Abstract
The genus Bipolaris includes important plant pathogens with worldwide distribution. Species recognition in the genus has been uncertain due to the lack of molecular data from ex-type cultures as well as overlapping morphological characteristics. In this study, we revise the genus Bipolaris based on DNA sequence data derived from living cultures of fresh isolates, available ex-type cultures from worldwide collections and observation of type and additional specimens. Combined analyses of ITS, GPDH and TEF gene sequences were used to reconstruct the molecular phylogeny of the genus Bipolaris for species with living cultures. The GPDH gene is determined to be the best single marker for species of Bipolaris. Generic boundaries between Bipolaris and Curvularia are revised and presented in an updated combined ITS and GPDH phylogenetic tree. We accept 47 species in the genus Bipolaris and clarify the taxonomy, host associations, geographic distributions and species’ synonymies. Modern descriptions and illustrations are provided for 38 species in the genus with notes provided for the other taxa when recent descriptions are available. Bipolaris cynodontis, B. oryzae, B. victoriae, B. yamadae and B. zeicola are epi- or neotypified and a lectotype is designated for B. stenospila. Excluded and doubtful species are listed with notes on taxonomy and phylogeny. Seven new combinations are introduced in the genus Curvularia to accomodate the species of Bipolaris transferred based on the phylogenetic analysis. A taxonomic key is provided for the morphological identification of species within the genus.
Key words: Brown spot of rice, Field crop diseases, Graminicolous fungi, Helminthosporoid genera, molecular phylogeny, Pleosporales, Southern corn leaf blight, Taxonomy
Introduction
The genus Bipolaris includes a number of significant plant pathogens with worldwide distribution. These species are commonly associated with leaf spots, leaf blights, melting outs, root rots, foot rots and other disease symptoms mainly in high value field crops in the family Poaceae, including rice, maize, wheat and sorghum and on various other host plants (Ellis 1971, Sivanesan 1987, Berbee et al. 1999). Devastating diseases caused by species of Bipolaris on staple crops such as rice and wheat have been the cause of historical famines resulting in the starvation of large human populations in several regions in the world. For example, the Bengal famine in India (1943–1944) was the result of a rice disease caused by Bipolaris oryzae (Ou 1985, Scheffer 1997). Although not resulting in human starvation, Southern corn leaf blight caused by Bipolaris maydis in the 1970s resulted in catastrophic losses in maize crops in the USA and UK (Ullstrup 1972, Carson 1998, Lev et al. 1999, Manamgoda et al. 2011). In the conference “Wheat for the National Warm Areas” held in Brazil in 1990, Bipolaris sorokiniana, a common root rot and leaf spot pathogen of wheat and barley, was declared the most economically important foliar pathogen of wheat in warm regions worldwide (Duveiller & Gilchrist 1994). In addition to a host association with Poaceae, species of Bipolaris are known to occur on at least 60 other genera in Anacardiaceae, Araceae, Euphorbiaceae, Fabaceae, Malvaceae, Rutaceae and Zingiberaceae as either saprobes or pathogens (Ellis 1971, Sivanesan 1987, Manamgoda et al. 2011). The global distribution of common phytopathogenic species of Bipolaris may have resulted from the transfer of agricultural commodities including plants and seeds across geographical borders (Farr & Rossman 2013, Zhang et al. 2013).
The genus Bipolaris belongs to Ascomycota, Dothideomycetes, Pleosporales, Pleosporaceae. Its sexual morph, the genus Cochliobolus typified by C. heterostrophus, is now linked with the type species of Bipolaris, B. maydis (Rossman et al. 2013a). The sexual morph of Bipolaris is not common in nature, but it is occasionally produced under laboratory conditions (Nelson 1964, Paul & Parbery 1966, Alcorn 1978, 1990, Tsuda & Ueyama 1985). Even though Cochliobolus (1934) is the oldest name, Bipolaris (1959) is more frequently used by plant pathologists in disease reports and widely applied in taxonomic literature. Based on these reasons, the proposed conservation of the generic name Bipolaris was supported by an online vote coordinated through the International Commission on the Taxonomy of Fungi (Rossman et al. 2013a). Similarly, the generic type species B. maydis (basionym: Helminthosporium maydis Y. Nisik. & C. Miyake) was proposed for conservation over Helminthosporium maydis Brond, with a neotype designated in order to ensure the stable taxonomy of the genus (Rossman et al. 2013b).
Species in Bipolaris were initially described in the genus Helmisporium Link (1809), which was typified by Helmisporium velutinum. Helmisporium was validated by Gray (1821). Persoon (1822) altered the spelling of the name to Helminthosporium. Link (1824) accepted the altered spelling as an appropriate orthographic variant and Helminthosporium has since been widely used. The graminicolous species described in Helminthosporium were determined to be different from the type species H. velutinum by Luttrell (1963) and Ellis (1971). Alcorn (1988) provided illustrations showing the morphological distinctiveness of graminicolous Helminthosporium. Nisikado (1928a) divided graminicolous Helminthosporium species into two subgenera Cylindro-Helminthosporium and Eu-Helminthosporium. Species with straight cylindrical conidia that germinate with one or more germ tubes from any cell were placed in the former subgenus Cylindro-Helminthosporium, whereas species with fusiform and curved conidia germinating only from end cells were placed in the latter (Nisikado 1929, Alcorn 1988). After several taxonomic refinements, graminicolous Helminthosporium were segregated into several genera including Bipolaris, Curvularia, Drechslera and Exserohilum (Sivanesan 1987).
Drechslera Ito (1930) accommodated fungi previously in subgenus Cylindro-Helminthosporium. Drechslera can be differentiated from all other graminicolous helminthosporoid genera by its ability to develop a germ tube from any of the cells in the distoseptate conidia (Sivanesan 1987, Alcorn 1988). Hilum morphology can also be used to differentiate Bipolaris and Drechslera. In Drechslera a flat scar exists within the lowest part of the basal cell, whereas in Bipolaris it is inconspicuous or very slightly protuberant (Alcorn 1988). The sexual morphs of Drechslera have been linked to Pyrenophora whereas the sexual morphs of Bipolaris were regarded as Cochliobolus (Drechsler 1934, Alcorn 1983a). Exserohilum Leonard & Suggs (1974) can be differentiated from other graminicolous helminthosporoid genera by a truncate, strongly protruding hilum, often with an enveloping bubble. Illustrations of the different hilum morphologies were given by Alcorn (1988). The sexual morphs of Exserohilum have been placed in Setosphaeria K.J. Leonard & Suggs (1974).
Subramanian & Jain (1966) amended the description of Drechslera to include all Bipolaris species and synonymised Drechslera and Bipolaris. Later authors did not accept this approach and claimed that generic differences are evident (Talbot 1973, Luttrell 1977, 1978). Molecular phylogenetic analysis based on ITS (internal transcribed spacers and intervening 5.8S nrDNA) and GPDH (partial glyceraldehyde-3-phosphate dehydrogenase) genes (Berbee et al. 2000) showed Drechslera and Bipolaris to be two distinct genera. Bipolaris and Curvularia Boedijn (1933) share many morphological similarities, and both genera have sexual morphs in Cochliobolus. According to molecular analyses of ITS and GPDH sequence data, some Bipolaris species clustered with Curvularia and resulted in two major clades referred to as Cochliobolus group 1 and Cochliobolus group 2 (Berbee et al. 1999). Similar results were obtained with a combined analysis of ITS, GPDH, TEF (partial translation elongation factor 1-alpha gene) and LSU (partial 28S nrRNA gene) sequence data (Manamgoda et al. 2012). Group 1 includes the type of the genus Bipolaris, B. maydis, and group 2 includes the generic type of Curvularia, C. lunata. Based on the phylogenetic data, Bipolaris sensu stricto was applied to group 1 and Curvularia to group 2. Following the reclassification of Bipolaris and Curvularia by Manamgoda et al. (2012), a number of important plant pathogens are included in Bipolaris, while some species, especially those known as human pathogens, are now included in Curvularia (da Cunha et al. 2013, Madrid et al. 2014).
The genus Pseudocochliobolus was described by Tsuda et al. (1977) to accommodate Cochliobolus species in which the ascomata develop on columnar or flat stromata firmly adhering to the substrate at the base and having parallel to loosely coiled ascospores. The type species, Pseudocochliobolus nisikadoi, which is described based on the sexual morph in culture, is now regarded as Curvularia coicis (Manamgoda et al. 2012). All other species previously included in Pseudocochliobolus are now excluded from Bipolaris and are regarded as Curvularia spp. Therefore, the genus name Pseudocochliobolus is no longer regarded as a distinct genus as the type is synonymised under Curvularia.
Lack of ex-type or authenticated sequences in public databases is a drawback in the accurate molecular identification of Bipolaris species (Cai et al. 2011, Manamgoda et al. 2012). Some species of Bipolaris have been used widely in biotechnological applications and genetic manipulation because of their significance as plant pathogens on important crops. The understanding of virulent genes and infection strategies is important in disease control and related research. Whole genomes have been sequenced for the isolates of Bipolaris sorokiniana (as Cochliobolus sativus) (Ohm et al. 2012), B. victoriae (as C. victoriae), B. zeicola (as C. carbonum) and B. maydis (as C. heterostrophus) (Condon et al. 2013). Genetically improved maize varieties have been developed to resist B. maydis, B. oryzae and B. sorokiniana (Panchi & Xiaoqing, 1993, Aiguo & Chenghe 1997, Mehta & Angra 2000, Badu-Apraku et al. 2009, Yaqoob et al. 2012, Zhang et al. 2012). Functional genomics studies of Bipolaris species have determined fungal-host interactions and the molecular basis of toxin production (Lorang et al. 2007). Genetic manipulation such as insertion mutagenesis, targeted disruption of specific genes, and studies on over-expression of functional genes have also been conducted using several species of Bipolaris (Inagaki et al. 2012, Nizam et al. 2012, Suzuki et al. 2012). The applications of the species of Bipolaris also emphasise the need for accurate identification and availability of reference isolates since the names are the key to the accessing accumulated knowledge (Rossman & Palm-Hernandez 2008, Hyde et al. 2010, Hawksworth 2011).
The objectives of this study are 1) to establish a phylogenetic species concept for Bipolaris providing DNA sequence data for ex-type isolates including epitypes or neotypes designated wherever possible, and 2) to provide modern descriptions and illustrations of species resulting in a modern monographic treatment for the genus. A key to species in Bipolaris is provided for morphological identification. DNA sequence data linked to the reference isolates defined in this study will be a major resource for identification and determination of species limits in future assessments.
Materials and methods
Specimens and isolates
Fresh Bipolaris isolates were obtained from northern Thailand and the USA (Florida, Indiana and Maryland) from various host plants and as saprobes. Strains were obtained by single spore isolation as described in Manamgoda et al. (2012). Additional cultures from other geographic locations were obtained from various contributors and public culture collections including ATCC (USA), CBS (The Netherlands), DAOM (Canada), ICMP (New Zealand), MFLUCC (Thailand) as well as cultures housed at the Systematic Mycology and Microbiology Laboratory, USDA-ARS (USA) as listed in Table 1. Herbarium specimens were obtained from international fungaria including BPI, BRIP, CUP, K, PREM and WSP.
Table 1.
GenBank and culture collection accession numbers of strains used in this study.
| Species | Strain no.1 | Host | Country | Collector | GenBank accession no.2 |
References | |||
|---|---|---|---|---|---|---|---|---|---|
| ITS | GPDH | TEF | LSU | ||||||
| Alternaria alternata | EGS 34.0160 | Arachis hypogaea | India | — | AF071346 | AF081400 | — | — | Berbee et al. (1999) |
| Bipolaris bicolor | CBS 690.96 | — | — | R.F. Castañeda | KJ909762 | KM042893 | KM093776 | KM243287 | This study |
| B. chloridis | CBS 242.77 | Chloris gayana | Australia | J.L. Alcorn | JN192372 | JN600961 | — | — | Manamgoda et al. (2011) |
| B. clavata | BRIP 12530 | Dactyloctenium radulan | Australia | J.L. Alcorn | KJ415524 | KJ415422 | KJ415471 | KJ415477 | Tan et al. (2014) |
| B. coffeana | BRIP 14845 | Coffea arabica | Kenya | I. Furtado | KJ415525 | KJ415421 | KJ415470 | KJ415478 | Tan et al. (2014) |
| C 12.04 | Cynodon dactylon | USA | — | KM230385 | KM034837 | KM093781 | — | This study | |
| MFLUCC 12-0185 | Digitaria sp. | Thailand | D.S. Manamgoda | KJ922385 | KM034841 | KM093784 | — | This study | |
| M 1129 | Bouteloua gracilis | USA | M. Barkworth | KJ922384 | KM034836 | KM093780 | — | This study | |
| M 1130 | B. gracilis | USA | M. Barkworth | KM230387 | KM034835 | KM093779 | — | This study | |
| MFU0090 | Poaceae | Thailand | D.S. Manamgoda | KM230386 | KM034840 | KM093783 | KM243293 | This study | |
| ICMP 6128 | C. dactylon | New Zealand | E.H.C. McKenzie | JX256412 | KM034839 | JX266581 | JX256380 | Manamgoda et al. (2011) / This study | |
| B. cookei | AR 5185 | Sorghum sp. | Japan | T. Tsukiboshi | KJ922391 | KM034833 | KM093777 | — | This study |
| MAFF 51191 | Sorghum bicolor | Japan | N. Nishihara | KJ922392 | KM034834 | KM093778 | — | This study | |
| B. crotonis (= B. eleusines) | CBS 274.91 | Eleusine indica | Australia | J.L. Alcorn | KJ909768 | KM034820 | KM093758 | KM243289 | Berbee et al (1999) |
| B. crotonis | BRIP 14838 | Croton sp. | Samoa | — | KJ415526 | KJ415420 | KJ415479 | KJ415469 | Tan et al. (2014) |
| B. cynodontis | CBS 109894 | C. dactylon | Hungary | J. Bakonyi | KJ909767 | KM034838 | KM093782 | KM243288 | This study |
| B. drechsleri | CBS 136207 | Microstegium vimineum | USA | N. Kleczewski | KF500530 | KF500533 | KM093760 | — | Crous et al. (2013) / This study |
| MUS0028 | M. vimineum | USA | N. Kleczewski | KF500532 | KF500535 | KM093761 | — | Crous et al. (2013) / This study | |
| FIP 373 | Ornamental grass | USA | O'Neil | KF500531 | KF500534 | KM093759 | — | Crous et al. (2013) | |
| B. heliconiae | BRIP 17186 | Heliconia psittacorum | Australia | J.D. Duff | KJ415530 | KJ415417 | KJ415465 | KJ415483 | Tan et al. (2014) |
| B. heveae | CBS 241.92 | Hevea sp. | Nigeria | J.H. Simmond | KJ909763 | KM034843 | KM093791 | KM243294 | This study |
| B. gossypina | BRIP 14840 | Gossypium sp. | Kenya | M.H. White | KJ415528 | KJ415418 | KJ415467 | KJ415481 | |
| B. luttrellii | BRIP 14643 | D. aegypticum | Australia | R.A. Peterson | AF071350 | AF081402 | — | — | Berbee et al. (1999) |
| B. maydis | CBS 137271/ C5 | Zea mays | USA | G. Turgeon | AF071325 | KM034846 | KM093794 | KM243280 | Berbee et al. (1999) / This study |
| AR 5182 | S. bicolor | Japan | N. Nishihara | KM230388 | KM034844 | KM093792 | — | This study | |
| AR 5183 | S. bicolor | Japan | T. Tsukiboshi | KM230390 | KM034848 | KM093796 | KM243274 | This study | |
| M 1122/ C4 | Z. mays | USA | G. Turgeon | KM230389 | KM034847 | KM093795 | — | This study | |
| CBS 136.29 | Z. mays | Japan | Y. Nisikado | KJ909769 | KM034845 | KM093793 | KM243279 | This Study | |
| B. microlaenae | BRIP 15613 | Microlaena stipoides | Australia | J.L. Alcorn | JN601032 | JN600974 | JN601017 | JN600995 | Manamgoda et al. (2011) |
| B. microstegii | CBS 132550 | M. vimineum | USA | N.M. Kleczewski | JX089579 | JX089575 | KM093756 | JX100808 | This study |
| AR 5192 | M. vimineum | USA | W. L. Bruckart | KM230391 | KM034819 | KM093757 | — | This study | |
| B. oryzae | MFLUCC 100715 | Oryza sativa | Thailand | D.S. Manamgoda | JX256416 | JX276430 | JX266585 | JX256384 | Manamgoda et al. (2012) |
| MFLUCC 100733 | O. sativa | Thailand | D.S. Manamgoda | JX256417 | KM042898 | KM093790 | JX256385 | Manamgoda et al. (2012) | |
| MAFF 235499 | O. sativa | Japan | T. Aoki | KJ922383 | KM042897 | KM093789 | — | This study | |
| AR3797 | Panicum virgatum | USA | J. Krupinsky | KM230392 | KM042894 | KM093786 | — | This study | |
| AR 5204 | P. virgatum | USA | K. Craven | KM230393 | KM042895 | KM093787 | KM243277 | This study | |
| B. panici-miliacei | CBS 199.29 | P. miliaceum | Japan | Y. Nisikado | KJ909773 | KM042896 | KM093788 | KM243281 | This study |
| B. peregianensis | DAOM 221998 | C. dactylon | Australia | J.L. Alcorn | KJ922393 | KM034849 | KM093797 | — | This study |
| BRIP 12790 | C. dactylon | Australia | J.L. Alcorn | JN601034 | JN600977 | JN601022 | JN601000 | Manamgoda et al. (2011) | |
| B. pluriseptata | BRIP 14839 | E. coracana | Zambia | — | KJ415532 | KJ415414 | KJ415461 | KJ415486 | Tan et al. (2014) |
| B. sacchari | ICMP 6227 | Oplismenus imbecillis | New Zealand | E.H.C. McKenzie | KJ922386 | KM034842 | KM093785 | — | This study |
| B. salkadehensis | Bi 4 | Cladium mariscus | Iran | A. Ahmadpour | AB675491 | — | — | — | Ahmadpour et al. (2012) |
| B. salviniae | IMI 228224 | Salvinia auriculata | Brazil | J.J. Muchovej | KJ922390 | KM034829 | KM093772 | KM243283 | This study |
| B. salviniae (= B. melinidis) | BRIP 12898 | Melinis minutiflora | Australia | J.L. Alcorn | JN601035 | JN600972 | KM093771 | JX256411 | Manamgoda et al. (2011) |
| B. secalis | BRIP 14453 | Secale cereale | Argentina | M.N. Sisterna | KJ415537 | KJ415409 | KJ415455 | KJ415492 | Tan et al. (2014) |
| B. sorokiniana (= B. multiformis) | CBS 120.24 | — | Italy | L. Montemartini | KJ909776 | KM034821 | KM093762 | KM243278 | This study |
| CBS 110.14 | Hordeum sp. | USA | A.L. Bakke | KJ922381 | KM034822 | KM093763 | — | This study | |
| FIP 499 | Phalaris arundinaceae | USA | — | KJ922382 | KM034828 | KM093769 | — | This study | |
| MAFF 236448 | Z. mays | Japan | T. Aoki | KJ909792 | KM034826 | KM093767 | — | This study | |
| MAFF 235500 | Paddy field soil | Japan | T. Aoki | KJ909789 | KM034823 | KM093764 | — | This study | |
| MAFF 235501 | Z. mays | Japan | T. Aoki | KJ909791 | KM034825 | KM093766 | — | This study | |
| MAFF 238877 | Hordeum vulgare | Japan | T. Furukawa | KJ909790 | KM034824 | KM093765 | — | This study | |
| CBS 480.74 | Tribulus terrestris | South Africa | W.F.O. Marasas | KJ909771 | KM034827 | KM093768 | KM243282 | This study | |
| B. urochloae | ATCC 58317 | Urochloa panicoides | Australia | J.L. Alcorn | KJ922389 | KM230396 | KM093770 | — | This study |
| B. victoriae | CBS 327.64 | Avena sativa | USA | R.R. Nelson | KJ909778 | KM034811 | KM093748 | KM243271 | This study |
| DAOM 147449 | A. sativa | USA | KJ909785 | KM034812 | KM093749 | — | This study | ||
| B. yamadae | DAOM 147441 | Saccharum officinarum | Cuba | E.S. Luttrell | KJ922388 | KM034831 | KM093774 | — | This study |
| MAFF 235507 | Z. mays | Japan | T. Aoki | KJ922387 | KM034832 | KM093775 | — | This study | |
| CBS 202.29 | P. miliaceum | Japan | Y. Nisikado | KJ909779 | KM034830 | KM093773 | KM243275 | This study | |
| B. zeae | AR 3795 | P. virgatum | USA | J. Krupinsky | KJ909786 | KM034816 | KM093753 | — | This study |
| AR 5181 | S. bicolor | Japan | N. Nishihara | KM230394 | KM034817 | KM093754 | — | This study | |
| DAOM 211267 | Triticum sp. | Canada | R.M. Clear | KJ909787 | KM034818 | KM093755 | — | This study | |
| B. zeicola | AR 5166 | Sorghum sp. | USA | D. Funnell-Harris | KJ909788 | KM034813 | KM093750 | — | This study |
| AR 5168 | Sorghum sp. | USA | D. Funnell-Harris | KM230397 | KM034814 | KM093751 | — | This study | |
| FIP 532 | Z. mays | USA | R. Hite | KM230398 | KM034815 | KM093752 | — | This study | |
| Curuvlaria australis | BRIP 12525 | Sporobolus caroli | Australia | J.L. Alcorn | AF081448 | AF081409 | — | — | Berbee et al. (1999) |
| C. brachyspora | CBS 186.50 | Soil | Java | K.B. Boedijn & J.R. Reitsma | KJ922372 | KM061784 | KM230405 | KM243268 | This study |
| C. buchloës comb. nov. | CBS 246.49 | Buchloe dactyloides | USA | C.L. Lefebvre & A.G. Johnson | KJ909765 | KM061789 | KM196588 | KM243272 | This study |
| C. crustacea | 8225-1 | — | — | — | AF163070 | — | — | AF163977 | Goh et al. (1998) |
| C. dactyloctenii | BRIP 12913 | Dactyloctenium | Australia | J.L. Alcorn | AF071322 | AF081376 | — | — | Berbee et al. (1999) |
| C. ellisii | IMI 75862 | Air | Pakistan | M.S. Quraishi | KJ922379 | KM061792 | KM230406 | — | This study |
| CBS 193.62 | Air | Pakistan | M.S. Quraishi | AF081447 | AF081410 | JN601006 | JN600984 | Manamgoda et al. (2011) | |
| C. geniculata | CBS 187.50 | Andropogon sorghum | Indonesia | K.B. Boedijn & J.R. Reitsma | KJ909781 | KM083609 | KM230410 | KM243260 | This study |
| C. gladioli | ICMP 6160 | Gladiolus sp. | New Zealand | E.H.C. McKenzie | JX256426 | JX276438 | JX266595 | JX256393 | Manamgoda et al. 2012 |
| C. hawaiiensis | BRIP 15933 | C. gayana | Australia | J.L. Alcorn | JN601028 | JN600965 | JN601009 | JN600987 | Manamgoda et al. 2011 |
| C. heteropogonis | CBS 284.91 | Heteropogon contortus | Australia | J.L. Alcorn | JN192379 | JN600969 | JN601013 | JN600990 | Manamgoda et al. (2012) |
| C. homomorpha | DAOM 63822 | H. vulgare | USA | E.S. Luttrell & C.T. Rogerson | KM257055 | KM257058 | — | — | This study |
| C. inaequalis | CBS 102.42 | Sand dune soil | France | F. Moreau | KJ922375 | KM061787 | KM196574 | KM243261 | This study |
| C. ischaemi | ICMP 6172 | Ischaemum indicum | New Zealand | E.H.C. McKenzie | JX256428 | JX276440 | — | JX256395 | Manamgoda et al. (2012) |
| C. kusonoi comb. nov. | CBS 137.29 | Eragrostis major | Japan | Y. Nisikado | JN192381 | — | KM196592 | JN600993 | Manamgoda et al. (2011) |
| C. lunata | CBS 730.96 | Human lung biopsy | USA | — | JX256429 | JX276441 | JX266596 | JX256396 | Manamgoda et al. (2012) |
| C. miyakei | CBS 197.29 | E. pilosa | Japan | Y. Nisikado | KJ909770 | KM083611 | KM243265 | — | This study |
| C. neoindica comb. et nom. nov. | BRIP 17439 | Trianthema portulacastrum | Australia | K.D. Hyde | AF081449 | AF081406 | — | — | Berbee et al. (1999) |
| C. neergaardii | DAOM 228085 | Desert soil | Chile | E. Piontelli | KJ909784 | KM083615 | KM196593 | — | This study |
| C. nicotiae | CBS 655.74 | Desert soil | Algeria | J. Mouchacca | KJ909772 | KM083614 | — | KM243291 | This study |
| C. nodulosa comb. nov. | CBS 160.58 | E. indica | USA | E.S. Lutrell | JN601033 | JN600975 | JN601019 | JN600997 | Manamgoda et al. (2011) |
| C. ovariicola | CBS 470.90 | E. interrupta | Australia | J.L. Alcorn | JN192384 | JN600976 | JN601020 | JN600998 | Manamgoda et al. (2011) |
| C. pallescens | CBS 156.35 | Air | Java | H.J. Toxopeus | KJ922380 | KM083606 | KM196570 | KM243269 | This study |
| C. papendorfii | CBS 308.67 | Acacia karroo | South Africa | M.C. Papendorf | KJ909774 | KM083617 | KM196594 | KM243290 | This study |
| C. perotidis | CBS 350.90 | Perotis rara | Australia | J.L. Alcorn | JN192385 | JN601021 | KM230407 | JN600999 | Manamgoda et al. (2011) |
| C. portulacae | CBS 239.48 | Portulaca oleracea | USA | W.E. Rader | KJ909775 | KM083616 | KM230404 | KM243292 | This study |
| C. prasadii | CBS 143.64 | Jasminum sambac | India | R.L. Mathur | KJ922373 | KM061785 | KM230408 | — | This study |
| C. protuberata | CBS 376.65 | Deschampsia flexuosa | UK: Scotland | R.R. Nelson | KJ922376 | KM083605 | KM196576 | KM243264 | This study |
| C. ravenellii | BRIP 13165 | S. fertilis | Australia | J.L. Alcorn | JN192386 | JN600978 | JN601024 | JN601001 | Manamgoda et al. (2011) |
| C. ryleyi | CBS 349.90 | S. creber | Australia | J.L. Alcorn | KJ909766 | KM083612 | KM196567 | KM243267 | This study |
| C. robusta | CBS 624.68 | Dichanthium annulatum | USA | E.S. Luttrell | KJ909783 | KM083613 | KM196577 | KM243297 | This study |
| C. sesuvii comb. nov. | Bp Zj 01 | Sesuvium portulacastrum | China | J.Z. Zhang | EF175940 | — | — | — | Zhang and Li (2009) |
| C. spicifer | DAOM 575355 | B. gracilis | USA | R. Sprague & G.W. Fisher | KJ922377 | KM061788 | KM196589 | — | Manamgoda et al. (2011) |
| C. spicifer | CBS 274.52 | Soil | Spain | J. Nicot | JN192387 | JN600979 | JN601023 | JX256400 | Manamgoda et al. (2011) |
| C. subpapendorfii | CBS 656.74 | Desert soil | Egypt | J. Mouchacca | KJ909777 | KM061791 | KM230403 | KM243266 | This study |
| C. trifolii | ICMP 6149 | Setaria glauca | New Zealand | E.H.C. McKenzie | KM230395 | KM083607 | — | KM243262 | This study |
| C. tripogonis | BRIP 12375 | — | Australia | J.L. Alcorn | JN192388 | JN600980 | JN601025 | JN601002 | Manasmgoda et al. (2011) |
| C. tuberculata | CBS 146.63 | Z. mays | India | R.L. Mathur & B.L. Jain | JX256433 | JX276445 | JX266599 | JX256401 | Manamgoda et al. (2011) |
| Curvularia sp. | DAOM 20022 | Pisum sativum | Canada | — | KJ922374 | KM061786 | KM196575 | — | This study |
| Curvularia sp. | MAFF 236750 | Rhodes grass | Japan | M. Tsuda | KJ922378 | KM061790 | KM230409 | — | This study |
| Drechslera brizae | CBS 190.29 | Briza minor | Japan | Y. Nisikado | KM257054 | KM257057 | — | KM243296 | — |
| Johnalcornia aberrans (= B. aberrans) | CBS 510.91 | E. paviflora | Australia | J.L. Alcorn | KM257053 | KM257056 | KM243286 | — | This study |
AR, FIP, MFU, MUS: Isolates housed in Systematic Mycology and Microbiology Laboratory, United States Department of Agriculture, Agricultural Research Service, Beltsville, Maryland. ATCC: American Type Culture Collection, Virginia, USA; BRIP: Plant Pathology Herbarium, Department of Primary Industries, Queensland, Australia; CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; DAOM: Plant Research Institute, Department of Agriculture (Mycology), Ottawa, Canada; E.G.S.: Collection of E.G. Simmons; ICMP = PDDCC: International Collection of Micro-organisms from Plants, Landcare Research, Private Bag 92170, Auckland, New Zealand; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, United Kingdom; MAFF: Ministry of Agriculture, Forestry and Fisheries, Tsukuba, Ibaraki, Japan; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; GPDH: partial glyceraldehyde-3-phosphate dehydrogenase gene; TEF: partial translation elongation factor 1-alpha gene; LSU: partial 28S nrRNA gene.
Morphology
Digital images of the ascomata and conidiophores were captured using a Discovery v20 stereomicroscope and AxioCam HRc digital camera (Carl Zeiss Microscopy, Thornwood, NY, USA). To observe the ascomatal wall and arrangement of asci within ascomata, structures were rehydrated with 5 % KOH and sectioned using a freezing microtome. The asci and ascospores were observed by rehydrating the fruiting bodies with 5 % KOH. To observe the bitunicate form of asci, they were stained with Melzer’s reagent. In order to observe conidia and conidiophores, living cultures were sporulated on sterilised Zea mays leaves placed on 1.5 % water agar (WA) or slide cultures of half-strength potato dextrose agar (PDA). The sexual morph was induced by pairing compatible isolates in Sach's agar with sterilised rice or wheat straw at 25 °C. Conidia and conidiophores were mounted in distilled water and observed with a Carl Zeiss Axioplan2 compound light microscope. Conidial width measurements were taken from the widest part of each conidium. The lengths and widths were measured using Axiovision Rel. v. 4.8 software (Carl Zeiss Microscopy, Thornwood, NY, USA). Whenever possible, more than 30 measurements were made. For morphological structures mean, minimum, maximum and standard deviation were calculated. Conidial and conidiophore length and width ranges are reported as mean ± standard deviation. As conidial length shows a high standard deviation, those ranges are rounded into the nearest multiple of five. Extreme measurements are given in parentheses with mean and standard deviation. The conidial germination, septum ontogeny and sexual characters of several species are based on Ellis (1971), Sivanesan (1987) or protologues where these characters could not be verified based on dry specimens otherwise the living cultures observed. Three sets of duplicate cultures of each isolate were measured to determine colony characters on PDA at 25 °C in the dark. After 1 wk, colony size and colour using Rayner (1970) and zonation were recorded. All herbarium materials listed were observed by the authors unless stated otherwise.
DNA extraction, PCR and sequencing
For genomic DNA extractions, isolates were grown on PDA at 25 °C in the dark for 2 d. Mycelial scrapings (50–60 mg) were obtained from the leading edge of cultures. Harvested mycelium was lysed in tubes containing 500 μm garnet media and a 6 mm zirconium bead (OPS Diagnostics, Lebanon, NJ, USA) with the Fast Prep FP120 (Fischer Scientific Inc, Waltham, MA, USA) for 20 s. A DNeasy Plant Mini Kit (Qiagen, Inc., Valencia, CA, USA) was used to extract DNA as described in Udayanga et al. (2014).
The ITS, GPDH, TEF and LSU regions were amplified using the PCR primers and conditions listed in Manamgoda et al. (2012) on a Bio-Rad Dyad Peltier thermal cycler in a 25 μL reaction volume: 10–15 ng genomic DNA, 12.5 μL Quick-Load Taq 2× Master Mix (New England BioLabs, Ipswich, MA, USA), 1 μL 10 mM of each primer and 1 % DMSO with volumes adjusted to 25 μL with nuclease-free water. PCR products were visualised by electrophoresis in 1 % agarose gels stained with SYBR Safe DNA Gel Stain (Invitrogen, Eugene, OR, USA). Excess primers and dNTPs were removed from PCR amplification mixtures with ExoSAP-IT (USB Corp., Cleveland, OH, USA) according to the manufacturer’s instructions. Amplicons were sequenced using the BigDye Terminator v. 3.1 Cycle Sequencing kit (Life Technologies, Grand Island, NY, USA) on an Applied Biosystems 3130xl Genetic Analyser using the same primers used to amplify each of the gene regions except an additional primer EF1-1567R (Rehner 2001) was used for sequencing the TEF region.
Sequence alignment, phylogenetic analyses and species recognition
Raw sequences were assembled with Sequencher v. 4.9 for Windows (Gene Codes Corp., Ann Arbor, MI, USA). The assembled consensus sequences were initially aligned with ClustalW and optimised with MAFFT v. 7 using default settings (http://mafft.cbrc.jp/alignment/server/) and adjusted manually where necessary (Katoh & Standley 2013). Newly generated ITS, GPDH, TEF and LSU sequences were analysed separately with all available type-derived sequences listed in Manamgoda et al. (2011, 2012) to determine preliminary identifications of the isolates. To fully resolve closely related species, all isolates were subjected to a multi-gene combined analysis. PAUP v. 4.0b10 (Swofford 2003) was used to perform maximum parsimony (MP) analyses. Trees were inferred using the heuristic search option with 1000 random sequence additions. Maxtrees were unlimited, branches of zero length were collapsed and all multiple equally most parsimonious trees were saved. Descriptive tree statistics for parsimony [Tree Length (TL), Consistency Index (CI), Retention Index (RI), Related Consistency Index (RC) and Homoplasy Index (HI)] were calculated for trees generated in the parsimony analysis.
Evolutionary models for phylogenetic analyses were selected independently for each locus using MrModeltest v. 2.3 (Nylander 2004) under the Akaike Information Criterion (AIC) implemented in both PAUP v. 4.0b10 and MrBayes v. 3. Phylogenetic reconstructions of concatenated and individual gene trees were performed using both Bayesian Inference (BI) Markov Chain Monte Carlo and Maximum Likelihood (ML) criteria. Bayesian reconstructions were performed using MrBayes v. 3.1.2 (Huelsenbeck & Ronquist 2001). Two simultaneous analyses, each consisting of six Markov chains, were run for 1 000 000 generations with trees sampled every 100 generations resulting in 20 000 total trees. The first 2 000 trees, representing the burn-in phase of the analyses were discarded from each run and the remaining trees (a total of 16 000) were used for calculating posterior probabilities (PP) in the majority rule consensus tree.
Maximum likelihood trees were generated using the RAxML v. 7.4.2 Black Box (Stamatakis et al. 2008) in the CIPRES Science Gateway platform (Miller et al. 2010). For the combined dataset all free modal parameters were obtained using RAxML with ML estimate of 25 per site rate categories. The combined three-gene (ITS, GPDH and TEF) dataset was partitioned by gene region. The RAxML software accommodated the GTR model of nucleotide substitution with the additional options of modeling rate heterogeneity (Γ) and proportion invariable sites (I).
In order to determine the species limits, we applied the criteria of Genealogical Concordance Phylogenetic Species Recognition (GCPSR) (Taylor et al. 2000, Dettman et al. 2003). Dettman et al. (2003) emphasise that species should be recognised if they satisfy one of two criteria: genealogical concordance or genealogical non-discordance. Clades were genealogically concordant if they were present in at least some of the gene trees and genealogically non-discordant if they were strongly supported (MP ≥ 70 %; ML ≥ 70 %) in a single gene and not contradicted at or above this level of support in any other single gene tree. This criterion prohibits poorly supported non-monophyly at one locus from undermining well-supported monophyly at another locus (Dettman et al. 2003). Phylogenetic trees were viewed in MEGA v. 5 (Tamura et al. 2011), TreeView v. 1.6.6 (Page 1996) and FigTree v. 1.4 (Rambaut & Drummond 2008). All sequences generated were deposited in GenBank (Table 1), alignments and trees in TreeBASE (Study 16163, 16165), taxonomic novelties (MB809648, MB809649, MB809652 – MB809655, MB810140) and novel typifications (MBT197968, MBT197970 – MBT197980, MBT198049 – 198051, MBT198292, MBT198401 – 198402) in MycoBank (Crous et al. 2004).
Results
Phylogenetic analysis
A total of 221 new sequences were generated in this study with additional sequences downloaded from GenBank, including 63 sequences from our previous studies (Manamgoda et al. 2011, 2012). Maximum parsimony analysis of combined ITS, GPDH and TEF loci for Bipolaris included 63 isolates with the outgroup taxon. The concatenated alignment consisted of 1 908 positions of which 1 561 were constant, 133 were parsimony uninformative and 214 were parsimony informative. Four equally most parsimonious trees were generated and one of them was used to represent the molecular phylogeny of the genus Bipolaris as Fig. 1 (RI = 0.840; CI = 0.645; RC = 0.542 HI = 0.355; Tree length = 645). The BI and ML trees were similar to the MP tree in terms of major clades and topology of the tree. Topologies of the individual gene trees were determined to be congruent and no conflicts were observed in species delimitation. The alignment properties for the individual genes are shown in the Table 2. The combined gene phylogeny of Bipolaris (Figs 1, 2) resolved 29 species with high bootstrap support values at the terminal nodes. Closer inspection of the sequence alignment revealed that some species pairs such as Bipolaris cynodontis and B. coffeana as well as B. oryzae and B. panici-miliacei show only a few variable characters. These species were treated as distinct taxa at this time based on the applications of GCPSR.
Fig. 1.
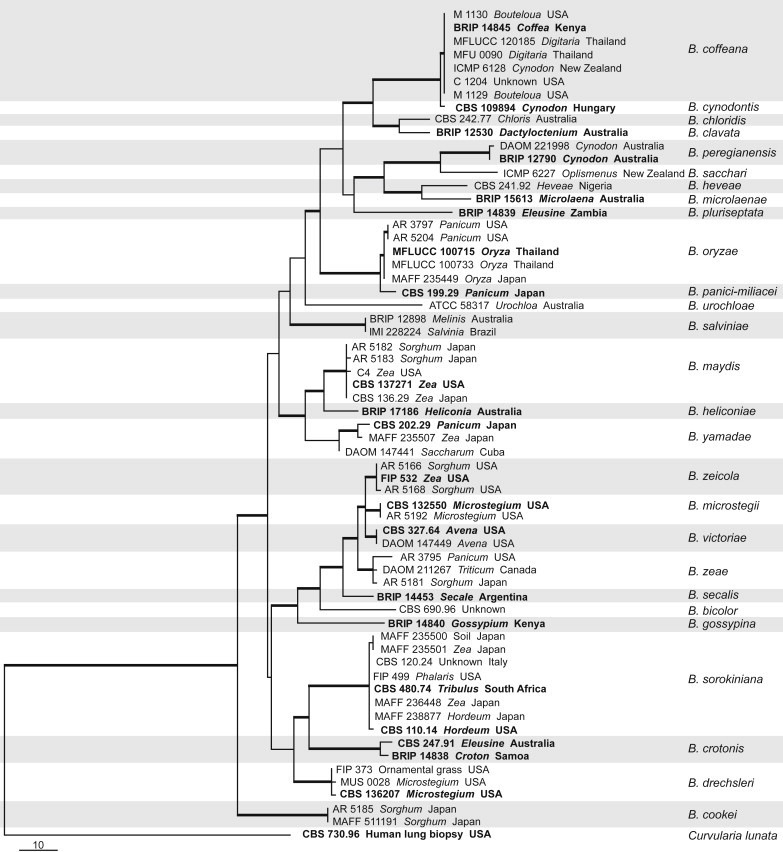
One of the four equally most parsimonious trees generated for Bipolaris from the parsimony analysis based on the combined alignment of ITS, GPDH and TEF sequences. The thickened branches correspond to parsimony and maximum likelihood bootstrap support values ≥60 % and Bayesian posterior probability values ≥0.7. All ex-type cultures are printed in bold. The tree is rooted with Curvularia lunata (CBS 730.96).
Table 2.
Alignment properties and nucleotide substitution models per locus1 and combined.
| ITS | GPDH | TEF | Combined ITS, GPDH and TEF | |
|---|---|---|---|---|
| Number of characters included in analysis (including gaps) | 509 | 496 | 899 | 1908 |
| Number of constant characters | 426 | 354 | 799 | 1561 |
| Number of parsimony informative characters (%) | 52 (10 %) | 96 (19 %) | 55 (6 %) | 214 (11 %) |
| Number of uninformative and variable characters | 31 | 46 | 45 | 133 |
| Nucleotide substitution model | HKY + I + G | GTR + G | GTR + I + G | GTR + I + G |
ITS: internal transcribed spacers and intervening 5.8S nrDNA; GPDH: partial glyceraldehyde-3-phosphate dehydrogenase gene; TEF: partial translation elongation factor 1-alpha gene.
Fig. 2.
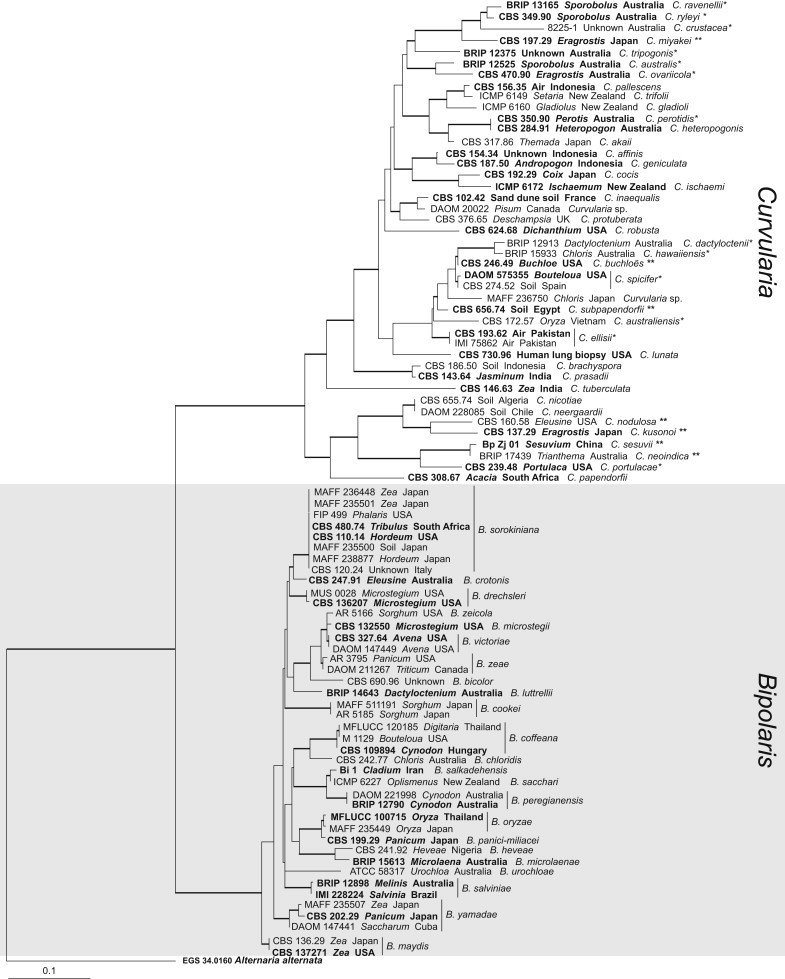
Phylogram generated for Bipolaris and Curvularia from a maximum likelihood analysis based on the combined ITS and GPDH alignment. The thickened branches corrospond to parsimony and maximum likelihood bootstrap support values ≥60 % and Bayesian posterior probability values ≥0.7. All ex-type cultures are printed in bold. Novel combinations are indicated with two asterisks and species transferred from Bipolaris are indicated with a single asterisk. The tree is rooted with Alternaria alternata (EGS 34.0160).
The combined ITS and GPDH sequence alignment containing 86 isolates of Bipolaris and Curvularia (85 ingroup and 1 outgroup) includes 1 018 positions of which 661 are constant, 84 are variable and parsimony uninformative and 280 are parsimony informative. The BI, ML and MP trees were similar in terms of major clades and topology. The ML tree generated from RAxML was used to illustrate the phylogenetic relationships of the sister genera Bipolaris and Curvularia. Two major clades representing Bipolaris and Curvularia are supported by 100 %, 100 % Maximum parsimony, 85 %, 75 % ML bootstrap and 1.00, 1.00 BI posterior probability values, respectively. In the single gene LSU alignment for Bipolaris and Curvularia, the alignment contained 106 isolates (105 in-group; 1 out-group) with 851 positions of which 790 are constant and 18 are variable and parsimony uninformative while 43 are parsimony informative (trees not shown here; see general Discussion).
Taxonomy
In this section we provide a modern generic circumscription for Bipolaris with 47 species treated within the genus based on the current concept. The species descriptions are given with current nomenclature, host range, geographic distribution and notes on taxonomy and phylogeny. Disease symptoms on hosts are given separately, when available, from specimens observed or on the protologue in order to assist in field identifications. Hosts are listed based on the specimens observed by authors indicated in protolgues and the host records are extracted from the database of the Systematic Mycology and Microbiology Laboratory (Farr & Rossman 2013) or the cited literature. Host records that are not linked to specimens in this paper are listed as “Also reported from”. Poaceous and non-poaceous hosts are listed separately if species occur on both poaceous and non-poaceous hosts. If molecular data of the type or a well-authenticated culture suggests that a species does not belong in Bipolaris, that species is listed under excluded names. Species are listed as doubtful if the data are inadequate or if it has distinct morphological characteristics indicative of another known genus. Seven new combinations are made for species previously in Bipolaris that should be placed in the genus Curvularia.
Generic description
Bipolaris Shoemaker, Canad. J. Bot. 37: 882. 1959.
Synonym: Cochliobolus Drechsler, Phytopathology 24: 973. 1934.
Asexual morph on PDA: Hyphae hyaline, pale to dark brown or grey. Conidiophores pale to dark brown, single, branched, sometimes arranged in small groups, straight to flexuous or geniculate. Conidiogenous nodes smooth to slightly verruculose. Conidia mostly curved, canoe-shaped, fusoid or obclavate, rarely straight, 3–14-distoseptate (usually more than 6), hyaline, pale or dark brown, reddish brown or pale to deep olivaceous, germinating by production of one or two germination tubes by polar cells. Hilum often slightly protruding or truncate, sometimes inconspicuous. Septum ontogeny first septum median to sub-median, second septum delimits basal cell and third delimits distal cell. Sexual morph on Sach's agar and sterilised plant material in culture: Ascomata brown or black, immersed, erumpent, partially embedded or superficial, free or on flat stroma, mostly globose to ellipsoidal, sometimes flask-shaped or flattened on hard substrata, smooth or covered with vegetative filaments. Ostiole central, papillate or with a sub-conical, conical, paraboloid or cylindrical neck. Peridium comprising pseudoparenchymatous cells of equal thickness or slightly thickened at apex. Hamathecium comprising septate, filiform, branched pseudoparaphyses. Asci 2–8-spored, clavate, cylindrical-clavate or broadly fusoid, straight or slightly curved, thin-walled, bitunicate, fissitunicate, often becoming more or less distended prior to dehiscence, short pedicellate, rounded at apex. Ascospores fasciculate, filiform or flagelliform, hyaline or sometimes pale yellow or pale brown at maturity, septate, helically coiled within ascus, degree of ascospore coiling moderate to very strongly coiled, sometimes with free ends, often with a thin mucilaginous sheath (modified from Manamgoda et al. 2012).
Cultural characteristics: Colonies white or pale grey when young, becoming brown or dark grey with maturity, fluffy, cottony, raised or convex with papillate surface, margin lobate, undulate, entire or sometimes rhizoid.
Type species: Bipolaris maydis (Y. Nisik. & C. Miyake) Shoemaker
Species descriptions
Bipolaris arizonica R. Sprague, Mycologia 52: 358. 1960. Fig. 3.
Fig. 3.

