Wang Y.C., Zheng X.B. Genetic analysis and molecular mapping of the avirulence gene PRE1, a gene for host-species specificity in the blast fungus Magnaporthe grisea. Genome. 2006;49:873–881. doi: 10.1139/g06-043. [DOI] [PubMed] [Google Scholar]
- Chen W., Shearer C.A., Crane J.L. Phylogeny of Ophioceras spp. based on morphological and molecular data. Mycologia. 1999;91:84–94. [Google Scholar]
- Choi J., Park S.-Y., Kim B.-R. Comparative analysis of pathogenicity and phylogenetic relationship in Magnaporthe grisea species complex. PLoS One. 2013;8:e57196. doi: 10.1371/journal.pone.0057196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuma I., Shinogi T., Hosogi N. Cytological characteristics of microconidia of Magnaporthe oryzae. Journal of General Plant Pathology. 2009;75:353–358. [Google Scholar]
- Constantinescu O. Deightoniella on Phragmites. Proceedings van de Koninklijke Nederlandse Akademie van Wetenschappen Section C. 1983;86:137–141. [Google Scholar]
- Couch B.C., Fudal I., Lebrun M.-H. Origins of host-specific populations of the blast pathogen Magnaporthe oryzae in crop domestication with subsequent expansion of pandemic clones on rice and weeds of rice. Genetics. 2005;170:613–630. doi: 10.1534/genetics.105.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch B.C., Kohn L.M. A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia. 2002;94:683–693. doi: 10.1080/15572536.2003.11833196. [DOI] [PubMed] [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Crous P.W., Groenewald J.Z., Shivas R.G. Fungal Planet Description Sheets: 69–91. Persoonia. 2011;26:108–156. doi: 10.3767/003158511X581723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Verkley G.J.M., Groenewald J.Z. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2009. Fungal biodiversity. CBS Laboratory manual 1. [Google Scholar]
- Crous P.W., Wingfield M.J., Guarro J. Fungal Planet description sheets: 154–213. Persoonia. 2013;31:188–296. doi: 10.3767/003158513X675925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Mansilla J.P. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology. 2006;55:99–131. doi: 10.3114/sim.55.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R.A., Talbot N.J., Ebbole D.J. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 2005;434:980–986. doi: 10.1038/nature03449. [DOI] [PubMed] [Google Scholar]
- Ellis M.B. CMI; Kew, England: 1971. Dematiaceous Hyphomycetes. [Google Scholar]
- Ellis M.B. CMI; Kew, England: 1976. More Dematiaceous Hyphomycetes. [Google Scholar]
- Faivre-Rampant O., Thomas J., Allégre M. Characterization of the model system rice-Magnaporthe for the study of nonhost resistance in cereals. New Phytologist. 2008;180:899–910. doi: 10.1111/j.1469-8137.2008.02621.x. [DOI] [PubMed] [Google Scholar]
- Gams W. Phialophora and some similar morphologically little-differentiated anamorphs of divergent ascomycetes. Studies in Mycology. 2000;45:187–199. [Google Scholar]
- Hashioka Y. Notes on Pyricularia II. Four species and one variety parasitic to Cyperaceae, Gramineae and Commelinaceae. Transactions of the Mycological Society of Japan. 1973;14:256–265. [Google Scholar]
- Hirata K., Kusaba M., Chuma I. Speciation in Pyricularia inferred from multilocus phylogenetic analysis. Mycological Research. 2007;111:799–808. doi: 10.1016/j.mycres.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Hoog G.S. de. Taxonomy of the Dactylaria complex, IV. Dactylaria, Neta, Subulipora and Scolecobasidium. Studies in Mycology. 1985;26:1–60. [Google Scholar]