Bipolaris arizonica (WSP 46123). A. Conidia and conidiophores on a diffuse leaf spot on the host Muhlenbergia wrightii. B. Conidia attached to conidiophore. C. D. Conidiophores. E–J. Conidia. Scale bars: A = 50 μm, B = 10 μm, C–J = 5 μm.
≡ Drechslera arizonica (R. Sprague) Subram. & B.L. Jain, Curr. Sci. 35: 353. 1966.
Type material: USA, Arizona, on Muhlenbergia wrightii, 3 Sep. 1957, R. Sprague, WSP 46123, holotype.
Asexual morph on Muhlenbergia wrightii: Leaf spots emarginated, diffuse, dry, grey. Conidiophores (56–)70–105(–112) × 5–7(–8) μm (av. = 89, SD = 16, n = 13; av. = 6, SD = 1, n 13), smooth, usually arising singly or sometimes in pairs, simple, unbranched, 4–10-septate, geniculate towards apex, pale olivaceous brown to dark brown, hyaline or pale brown at apex scattered throughout leaf spots. Conidia (32–)35–50(–58) × 10–14 μm (av. = 41, SD = 7, n 31; av. = 12, SD = 2, n = 31), smooth, straight, fusiform or cylindrical widest between second and third or third and fourth septum in long conidia, olivaceous to pale brown, (3–)5(–6)-distoseptate, hilum truncate, enclosed in cell wall, spores germinating from both ends.
Host: Muhlenbergia wrightii (Poaceae).
Distribution: USA (AZ), known only from the type.
Notes: According to Sprague (1960) this fungus produces a slightly cobwebby nearly black growth in pure culture on PDA. Although this species is known only from the type specimen and no molecular data exist, it is accepted in Bipolaris based on the characteristic hilum structure of the conidia. Bipolaris cynodontis has been reported from Muhlenbergia hosts but B. cynodontis usually produces longer conidia (40–80 μm) than B. arizonica.
Bipolaris bicolor (Mitra) Shoemaker, Canad. J. Bot. 37: 884. 1959. Fig. 4.
Fig. 4.
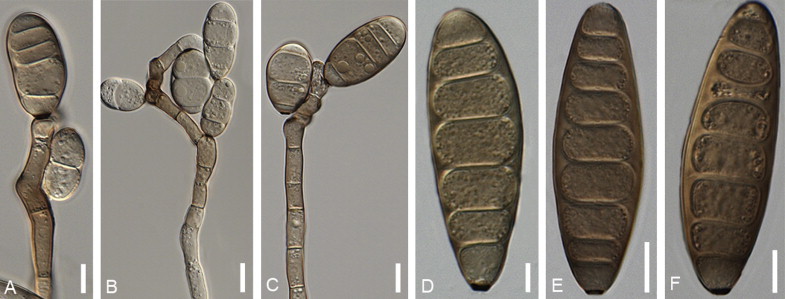
Bipolaris bicolor (CBS 690.96). A–C. Conidiophore with conidia. D–F. Conidia. Scale bars: A–C = 10 μm, D = 5 μm, E–F = 10 μm.
Basionym: Helminthosporium bicolor Mitra, Trans. Brit. Mycol. Soc. 15: 286. 1931.
≡ Drechslera bicolor (Mitra) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
= Cochliobolus bicolor A.R. Paul & Parbery, Trans. Brit. Mycol. Soc. 49: 386. 1966. (fide Paul & Parbery 1966).
= Drechslera bhawanii O. Prakash & A.P. Misra (as “bhawani”), Sydowia 33: 242. 1980. (fide Sivanesan 1987).
Type material: India, Poona, associated with wheat foot-rot (Bipolaris bicolor), not seen; Australia, Queensland, and New South Wales, Bega, dry culture on Sach’s agar + pieces of Danthonia spikelet and pedicel, produced by pairing two isolates from Pennisetum clandestinum from Toowoomba, Queensland, Australia, 1966, A.R. Paul & Parbery, MELU F2220 (Cochliobolus bicolor), not seen.
Asexual morph on PDA: Conidiophores (78–)150–490(–596) × 5–7 μm (av. = 317, SD = 169, n = 15; av. = 6, SD = 1, n = 15), arising singly or in small groups, simple or branched, septate, straight or flexuous, greyish brown, on slide cultures of PDA. Conidia (23–)40–70(–80) × 15–19 μm (av. = 55, SD = 15, n = 47; av. = 17, SD = 2, n = 46), smooth, straight, rarely curved, usually cylindrical sometimes obclavate, tapering towards rounded ends, greyish to dark greyish brown, hyaline when immature, at maturity usually two end cells lighter than middle cells, sometimes sub-hyaline, (2–)6(–9)-distoseptate. Hilum conspicuous, 3–5 μm wide. Sexual morph on Sach’s agar + pieces of Danthonia sp.: Ascomata 260–530 × 240–470 μm in diameter, brown to black, globose to elliptical. Ostiole 40–140 μm wide, when mature, few hyaline cells cover ostiole. Asci 132–208 × 14–23 μm, straight or slightly curved, bitunicate with a short stipe, 1–8-spored, some asci with only 2–4 spores, then width greater (5–6 μm), but length and septation same. Ascospores 165–242 × 4–7 μm, closely coiled together filiform, tapering at both ends, particularly at base, hyaline.
Cultural characteristics: Colonies on PDA, whitish grey, velvety, raised with entire or undulate margin.
Hosts: Pennisetum clandestinum, Zea mays. (Poaceae). Also reported from: Andropogon aciculatus, Apluda aristata, Brachiaria ruziziensis, Eleusine coracana, Eragrostis japonica, Melanocenchris abyssinica, Oryza sativa, Panicum maximum, Pennisetum sp., P. glaucum, P. typhoides, Setaria sp., Sorghum sp., S. vulgare, Triticum aestivum, Urochloa panicoides, Zizania aquatica (Poaceae). Also reported from non-Poaceae hosts: Quercus sp. (Fagaceae) (Farr & Rossman 2013)
Distribution: Australia, India. Also reported from: Africa, Brazil, Canada, Cote d'Ivoire, Denmark, New Zealand, Nigeria, Swaziland, Zimbabwe (Farr & Rossman 2013).
Additional material examined: Unknown location and host, Jun. 1996, R.F. Castañeda, identified by R. Shoemaker, CBS 690.96 = INIFAT C95/100.
Notes: This species is relatively common in warm temperate and tropical regions and occurs on a variety of grasses as well as one report from Quercus in Mexico (Mena-Portales et al. 1995, Farr & Rossman 2013). It is considered to be a seed-borne pathogen (Lau & Sheridan 1975, Wu 1983, Richardson 1990) and reported as the cause of foot rot of wheat and leaf spot of Pennisetum (Sivanesan 1987). There is no available ex-type culture for this species. The culture CBS 690.96 was identified by R. Shoemaker as Bipolaris bicolor and is presently used as representative strain (Berbee et al. 2000).
Bipolaris chloridis (Alcorn) Alcorn, Mycotaxon 16: 373. 1983. Fig. 5.
Fig. 5.

Bipolaris chloridis (IMI 213865). A. Ascoma on Chloris gayana. B. Cross section of ascoma. C. Asci and pseudoparaphyses. D–F. Asci. G. Ascoma with conidiophores on surface. H, I. Conidiophores. J, K. Conidia. Scale bars: A, B, H = 100 μm, C, E, F, I, H = 20 μm, D = 10 μm, J, K = 5 μm, B–F stained with Melzer’s reagent.
Basionym: Drechslera chloridis Alcorn, Trans. Brit. Mycol. Soc. 67: 148. 1976.
= Cochliobolus chloridis Alcorn, Trans. Brit. Mycol. Soc. 70: 61. 1978. (fide Alcorn 1978).
Type material: Australia, Queensland, Booie prop, Kingaroy, on Chloris gayana, 21 Dec. 1972, J.L. Alcorn, BRIP 10965 (Bipolaris chloridis), holotype, not seen; ibid., IMI 181067, isotype; ibid., on C. gayana, 21 Mar. 1977, J.L. Alcorn 7781, IMI 213865 (Cochliobolus chloridis), holotype; ibid., BRIP 12106a, isotype.
Asexual morph on PDA: Conidiophores (79–)110–320(–383) × (5–)6–10(–13) μm (av. = 218, SD = 105, n = 20; av. = 8, SD = 2, n = 20), arising singly or in small groups, simple, rarely branched, septate, straight or flexuous, geniculate at upper part, pale brown to reddish brown. Conidiogenous nodes dark brown, distinctly swollen. Conidia (42–)65–90(–93) × (12–)13–20(–26) μm (av. = 79, SD=14, n = 34; av. = 17, SD = 4, n = 34), smooth, straight or curved, ellipsoid, tapering toward rounded ends, pale brown to reddish brown, (3–)5(–7)-distoseptate. Sexual morph on sterile leaves of Chloris gayana in Sach’s agar medium: Ascomata 265–350(–366) μm in diameter (av. = 308, SD = 42, n = 15), black, globose, with a short ostiole 100–120 × 60–70 μm (av. = 116, SD = 5, n = 20; av. = 65, SD = 5, n = 20). Conidiophores developing on ascomata wall. Asci (112–)135–165(–181) × (17–)18–22(–24) μm (av. = 150, SD = 16, n = 33; av. = 20, SD = 2, n = 33), with 1–8 ascospores arranged into a tightly coiled helix, bitunicate, sub-cylindrical, obclavate or fusiform, tapering to a rounded apex, sessile or shortly pedicellate. Ascospores 130–165 × 3–5 μm (av. = 148, SD = 17, n = 15; av. = 4, SD = 1, n = 15), hyaline, filiform, septate.
Cultural characteristics: Colonies on PDA, greyish white to greyish, effuse, producing abundant conidia in 1 wk.
Host: Chloris gayana (Poaceae).
Distribution: Australia. Also reported from India, Kenya, Malawi, Tanzania, Zambia (Sivanesan 1987).
Additional material examined: Australia, Queensland, Booie prop, Kingaroy, on Chloris gayana, 11 Apr. 1976, J.L. Alcorn, CBS H-12218 (Bipolaris chloridis), culture CBS 242.77 = ATCC 34706 = IMI 208338.
Notes: The conidial morphology of B. chloridis is similar to that of B. cookei in dimensions and septation. However, B. cookei often produces secondary conidiophores and secondary conidia; these are generally lacking in B. chloridis (Alcorn 1983b, Sivanesan 1987). The phylogenetic analysis confirmed that B. chloridis and B. cookei are two distinct species (Fig. 1).
Bipolaris clavata Alcorn, Mycotaxon 15: 15. 1982. Fig. 6.
Fig. 6.

Bipolaris clavata (IMI 264352). A. Culture on filter paper. B. Conidiophores. C. Conidia. D. Conidia and conidiophores. E–I. Conidia. Scale bars: A = 1000 μm, B, D–F, I = 20 μm, C, H = 10 μm.
Type material: Australia, Queensland, on Dactyloctenium radulans, 12 May 1977, J.L. Alcorn 77144C, BRIP 12530, holotype, not seen; ibid., ex-holotype culture Alcorn 77144c; ibid., J.L. Alcorn 77140, IMI 264352, DAR 35054, isotypes.
Asexual morph on Dactyloctenium radulans: Conidiophores 125–185(–190) × 5–9 μm (av. = 157, SD = 29, n = 7; av. = 7, SD = 2, n = 7), arising singly or in small groups of few, septate, straight or flexuous, geniculate at upper fertile part, brown to reddish brown. Conidiogenous nodes distinct, apex of conidiogenous cell sometimes swollen. Conidia (70–)80–100(–106) × (11–)14–18(–20) μm (av. = 88, SD = 9, n = 30; av. = 16, SD = 2, n = 30), smooth, straight or mostly curved, cylindrical, sub-cylindrical, narrowly clavate to fusoid, pale reddish to pale brown, (3–)4(–5)- distoseptate. Hilum inconspicuous.
Host: Dactyloctenium radulans (Poaceae).
Distribution: Australia.
Notes: This species is known to cause leaf spots on Dactyloctenium radulans. According to Alcorn (1982) conidial dimorphism in B. clavata has been observed. The ex-type culture of B. clavata produced two types of conidia, specifically large, curved-clavate conidia typical of Bipolaris and small cylindrical conidia. Conidial dimorphism is a rare phenomenon within the genus Bipolaris, only recorded in this particular species. Dimorphic conidia were not observed associated with the isotype specimens. This species is accepted in the genus Bipolaris based on the conidial morphology and phylogenetic evidence (Fig. 1). A sexual morph is not known for this species.
Bipolaris coffeana Sivan., Trans. Brit. Mycol. Soc. 84: 404. 1985. Fig. 7.
Fig. 7.

Bipolaris coffeana (IMI 144159). A, B. Conidiophores. C–H. Conidia. Scale bars = 10 μm.
Type material: Kenya, on Coffea arabica, 31 Oct. 1969, I. Furtado, IMI 144159, holotype; ibid., ex-isotype BRIP 14845
Asexual morphology on PDA: Hyphae pale brown, smooth, septate. Conidiophores 170–230(–250) × 4–6 μm (av. = 202, SD = 29, n = 12; av. = 5, SD = 1, n = 12), arising in small groups, simple, cylindrical, septate, distinctly geniculate, with a swollen base, pale brown to dark brown. Conidia 35–50(–69) × 14–20(–23) μm (av. = = 42, SD = 7, n = 31; av. = 17, SD = 3, n = 31), smooth, usually straight, rarely curved, oblong, ellipsoidal or broadly fusoid, pale brown to dark brown, (4–)6(–7)-distoseptate. Hilum slightly conspicuous, truncate. Septum ontogeny first septum in conidia median, second septum delimiting basal cell and third septum distal; germination bipolar.
Cultural characteristics: Colonies on PDA, mid to dark brown, sporulating abundantly.
Hosts: Bouteloua gracilis, Cynodon dactylon, Digitaria sp. (Poaceae), on leaves of Coffea arabica (Rubiaceae).
Distribution: Kenya, New Zealand, Thailand, USA.
Additional materials examined: New Zealand, Auckland, Mt Albert, on Cynodon dactylon, E.H.C. McKenzie, ICMP 6128; Thailand, Chiang Rai, Mae Fah Luang University garden, on Digitaria sp., 31 Jun. 2011, D.S. Manamgoda M1010; Chiang Rai, Mae Fah Luang University garden, on Digitaria sp., 28 Jun. 2011, D.S. Manamgoda, MFLUCC 12-0185; USA, Utah, on Bouteloua gracilis, Apr. 2012, M. Barkworth, M 1128 – M 1132; ibid., M 1134; ibid., M 1137– M 1141.
Notes: This species is known only from the type specimen and according to phylogenetic (Fig. 1) and morphological evidence, placement in the genus Bipolaris is confirmed. This species was known only from Coffea arabica before; in this study we report B. coffeana on Bouteloua gracilis from USA, on Cynodon dactylon from New Zealand and on Digitaria sp. from Thailand for the first time. Bipolaris tropicalis and B. zeicola are also reported on Coffea. However Bipolaris coffeana has smaller conidia compared to B. zeicola (65–90 μm). Production of stromata was observed in the culture of B. tropicalis and such formation is not recorded on B. coffeanae Phylogenetically B. coffeana is similar to B. cynodontis. A sexual morph is not found in association with this species, and the conidial germination is known only from Sivanesan (1985).
Bipolaris colocasiae (M.P. Tandon & Bhargava) Alcorn, Mycotaxon 17: 67. 1983. Fig. 8.
Fig. 8.

Bipolaris colocasiae (IMI 177992). A. Conidiophores and conidia on the surface of the host Colacasia esculenta. B, C. Conidiophores and conidia. D–I. Conidia. Scale bars: A = 50 μm, B, C = 20 μm, D–I = 10 μm.
Basionym: Drechslera colocasiae M.P. Tandon & Bhargava (as “colocaseae”), Curr. Sci. 49: 76. 1980.
Type material: India, isolated from Colocasia esculenta, dried culture on PDA, 12 Feb. 1973, M.P. Tandon, IMI 177992, holotype.
Leaf spots on Colocasia esculenta: 0.5–1.5 cm diameter, dull yellow. Asexual morph in PDA: Hyphae pale brown, smooth, and septate. Conidiophores 65–160(–189) × 3–5(–6) μm (av. = 112, SD = 48, n = 12; av. = 4, SD = 1, n = 12), arising singly, simple, septate, flexuous, distinctly geniculate at apex, basal cell usually swollen, pale brown to reddish dark brown. Conidia 25–35 × 7–11 μm (av. = 30, SD = 5, n = 32; av. = 9, SD = 2, n = 32), smooth, straight or somewhat curved, oblong to fusoid pale brown to dark brown, (3–)5(–7)-distoseptate. Hilum conspicuously truncate, sometimes slightly protruding.
Cultural characteristics: Colonies on PDA, velvety, effuse, abundantly sporulating, dark brown.
Hosts: Colocasia esculenta (Arecaceae). Also reported from: Cymbopogon martini, Pennisetum americanum, a hybrid of Hordeum/Triticum (Poaceae). Also reported from non-Poaceae hosts: Brassica juncea (Brassicaceae), Phaseolus vulgarias (Fabaceae), Cicer arietinum (Leguminosae), Hibiscus abelmoschi (Malvaceae), Phlox drummondii (Polemoniaceae) (Sivanesan 1987).
Distribution: India.
Notes: According to Tandon & Bhargava (1980), Bipolaris colocasiae causes a disease that resulted in 8–10 % crop loss. It was also reported to cause reddish brown leaf spots on Cymbopogon martini (Sivanesan 1987). The species is morphologically similar to Curvularia hawaiiensis but conidia of this species are longer and narrower than in C. hawaiiensis. Bipolaris colocasiae has relatively shorter conidia compared to other species of the genus Bipolaris. However, based on septation and hilum morphology, this species is accepted in the genus Bipolaris. A sexual morph is not found in association with this species.
Bipolaris cookei (Sacc.) Shoemaker, Canad. J. Bot. 37: 884. 1959. Fig. 9.
Fig. 9.