- Hoog G.S. de, Gerrits van den Ende A.H.G. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses. 1998;41:183–189. doi: 10.1111/j.1439-0507.1998.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Hornby D., Slope D.B., Gutteridge R.J. Gaeumannomyces cylindrosporus, a new ascomycete from cereal roots. Transactions of the British Mycological Society. 1975;69:21–25. [Google Scholar]
- Huhndorf S.H., Greif M., Mugambi G.K. Two new genera in the Magnaporthaceae, a new addition to Ceratosphaeria and two new species of Lentomitella. Mycologia. 2008;100:940–955. doi: 10.3852/08-037. [DOI] [PubMed] [Google Scholar]
- Kang S., Sweigard J.A., Valent B. The PWL host specificity gene family in the blast fungus Magnaporthe grisea. Molecular Plant Microbe Interactions. 1995;8:939–948. doi: 10.1094/mpmi-8-0939. [DOI] [PubMed] [Google Scholar]
- Kato H., Yamamoto M., Yamaguchi-Ozaki T. Pathogenicity, mating ability and DNA Restriction fragment length polymorphisms of Pyricularia populations isolated from Gramineae, Bambusideae and Zingiberaceae plants. Journal of General Plant Pathology. 2000;66:30–47. [Google Scholar]
- Kohlmeyer J., Volkmann-Kohlmeyer B. Fungi on Juncus roemerianus. I. Trichocladium medullare sp. nov. Mycotaxon. 1995;53:349–353. [Google Scholar]
- Kohlmeyer J., Volkmann-Kohlmeyer B., Eriksson O.E. Fungi on Juncus roemerianus. 4. New marine ascomycetes. Mycologia. 1995;87:532–542. [Google Scholar]
- Kotani S., Kurata M. Black blotch of ginger rhizome by Pyricularia zingiberi Nishikado. Annals of the Phytopathological Society of Japan. 1992;58:469–472. [Google Scholar]
- Lombard L., Crous P.W., Wingfield B.D. Phylogeny and systematics of the genus Calonectria. Studies in Mycology. 2010;66:31–69. doi: 10.3114/sim.2010.66.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Zhang N. Magnaporthiopsis, a new genus in Magnaporthaceae (Ascomycota) Mycologia. 2013;105:1019–1029. doi: 10.3852/12-359. [DOI] [PubMed] [Google Scholar]
- Luttrell E.S. An undescribed species of Pyricularia on sedges. Mycologia. 1954;46:810–814. [Google Scholar]
- Mackill A.O., Bonham J.M. New hosts of Pyricularia oryzae. Plant Disease. 1986;70:125–128. [Google Scholar]
- Mason-Gamer R.J., Kellogg E.A. Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae) Systematic Biology. 1996;45:524–545. [Google Scholar]
- Murata N., Aoki T., Kusaba M. Various species of Pyricularia constitute a robust clade distinct from Magnaporthe salvinii and its relatives in Magnaporthaceae. Journal of General Plant Pathology. 2014;80:66–72. [Google Scholar]
- Nirenberg H.I. Untersuchungen über die morphologische und biologische differenzierung in der Fusarium-Section Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft (Berlin-Dahlem) 1976;169:1–117. [Google Scholar]
- Nishikado Y. Studies on the rice blast fungus, (I) Berichte des Ohara Instituts für Landwirtschaftliche Forschungen. 1917;1:171–218. [Google Scholar]
- O'Donnell K. Fusarium and its near relatives. In: Reynolds D.R., Taylor J.W., editors. The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International; Wallingford, UK: 1993. pp. 225–233. [Google Scholar]
- Ou S.H. CAB International; Wallingford, UK: 1985. Rice diseases. [Google Scholar]
- Pappas A.C., Paplomatas E.J. Pyricularia leaf spot: a new disease of ornamental plants of the family Marantaceae. Plant Disease. 1998;82:465–469. doi: 10.1094/PDIS.1998.82.5.465. [DOI] [PubMed] [Google Scholar]
- Park M.J., Shin H.D. A new species of Pyricularia on Commelina communis. Mycotaxon. 2009;108:449–456. [Google Scholar]
- Prasada R., Goyal J.P. A new species of Pyricularia on Bajra. Current Science. 1970;39:287–288. [Google Scholar]
- Rayner R.W. CMI and British Mycological Society; Kew, Surrey, England: 1970. A mycological colour chart. [Google Scholar]