Bipolaris cookei (BPI 428852, BPI 430358, AR 5185). A. Symptom on Sorghum sudanense. B, C. Conidiophores. D–F. Conidia. G. Conidia and conidiophores on slide culture. H. Conidia attached to conidiophores. I–K. Conidiophores. L–O. Conidia with secondary sporulation. P–Z. Conidia. Scale bars: A = 500 μm, B = 10 μm, C, E, F, H = 5 μm, I–K = 10 μm, L–O = 5 μm, P–Z = 10 μm.
Basionym: Helminthosporium cookei Sacc., Syll. Fung. 4: 420. 1886.
≡ Helminthosporium sorghi Cooke, Grevillea 6: 141 (1878) non Schwein. Trans. Am. Phil. Soc 4: 279. 1832.
= Helminthosporium sorghicola Lefebvre & Sherwin, Mycologia 40: 714. 1949. (fide Shoemaker 1959).
≡ Drechslera sorghicola (Lefebvre & Sherwin) M.J. Richardson & E.M. Fraser, Trans. Brit. Mycol. Soc. 51: 148. 1968.
≡ Bipolaris sorghicola (Lefebvre & Sherwin) Alcorn, Mycotaxon 17: 69. 1983.
Type material: USA, South Carolina, Aiken, on culms of Sorghum sp., (1874) Ravenel, American Fungi no. 167, BPI 430300 (Heminthosporium cookei), holotype; Georgia, Tifton, on Sorghum sudanense, 1 Aug. 1943, C.L. Lefebvre, BPI 428852 (Helminthosporium sorghicola), holotype; ibid., BPI 430372; ibid., BPI 430369, paratypes.
Leaf spots on Tift Sudan grass (common Sudan grass × Leoti Sorghum hybrid): Round to elliptic, parallel to leaf veins, zonate, alternating pale tan with darker narrower bands. On common Sudan grass (Sorghum bicolor): Cause of target leaf spot disease. Lesions less zonate, often straw coloured in centre surrounded by a reddish purple border (Lefebvre & Sherwin 1949). Asexual morph on PDA: Hyphae hyaline to brown. Conidiophores 40–520(–690) × 5–7 μm (av. = 280, SD = 240, n = 30; av. = 6, SD = 1, n = 30), arising singly or in small groups of 2–4, simple, occasionally branching, septate, straight or flexuous, geniculate at upper part, greyish brown. Conidiogenous nodes smooth, dark brown. Conidia (28–)40–75(–100) × (11–)14–18(–20) μm (av. = 59, SD = 16, n = 117; av. = 16, SD = 2, n = 117), usually slightly curved, sometimes straight, ellipsoidal or obclavate, widest at middle, tapering towards broadly rounded ends, olivaceous to golden brown, (3–)5(–7)-distoseptate. Hilum 3–4 μm wide, inconspicuous, germinating with two polar germ tubes. Secondary conidiophores formed readily under moist conditions, sometimes repeatedly sporulating when conidia attached to primary conidiophores, forming chains of conidia.
Cultural characteristics: Colonies on PDA, velvety, aerial mycelium white when young, becoming greyish brown at maturity, colony appears greyish olive when sporulating.
Hosts: Sorghum halapense, S. sudanense, S. vulgare, Sorghum sp., Zea mays. Reported from: Chloris gayana, Eriochloa procera, Oryza sativa, Sorghum bicolor (Poaceae) (Farr & Rossman 2013).
Distribution: Japan, USA (AZ, FL, GA, KS, MD, PA, TX, VA). Also reported from: Australia, Bolivia, Brazil, China, Cuba, Guyana, India, Korea, Nigeria, Malaysia, Papua New Guinea, Pakistan, Saudi Arabia, Solomon Islands, Sri Lanka, Sudan, Taiwan, Togo, Yemen, Zimbabwe (Farr & Rossman 2013).
Additional materials examined: Japan, on Sorghum halepense, Sep. 1990, T. Tsukiboshi, culture AR 5185; ibid., culture MAFF 511191. USA, Arizona, Nogales, on S. halepense, 19 Aug. 1963, J. Alice Watson, BPI 430336; Florida, Gainesville, on S. sudanense, 4 Jul. 1944, C.L. Lefebvre, BPI 430360; Florida, Gainesville, on S. sudanense, Aug. 1944, G.E. Ritchey, BPI 430361; Georgia, Cordele, on S. halepense, 22 Aug. 1945, C.L. Lefebvre, BPI 430335; Kansas, Rooks Co., on Zea mays, 06 Jul. 1898, BPI 428853; Maryland, Beltsville, on S. halepense, 12 Mar. 1946, C.L. Lefebvre, BPI 430337; ibid., 25 Mar. 1948, C.L. Lefebvre, BPI 430338; ibid., 18 Mar. 1948, C.L. Lefebvre, BPI 430339; ibid., 20 Apr. 1948, C. L. Lefebvre & H. Sherwin, BPI 430340; ibid., Mar. 1948, C.L. Lefebvre & H. Sherwin, BPI 430343; ibid., 20 Apr. 1948, C.L. Lefebvre & H. Sherwin, BPI 430344; ibid., 18 Feb. 1948, C.L. Lefebvre & H. Sherwin 1680, BPI 430362; ibid., 20 Apr. 1948, C.L. Lefebvre & H. Sherwin 1724, BPI 430363; ibid., BPI 430352; Maryland, Beltsville, on S. vulgare, 5 May 1948, C.L. Lefebvre & H. Sherwin 1673, BPI 430364; ibid., 1674, BPI 430365; ibid., BPI 430350; Maryland, Beltsville, on S. sudanense, 18 Mar. 1946, C.L. Lefebvre, BPI 430351; ibid., 18 Aug. 1944, C.L. Lefebvre, BPI 430356; ibid., 24 Aug. 1944, C.L Lefebvre, BPI 430359; ibid., 3 Oct. 1945, C.L. Lefebvre, BPI 430370; ibid., 18 Aug. 1944, C.L. Lefebvre, BPI 430375; ibid., 3 Oct. 1946, C.L. Lefebvre, BPI 430376; ibid., 18 Aug. 1944, C.L. Lefebvre, BPI 430380; ibid., 28 Nov. 1944, C.L. Lefebvre, BPI 430383; ibid., 28 Nov. 1946, C.L. Lefebvre, BPI 430384; ibid., 28 Nov. 1944, BPI 430390; ibid., BPI 430342; Maryland, Beltsville, on S. vulgare, H. Sherwin 1847, BPI 430345; Maryland, Finksburg, on S. sudanense, 29 Aug. 1945, H.W. Johnson, BPI 430368; Mississippi, Stoneville, on S. vulgare, 10 Sep. 1949, H.W. Johnson, BPI 430366; ibid., BPI 430358; North Carolina, Etheridge, on Sorghum sp., 14 Aug. 1905, F.L. Stevens, BPI 430301; Texas, Austin, on S. halepense, Aug.–Nov. 1900, BPI 430303; Texas, Austin, on S. halepense, Aug.–Nov. 1900, W.H. Long, BPI 428849; Texas, Bastrop, on S. halepense, 12 Aug. 1909, F.D. Heald, BPI 428848; Texas, College Station, on S. halepense, Autumn 1889, H.S. Jennings, BPI 428850; ibid., 10 Jan. 1890, H.S. Jennings, BPI 428851; Texas, Austin, on S. sudanense, 27 Jun. 1946, C.L Lefebvre, BPI 430347; Texas, Weslaco, on S. sudanense, 27 Jun. 1946, C.L. Lefebvre, BPI 430348; Texas, San Antonio, on S. halepense, 29 Jul. 1910, W.P.C., Detr: Charles Vera K., BPI 428847; Virginia, Montgomery Co., Rt. 603, on S. halepense, 22 Jun. 2008, C.W. Roane, BPI 882540; Wisconsin, Madison, on S. sudanense, 30 Aug. 1935, L.A. Henry, BPI 430341.
Notes: Helminthosporium sorghi was originally described by Schweinitz (1832) from the decaying leafs of Sorghum in Pennsylvania, USA. Later Cooke (1878) applied the same name to a different fungus isolated from Sorghum sp. in USA. Saccardo (1886) established another name, H. cookei Sacc., for the later homonym of Helminthosporium sorghi Cooke. Lefebvre & Sherwin (1949) described H. sorghicola on Sorghum sudanense. Shoemaker (1959) regarded H. sorghicola Cooke as a synonym of Bipolaris cookei.
The type specimen of H. cookei (Ravenel, American fungi exsiccati No. 167), was examined but no conidia or conidiophores of Bipolaris were found. Saccardo (1886) found a few conidia and conidiophores on a specimen with the same exsiccati number. The description provided by Saccardo (1886) is similar to the description of B. sorghicola in spore dimension and septation. We examined all specimens held in BPI under the name B. cookei and found that all were morphologically and symptomatically similar to B. sorghicola. Based on our examination of the type specimens we agree with Saccardo (1886) that H. sorghicola is a synonym of Bipolaris cookei.
We examined the isotype material of H. sorghi Schwein. but were unable to find conidia. However, the leaf spots on the host were similar to the leaf spots on common Sudan grass (Sorghum bicolor) caused by B. sorghicola as described by Lefebvre & Sherwin (1949). Helminthosporium sorghi Schwein and B. cookei may also be conspecific. Unfortunately the type specimens were not in a good enough condition to determine this. Lefebvre & Sherwin (1949) observed the holotype material of H. sorghi Schwein and did not find conidia. Consequently, Sivanesan (1987) listed Helminthosporium sorghi Schwein. as a doubtful species.
Bipolaris cookei has been known to cause serious loss in the production of Sorghum halepense (Zummo & Gourley 1987). No sexual morph is known for this species.
Bipolaris costina Sivan., R.S. Shukla, K.P. Singh & A. Husain, Trans. Brit. Mycol. Soc. 84: 404. 1985. Fig. 10.
Fig. 10.

Bipolaris costina (IMI 256417). A. Conidiophores grown on the stem of Cheilocostus speciosus. B. Conidiophore. C. Conidia with secondary sporulation. D–I. Conidia. Scale bars: A = 500 μm, B = 10 μm, D–E = 10 μm, C, F–I = 20 μm.
Type material: India, Lucknow, on Cheilocostus speciosus (as Costus speciosus), 9 Mar. 1981, K.P. Singh 113, IMI 256417, holotype.
Asexual morph on WA + wheat straw media: Hyphae olive green to dark brown, branched, smooth, septate, Conidiophores (70–)170–230(–285) × 6–8 μm (av. = 200, SD = 30, n = 15; av. = 7, SD = 1, n = 15), arising singly or in groups, simple, flexuous, septate, geniculate, hyaline, brown towards apex. Conidiogenous nodes distinct with verruculose surface. Conidia (58–)70–105 × 14–22 μm (av. = 85, SD = 18, n = 35; av. = 18, SD = 4, n = 35) smooth, straight, somewhat curved, ellipsoidal to obclavate, pale brown to reddish brown, (7–)9(–10)-distoseptate. Secondary sporulation observed. Hilum distinct, truncate, slightly protruding. Septum ontogeny first septum median, second septum delimiting basal cell and third septum distal, germination bipolar.
Cultural characteristics: On WA + wheat straw media, colonies dark brown, velvety.
Host: On decaying leaves of Cheilocostus speciosus (≡ Costus speciosus) (Costaceae), known only from type.
Distribution: India.
Notes: Bipolaris costina was collected several times on Cheilocostus speciosus at the type locality in India but has not been reported since the original description. Based on the conidial and hilum morphology, this species appears to belong in Bipolaris. A sexual morph has not been recorded in association with this species.
Bipolaris crotonis Sivan., Trans. Brit. Mycol. Soc. 84: 404. 1985. Fig. 11.
Fig. 11.

Bipolaris crotonis (IMI 223682). A. Aerial view of culture. B. Conidia and conidiophores. C, D. Conidiophores. E–K. Conidia. Scale bars: A = 200 μm, B–K = 10 μm.
[= Bipolaris eleusines Alcorn & R.G. Shivas, in Alcorn, Mycotaxon 39: 369. 1990, non Bipolaris eleusines Peng & Lu J. Nanjing Agric. Univ. 12(4): 47. 1989 (fide Tan et al. 2014).]
= Cochliobolus eleusines Alcorn, Mycotaxon 39: 367. 1990. (fide Tan et al. 2014).
Type material: Australia, Queensland, Goldsborough, from leaf spot of Eleusine indica, 1 May 1987, J.L. Alcorn 8786a, BRIP 15875 (Bipolaris eleusines Alcorn & R.G. Shivas), holotype; ibid., IMI 335212 isotype; ibid., ex-isotype culture CBS 274.91. Samoa, on Croton sp., 21 Nov. 1977, G.F. Laundon, LEV 12488, IMI 223682 (Bipolaris crotonis), holotype.
Asexual morph on WA + wheat straw media: Conidiophores (50–)110–230(–260) × 6–8 (–10) μm (av. = 172, SD = 60, n = 13; av. = 7, SD = 1, n = 13), arising singly, terminally or laterally, simple or branched, septate, straight or flexuous, distinctly geniculate at upper part, pale brown to dark brown, paler towards upper part. Conidiogenous node surface dark brown, verrucose. Conidia (51–)60–110(–138) × (14–)20–25(–32) μm (av. = 88, SD = 25, n = 44; av. = 22, SD = 3, n = 44), smooth, straight, broadly ellipsoidal or obclavate, subhyaline to dark brown, sometimes septum near hilum paler, (4–)7(–11)-distoseptate. Hilum truncate, slightly protruding. Conidial septum ontogeny first septum median, second septum often delimiting basal cell, but sometimes formed in distal third; germination bipolar. Sexual morph on Sach's agar: Ascomata black, globose, glabrous, (278–)360–635(–659) μm (av. = 498, SD = 138, n = 16) diam, with short truncate conic or cylindrical ostiolar beak, 50–205 μm long and 95–205 μm wide at base. Asci (100–)110–180(–200) × (15–)18–24(–25) μm (av. = 145, SD = 32, n = 21; av. = 21, SD = 3, n = 21), fusoid, cylindrical or narrowly obclavate, short pedicellate, straight or curved, bitunicate. Ascospores 115–270 × 6–9 μm, strongly coiled into a helix, tapering towards obtuse, subacute ends, hyaline, filiform, 3–10-septate.
Cultural characteristics: Colonies on WA + wheat straw, cottony, velvety, dark grey-black. Hyphae subhyaline to pale brown, smooth, septate, branched.
Hosts: On decaying leaves of Croton sp. (Euphorbiaceae), Eleusine indica (Poaceae).
Distribution: Samoa.
Notes: According to morphological and molecular data (Fig. 1) this species is included in the genus Bipolaris. The name Bipolaris eleusines Alcorn & R.G. Shivas is a later homonym of B. eleusines Peng & Lu (1989) and therefore is nomenclaturally illegitimate. Tan et al. (2014) reported that Bipolaris eleusines Alcorn & R.G. Shivas is phylogenetically similar to B. crotonis. Cochliobolus eleusines Alcorn is synonymized under B. crotonis based on priority.
Bipolaris cynodontis (Marignoni) Shoemaker, Canad. J. Bot. 37: 883. 1959. Fig. 12.
Fig. 12.

Bipolaris cynodontis (BPI 626389, CBS 109894). A. Ascoma on host. B. Ascoma. C. Conidiophores and conidia on host. D. Conidiophore. E–H. Conidia. I. Conidiophores on culture. J. Conidia attached to conidiophores. K–M. Conidiophores. N. Germinating conidia. O–S. Conidia. Scale bars: A–C = 100 μm, D–H = 10 μm, I = 500 μm, J–S = 10 μm.
Basionym: Helminthosporium cynodontis Marignoni, Micromiceti di Schio: 27. 1909.
≡ Drechslera cynodontis (Marignoni) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
= Cochliobolus cynodontis R.R. Nelson, Mycologia 56: 67. 1964. (fide Nelson 1964).
Type material: Italy, on Cynodon dactylon, iconotype designated here figure in Marignoni (1909), Micromiceti di Schio: 27, J.A. Stevenson Mycology Library, USDA-ARS, Beltsville, Maryland, USA (Helminthosporium cynodontis) “MBT197968”. Hungary, Keszthely, on Cynodon dactylon, 1992, J. Bakonyim epitype designated here BPI 892949 dried culture specimen (Bipolaris cynodontis) “MBT198051”, ex-epitype culture CBS 109894. USA, dried culture on Sach's agar with Zea mays, Nov. 1962, R.R. Nelson 85101, BPI 626389 (Cochliobolus cynodontis), holotype.
Leaf spots on Cynodon dactylon: Small, punctiform brown lesions. Asexual morph on PDA: Conidiophores (43–)60–145(–160) × 4–6(–8) μm (av. = 103, SD = 42, n = 35; av. = 5, SD = 1, n = 35), arising singly or in small groups, branched or simple, septate, straight or flexuous, geniculate at upper part, brown to olivaceous brown. Conidiogenous nodes dark brown, distinct, slightly verruculose below nodes. Conidia (27–)40–80(–100) × (10–)12–18(–20) μm (av. = 58, SD = 20, n = 120; av. = 15, SD = 3, n = 120), smooth, slightly curved or straight, cylindrical to ellipsoidal, hyaline when immature, turning olivaceous green, then brown or golden brown when mature, (3–)7(–9)-distoseptate. Hilum inconspicuous or slightly protuberant 3–4 μm wide. Septum ontogeny first septum usually basal, rarely median, second septum distal. Conidial germination bipolar, end cells sometimes swelling to form a globose, thin vesicle where germ tube originates. Sexual morph on Sach’s agar with Zea mays: Ascomata 300–450 × 200–400 μm (av. = 375, SD = 75, n = 15; av. = 300, SD = 100, n = 15), black, globose or ellipsoidal, with long brown setae and conidiophores with conidia developing on upper part of ascoma. Ostiolar beak subconical 30–90 μm long (av. = 60, SD = 30, n = 10), with a mass of hyaline cells on upper part. Pseudoparaphyses, hyaline, septate, filamentous. Asci 130–210 × 16–28 μm (av. = 175, SD = 45, n = 8; av. = 22, SD = 6, n = 8), produced among pseudoparaphyses, arising from base of locule. Asci 160–320 × 5–10 μm (n = 7), with 1–8 ascospores coiled in a tight helix, cylindrical or clavate, straight or slightly curved. Ascospores filiform to flagelliform, somewhat tapered at ends, mature ascospores typically hyaline, 3–9-septate upon release, ascospores often surrounded with a thin mucous envelope, germination either terminal or lateral.
Cultural characteristics: Colonies on PDA, white when young, becoming greyish black when mature.
Hosts: Cynodon dactylon, Echinochloa crus-galli, Eragrostis pectinacea, Miscanthus sinensis, Muhlenbergia mexicana, Panicum philadelphicum, Zea mays (Poaceae). Also reported from: Arthraxon affinis, A. hispidus, Brachiaria brizantha, B. platyphylla, Cynodon bradleyi, C. plectostachyus, C. transvaalensis, Cynosurus cristatus, Dactylis glomerata, Dactyloctenium aegyptium, Eleusine indica, Elymus riparius, Eragrostis pectinacea, Festuca sp., Hordeum sp., Hordeum vulgare, Heteropogon contortus, Leptochloa fascicularis, Lolium multiflorum, L. × multiflorum-perenne, Microstegium vimineum, Muhlenbergia schreberi, M. sylvatica, M. tenuiflora, Oryza sativa, Panicum maximum, Paspalum conjugatum, Pennisetum clandestinum, P. purpureum, P. typhoides, Phyllostachys sp., Saccharum officinarum, Secale cereal, Setaria geniculate, S. glauca, S. pumila, Sorghum arundinaceum, S. halepense, Triticum sp. (Poaceae). Also reported from non-Poaceae hosts: Senecio mesogrammoides (Asteraceae), Eucalyptus sp. (Myrtaceae), Ligustrum lucidum (Oleaceae), Pinus caribaea (Pinaceae), Cardiospermum corindum (Sapindaceae), Rosa sp. (Rosaceae) (Farr & Rossman 2013).
Distribution: Italy, Hungary, New Zealand, Thailand, USA; Also reported from: Argentina, Australia, Bangladesh, Brazil, Brunei Darussalam, Ghana, Guinea, India, Kenya, Malaysia, Myanmar, New Guinea, Nicaragua, Pakistan, Papua New Guinea, South Africa, Tanzania, Turkey, Venezuela, Zambia, Zimbabwe, Yugoslavia (Farr & Rossman 2013).
Additional material examined: USA, Florida, Fort Myers, on Cynodon dactylon, 20 Feb.1921, C. Drechsler, BPI 428876; Florida, Gainesville, University of Florida, on C. dactylon, 16 Oct. 1968, J.E. Mabry, BPI 428880; Kansas, Manhattan, Riley Co. Kansas State College campus, on C. dactylon, 7 Oct. 1955, C.T Rogerson, BPI 428877; Maryland, Glen Burnie, on C. dactylon, 25 Sep. 1928, C. Drechsler, BPI 428875; Maryland, Kenilworth, on C. dactylon, 25 Jul. 1925, C. Drechsler, BPI 427878; Virginia, Vienna, on C. dactylon, 16 Oct. 1968, J. Harper, Forage Crop Herbarium 2062 = BPI 428879; Virginia, Montgomery Co. Blacksburg Town Park, Behind Roane's lot, 607 Lucas Dr., on Muhlenbergia mexicana, 12 Nov. 2003, C.W. Roane, BPI 480866A; Virginia, Montgomery, on Eragrostis pectinacea, 14 Aug. 2004, BPI 880257A; Virginia, Montgomery Co., at Montgomery Tunnels, on leaf spots of C. dactylon, 11 Jun. 2004, C.W. Roane, BPI 880234; Virginia, Roanoke Co., on Echinochloa crus-galli, 13 Sep. 2009, C.W. Roane, BPI 882557A; Virginia, Roanoke Co., on the leaf spots of Miscanthus sinensis var. purpurescens, 9 Sep. 2004, C.W Roane, BPI 880269; Virginia, Giles Co., about 25 yards above Rich Ck. boat ramp, shore of New River, on Panicum philadelphicum, 14 Nov. 2010, C.W. Roane, BPI 882619.
Notes: Bipolaris cynodontis is considered a pathogen, secondary invader or saprobe on a wide range of hosts. It is not known to cause serious disease, although infection can produce leaf spots on Cynodon dactylon (Datnoff & Rutherford 2004, Hagan 2005). A phytotoxin named bipolaroxin is produced by a strain of this species (Sugawara et al. 1985). Bipolaris cynodontis shows a wide range of conidial and conidiophore measurements. Swollen end cells of germinating spores are a characteristic feature. According to phylogenetic data B. cynodontis is highly similar to B. coffeana. However the ex-epitype culture of B. cynodontis from Hungary is represented by a singleton. Therefore to avoid confusion we treat them as two distinct species.
Bipolaris drechsleri Manamgoda & Minnis., Persoonia 31: 293. 2013. Fig. 13.
Fig. 13.

Bipolaris drechsleri (CBS 136207). A. Infected leaf of Microstegium vimineum in the field (Photo credit: Nathan M. Kleczewski). B. Conidiophores and conidia. C, D. Conidiophores. E–I. Conidia. Scale bars: B = 20 μm, C–I = 5 μm.
Type material: USA, Indiana, Big Oaks Wildlife Refuge, on living leaves of Microstegium vimineum, 2010, N. Kleczewski, BPI 892682, holotype; ex-holotype culture CBS 136207.
Leaf spots on Microstegium vimineum: Irregular, small, distinct, purplish, with dark margin, surrounded by a chlorotic halo. Asexual morph on PDA: Conidiophores (74–)95–300(–602) × 4–6 μm (av. = 250, SD = 152, n = 35; av. = 5, SD = 1, n = 35), arising singly or in groups of two to three, simple or with one branch, septate, straight to flexuous, geniculate in upper part, cylindrical, smooth-walled, pale brown. Conidiogenous cells integrated, intercalary, with sympodial proliferation, dark brown, with circular scars. Conidia (39–)50–80(–102) × (10–)13–19(–20) μm (av. = 66, SD = 14, n = 125; av. = 16, SD = 3, n = 125), smooth, curved or straight, ellipsoidal, obclavate, obclavate-ellipsoidal, rostrate, rarely obovoid, apex and base obtuse, pale to dark golden-brown, sometimes paler in end cells, (3–)7(–10)-distoseptate. Hilum inconspicuous or slightly protuberant, dark brown to black, germinating with a germ tube at each end of conidia.
Cultural characteristics: Colonies on PDA, white when young, becoming whitish grey at maturity; margin irregular, effuse, velvety, concolorous. Reverse black, with white margin.
Host: Microstegium vimineum (Poaceae).
Distribution: USA (IN, MD, WV).
Additional material examined: USA, Maryland, Montgomery Co., Wheaton, Brookside Garden, on an unidentified ornamental grass, Oct. 1995, N. O’Neil N395, BPI 892684, culture CBS 163245; West Virginia, Arnoldsburg, on living leaves of Microstegium vimineum, N. Kleczewski, BPI 892683, culture CBS 136208.
Notes: There are two species known from Microstegium vimineum in the USA, namely, B. microstegi and B. drechsleri (Crous et al. 2012, 2013). Bipolaris drechsleri has conidial dimensions similar to B. microstegii, but B. drechsleri has shorter conidiophores, which have more proliferations than those of B. microstegii. The combined gene analysis revealed that B. microstegii is phylogenetically close to B. victoriae and B. zeicola, but the latter two species do not show a close phylogenetic relationship with B. drechsleri. There is no sexual morph found in association with B. drechsleri.
Bipolaris eragrostidis (Henn.) Shoemaker, Canad. J. Bot. 37: 883. 1959. Fig. 14.
Fig. 14.

Bipolaris eragrostidis (BPI 428930A). A. An infected spikelet of Eragrostis interrupta. B. Conidia on the host. C–G. Conidia. Scale bars: A = 2 cm, B = 100 μm, C–G = 5 μm.
Basionym: Helminthosporium eragrostidis Henn., Annals du Musée du Congo. Botanique Série. 5: 231. 1908.
≡ Drechslera eragrostidis (Henn.) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Type material: Democratic Republic of Congo, Quwango, on a spikelet of Eragrostis ciliaris, Jun. 1906, P. Hennings, IMI 296812, holotype.
Asexual morph on Eragrostis ciliaris: Conidiophores (25–)30–90(–120) × 5–9 μm (av. = 60, SD = 30, n = 24; av. = 7, SD = 2, n = 24), arranged in dense groups, branched, septate, geniculate at upper part, verruculose, apex rounded, olivaceous to pale brown. Conidiogenous nodes flattened, darkened. Conidia 45–70(–79) × (13–)16–22(–24) μm (av. = 58, SD = 11, n = 45; av. = 19, SD = 3, n = 45), smooth, straight, ellipsoid, obclavate-ellipsoidal, often rostrate, base and apex rounded, pale brown to reddish brown, cells concolorous, sometimes rostrate portion paler, often not accentuated, occasionally with a transverse line, (2–)4(–7)-distoseptate. Hilum 1–3 μm long, conspicuous.
Hosts: Eragrostis ciliaris, E. interrupta (Poaceae).
Distribution: Democratic Republic of Congo, Myanmar.
Additional material examined: Myanmar, on Eragrostis interrupta, BPI 428930A = IMI 9779; ibid., BPI 428930B.
Notes: Although no molecular data exist for B. eragrostidis, this species is retained in Bipolaris based on the characteristic hilum structure and conidial morphology. Among the numerous species of Bipolaris occurring on Eragrostis, B. eragrostidis is unique in having rostrate conidia. No sexual morph is recorded in association with this species.
Bipolaris eragrostiellae (A.P. Misra & R.A. Singh) Sivan., Mycol. Pap. 158: 80. 1987. Fig. 15.
Fig. 15.

Bipolaris eragrostiellae (IMI 155931). A. Conidiophores and conidia on Eragrostiellam bifaria causing sooty heads on the inflorescence. B. Conidiophores arranged in a fascicle. C–G. Conidia. Scale bars: A = 100 μm, B–E, G = 10 μm, F = 5 μm.
Basionym: Drechslera eragrostiellae A.P. Misra & R.A. Singh, Sydowia 32: 185. 1980. (1979)
Type material: India, on Eragrostiella bifaria, A.P. Misra S1, IMI 155931, holotype.
Symptoms on Eragrostiella bifaria: Inflorescences covered with a compact black mass giving it a smutted or sooty appearance. Severe infections resulting in the suppression of seed formation. Asexual morph on Eragrostiella bifaria: Conidiophores (53–)110–170 × 4–6 μm (av. = 140, SD = 30, n = 10; av. = 5, SD = 1, n = 10), emerging singly or in groups of 2–10, occasionally more, branched, septate, geniculate towards apex rounded, slightly swollen at end, pale brown to dark olive. Conidia (45–)50–75(–86) × (8–)10–11(–13) μm (av. = 63, SD = 13, n = 31; av. = 12, SD = 1, n = 31), straight or slightly curved, slightly tapering towards rounded ends, yellowish brown or olive brown. Hilum inconspicuous. Conidial germination described as both unipolar and bipolar by Misra (1979).
Host: Eragrostiella bifaria (Poaceae).
Distribution: India.
Notes: Based on the characteristic hilum on the conidia, this species is retained in Bipolaris despite the lack of molecular data. No sexual morph recorded in association with this species.
Bipolaris euchlaenae (Zimm.) Shoemaker, Canad. J. Bot. 37: 883. 1959.
Basionym: Helminthosporium euchlaenae Zimm., Ber. Land Forstw. Deutsch-Ostafr.: 18. 1904.
≡ Drechslera euchlaenae (Zimm.) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Type material: Africa, African Great Lakes Region (previously German East Africa), on Euchlaena mexicana, not seen.
Leaf spots: dark brown, elongate, up to 2 mm long by 2 mm wide. Asexual morph on Euchlaena mexicana: Conidiophores up to 150 μm long, 5–7 μm thick, arising in small groups, smooth, septate, geniculate above, with a swollen base, brown. Conidia 50–60 × 13–15 μm, straight or slightly curved, cylindrical, brown, up to 7 distoseptate.
Host: Euchlaena mexicana (Poaceae), known only from the type.
Distribution: Central East Africa (previously German East Africa).
Notes: Based on the conidial shape and septation, this species is accepted in the genus Bipolaris despite the lack of molecular evidence. The type specimen for Bipolaris euchalenae could neither be located for this study nor by Sivanesan (1987) and the species has not been recorded since it was described. There are two other species of Bipolaris reported on Euchlaena mexicana, i.e. B. zeicola and B. maydis (Sivanesan 1987). Although the conidial size for B. zeicola, 30–100 × 12–18 μm, overlaps with that of B. euchlaenae, the secondary metabolite cynodontin was reported from B. euchlaenae (Sivanesan 1987) but is not known from B. zeicola. Bipolaris maydis produces cynodontin and the conidia are 70–160 μm long, exceeding those of B. euchlaenae. A sexual state is not recorded in association with B. euchlaenae whereas a sexual state has been produced for B. zeicola and B. maydis.
Bipolaris euphorbiae (Hansf.) J.J. Muchovej & A.O. Carvalho, Mycotaxon 35: 160. 1989.
Basionym: Helminthosporium euphorbiae Hansf., Proc. Linn. Soc. Lond. 155: 49. 1943. (1942–1943).
≡ Drechslera euphorbiae (Hansf.) M.B. Ellis, Dematiaceous Hyphomycetes (Kew): 440. 1971.
Type material: Brazil, Viçosa, on Euphorbia sp., Mar. 1988, BRIP 16567, neotype, not seen, ex-neotype culture ATCC 64939.
Symptoms on Euphorbia sp.: Leaf spots and defoliation.
Description: A full description is available in Muchovej & Carvalho (1989).
Host: Euphorbia sp. (Euphorbiaceae).
Distribution: Brazil.
Notes: The species was originally described in Helminthosporium. Ellis (1971) placed this species in Drechslera. Based on the bipolar germination and hilum structure, Muchovej & Carvalho (1989) placed the species in Bipolaris. As the holotype specimen was not available, Muchovej & Carvalho (1989) designated a neotype. The conidia were reported as 63–96(–145) × 15–18 μm, 7–8-distoseptate (Muchovej & Carvalho 1989). According to the morphological features such as the shape of conidia, septation of conidia and hilum structure, we accept this species in the genus Bipolaris despite the lack of molecular data.
Bipolaris gossypina Sivan., Trans. Brit. Mycol. Soc. 84: 404. 1985. Fig. 16.
Fig. 16.

Bipolaris gossypina (IMI 123377). A. Mycelium on the host seed. B–G. Conidiophores and conidia. H–M. Conidia. Scale bars: A = 500 μm, B–M = 10 μm.
Type material: Kenya, on Gossypium sp., 1966, W.H. White, IMI 123377, holotype.
Asexual morph on PDA: Conidiophores (100–)130–250(–288) × 8–10 μm (av. = 192, SD = 59, n = 26; av. = 9, SD = 1, n = 26), arranged singly, in pairs or in small groups, simple, septate, basal cell rounded, enlarged; smooth to slightly verruculose, pale brown to dark brown. Conidiogenous scars dark brown. Conidia (50–)55–80(–80) × 14–18 μm (av. = 69, SD = 11, n = 44; av. = 16, SD = 2, n = 44), smooth, straight, obclavate, sub-cylindrical, sometimes rostrate, olivaceous brown to mid reddish brown, (7–)8(–10)-distoseptate. Hilum truncate, slightly protruding. Conidial septum ontogeny first septum median, second septum often delimiting basal cell, but some formed in distal third; germination bipolar.
Host: On seeds and leaves of Gossypium sp. (Malvaceae), known only from type.
Distribution: Kenya.
Notes: This is the only Bipolaris species recorded from Gossypium. Bipolaris gossypina differs from Helminthosporium gossypii Tucker, the only other bipolaris-like species on Gossypium by the shape, and bipolar germination. Helminthosporium gossypii produces elliptic conidia, which can germinate from any cell (Tucker 1926). No sexual morph is reported in association with this species. The placement of B. gossypina in the genus Bipolaris is confirmed with morphological and molecular data (Fig. 1).
Bipolaris hadrotrichoides (Ellis & Everh.) Luttr., Mycologia 61: 1035. 1970 (1969). Fig. 17.
Fig. 17.

Bipolaris hadotrichoides (BPI 429013). A. Conidia and conidiophores on Eleusine indica. B. Conidiophores inside the plant tissue. C, D, F, G. Conidiophores. E. Penetration of the B. hadrotrichoides hyphae through the plant tissue. H–J. Conidia. Scale bars: A = 200 μm, B = 100 μm, C–J = 20 μm.
Basionym: Helminthosporium hadotrichoides Ellis & Everh., J. Mycol. 4: 44. 1888.
Type material: USA, Delaware, Faulkland, on leaves of Eragrostis major, Sep. 1887, A. Commons, No. 347, K-Ellis and Everhardt North American Fungi 2186 = BPI 429028, holotype; ibid., BPI 429027, isotype; ibid., IMI 296473, lectotype designated by Sivanesan (1987).
Leaf spots on Eragrostis sp.: Pale brown to whitish spots and grey linear streaks (Sivanesan 1987). Asexual morph on Eragrostis major: Conidiophores (21–)35–105(–121) × (6–)8–10(–12) μm (av. = 71, SD = 34, n = 30; av. = 9, SD = 1, n = 30), arranged in small groups on flat stromata, simple, geniculate, septate, pale brown, becoming paler towards apex. Conidiogenous nodes distinctly swollen, dark brown, flattened at apex. Conidia (35–)40–70(–75) × 15–21(–25) μm (av. = 55, SD = 11, n = 33; av. = 18, SD = 3, n = 33), usually minutely echinulate, straight, ovoid, end cell and basal cell ellipsoidal, widest at second or third septum from base, pale to reddish brown, (3–)5(–6)-distoseptate. Hilum inconspicuous.
Hosts: On leaves of Eragrostis cilianensis, E. indica, E. major, and E. pectinacea (Poaceae).
Distribution: USA (DE, KS, MD, MO, WI, WV).
Additional material examined: USA, Kansas, Riley Co., Manhattan, Kansas State College, 18 Jul. 1956, C.T. Rogerson, BPI 429014; ibid., 18 Jul. 1957, C.T. Rogerson, BPI 429020; Maryland, Glen Burnie, on Eragrostis major, 25 Sep. 1928, C. Drechsler, BPI 429022; Maryland, Hurlock, 16 Aug. 1923, C. Drechsler, BPI 429025; Maryland, Glen Burnie, on E. major, 11 Aug. 1923, C. Drechsler, BPI 429026; Maryland, Beltsville, on E. cilianensis, 30 Jul. 1942, C.L. Lefebvre 759, BPI 429017; Missouri, Kennett, 25 Aug. 1924, C. Drechsler, BPI 429022; Montana, Miles City, 13 Aug. 1941, R. Sprague, BPI 429021; West Virginia, Morgantown Agronomy Farm, on Eleusine indica, 8 Aug. 1955, E.S. Elliott, BPI 429013; Wisconsin, Madison, 15 Sep. 1943, H.C. Green, C.L. Lefebvre 949, BPI 429018; Wisconsin, Madison, 11 Aug. 1947, H.C. Greene, BPI 429019.
Notes: Among the 13 species of Bipolaris occurring on Eragrostis, B. hadrotrichoides is unique in its arrangement of conidiophores, which are comparatively short, always found in groups with a flattened apex, and minutely echinulate conidia. Bipolaris hadrotrichoides was placed in the nodulose Bipolaris group (Luttrell 1969) based on its close resemblance to species such as B. coicis, B. kusanoi and B. nodulosa. Those three species do not cluster in the genus Bipolaris according to molecular data. Based on currently available morphological characters, we retain B. hadrotrichoides in the genus Bipolaris. A sexual morph is not known in association with this species.
Bipolaris halepense M.Y. Chiang, K.J. Leonard & Van Dyke, Mycologia 81: 537. 1989. Fig. 18.
Fig. 18.

Bipolaris halepensis (BPI 1103129). A. Leaf spots on host. B. Conidiophores on the host. C, D. Conidiophores. E–H. Conidia. Scale bars: A = 1 cm, B = 200 μm, C–H = 10 μm.
Type material: USA, North Carolina, Iredell Co, Mooresville, on Sorghum halapense, 27 May 1988, C. Mou-yen 85121, BPI 1103129, holotype.
Leaf spots on Sorghum halepense: Ovoid, 5–10 × 2–4 mm, with a broad, blackened margin. Asexual morph on Sorghum halapense: Conidiophores (100–)150–290(–300) × 7–9 μm (av. = 217, SD = 70, n = 10; av. = 8, SD = 1, n = 10), arising mostly singly or in pairs, mostly simple, septate, sometimes geniculate near apex, basal cell often enlarged, olivaceous brown to dark brown. Conidia (41–)60–110(–138) × (9–)13–19(–24) μm (av. = 85, SD = 25, n = 45; av. = 16, SD = 3, n = 45), smooth, straight or moderately curved, cylindrical or ellipsoidal, olivaceous brown to dark brown, concolorous, (4–)7(–12)-distoseptate. Hilum inconspicuous. Conidial germination bipolar.
Host: Sorghum halepense (Poaceae), known only from the type.
Distribution: USA (NC).
Notes: Bipolaris halepense is distinct from B. cookei, the common pathogen of Sorghum halepense, by conidial morphology and pathogenicity. Bipolaris cookei produces slightly smaller conidia (40–70 μm) than B. halepense (60–110 μm). Secondary conidiophores are found in association with B. cookei, but such formation is not recorded for B. halepense. In addition the lesions of B. halepense on Johnson grass (Sorghum halepense) lack the zonate pattern, which is typical for the lesions caused by B. sorghicola. Based on the conidial morphology and hilum structure, this species is accepted in the genus Bipolaris despite lack of molecular evidence. There is no sexual morph observed in association with this species.
Bipolaris heliconiae Alcorn, Austral. Syst. Bot. 9: 814. 1996.
Type material: Australia, Northern Territory, Batchelor, on Heliconia psittacorum, BRIP 17186 (Bipolaris heliconiae), holotype (not seen); ibid., on Heliconia sp., 18 Jan. 1991, J.L. Alcorn, BRIP 17349 (Cochliobolus heliconiae), holotype.
Asexual morph on Heliconia sp.: Conidiophores up to 600 μm long, simple, multi-septate, straight to flexuous in lower part, geniculate in fertile region, olivaceous brown below, paler apically, base swollen. Conidiogenous nodes distant, verruculose, Conidia 65–150 × 15–19 μm, fusoid to clavate fusoid, often slightly wider in upper half, curved, olivaceous to slightly reddish brown, 7–10-distoseptate, basal cell hemi-ellipsoidal. Hilum inconspicuous or slightly truncate. Primary septum in developing conidia, second septum delimits basal cell. Sexual morph produced on Sach's agar medium. Ascomata (280–)310–530(–568) μm (av. = 423, SD = 109, n = 15), black, globose, setose, sometimes flattened across base. Ostiolar beak (80–)85–140(–155) μm (av. = 114, SD = 26, n = 11) long and 90–140(–160) μm (av. = 115, SD = 23, n = 11) wide at end of ostiolar beak, conical. Asci 100–245 × 25–60 μm (n = 10), fusoid, obclavate–fusoid, cylindrical to ellipsoidal or obpyriform, often with a pedicel 15–45 × 7–10 μm (n = 10). Ascospores 310–650 × 6–11 μm hyaline, filiform, scarcely tapered to apex and gradually tapered to base, strongly coiled for length of ascus or sometimes irregularly looped, thin-walled, 5–24-septate.
Host: Heliconia sp. (Heliconiaceae).
Distribution: Australia.
Notes: This species is placed in the genus Bipolaris based on the conidial morphology, hilum characteristics and phylogenetic evidence (Fig. 1). Other Bipolaris species found on Heliconia sp. are B. cynodontis, B. incurvata, B. salviniae and B. setariae. Bipolaris cynodontis and B. setariae produce smaller spores than B. heliconiae. Bipolaris incurvata produces wider conidia compared to B. heliconiae. Conidia of B. salviniae are usually cylindrical whereas B. heliconiae produces fusoid to clavate conidia. Bipolaris heliconiae is phylogenetically closely related to B. maydis (Fig. 1).
Bipolaris heveae (Petch) Arx, Nova Hedwigia, Beih. 87: 288. 1987. Fig. 19.
Fig. 19.

Bipolaris heveae (K (M) 181465, CBS 241.92). A. Leaf spots on Hevea brasiliensis. B. Conidiophores and conidia on the leaf spots. C. Conidiophores. D. Young conidia attached to conidiophores. E. Matured conidia attached to conidiophores. F–I. Conidia. Scale bars: A = 300 μm, B = 75 μm, C–E = 10 μm, F–I = 5 μm.
Basionym: Helminthosporium heveae Petch, Ann. Roy. Bot. Gard. (Peradeniya) 3: 396. 1906.
≡ Drechslera heveae (Petch) M.B. Ellis, Dematiaceous Hyphomycetes (Kew): 451. 1971.
Type material: Sri Lanka, Gampaha, Henarathgoda, on Hevea brasiliensis, Mar. 1917, T. Petch 5030, K(M) 181465, holotype.
Leaf spots on Hevea brasiliensis: Circular, semi-transparent, bordered by a purplish brown line. Asexual morph on Hevea brasiliensis: Conidiophores (92–)100–325(–335) × 4–6(–9) μm (av. = 212, SD = 112, n = 23; av. = 5, SD = 1, n = 23), arising solitary or usually in small groups, simple, septate, straight, flexuous, sometimes geniculate at upper part, pale brown to olivaceous brown. Conidiogenous scars dark brown. Conidia (62–)80–105(–130) × (13–)15–21 μm (av. = 92, SD = 12, n = 61; av. = 18, SD = 3, n = 61), smooth, curved, navicular or fusiform, hyaline when immature, becoming mid golden to olivaceous brown when mature, (5–)7(–9)-distoseptate. Hilum inconspicuous or slightly protuberant, 3–4 μm. Conidial germination bipolar, first septum usually median, rarely basal, second septum distal.
Host: Hevea brasiliensis (Euphorbiaceae).
Distribution: Cambodia, Dominican Republic, Ghana, Guatemala, Haiti, Honduras, Indonesia, Mexico, Nigeria, Philippines, Sri Lanka, USA (FL).
Additional material examined: Cambodia, on Hevea brasiliensis, 1959, S.C. Litzenberger, BPI 429063; Kampong Cham, on H. brasiliensis, 31 Aug. 1958, S.C. Litzenberger, BPI 429069. Dominican Republic, Piedras Blancas, on H. brasiliensis, 15 Mar. 1941, R.C. Lorenz, BPI 429048; Piedra Blanca, on H, brasiliensis, 22 Oct. 1943, H.F. Allard, BPI 429052. Ghana, Aiyanasi, Rubber Nursery, on H. brasiliensis, Apr. 1960, Mofft, BPI 429066. Guatemala, Bananera, on H. brasiliensis, Jun. 1943, L.E. Letsinger, BPI 429054; Cuyotenango, on H. brasiliensis, 12 Nov. 1946, W.J. Martin, BPI 429043; Entre Rio, on H. brasiliensis, 25 Feb. 1949, R.D Rands, BPI 429041; on H. brasiliensis, Aug. 1945, D. Randa, BPI 429042. Haiti, Bayeux, Nursery, on H. brasiliensis, 1 Dec. 1925, Jenkins, BPI 429062A; ibid., BPI 429062B; Source Chaude, on H. brasiliensis, 8 Nov. 1944, R.D. Rands, BPI 429044; Source Chaude, on H. brasiliensis, Aug. 1942, M. Bradshaw, BPI 429051. Honduras, Lancetilla, on H. brasiliensis, 17 Feb. 1941, T.J. Grant, BPI 429046; ibid., BPI 429047; Lancetilla, on H. brasiliensis, 31 Jul. 1941, E.T. Stanwood, BPI 429050; ibid., BPI 429055; ibid., BPI 429059; ibid., 23 Apr. 1941, E.T. Stanwood, BPI 429053; ibid., BPI 429057; ibid., BPI 429058; ibid., BPI 429056; La Mesa. Farm, on H. brasiliensis, E.T. Stanwood, BPI 429045. Indonesia, Java, Bogor, on H. brasiliensis, 21 Apr. 1955, A. Kurnadi, BPI 429067. Mexico, El Palmar, Veracruz, on H. brasiliensis, 30 Nov. 1944, W.J. Martin, BPI 429049. Nigeria, on H. brasiliensis, 1971, J.H. Simmonds, CBS 241.92. USA, Florida, Coconut Grove, on H. brasiliensis, 9 Jan.1926, A. Keys, BPI 429061. Philippines, Los Banos, Laguna, on H. brasiliensis, Mar. 1920, O.A. Reinking, BPI 429065, Mindanao., Strong Nursery, on H. brasiliensis, 5 May 1918, O.A. Reinking, BPI 429068; ibid., BPI 429064. USA, Florida, Coconut Grove, on H. brasiliensis, 5 Feb. 1925, V.K. Charles, BPI 429060.
Notes: Bipolaris heveae is known to cause diseases on rubber trees in the rubber growing countries in the tropics. Bipolaris heveae is phylogenetically closely related to B. microlenae. However, a sexual morph of B. microlenae is known while none is known for B. heveae. In addition B. microlenae has only been reported from Australia whereas B. heveae is found in the tropics. Most other Bipolaris species have been found in association with grasses (Poaceae). Bipolaris heveae has never been reported from grass species and is restricted to its specific host Hevea brasiliensis.
Bipolaris incurvata (C. Bernard) Alcorn, Mycotaxon 17: 68. 1983.
Basionym: Helminthosporium incurvatum C. Bernard, Bull. Dept. agric. Indes Néerland 2: 31. 1906.
Leaf spots on Cocos sp.: Intially small, oval, brown, later enlarging becoming pale buff in centre with broad dark margins (Ellis 1971).
Description: See Ellis (1971).
Hosts: Cocos nucifera, Chrysalidocarpus lutescens, Neodypsis sp. (Arecaceae).
Distribution: Australia, Brazil, Brunei Darussalam, Fiji, French Polynesia, Guadalcanal, Java, New Caledonia, New Hebrides, Malaysia, Papua New Guinea, Philippines, Sabah, Seychelles, Sri Lanka, Thailand, USA (FL), Vanuatu (Ellis 1971, Farr & Rossman 2013).
Notes: According to the descriptions given in Bernard (1906) and Ellis (1971), this species produces conidia 100–150 × 19–22 μm with 8–13-distoseptae. The conidial morphology is similar to B. maydis, the type species of the genus, but the conidia of B. incurvata are longer than those of B. maydis. Unfortunately the type specimen of B. incurvata could not be located and no molecular data are available. Based on the conidial morphology and hilum structure, we retain this species in the genus Bipolaris. This species had been only reported from Arecaceae and no records are found in association with Poaceae. A sexual morph is not recorded.
Bipolaris leersiae (G.F. Atk.) Shoemaker, (as “leersii”) Canad. J. Bot. 37: 883. 1959. Fig. 20.
Fig. 20.

Bipolaris leersiae (BPI 429499). A. Conidiophores on host. B. Conidia on host. C–F. Conidiophores. G–L. Conidia. Scale bars: A, B = 50 μm, C–L = 5 μm.
Basionym: Helminthosporium leersiae G.F. Atk., Bull. Cornell Univ. (Science) 3: 47. 1897.
≡ Drechslera leersiae (G.F. Atk.) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Type material: USA, Alabama, Auburn, on Leersia virginica, 13 Sep. 1891, G.F. Atkinson 2103, CUP, holotype.
Leaf spots on Leersia hexandra: Small, indefinite, dark brown, eye spots. Asexual morph on Leersia virginica: Conidiophores (50–)65–170(–250) × 6–8 μm (av. = 118, SD = 52, n = 30; av. = 7, SD = 1, n = 30), arising singly or in small groups, simple, septate, sometimes geniculate at upper part, with a swollen basal cell, dark brown. Conidia (54–)65–105 × 15–20 μm (av. = 84, SD = 19, n = 30; av. = 18, SD = 2, n = 30), usually curved, sometimes straight, elliptical, sometimes obclavate, widest at or just below middle, tapering toward ends, pale brown to dark brown, (5–)8(–10)-distoseptate. Sometimes end cells of conidia swell to form more or less global vesicles from which germ tubes originate.
Hosts: Leersia hexandra, L. oryzoides, L. virginica (Poaceae). Also reported from: Setaria sp. (Poaceae) (Sivanesan 1987).
Distribution: USA (AL, DC, IL, KS, NC, VA). Reported from Australia (Farr & Rossman 2013).
Additional material examined: USA, Connecticut, Meriden, on Leersia virginica, 7 Sep. 1920, BPI 429501; ibid., BPI 429504; District of Columbia, Georgetown, on L. virginica, 18 Oct. 1926, C. Drechsler, BPI 429500; Illinois, Carmi, on L. oryzoides, 29 Aug. 1924, C. Drechsler, BPI 429498; Kansas, Wildcat Creek, on L. virginica, 15 Oct. 1954, C.T. Rogerson, BPI 429499; ibid., BPI 429505; North Carolina, Flat Rock, on L. virginica, 16 Aug. 1925, C. Drechsler, BPI 429502; Virginia, West Falls Church, on L. virginica, 4 Sep. 1925, C. Drechsler, BPI 429503.
Notes: Bipolaris leersiae is known primarily on Leersia spp. in the USA and Australia. The report of this species on Bromus in Oklahoma (Preston 1945) and Urochloa in South Africa (Doidge 1950) could not be confirmed with specimens.
Bipolaris luttrellii Alcorn, Mycotaxon 39: 378. 1990. Fig. 21.
Fig. 21.