- Réblová M. Molecular systematics of Ceratostomella sensu lato and morphologically similar fungi. Mycologia. 2006;98:68–93. doi: 10.1080/15572536.2006.11832714. [DOI] [PubMed] [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh D., Milazzo J., Adreit H. South-East Asia is the center of origin, diversity and dispersion of the rice blast fungus, Magnaporthe oryzae. New Phytologist. 2014;201:1440–1456. doi: 10.1111/nph.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson R.A., Houbraken J., Frisvad J.C. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2010. Food and indoor fungi. CBS Laboratory Manual 2. [Google Scholar]
- Seifert K.A., Morgan-Jones G., Gams W. CBS-KNAW Fungal Biodiversity Centre; Utrecht: 2011. The Genera of Hyphomycetes. CBS Biodiversity Series no. 9. [Google Scholar]
- Serghat S., Mradmi K., Touhami A.O. Rice leaf pathogenic fungi on wheat, oat, Echinochloa phyllopogon and Phragmites australis. Phytopathologia Mediterranea. 2005;44:44–49. [Google Scholar]
- Shearer C.A. Pseudohalonectria (Lasiosphaeriaceae), an antagonistic genus from wood in freshwater. Canadian Journal of Botany. 1989;67:1944–1955. [Google Scholar]
- Shearer C.A., Crane J.L., Chen W. Freshwater ascomycetes: Ophioceras species. Mycologia. 1999;91:145–156. [Google Scholar]
- Skamnioti P., Gurr S.J. Against the grain: safeguarding rice from rice blast disease. Trends in Biotechnology. 2009;27:141–150. doi: 10.1016/j.tibtech.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Soares D.J., Rocha F.B., Barreto R.W. Pyriculariopsis calatheae anam. nov. a novel fungus from the Atlantic rainforest of Brazil associated with leaf spots on Calathea sp., with a key of Pyriculariopsis spp. Mycological Progress. 2011;10:315–321. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts, USA: 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. [Google Scholar]
- Tamura K., Peterson D., Peterson N. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongkantha S., Jeewon R., Vijaykrishna D. Molecular phylogeny of Magnaporthaceae (Sordariomycetes) with a new species Ophioceras chiangdaoense from Dracaena loureiroi in Thailand. Fungal Diversity. 2009;34:157–173. [Google Scholar]
- Tosa Y., Chuma I. Classification and parasitic specialization of blast fungi. Journal of General Plant Pathology. 2014;80:202–209. [Google Scholar]
- Tsurushima T., Don L.D., Kawashima K. Pyrichalasin H production and pathogenicity of Digitaria-specific isolates of Pyricularia grisea. Molecular Plant Pathology. 2005;6:605–613. doi: 10.1111/j.1364-3703.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. Gaeumannomyces, Linocarpon, Ophiobolus and several other genera of scolecospored ascomycetes and Phialophora conidial states, with a note on hyphopodia. Mycotaxon. 1980;11:1–129. [Google Scholar]
- Wang G.L., Mackill D.J., Bonman J.M. RFLP mapping of genes conferring complete and partial resistance to blast in a durably resistant rice cultivar. Genetics. 1994;136:1421–1434. doi: 10.1093/genetics/136.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR protocol: a guide to methods and applications. Academic Press; San Diego, CA, USA: 1990. [Google Scholar]
- Yaegashi H., Nihihara N. The taxonomical identity of the perfect state of Pyricularia grisea and its allies. Canadian Journal of Botany. 1978;56:180–183. [Google Scholar]
- Zhang H., Wu Z., Wang C. Germination and infectivity of microconidia in the rice blast fungus Magnaporthe oryzae. Nature Communications. 2014;5:4518. doi: 10.1038/ncomms5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Zhao S., Shen Q. A six-gene phylogeny reveals the evolution of mode of infection in the rice blast fungus and allied species. Mycologia. 2011;103:1267–1276. doi: 10.3852/11-022. [DOI] [PubMed] [Google Scholar]