Bipolaris luttrellii (IMI 345516). A, B. Ascomata. C–G, K–M, J. Asci. H, I. Ascospores. Scale bars: A = 400 μm, B = 20 μm, C–M = 20 μm.
= Cochliobolus luttrellii Alcorn, Mycotaxon 39: 377. 1990.
Type material: Australia, on Dactyloctenium aegyptium, 3 Jun. 1985, J.L. Alcorn, dried culture BRIP 14791 (Cochliobolus luttrellii), holotype; ibid., IMI 335215, isotype; Northern Territory, on Dactyloctenium aegyptium, 31 Mar. 1985, R.A. Peterson, BRIP 14643 (Bipolaris luttrelii), holotype; ibid., IMI 332216, isotype, not seen.
Asexual morph on Dactyloctenium aegyptium: Conidiophores 95–300 μm long, base swollen 7–15 μm diam, 5–9 μm diam above base, 4–6 μm at apex, single or in groups of two or three, septate, straight or flexuous, multi-septate, pale to mid olivaceous brown. Conidiogenous nodes prominent, verruculose. Conidia 38–103 × 10–19 μm, smooth, fusoid to obclavate fusoid, curved, sometimes constricted at basal septum, less commonly in upper part of conidia, end cells ellipsoidal, pale to mid olivaceous brown, sometimes concolorous or in darker conidia with end cells paler, 4–9-distoseptate. Hilum 2.5–4 μm diam, inconspicuous, in some cases slightly truncate with wall projecting. Secondary sporulation observed occasionally. Conidial germination bipolar, with basal germ tubes semi axial and displacing hilum strongly. Septum ontogeny: primary septum in developing conidia sub-median, second septum delimits basal cell, third septum distal (modified from Alcorn 1990). Sexual morph on Sach's agar medium: Ascomata (191–)260–370(–389) μm (av. = 316, SD = 56, n = 31) diam, dark brown to black, globose or sometimes slightly flattened. Ostiolar beak conical to campanulate, (45–) 55–90 (–95) μm high (av. = 74, SD = 17, n = 21) × (41–)50–90(–96) μm wide (av. = 73, SD = 20, n = 21) near base, ostiolar beak and upper part of ascomata covered by densely arranged setae. Asci (131–)140–205(–255) × 18–26(–31) μm (av. = 178, SD = 39, n = 31; av. = 22, SD = 4, n = 31), obclavate fusoid or sub-cylindrical, straight or curved, sometimes pedicellate. Ascospores (144–)180–285 × 6–8 μm (av. = 235, SD = 53, n = 12; av.= 7, SD = 1, n = 12), filiform, hyaline, tapered slightly towards apex and base, tightly coiled inside ascus, sometimes slightly coiled to straight at upper most part, (7–)8(–12)-distoseptate.
Host: On leaves of Dactyloctenium aegyptium (Poaceae).
Distribution: Australia.
Additional material examined: Australia, Queensland, on Dactyloctenium aegyptium, Apr. 1985, J.L. Alcorn, IMI 345516.
Notes: According to the ITS and GPDH sequence of the type provided by Berbee et al. (1999), this species belongs to the genus Bipolaris (Fig. 2). Morphologically B. luttrellii is similar to B. setariae but differs from the latter species by having fewer conidiogenous loci on the conidiophores. Also B. luttrellii usually produces darker conidia with paler end cells while the conidia of B. setariae are always concolorous.
Bipolaris maydis (Y. Nisik. & C. Miyake) Shoemaker, Canad. J. Bot. 33: 882. 1959. Fig. 22.
Fig. 22.

Bipolaris maydis (BPI 626700, CBS 241.92). A, B. Ascomata. C. Asci. D. Fissitunicate releasing state of asci. E. Arrangement of ascospores. F–I. Conidiophores. J. Secondary sporulation. K–P. Conidia. Scale bars: A = 300 μm, B = 50 μm, C = 20 μm, D = 10 μm, E = 20 μm, F = 5 μm, G = 5 μm, H = 5 μm, I = 10 μm, J = 10 μm, K–P = 5 μm.
Basionym: Helminthosporium maydis Y. Nisik. & C. Miyake, Ber. Ohara Inst. landw. Biol. 3: 243. 1926.
≡ Drechslera maydis (Y. Nisik. & C. Miyake) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
= Helminthosporium maydis Brond., Ill. Iconogr. Microscop. Cryptog. France 15. 1856–1857 (as “Helmisporium”), nom. rej. prop. (Rossman et al. 2013b).
= Ophiobolus heterostrophus Drechsler, J. Agric. Res. 31: 723. 1925, nom. rej. prop (Rossman et al. 2013b).
≡ Cochliobolus heterostrophus (Drechsler) Drechsler, Phytopathology 24: 973. 1934.
Type material: USA, North Carolina, isolated from Zea mays, Olin Yoder C5, resulting from six crosses, culture sporulating on Z. mays BPI 892696 (Bipolaris maydis), neotype, dried culture ATCC 48332, ex-neotype culture CBS 137271; Florida, Sanford, on Z. mays, 22 Sep. 1923, C. Drechsler, BPI 626700 (Cochliobolus heterostrophus), holotype.
Symptoms on Zea mays: Causes Southern corn leaf blight, leaf lesions brown, sometimes with purplish tinge or reddish brown margin, occasionally zonate, coalescing and becoming grey, 2.5 cm long, elliptic at first and then elongate longitudinally; becoming rectangular when spots are restricted by veins. Asexual morph on PDA: Conidiophores (90–)105–470(–712) × 5–7 μm (av. = 286, SD = 182, n = 50; av. = 6, SD = 1, n = 50), usually arising singly or in small groups, simple or rarely branched, septate, straight or flexuous, geniculate at upper part, olivaceous brown. Conidiogenous nodes dark brown, distinct. Conidia (59–)66–102(–160) × (12–)14–18 (–20) μm (av. = 94, SD = 28, n = 100; av. = 16, SD = 2, n = 100) μm, pale to mid dark brown, smooth, slightly curved, fusiform, (5–)8(–11)-distoseptate. Hilum distinct, 3–5 μm wide, germination tubes arising from both ends of conidia. Secondary sporulation occasionally observed. Sexual morph on Sach's agar medium: Ascomata (198–)225–459 (–600) μm (av. = 342, SD = 117, n = 20) diam, superficial or slightly immersed, black, sub-globose to ellipsoidal. Ostiolar beak up to 150 μm wide and 30–150 μm (n = 10) long, sub-conical to paraboloid. Pseudoparaphyses filiform, hyaline, septate, branched. Asci 150–180 μm × 25–30 μm (n = 10), 1–8-spored, bitunicate, fissitunicate, hyaline, sub-cylindrical, short stalked. Ascospores 130–340 × 6–9 μm filiform, hyaline or pale yellow, attenuate at ends, 5–9-septate, tightly coiled inside the asci. Ascospores germinate either laterally or terminally.
Cultural characteristics: Colonies on PDA greyish white when young, becoming blackish when mature.
Hosts: Sorghum sp., Zea mays. Also reported from: Antirrhinum majus, Bothriochloa insculpta, Brachiaria foliosa, Chloris gayana, C. virgate, Coix lacryma-jobi, Cymbopogon citratus, C. martini, Cynodon dactylon, Dactyloctenium aegyptium, Dianthus caryophyllus, Digitaria ciliaris, Echinochloa colonum, E. crus-galli, Euchlaena mexicana, Eleusine indica, Eriochloa procera, Oryza sativa, Panicum bisulcatum, P. maximum, P. miliaceum, P. palmifolium, Paspalum scrobiculatum, Pennisetum maximum, P. typhoides, Perotis indica, Populus deltoids, Rottboellia exaltata, Saccharum officinarum, Salacca wallichiana, Setaria barbata, S. viridis, S. homonyma, S. sphacelata, Sorghum bicolor, S. halepense, S. vulgare, Sporobolus poiretiana, Triticum sp. (Poaceae) (Farr & Rossman 2013).
Distribution: Japan, USA (AL, AR, CT, DE, FL, GA, HI, IA, IL, IN, KS, LS, MA, MD, ME, MI, MN, MS, NB, NC, NE, NJ, NY, OK, OH, PA, RI, SC, TN, TX, VA, WI, WV). Also reported from: Australia, Bahamas, Bhutan, Bolivia, Brazil, Brunei Darussalam, China, Denmark, Egypt, Gambia, Ghana, Hong Kong, India, Jamaica, Malawi, Malaysia, Myanmar, Nepal, New Zealand, Nicaragua, Nigeria, Pakistan, Papua New Guinea, Portugal, Samoa, Sierra Leone, Solomon Islands, South Africa, Sudan, Swaziland, Taiwan, Thailand, Trinidad, Tobago, Zambia. (Farr & Rossman 2013).
Additional specimens examined: Japan, Kogoshima, on Sorghum bicolor, Sep. 1990, T. Tsukiboshi, AR 5184; On Zea mays, Nov. 1929, Y. Nisikado, CBS 136.29; Miyacaki, on S. bicolor, Sep. 1990, N. Nishihara, culture AR 5182; Miyacaki, on S. bicolor, Sep. 1990, T. Tsukiboshi, AR 5183. USA, Florida, Miami, on Zea mays, Nov. 1946, C. L. Lefebvre & H. Sherwin, BPI 626699; Indiana, on Z. mays, 28 Aug. 1928, C. Drechsler, BPI 626698; Oregon, on Z. mays, 19 Mar. 1977, J. Leach, BPI 1107544; On Zea mays, 7 Oct. 1924, BPI 626696; Washington DC, on Z. mays, 7 Oct. 1924, C. Drechsler, BPI 626697.
Notes: Bipolaris maydis causes an economically important disease, Southern leaf blight in maize, especially on the Texas male sterile plants (cms-T) inbred line. Several pathological races of the species, T, O and C, have been documented in previous studies (Leonard 1977, Wei et al. 1988). Accordingly, race T was responsible for the Southern corn leaf blight epidemic and found in association with cms-T maize. Also, two mating types “A” and “a” are known within the population of the species. The complete genome of B. maydis has been sequenced (Turgeon & Baker 2007, Ohm et al. 2012) and is now available in public databases. Based on the common use of B. maydis in most of the phytopathological literature, the conservation of the basionym Helminthosporium maydis Y. Nisik. & C. Miyake over the two other available synonyms, Helmisporium maydis Brond. and Ophiobolus heterostrophus Drechsler, was proposed (Rossman et al. 2013b).
Bipolaris mediocris (V.A. Putterill) Shoemaker, Canad. J. Bot. 37: 884. 1959.
Basionym: Helminthosporium mediocre V.A. Putterill, Bothalia 6: 354. 1954.
≡ Drechslera mediocris (V.A. Putterill) Subram. & B.L. Jain (as “mediocre”), Curr. Sci. 35: 354. 1966.
Type material: South Africa, on Pennisetum clandestinum, Jun. 1939, L.L.C. Liebenberg 30756, IMI 9833, holotype, not seen.
Leaf spots on Pennisetum clandestinum: Brown, 0.2–1.5 × 1–2 mm, coalescing to form larger spots that are parallel to mid vein (Sivanesan 1987).
Description: Putterill (1954) and Sivanesan (1987).
Host: Pennisetum sp. (Poaceae).
Distribution: Ethiopia, Guinea, South Africa (Farr & Rossman 2013).
Notes: Despite the lack of molecular data, this species is accepted in the genus Bipolaris based on the size of the conidia (40–108 × 13–18 μm) and the hilum structure.
Bipolaris microlaenae Alcorn, Mycotaxon 39: 382. 1990. Fig. 23.
Fig. 23.

Bipolaris microlaenae (IMI 338218). A–C. Ascomata. D–G. Asci. H. Ascospores. C–H stained with Melzer’s reagent. Scale bars: A = 100 μm, B = 100 μm, C–H = 10 μm.
= Cochliobolus microlaenae Alcorn, Mycotaxon 39: 381. 1990.
Type material: Australia, Queensland, Highfields, on Microlaena stipoides, 4 Mar. 1987, J.L. Alcorn 8705, BRIP 15613 (Bipolaris microlaenae), holotype, culture ex-type CBS 280.91; ibid., IMI 335218, isotype; Queensland, on M. stipoides, 6 Jul. 1988, J.L. Alcorn, developing in culture BRIP 16363 (Cochliobolus microlaenae), holotype; ibid., IMI 335217, isotype.
Leaf spots on Microlaena stipoides: Small dark brown. Asexual morph on Microlaena stipoides: Conidiophores (155–)215–490(–600) × 8–12 μm (av. = 352, SD = 135, n = 15; av. = 10, SD = 2, n = 15), single or rarely in small groups, simple, septate, straight or slightly curved below, geniculate at upper part, conidiophore base swollen, pale brown to dark olivaceous brown. Conidiogenous nodes verruculose. Conidia (63–)80–150(–185) × 15–19(–22) μm (av. = 117, SD = 36, n = 30; av. = 17, SD = 2, n = 30), smooth, apex ellipsoidal, base ellipsoidal to truncate, straight or curved, pale brown to dark olivaceous brown, usually concolorous, sometimes apical cells paler, 6–14-distoseptate. Hilum inconspicuous or sometimes slightly conspicuous. Germination predominantly bipolar, apical germ tube axial, basal germ tube semi axial, displacing hilum slightly. Septum ontogeny primary septum sub-median or delimiting basal cell, second septum visa versa, and third septum distal (modified from Alcorn 1990). Sexual morph on Sach's agar medium: Ascomata (395–)455–745(–800) × (237–)350–580(–600) μm (av. = 601, SD = 146, n = 10; av. = 466, SD = 116, n = 10), black, globose or flattened on hard surfaces. Ostiolar beak 145–210 μm long × (85–)90–160(–200) μm wide at base (av. = 179, SD = 33, n = 10; av. = 125, SD = 36, n = 18), cylindrical to tapered towards apex, body densely hairy, less hairy near ostiole. Asci fusoid, obclavate fusoid or cylindrical, straight or curved, gradually tapered towards apex and base, short pedicellate. Ascospores fusoid, gradually tapered towards ends, very closely coiled over large portion of length of ascus, apical segments of ascospores less tightly coiled, 163–217(–233) × 8–12 μm (av. = 190, SD = 27, n = 10; av. = 10, SD = 2, n = 10), (5–)9(–12)-septate.
Host: Microlaena stipoides (Poaceae).
Distribution: Australia.
Notes: Bipolaris microlaenae is distinguished from several species found in Australia based on morphological characters of both sexual and asexual morphs (Alcorn 1990). It is phylogenetically closely related to B. heveae, which is isolated from Hevea sp., and only known from the asexual morph. In addition B. heveae is reported in tropical countries whereas B. microlaenae is only known from Australia.
Bipolaris microstegii Minnis et al., Persoonia 29: 151. 2012. Fig. 24.
Fig. 24.

Bipolaris microstegii (BPI 883728). A–E. Conidiophores. F. Immature conidia attached to conidiophores. G–K. Conidia. L–N. Germinating conidia. Scale bars = 5 μm.
Type material: USA, West Virginia, near Arnoldsburg, Crummies Creek Tree Farm, on living leaves of Microstegium vimineum, Aug. 2009, R. Richardson Bipolaris 4, BPI 883727, holotype, culture ex-type CBS 132550.
Leaf spots on Microstegium: Ellipsoid to irregular, brown with dark brown or black border. Asexual morph on PDA: Conidiophores 300–700(–750) × (4–)7–9(–10) μm (av. = 501, SD = 198, n = 30; av. = 8, SD = 1, n = 30), usually arising singly or in small groups, simple or with a single dichotomous branch, septate, straight or flexuous, smooth, pale to dark brown. Conidiogenous nodes distinct, dark brown. Conidia (32–)45–86(–97) × (12–)14–18(–20) μm (av. = 66, SD = 20, n = 71; av. = 16, SD = 2, n = 71) curved or straight, cylindrical, ellipsoidal or obclavate, slightly tapering towards obtuse ends, olivaceous brown to dark golden brown, (3–)7(–9)-distoseptate, septa accentuated at maturity, hilum inconspicuous. Germination at both ends. Secondary sporulation common in culture.
Cultural characteristics: Colonies on PDA, dull green to greenish grey, irregular, lobed, effuse, velvety.
Host: Microstegium vimineum (Poaceae).
Distribution: USA (IN, WV).
Additional material examined: USA, Maryland, near Frederick, on Microstegium vimineum, 1 Apr. 2013, W.L. Bruckart, culture AR 5192; West Virginia, Calhoun Co., Crummies Creek Tree Farm, Cove, near Arnoldsburg, on M. vimineum, Aug. 2009, R. Richardson, BPI 883728; ibid., BPI 883729; ibid., culture AR 4838; ibid., culture AR 4839.
Notes: Bipolaris microstegii is phylogenetically closely related to two serious plant pathogens, B. victoriae and B. zeicola. The host plant Microstegium vimineum is native to Asia and is considered to be an invasive weed in several states in the USA. Several species of Bipolaris are known from Microstegium in Asia (Shimizu et al. 1998), but the origin of B. microstegii is unknown (Crous et al. 2012). A sexual morph is not recorded in association with this species.
Bipolaris musae-sapientium (Hansf.) B.A. Khasanov, (as “musae-sapienti”) Opredelitel' Gribov-Vozbuditeleĭ 'Gel'mintosporiozov' Rasteniĭ iz Rodov Bipolaris, Drechslera, Exserohilum (Tashkent): 68. 1992. Fig. 25.
Fig. 25.
Bipolaris musae-sapientium (K (M) 181466). A. Leaf spots on Musa sapientium. B. Conidia and conidiophores on leaf spots. C, D. Conidiophores. E, F. Conidia. Scale bars: A = 1000 μm, B = 500 μm, C–F = 5 μm.
Basionym: Helminthosporium musae-sapientium Hansf., (as “musae-sapientum”) Proc. Linn. Soc. London 155: 49. 1943.
≡ Drechslera musae-sapientium (Hansf.) M.B. Ellis, (as “musae-sapientum”) Dematiaceous Hyphomycetes (Kew): 451. 1971.
Type material: Uganda, on living leaves of Musa sapientium, Feb. 1915, Hansford, K (M) 181466, holotype.
Leaf spots on Musa sapientium: Oval or irregular, very pale, 0.2–1 cm × 0.1–0.5 cm, each spot surrounded by a thick black border. Asexual morph on Musa sapientium: Conidiophores (81–)110–210(–240) × 7–9(–10) μm (av. = 160, SD = 49, n = 11; av. = 8, SD = 1, n = 1), arising singly or few together, simple, septate, straight or flexuous, sometimes geniculate at upper part, pale brown to olivaceous brown. Conidiogenous nodes distinct, smooth. Conidia (50–)55–11 (–120) × 15–25 μm (av. = 84, SD = 26, n = 20; av. = 20, SD = 5, n = 20), smooth, slightly curved, sub-cylindrical, obclavate or ellipsoidal, tapering towards rounded ends, pale brown to olivaceous brown, (6–)7(–12)-distoseptate. Hilum inconspicuous or slightly protuberant, 3–4 μm wide.
Host: Musa sapientium (Musaceae).
Distribution: Uganda. Also reported from: Myanmar, Sudan (Farr & Rossman 2013).
Notes: Bipolaris musae-sapientium is one of three species of Bipolaris known on Musa. Bipolaris triticicola was recorded from the Windward Islands (Sivanesan 1987) and B. cynodontis from South Africa (Manamgoda et al. 2011). These two species can be distinguished from B. musae-sapientium by the much longer conidia of B. triticicola (80–185 × 18–21 μm) and shorter conidia of B. cynodontis (30–75 × 10–16 μm). Bipolaris cynodontis also produces a globose-shaped structure from the end cells during germination. A sexual morph is not recorded associated with Bipolaris musae-sapientium.
Bipolaris obclavata Sivan., Mycol. Res. 96: 485. 1992. Fig. 26.
Fig. 26.

Bipolaris obclavata (IMI 331725). A. Conidia and conidiophores on sterilised Zea mays leaf. B. Conidiophores on Zea mays leaf. C–E. Conidiophores. F–M. Conidia. Scale bars: A = 100 μm, B = 5 μm, C–M = 5 μm.
Type material: India, Warangal, on unknown plant, Sep. 1987, N.D Sharma 5, IMI 331725, holotype.
Asexual morph on PCA, Conidiophores 140–300(–320) × 5–7 μm (av. = 220, SD = 76, n = 22; av. = 6, SD = 1, n = 22), arising singly or in small groups, branched, septate, usually flexuous, sometimes straight, geniculate at upper part, mid brown to dark brown. Conidiogenous nodes dark brown, slightly verruculose. Conidia (27–) 35–55(–60) × (9–)11–15(–18) μm (av. = 45, SD = 9, n = 45; av. = 13, SD = 2, n = 45), straight, usually obclavate, tapering towards rounded ends, olivaceous brown to golden brown, (3–)4(–5)-distoseptate. Hilum inconspicuous, enclosed in cell wall.
Cultural characteristics: Colonies on PCA brown, velvety, effuse, hyphae pale brown to dark brown, septate, branched, smooth.
Host: Isolated from unknown plant material, known only from type.
Distribution: India.
Notes: This species has not been recorded after it is original description. Bipolaris obclavata is morphologically similar to B. arizonica in conidial dimensions. However, neither of these species have type sequences, therefore, we are unable to determine their phylogenetic relationships. Bipolaris arizonica and B. obclavata have been recorded only from USA and India, respectively. Based on the available data, we retain these as two separate species. A sexual morph is not recorded in association with this species.
Bipolaris oryzae (Breda de Haan) Shoemaker, Canad. J. Bot. 37: 883. 1959. Fig. 27.
Fig. 27.

Bipolaris oryzae (MFLUCC 10-0715, MFLUCC 10-0733). A. Surface view of infected seeds. B. Conidiophores and conidia. C–E. Conidiophores. F–M. Conidia. N. Bipolar germination of the conidia. Scale bars: A = 500 μm, B = 200 μm, C = 10 μm, D–N = 5 μm.
Basionym: Helminthosporium oryzae Breda de Haan, Bull. Inst. Bot. Buitenzorg 6: 11. 1900.
≡ Drechslera oryzae (Breda de Haan) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
≡ Luttrellia oryzae (Breda de Haan) Gornostaĭ (as “Lutrellia”), in Azbukina et al. (eds), Vodorosli, Griby i Mkhi Dal'nego Vostoka (Algae, Fungi and Mosses of the Soviet Far-East) (Vladivostok): 81. 1978.
= Ophiobolus miyabeanus S. Ito & Kurib., Ann. Phytopathol. Soc. Japan. 2: 1. 1927. (fide Ito & Kuribayashi, 1927).
≡ Cochliobolus miyabeanus (S. Ito & Kurib.) Drechsler ex Dastur, Indian J. Agric. Sci. 12: 733. 1942. (fide Sivanesan 1987).
= Helminthosporium zizaniae Y. Nisik., Rep. Ohara Inst. Agric. Res. 4: 122. 1929.
≡ Bipolaris zizaniae (Y. Nisik.) Shoemaker, Canad. J. Bot. 37: 885. 1959.
≡ Drechslera zizaniae (Y. Nisik.) Subram. & B.L. Jain, Curr. Sci. 35: 355. 1966.
= Helminthosporium oryzae Miyabe & Hori in Hori, Rept. Imp. Centr. Agric. Exp. Stn. Nishigohara, Japan 18: 79. 1901. (fide Sivanesan 1987).
Type material: Japan, on Oryza sativa, iconotype designated here Ito & Kuribayashi (1927), Ann. Phytopathol. Soc. Japan 2: 9 plate I, J.A. Stevenson Mycology Library, USDA-ARS, Beltsville, Maryland, USA (Ophiobolus miyabeanus) “MBT197970”. Thailand, Chiang Rai, near Khunkoon waterfall, on seeds of Oryza sativa, May 2010, D.S. Manamgoda, neotype designated here BPI 892948 (Bipolaris oryzae) “MBT198050”, ex-neotype culture MFLUCC 10-0715.
Symptoms on Oryza sativa: Brown spots on leaves and seeds. Leaf spots ovoid up to 1 cm long. Initially usually brown, sometimes purplish, later forming white to grey centres, often coalescing when leaf withers (Sivanesan 1987). Seeds spotted, becoming black, velvety with sporulation. Asexual morph on PDA: Conidiophores (150–)405–625(–620) × 6–8 μm (av. = 515, SD = 110, n = 20; av. = 7, SD = 1, n = 20), arising singly or in groups, branched or simple, multi-septate, flexuous, sometimes upper part geniculate, brown to black. Conidiogenous nodes dark brown, smooth or slightly verruculose. Conidia (50–)68–108(–155) × (10–)14–20(22) μm (av. = 17, SD = 3, n = 37), usually curved, rarely straight, navicular, fusiform, obclavate or almost cylindrical, hyaline when immature, becoming slightly brown when mature, (6–)10(–12)-distoseptate. Hilum minute, slightly protruding. Germinating at both ends of conidia. Secondary sporulation in some conidia. Sexual morph on Sach's agar + sterilised rice stem: Ascomata 370–760 × 360–780 μm black, globose. Ostiolar beak 98–200 × 55–110 μm, cylindrical to conical. Pseudoparaphyses filiform, hyaline, septate and branched. Asci 140–235 × 21–26 μm, 1–8-spored, most commonly 4 or 8, clavate or broadly fusoid. Ascospores 235–470 × 4–9 μm hyaline, tapering from ends, with a thin mucilaginous sheath visible in water mounts, 8–12-septate.
Cultural characteristics: Colonies on PDA white when young, becoming slightly grey when mature, fluffy, cottony.
Hosts: Oryza sativa, Panicum maximum, Zizania latifolia (Poaceae). Also reported from: Alopecurus aequalis, Chikusichloa aquatic, Cordia trichotoma, Eleusine indica, Leersia hexandra, Oryza australiensis, O. latifolia, Panicum colonum, P. virgatum, Setaria italica, Triticum aestivum, Zizania palustris (Poaceae) (Krupinsky et al. 2004, Farr & Rossman 2013).
Distribution: Japan, Thailand, USA (CA, FL, MI, NY, OK). Also reported from: Australia, Bangladesh, Bhutan, Bolivia, Brazil, Brunei Darussalam, China, Colombia, Egypt, Fiji, Gambia, Ghana, Guinea, India, Indonesia, Iran, Jamaica, Korea, Malawi Malaysia, Mauritius, Mexico, Myanmar, Nepal, New Zealand, Nicaragua, Nigeria, Pakistan, Panama, Papua New Guinea, South Africa, Venezuela, Yugoslavia, Zambia, Zimbabwe (Farr & Rossman 2013).
Additional material examined: Thailand, Chiang Rai, Muang, on seeds of Oryza sativa, Jun. 2010, D.S. Manamgoda, culture MFLUCC 10-0733; ibid., culture MFLUCC 10-0714. USA, North Dakota, on Panicum virgatum, J. Krupinsky, culture CBS 112775 = AR 3796; ibid., specimen BPI 842262; ibid., culture AR 3798.
Notes: Bipolaris oryzae was the causative agent of devastating diseases in rice leading to the 1943 Bengal famine in India (Scheffer 1997). Since the type specimen of this species could not be located, a neotype is designated here from a recent collection from Thailand. Considerable variation in conidial morphology has been reported within this species (Subramanian & Bhat 1978), and therefore molecular data is critical for its identification. Bipolaris oryzae also shows considerable genetic variation within the species, thus several biotypes and pathotypes may exist within the species (Cholil & de Hoog 1982).
Bipolaris panici-miliacei (Y. Nisik.) Shoemaker, Canad. J. Bot. 37: 883. 1959. Fig. 28.
Fig. 28.
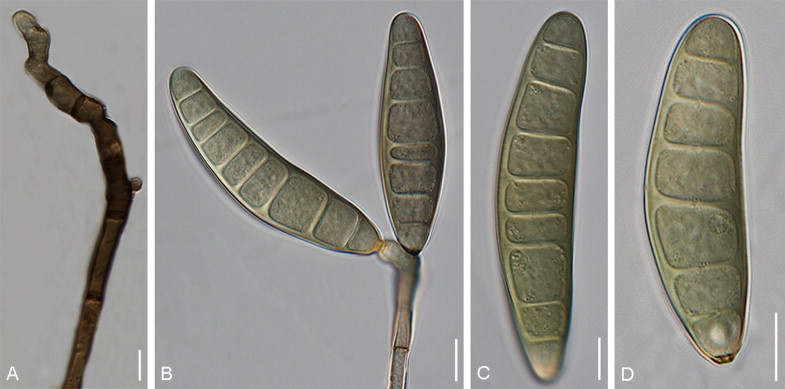
Bipolaris panici-miliacei (CBS 199.29). A. Conidiophores. B. Conidiphores with conidia. C–D. Conidia. Scale bars = 10 μm.
Basionym: Helminthosporium panici-miliacei Y. Nisik., Ber. Ohara Inst. Landw. Fursch. 4: 120. 1929.
≡ Drechslera panici-miliacei (Y. Nisik.) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Type material: Japan, on Panicum miliaceum, iconotype designated here Y. Nisikado, Ber. Ohara Inst. Landw. Fursch. 4:120 (1929): plate XIII, J.A. Stevenson Mycology Library, USDA-ARS, Beltsville, Maryland. USA, on Panicum miliaceum, Nov. 1929, Y. Nisikado, lectotype designated here CBS H-7031, ex-lectotype culture CBS 199.29 “MBT197971”.
Leaf spots on Panicum miliaceum: Long elliptic or fusiform, up to 30 cm long and 5 cm wide with indistinct margins, sometimes formed with pale yellowish halo (Nisikado 1929). Asexual morph on PDA: Conidiophores (120–)155–300(–400) × 6–8(–10) μm (av. = 228, SD = 72, n = 30; av. = 7, SD = 1, n = 30), arising singly or in groups, branched or simple, multi-septate, flexuous, sometimes upper part geniculate, dark olive green. Conidiogenous nodes dark brown, smooth or slightly verruculose. Conidia (80–)90–140(–175) × (15–)16–20(21) μm (av. = 112, SD = 31, n = 30; av. = 18, SD = 2, n = 30), curved or straight, navicular, fusiform, obclavate or almost cylindrical, hyaline when immature, becoming olive green when mature, (6–)10(–12)-distoseptate. Hilum minute, slightly protruding. Germinating at both ends of conidia.
Host: Panicum miliaceum (Poaceae).
Distribution: Japan.
Notes: The holotype specimen of this species could not be located, therefore, a lectotype is designated from available syntypes with a culture. Bipolaris panici-miliacei morphologically resembles B. oryzae. In the combined three-gene analysis these two species cluster as sister taxa and B. panici-miliacei is represented by a singleton. Based on a few variable characters observed in GPDH and TEF sequences between the ex-neotype of B. oryzae and the ex-lectotype B. panici-miliacei, these species are treated as distinct in this study.
Bipolaris peregianensis Alcorn, Mycotaxon 15: 9. 1982. Fig. 29.
Fig. 29.
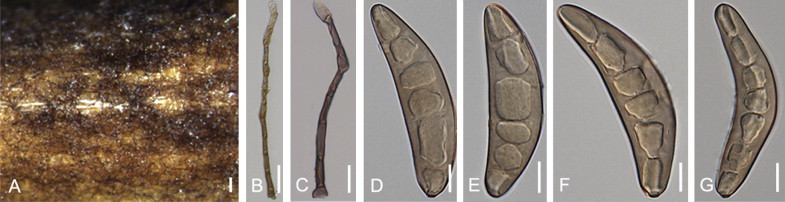
Bipolaris peregianensis (DAR 35057). A. Conidiophores on the host. B, C. Conidiophores. D–G. Conidia. Scale bars: A = 200 μm, B, C = 20 μm, D–G = 10 μm.
= Cochliobolus peregianensis Alcorn, Mycotaxon 15: 9. 1982.
Type material: Australia, Queensland, Oriunda, Perigian Beach, on leaf of Cynodon dactylon, 11 Nov. 1978, J.L. Alcorn 7848, BRIP 12790 (Bipolaris peregianensis), holotype, ex-holotype culture observed; IMI 264355, DAR 35057, isotypes; obtained by pairing cultures of B. peregianensis on leaves of Zea mays on Sach's agar, BRIP 11774 (Cochliobolus peregianensis), not seen, holotype.
Leaf spots on Cynodon dactylon: Small, punctiform brown lesions. Asexual morph on Cynodon dactylon leaf: Conidiophores (150–)170–285(–390) × 6–9 μm (av. = 228, SD = 58, n = 21; av. = 7, SD = 1, n = 21), arising singly or in small groups, simple, septate, straight at lower part, sometimes becoming geniculate at upper part, basal cell usually swollen, pale golden brown to reddish brown, paler towards apex. Conidiogenous nodes verruculose. Conidia (55–)60–75(–87) × 14–16(–19) μm (av. = 69, SD = 9, n = 35; av. = 15, SD = 1, n = 35), smooth, curved, fusoid, tapering towards broadly rounded end cells, mid olivaceous brown to reddish brown, cells concolorous, (4–)6(–8)-distoseptate, Hilum inconspicuous or slightly protuberant, 3–4 μm wide. Sexual morph on Sach's agar and sterilised Zea mays stem: Ascomata (390–)400–500(–520) × 350–450 μm (av. = 450, SD = 50, n = 10; av. = 400, SD = 50, n = 10), black, superficial, ampulliform, arising singly or in small groups. Ostiolar beak 80–120 μm (n = 6) long and 70–80 μm (n = 6) wide at opening, conical to paraboloid. Asci (130–)150–200(–215) × (14–)20–30(–35) μm (av. = 175, SD = 25, n = 10; av. = 25, SD = 5, n = 10), cylindrical to clavate, sometimes fusoid, apex rounded or flattened. Ascospores 170–200 × 5–8 μm coiled helically in ascus, filiform, rounded apex and bluntly pointed rounded base, with a hyaline sheath visible in water mounts, 5–8-septate.
Hosts: Cynodon dactylon. Also reported from: Rottboellia exaltata (Poaceae) (Lenne 1990).
Distribution: Australia, China (Wang et al. 2012, Farr & Rossman 2013).
Additional material examined: Australia, Queensland, on Cynodon dactylon, J.L. Alcorn, DAOM 221998.
Notes: Bipolaris peregianensis is less commonly found on Cynodon dactylon than B. cynodontis and is reported to cause small, elliptical, pale brown lesions (Wang et al. 2012). In the phylogenetic analysis they appear as two distinct species. Bipolaris peregianensis is more closely related to B. sacchari while B. cynodontis groups closer to B. chloridis and B. clavata (Fig. 1).
Bipolaris pluriseptata (Khetarpal, R. Nath & S.P. Lal) Alcorn, Mycotaxon 41: 329. 1991. Fig. 30.
Fig. 30.

Bipolaris pluriseptata (IMI 259810). A. Conidia on host Eleusine coracana. B. Conidiophore and conidia. C–F. Conidia. Scale bars: A = 20 μm, B–F = 10 μm.
Basionym: Drechslera pluriseptata Khetarpal, R. Nath & S.P. Lal, Indian Phytopath. 37: 320. 1984.
Type material: Zambia, on seeds of Eleusine coracana, Feb. 1981, IMI 259810, holotype; ibid., ITCC 3131, not seen, isotype; ibid., ex-isotype culture BRIP 14839.
Asexual morph on Eleusine coracana: Hyphae hyaline to pale brown, branched, septate. Conidiophores 40–220(–310) × 6–12 μm (av. = 131, SD = 90, n = 20; av. = 9, SD = 3, n = 20), arising singly or in groups of few, simple, septate, straight or flexuous, geniculate at upper part, smooth, size of cells decrease towards apex, basal cell distinctly swollen, pale brown to dark brown. Conidia (185–)190–280(–315) × (10–)12–16(–18) μm (av. = 234, SD = 47, n = 100; av. = 14, SD = 2, n = 100), often C-shaped, occasionally horse-shoe shaped or sigmoid, fusoid, rarely straight, tapering towards ends, reddish brown to dark brown, end cells sometimes lighter, very distinctly curved. Hilum usually inconspicuous, occasionally slightly protuberant.
Cultural characteristics: Colonies on PDA, effuse, brown to black, velvety.
Hosts: Eleusine coracana. Also reported from: Sorghum bicolor (Poaceae) (Yassin et al. 2010).
Distribution: Zambia. Also reported from: India, Saudi Arabia (Yassin et al. 2010, Farr & Rossman 2013).
Notes: Bipolaris pluriseptata has the longest conidia (up to 300 μm) compared to the average of about 100 μm for species in Bipolaris. These conidia are strongly curved in appearance. Sivanesan (1987) synonymised this species with B. curvispora, a name synonymised under B. salviniae in the present study. Alcorn (1990) found that the culture of B. pluriseptata was not fertile when paired with isolates of B. curvispora. Also Alcorn (1990) observed differences in morphology of B. pluriseptata. We observed type specimens of both species and the calculated mean of spore length of B. curvisopora and B. pluriseptata is 137 μm and 234 μm. In addition conidia of B. pluriseptata are more strongly curved than those of B. curvispora. According to our phylogenetic data these two species can clearly be identified as two separate species (Fig. 1). A sexual morph is not found in association with this species.
Bipolaris poae-pratensis H. Deng & T.Y. Zhang, Mycosystema 21: 328. 2002.
Type material: China, Hohhot, on Poa pratensis, Aug. 2000, H. Deng, HSAUP II01436, holotype.
Description: See Deng & Zhang (2002).
Host: Poa pratensis (Poaceae).
Distribution: China.
Notes: The only other Bipolaris species reported from Poa pratensis is B. sorokiniana. Bipolaris poa-pratensis has longer, narrower conidia (70–110 × 9.5–17.5 μm) than B. sorokiniana (40–70 × 15–25 μm). Based on the hilum morphology, distoseptation (7–14), conidial shape and dimensions, this species is accepted in the genus Bipolaris despite the lack of molecular data.
Bipolaris sacchari (E.J. Butler) Shoemaker, Canad. J. Bot. 17: 68. 1959. Fig. 31.
Fig. 31.
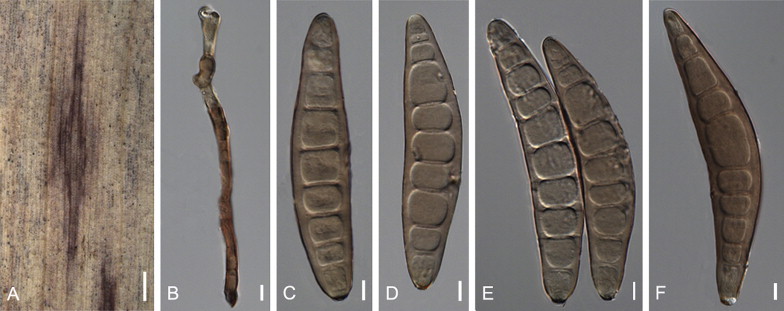
Bipolaris sacchari (BPI 430195). A. Leaf spots on Saccharum officinarum. B. Conidiophore. C–F. Conidia. Scale bars: A = 500 μm, B = 10 μm, C–F = 5 μm.
Basionym: Helminthosporium sacchari E.J. Butler, Memoirs of the Dept. Agric. India, Bot. Ser. 6: 207. 1913.
≡ Drechslera sacchari (E.J. Butler) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
= Cercospora sacchari Breda de Haan, Meded. Proefst. Kagok. 3: 15. 1892 (fide Putterill 1954).
≡ Bipolaris sacchari (Breda de Haan) Subram., Hyphomycetes (New Delhi): 769. 1971.
= Helminthosporium ocellum Faris, Phytopathology 18: 757. 1928. (fide Putterill 1954).
≡ Bipolaris ocella (Faris) Shoemaker, Canad. J. Bot. 37: 884. 1959.
≡ Drechslera ocella (Faris) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Type material: India, Pusa, on Saccharum officinarum, collection details unknown (Bipolaris sacchari) (Sivanesan 1987) (not seen); Cuba, on Saccharum officinarum, 1927, J.A. Faris, BPI 429720 (Helminthosporium ocellum), lectotype.
Leaf spots on Saccharum officinarum: Initially red, small, then elongating parallel to mid-vein, appearing as “eye spots” with straw-colored centre and red halo, 5–12 × 3–6 mm, coalescing. Asexual morph on Saccharum officinarum: Conidiophores 70–300 × 4–8 μm (n = 8), arising singly or in groups, simple, septate, usually straight, sometimes flexuous, geniculate at upper part, frequently swollen at base, pale brown to olivaceous brown. Conidiogenous nodes dark brown, distinct. Conidia (35–)55–90(–95) × (10–)12–14(–17) μm (av. = 74, SD = 19, n = 30; av. = 13, SD = 1, n = 30), usually curved, elliptic to elliptic fusiform, tapering towards rounded ends, pale brown to greyish brown, (5–)8(–9)-distoseptate. Hilum slightly protuberant, 2–3 μm wide.
Hosts: On Oplismenus imbecillus, Pennisetum purpureum, Saccharum officinarum, Saccharum sp. Also reported from: Cymbopogon citrates, Cynodon dactylon, Digitaria insularis, Echinochloa colona, Imperata arundinacea, I. cylindrica, Iseilema laxum, Leptocoryphium lanatum, Lygodium japonicum, L. microphyllum, Panicum fasciculatum, P. maximum, P. purpurascens, Pennisetum clandestinum, P. glaucum, P. typhoides, Tillandsia sp., Triticum aestivum, Zea mays (Poaceae) (Subramanian 1971, Farr & Rossman 2013).
Distribution: Brazil, Cook Islands, Cuba, India, Philippines, Puerto Rico, Uganda, USA. Also reported from: Australia, Costa Rica, Dominican Republic, El Salvador, Fiji, Guatemala, Honduras, Indonesia, Iran, Italy, Jamaica, Malawi, Malaysia, Myanmar, Nicaragua, Nigeria, Panama, Papua New Guinea, Senegal, Sierra Leone, Solomon Islands, Southern Africa, Sri Lanka, Taiwan, Trinidad, Venezuela, West Indies (Subramanian 1971, Farr & Rossman 2013).
Additional material examined: Brazil, Viçosa, Minas Gerais, Agricultural College, on Saccharum officinarum, 31 Mar. 1938, O.A. Drummond, BPI 430194; ibid., BPI 430194; on S. officinarum, Jun. 1933, A.S. Mueller, BPI 430188. Cook Islands, on S. officinarum, Feb. 1960, N.L.H. Krauss, BPI 43018. Cuba, Central Baragua, on S. officinarum, Apr. 1930, J.A. Faris, BPI 429719; Santiago de Las Vegas, Experiment Station Grounds, on S. officinarum, 5 Apr. 1915, R.A. Jehle & J.R. Johnston, BPI 430201. India, Abugodi, on S. officinarum, 10 Dec. 1966, Swaminathan & Raghunathan, BPI 430200; Hebbal, Bangalore, on S. officinarum, 15 Dec. 1966, V. Ragunathan, BPI 430196; Hebbal, Bangalore, on S. officinarum, 18 Jan. 1967, Swaminathan & Raghunathan BPI 430197. New Zealand, Auckland, on Oplismenus imbecillus, 1 May 1975, E.H.C. McKenzie, ICMP 6227. Philippines, Victorias, Negros Occidental, on S. officinarum, 3 Aug. 1929, P.W. Dwight, BPI 430193; on S. officinarum, 14 Aug. 1966, J. Pugat, BPI 430198. Puerto Rico, Rio Piedras, on S. officinarum, 1914, J.A. Stevenson, BPI 430186; Rio Piedras, on S. officinarum, 1918, J.A. Stevenson, BPI 430189. Uganda, Kampala, on S. officinarum, 1 Mar. 1921, J.D. Snowden, BPI 430185. USA, Florida, Canal Point, on S. officinarum, B.A. Bourne, BPI 430199; Florida, Gainesville, on Pennisetum purpureum, 24 Aug. 1943, C.L. Lefebvre, BPI 430179; Florida, Gainesville, on P. purpureum, 22 Aug. 1944, C.L. Lefebvre, BPI 430181; Florida, Canal Park, on S. officinarum, Dec. 1927, C. Drechsler, BPI 430195; Georgia, Tifton, on P. purpureum, 6 Aug. 1942, C.L. Lefebvre, BPI 430180; Hawaii, Waialua, Oahu, on S. officinarum, P.W. Dwight, BPI 430184; ibid., BPI 430190; ibid., BPI 430191; ibid., BPI 430192; Maryland, Beltsville, on P. purpureum, 5 Feb. 1945, C.L. Lefebvre, BPI 430183; Ibid., BPI 430182.
Notes: Eyespot disease on Sugar cane in India was attributed to Helminthosporium sacchari (Butler & Khan 1913). Breda de Haan (1892) described a disease on Sugar cane in Java caused by Cercospora sacchari, later identified as the eyespot disease of sugarcane (Krüger 1899). The type specimen of Cercospora sacchari was presumed to be lost (Sivanesan 1987). Illustrations of Cercospora sacchari were published by Wakker & Went (1899), later shown to be Helminthosporium rather than Cercospora (Faris 1928). Faris (1928) suggested that Cercospora sacchari was a Helminthosporium sp. different from the species described by Butler & Khan (1913) and thus provided it with a new name, Helminthosporium ocellum. Putterill (1954) was the first to consider that H. ocellum and H. sacchari were conspecific (Sivanesan 1987). Later Subramanian (1971) observed specimens of eyespot disease of sugarcane in India and synonymised Helminthosporium sacchari with H. ocellum, and placed it in the genus Bipolaris. Faris (1928) described a species collected from Cuba deposited in BPI, which is identical to the original description of Cercospora sacchari. After observing the specimen (BPI 429720), collected on 26 Nov. 1958, R.A. Shoemaker designated it as a lectotype for the Helminthosporium ocellum. According to morphological data we agree that Helminthosporium sacchari and H. ocellum are conspecific and the oldest epithet sacchari has priority, so the current species name should be Bipolaris sacchari. A sexual morph is not found in association with this species. Bipolaris sacchari is common on Sugar cane, but also infects other grass hosts. It is known to produce the toxin helminthosporoside (Sivanesan 1987). Another common disease on Sugar cane caused by a Bipolaris sp. is brown stripe caused by B. stenophila (conidia 70–105 μm), that usually has larger conidia than B. sacchari (55–90 μm).
Bipolaris salkadehensis Ahmadpour & Heidarian, Mycotaxon 120: 302. 2012.
Type material: Iran, West Azerbaijan, Khoy City, Salkadeh village, on infected leaves of Sparganium erectum, 20 Sep. 2010, A. Ahmadpour Bi-1, TUPP1366 (not seen), holotype.
Description: A recent description is available in Ahmadpour et al. (2012).
Hosts: Cladium mariscus (Cyperaceae), Sparganium erectum (Typhaceae).
Distribution: Iran.
Notes: The conidial dimensions of this species are reported as 50–70 × 10–15 μm (Ahmadpour et al. 2012), similar to B. cynodontis (40–80 × 12–18 μm). However, based on the available ITS sequence data, Bipolaris salkadehensis proved to be a distinct species closely related to B. sacchari (Fig. 2). A sexual morph is not found in association with this species.
Bipolaris salviniae (J.J. Muchovej) Alcorn, Mycotaxon 41: 331. 1991. Fig. 32.
Fig. 32.

Bipolaris salviniae (DAR 35056). A. Conidiophores and conidia on Melinis miniutiflorae. B. Conidiophores and conidia. C–E. Conidiophores. F–H, J, K. Conidia. I. Conidia with secondary sporulation. Scale bars: A = 200 μm, B–E = 20 μm, F–K = 10 μm.
Basionym: Drechslera salviniae J.J. Muchovej, Trans. Brit. Mycol. Soc. 72: 331. 1979.
= Bipolaris melinidis Alcorn, Mycotaxon 15: 7. 1982.
= Cochliobolus melinidis Alcorn, Mycotaxon 15: 5. 1982.
= Drechslera curvispora El Shafie 1982, Trans. Brit. Mycol. Soc. 78: 545. 1982. (fide Alcorn 1991).
≡ Bipolaris curvispora (El Shafie) Sivan., Mycol. Pap. 158: 47. 1987.
Type material: Australia, on Sach's agar + Melinis minutiflora, Nov. 1978, J.L. Alcorn, BRIP 12764a (Cochliobolus melinidis), holotype; Queensland, Maleny, on M. minutiflora, 24 May 1979, J.L. Alcorn, BRIP 12898 (Bipolaris melinidis), holotype; ibid., DAR 35056, isotype. Paraguay, on Triticum aestivum (as T. vulgare), 12 Dec. 1980, E.L. Shafie, IMI 253986 (Drechslera curvispora), holotype. Brazil, Minas Gerais, Viçosa, on Salvinia auriculata, Federal Viçosa herbarium (Bipolaris salviniae), not seen, holotype; ibid., 1978, J.J. Muchovej, BRIP 16571, lectotype, lecto type culture IMI 228224.
Asexual morph on PDA: Hyphae pale to mid brown, smooth, branched, septate. Conidiophores 170–520(–786) × 8–14(–18) μm (av. = 346, SD = 176, n = 46; av. = 11, SD = 3, n = 46), arising singly or in groups, simple, septate cylindrical, straight or geniculate at upper part, basal cell swollen, mid brown to dark reddish brown, sometimes paler towards apex. Conidiogenous nodes distinct, dark brown, smooth or slightly verruculose. Conidia (75–)100–170(–190) × (10–)13–17(–19) μm (av. = 137, SD = 33, n = 60; av. = 15, SD = 2, n = 60), distinctly curved, occasionally sigmoid or straight, cylindrical, sub-cylindrical or fusoid, end cells hemi-ellipsoidal or obconic, mid brown to reddish brown, concolorous, (6–)10(–14)-distoseptate. Hilum truncate, 3–4 μm. Germinating from both ends. Secondary sporulation observed. Sexual morph on Sach's agar: Ascomata 300–520 × 200–500 μm (av. = 10), black, globose, superficial or slightly embedded, short beaked, setose. Ostiolar beak 40–150 × 60–180 μm (av. = 10), conical or blunt. Setae mid brown to dark brown, straight, septate, tapered towards apex. Pseudoparaphyses hyaline, septate, straight or branched, filiform. Asci 120–200(–220) × 20–28(–30) μm (av. = 160, SD = 40, n = 20; av. = 24, SD = 4, n = 20), obclavate, pedicellate, bitunicate, 1–8 spored, hyaline, cylindrical to narrowly clavate. Ascospores 185–400 × 11–15 μm, hyaline, closely coiled inside ascus, tapered towards rounded ends, 5–12-septate, with a mucilaginous sheath up to 4 μm thick, visible in water mounts.
Cultural characteristics: Colonies on PDA, grey, velvety, effuse.
Hosts: Melinis minutiflora, Salvinia auriculata, Triticum aestivum. Also reported from: Panicum maximum var. trichoglume, Setaria anceps (Poaceae) (Farr & Rossman 2013).
Distribution: Australia, Brazil, Paraguay. Also reported from: India (Farr & Rossman 2013).
Notes: The synonymy of Bipolaris melinidis to Drechslera curvispora was proposed by Sivanesan (1987). Alcorn (1991) found that that these two species are interfertile and accepted this synonymy. The holotype specimen of Bipolaris salviniae was destroyed by insects and no isotype is preserved. Alcorn (1991) designated IMI 228224 as lectotype of D. salviniae with BRIP 16571 as isolectotype. Alcorn (1991) also stated that Bipolaris salviniae is morphologically similar to B. curvispora and B. melinidis. The ex-type cultures of B. salviniae and B. melinidis clustered as one species in our multigene phylogenetic trees (Figs 1, 2), thus both B. melinidis and B. curvispora are conspecific with Bipolaris salviniae.
Bipolaris secalis Sisterna Pl. Pathol. 38: 98. 1989.
Type material: Argentina, Buenos Aires, Los Hornos, from seed of Secale cereale, Aug. 1984, M.N. Sisterna, IMI 286591, lectotype; ibid., BRIP 14453, isolectotype (ex-isotype culture included).
Description and illustration: Available in Sisterna (1989).
Host: Secale cereale (Poaceae).
Distribution: Agentina.
Notes: In the first publication of this species (Sisterna 1989), two specimens are listed in different herbaria. Tan et al. (2014) recognised that those two specimens were duplicates of the same type specimen, and designated one of them as the lectotype. According to the phylogenetic data this species is accepted in the genus Bipolaris (Fig. 1).
Bipolaris setariae (Sawada) Shoemaker, Canad. J. Bot. 37: 884. 1959. Fig. 33.
Fig. 33.

Bipolaris setariae (BPI 880305B). A. Infected stem of Setaria faberi. B. Conidia attached to conidiophores on host. C–H. Conidiophores. I–R. Conidia. Scale bars: B = 50 μm, C–H = 20 μm, I–R = 10 μm.
Basionym: Helminthosporium setariae Sawada, Bull. Dept. Agric. Gov. Res. Inst. Formosa 64: 19. 1912.
≡ Drechslera setariae (Sawada) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
[= Helminthosporium setariae Lind, Danish Fungi (Copenhagen): 527 (1913) non Sawada 1912 (fide Shoemaker 1959)].
= Ophiobolus setariae S. Ito & Kurib., Proc. Imp. Acad. Japan 6: 352. 1930. (fide Sivanesan 1987).
≡ Cochliobolus setariae (S. Ito & Kurib.) Drechsler ex Dastur, Indian J. Agric. Res. 12: 733. 1942.
Type material: Iconotype designated here Lind (1913), Danish Fungi (Copenhagen): 527 (Helminthosporium setariae Lind.) “MBT197972”.
Leaf spots: Elongated, variable in size, white or pale grey centre (Sivanesan 1987). Asexual morph on Setaria italica: Conidiophores (96–)145–207(–218) × 4–6 μm (av. = 176, SD = 31, n = 20; av. = 5, SD = 1, n = 20), mid brown to olivaceous brown, arising singly or in small groups, simple, septate, straight or flexuous, sometimes geniculate at upper part, cylindrical, basal cell swollen. Conidiogenous nodes dark brown. Conidia (50–)65–100(–108) × (10–)13–16 μm (av. = 87, SD = 12, n = 30; av. = 15, SD = 1, n = 30), straight or curved, fusoid or navicular, pale brown to mid golden brown, (5–)8(–10)-distoseptate. Hilum inconspicuous or slightly protuberant. Germinating at both ends of conidia. Sexual morph on Sach's agar: Ascomata dark 240–505 × 220–315 μm, brown, globose or short ellipsoidal, pseudoparenchymatous. Ostiolar beak 60–125 × 50–110 μm, paraboloid, cylindrical. Asci 130–150 × 22–32 μm, numerous, fusiform straight or slightly curved, widest somewhat below middle, rounded at apex, shortly stipitate, hyaline, thin-walled, with 1–8 spores. Ascospores 200–315 × 6–7 μm, hyaline or olive coloured, filiform, obtusely pointed at both ends, 5–9-septate, coiled in a close helix (modified from Ito 1930).
Hosts: Setaria faberi, S. imberbis, S. italica, S. lutescens, S. macrostachya, and Pennisetum glaucum (Poaceae). Also reported from: Agrostis tenuis, Avena sativa, Brachiaria mutica, B. reptans, Cynodon sp., Desmostachya bipinnata, Digitaria granularis, Echinochloa colonum, Echinochloa sp., Eleusine coracana, Eragrostis sp., Hordeum sp., Hordeum vulgare, Ischaemum rugosum, Oryza sativa, Oryzopsis holciformis, Panicum clandestinum, P. fasciculatum, P. maximum, P. miliaceum, Paspalidium flavidum, Paspalum distichum, Pennisetum americanum, Saccharum officinarum, Setaria glauca, S. geniculata, S. tomentosa, Sorghum sp., Triticum sp., Zea mays. Also reported from non-Poaceae hosts: Caryota mitis, Chamaedorea elegans, C. seifrizii, Chrysalidocarpus lutescens (Arecaceae), Dianthus caryophyllus (Caryophyllaceae), Manihot esculenta (Euphorbiaceae), Persea americana (Lauraceae), Calathea sp., Maranta arundinacea, M. leuconeura (Marantaceae), Dendrobium sp. (Orchidaceae), Antirrhinum majus (Plantaginaceae), Rosa sp. (Rosaceae) (Farr & Rossman 2013).
Distribution: Australia, Canada, China, Egypt, Ethiopia, India, Korea, Myanmar, New Zealand, Pakistan, Peru, Sierra Leone, Taiwan, Turkey, Uganda, USA, Venezuela (Farr & Rossman 2013).
Additional material examined: USA, Maryland, Beltsville, on Setaria italica, 20 Sep. 1939, C.L. Lefebvre, BPI 430249; Maryland, ibid., 11 Jul. 1935, H.N. Vinall, BPI 430252; ibid., 28 Aug. 1935, A. G. Johnson, BPI 430253; ibid., BPI 430255; Maryland, ibid., 23 Jul. 1935, A.G. Johnson, BPI 430256; ibid., 17 Jul. 1935, A.G. Johnson, BPI 430257; ibid., BPI 430258; ibid., A.G. Johnson, BPI 430259; ibid., 17 Jul. 1935, A.G. Johnson & H.W. Johnson, BPI 430254; New Jersey, New Gretna, on S. italica, 23 Sep. 1941, F. Shropshire, BPI 430247; ibid., BPI 430248; Texas, Hulen on S. imberbis, 2 Jun. 1897, F.W. Mally, BPI 430243; Texas, San Antonio, on S. macrostachya, 30 Aug. 1930, B.F. Dana, BPI 430261; Virginia, Montgomery Co., Walton. Parking area, on S. faberi, 2 Aug. 2005, C.W. Roane, BPI 880305B; Virginia, Giles Co., Eggleston, on S. faberi, 8 Sep. 2001, C.W. Roane, BPI 880877B; Virginia, Arlington, Arlington Farm, on Pennisetum glaucum, 20 Feb. 1939, C.L. Lefebvre 107, BPI 430242; ibid., BPI 430244; ibid., 4 Mar. 1938, BPI 430245; ibid., 25 Aug. 1937, BPI 430246; ibid., 15 May 1940, BPI 430250; ibid., 27 Mar. 1938, BPI 430251; Virginia, Arlington, Arlington Farm Greenhouse, on S. lutescens, 4 Mar. 1938, C.L. Lefebvre, BPI 430260.
Notes: The type specimen of Helminthosporium setariae (Sawada) Shoemaker could not be located and there are no illustrations in the protologue (Sawada 1912). In the protologue of H. setariae Lind it is not indicated where the type specimen was deposited, thus, an illustration in the paper is chosen as iconotype. Ophiobolus setariae was introduced as the sexual morph of Helminthosporium setariae also without an indication of where the type is deposited. Based on the hilum morphology, septation and conidial shape, this species is accepted in the genus Bipolaris despite the lack of molecular data.
Bipolaris sorokiniana (Sorokin) Shoemaker, Canad. J. Bot. 37: 884. 1959. Fig. 34.
Fig. 34.

Bipolaris sorokiniana (BPI 882629A, BPI 430575, CBS 120.24, CBS 110.14). A. Conidia on the host. B. Conidiophore on the host. C–E, L–Q. Conidia. F–K. Conidiophore produced on PDA slide cultures. Scale bars = 5 μm.
Basionym: Helminthosporium sorokinianum Sorokin, Proc. Biol. Soc. Imp. Univ. Kazan 22: 29. 1890.
≡ Drechslera sorokiniana (Sorokin) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
[= Helminthosporium sorokinianum Sacc., Z. PflKrankh. 1: 238. 1891. non Sorokin 1890].
= Helminthosporium sativum Pammel, C.M. King & Bakke, Bull. Bot. Dept. State Agric. Coll. Ames. 116: 180. 1910. (fide Shoemaker 1959).
= Helminthosporium acrothecioides Lindf., Bot. Tidsskr. 12: 212. 1918. (fide Sivanesan 1987).
= Helminthosporium californicum Mackie & G.E. Paxton, Phytopathology 13: 562. 1923. (fide Shoemaker 1959).
≡ Bipolaris californica (Mackie & G.E. Paxton) Gornostaĭ (as “californicum”), in Azbukina et al. (eds), Vodorosli, Griby i Mkhi Dal'nego Vostoka (Algae, Fungi and Mosses of the Soviet Far-East) (Vladivostok): 80. 1978.
= Ophiobolus sativus S. Ito & Kurib., Trans. Sapporo Nat. Hist. Soc.10: 138. 1929. (fide Sivanesan 1987).
≡ Cochliobolus sativus (S. Ito & Kurib.) Drechsler ex Dastur, Indian J. Agric. Res. 12: 733. 1942.
= Drechslera multiformis Jooste, Bothalia 11: 511. 1975.
≡ Bipolaris multiformis (Jooste) Alcorn, Mycotaxon 17: 68. 1983.
Type material: Russia, iconotype designated here fig. 9 in Sorokin. (1890), Proc. Biol. Soc. Imp. Univ. Kazan 22: 21–32. (Helminthosporium sorokinianum) “MBT197973”. South Africa, Bloemfontein, on Tribulus terrestris, W.J. Jooste, PREM 44794 (Drechslera multiformis), holotype, ex-holotype culture CBS 480.74. USA, California, San Joaquin Co., Middle River, on Hordeum vulgare (as H. sativum) 28 May 1923, W.W. Mackie, BPI 428265 (Bipolaris californica), holotype.
Symptoms: Foliar spot blotch, root rot and black point on grains, head blight and seedling blight of wheat and barley. Infected seedlings develop dark brown necrotic lesions on roots, crowns, and lower leaf sheaths; infections develop as distinct oval to elongated light to dark brown blotches (modified from Kumar et al. 2002). Asexual morph on PDA: Conidiophores (52–)210–300(–310) × 6–8 μm (av. = 206, SD = 100, n = 30; av. = 7, SD = 1, n = 30), straight to flexuous, septate, sometimes geniculate at upper part, pale to mid brown arising singly or in small groups, simple or branched. Conidiogenous nodes smooth to verruculose, sometimes with a single terminal conidium on conidiophores. Conidia (31–)40–72(–100) × 15–25(–27) μm (av. = 56, SD = 16, n = 100; av. = 20, SD = 5, n = 100), curved or straight, obclavate, cylindrical, fusiform or broadly ellipsoidal, mostly tapering towards ends, dark olivaceous brown to golden brown, concolorous or slightly pale at ends, (3–)6(–12)-distoseptate. Hilum dark brown, 3–5 μm, inconspicuous or slightly protuberant, germination bipolar. Sexual morph on Sach's agar: Ascomata 340–370 × 370–530 μm, globose to ellipsoidal, dark brown or black. Ostiolar beak paraboloid to cylindrical, 90–150 × 80–110 μm, setose on upper surface. Pseudoparaphyses filiform, hyaline, septate, branched. Asci 110–225 × 32–45 μm, cylindrical to cylindric-clavate, short stalked, 1–8-spored, bitunicate, straight to slightly curved, rounded at apex. Ascospores 160–360 × 6–10 μm, hyaline, filiform or flagelliform, pointed towards ends, 6–14-septate, constricted at septum, closely coiled in a helix inside ascus, often surrounded by a hyaline, thin mucilaginous sheath.
Cultural characteristics: Colonies on PDA, velvety, grey, with an irregular or wavy margin. Sporulating abundantly after 1 wk. Reverse dark brown or black with concentric rings. Hyphae grey to brown, smooth or verruculose.
Hosts: Hordeum vulgare, Secale cereale, Tribulus terrestris, Zea mays (Poaceae) Also reported from: Aegilops cylindrica, Agropyron buonapartis, A. ciliare, A. cristatum, A. distichum, A. repens, A. trachycaulum var. trachycaulum, A. trachycaulum var. unilaterale, Agrostis capillaries, A. gigantea, A. palustris, Agrostis sp., A. stolonifera var. palustris, Alopecurus pratensis, Aneurolepidium chinense, Arrhenatherum elatius, Avena byzantina, A. sativa, Brachiaria plantaginea, Bromus inermis, B. japonicus, B. marginatus, B. uniloides, B. willdenowii, Buchloe dactyloides, Chloris virgata, Cynodon dactylon, C. transvaalensis, Dactylis glomerata, Dendrobium sp., Digitaria sanguinalis, Echinochloa crus-galli, Ehrharta calycina, Eleusine coracana, E. indica, Elymus breviaristatus, E. canadensis, E. riparius, E. sibiricus, E. trachycaulus, E. virginicus, Elytrigia intermedia, E. repens, Eragrostis cilianensis, Festuca arundinacea, F. ovina, F. pratensis, F. rubra, Glycine max, Holcus lanatus, Hordeum brevisubulatum, H. jubatum, H. leporinum, H. murinum, H. sativum, Hystrix patula, Leymus angustus, L. cinereus, Lolium multiflorum, L. perenne, Microlaena stipoides, Microstegium vimineum, Miscanthus sinensis var. zebrinus, Oryza sativa, Panicum dichotomiflorum, P. lacromanianum, P. virgatum, Paspalum notatum, Pennisetum clandestinum, Phalaris arundinacea, P. canariensis, Phleum pratense, Phleum sp., Poa annua, P. pratensis, P. sylvestris, P. trivialis, Psathyrostachys juncea, Roegneria hirsuta, Saccharum sp., Secale montanum, Setaria viridis, Sporobolus vaginiflorus, Stenotaphrum secundatum, Tribulus terrestris, Triticum aestivum, T. durum, T. secale, Triticum sp., T. sphaerococcum, T. vulgare, Zea mays, Zizania aquatica, Z. palustris (Poaceae). Also reported from non-Poaceae hosts: Allium sp. (Alliaceae), Helianthus annuus, Taraxacum kok-saghyz (Compositae), Calluna vulgaris (Ericaceae), Cicer arietinum, Lablab purpureus, Medicago sativa, Phaseolus vulgaris (Fabaceae), Linum usitatissimum (Linaceae), Lythrum salicaria (Lythraceae) Broussonetia papyrifera (Moraceae), Fagopyrum esculentum (Polygonaceae) (Farr & Rossman 2013).
Distribution: Canada, India, Italy, Japan, New Zealand, South Africa, USA (CO, IA, MN, OR, WV, WI). Also reported from Australia, Bhutan, Brazil, Cameroon, China, Costa Rica, Cyprus, Denmark, Egypt, Ethiopia, Israel, Nigeria, Nicaragua, Poland, UK, Yugoslavia, Zimbabwe (Farr & Rossman 2013).
Additional material examined: Canada, New Brunswick, Hartland, Field 164, on Hordeum vulgare, 17 Aug. 1937, I.L. Conners, BPI 430465; Ontario, Sudbury, on H. vulgare, 13 Aug. 1920, J.H. Faull, BPI 430467; Quebec, Lennoxville, on Hordeum sp., 8 Aug. 1939, I.L. Conners, BPI 626683A; ibid., BPI 626683B. India, Ratwara (Bihar), on H. vulgare, 16 Feb. 1969, R.A. Singh, BPI 430577. Italy, host unknown, Apr. 1924, L. Montemartini culture CBS 120.24. Japan, Hokkaidou, on Triticum aestivum, Jul. 1991, T. Aoki culture MAFF 236448; Hokkaidou, on Secale cereal, Jul. 2002, K. Kishi culture, MAFF 238877; Ibaraki, on Paddy field soil, Nov. 1990, T. Aoki, culture MAFF 235500; Ibaraki, on T. aestivum, May 1990, T. Aoki culture MAFF 235502; Okinawa, on T. aestivum, May 1991, T. Aoki culture MAFF 235501. New Zealand, Wanganui, Palmerston North, on Lolium perenne, 1 Mar. 1975, E.H.C. McKenzie, culture ICMP 6233. USA, on Phalaris arundinaceae, FIP 499; Colorado, Timnath, on H. vulgare, 29 Jun. 1923, C.D. Learn, BPI 430464; Illinois, Champaign Co., on H. vulgare, Jun. 1981, B. Jacobson, BPI 802093; Iowa, Ames, on seedlings of H. vulgare, Nov. 1909, A.G. Johnson, BPI 430463; Iowa, Clinton, on H. vulgare, 27 Jun. 1919, H.H. Plagge, BPI 430398A; ibid., BPI 430398B; Iowa, on Hordeum sp., Jun. 1914, A.L. Bakke, culture CBS 110.14; Minnesota, Calloway, on H. vulgare, 11 Aug. 1915, J.R. Holbert, BPI 430415; Oregon, Alsea Valley, on H. vulgare, Jun. 1932, S. Roderick, BPI 430462; Texas, Elsa, near Hidalgo Co., on H. vulgare, Feb. 1944, S.M. Pady, BPI 430468; West Virginia, Charleston, near Kanawha Co., on H. vulgare, 27 Oct. 1947, E.S. Elliott, BPI 430578; West Virginia, Greenbriar Co., on H. vulgare, 27 May 1937, C.R. Orton, BPI 430518; ibid., BPI 430517; Wisconsin, Madison, on H. vulgare, 1912, A.G. Johnson, BPI 430296; Wisconsin, Madison, on H. vulgare, 20 Oct. 1912, J. McMurphy, BPI 430469; ibid., BPI 430470.
Notes: Considerable morphological, physiological and genetic variation has been observed within B. sorkiniana (Christensen 1926, Misra 1979, Fetch & Steffenson 1994, Müller et al. 2005). Among 33 isolates of B. sorokiniana, Valjavec-Gratian & Steffenson (1997a,b) identified three pathotypes. A continuum of B. sorokiniana isolates differing in aggressiveness were found on Zea mays roots by Duveiller & Garcia-Altamirano (2000). Population-level genetic diversity within a pathogenic species is responsible for infection success and overcoming host resistance (Guseva et al. 1979, Müller et al. 2005).
Bipolaris sorokiniana infects small grain cereals and a wide range of grasses, although oats are less susceptible to infection (Zillinsky 1983, Farr & Rossman 2013). The mycotoxin prehelminthosporal is the most abundant and active compound known from B. sorokiniana whereas another mycotoxin, sorokinianin, was shown from a cultural filtrate to have inhibitory activity on seed germination (Nakajima et al. 1994).
Bipolaris multiformis was first described as Drechslera multiformis and is characterised by branched conidiophores, and a pigmented, verruculose area surrounding the hilum on the terminal conidia but the listed characters are also associated with B. sorokiniana. The conidiophore and conidial measurements in the type culture of B. multiformis overlap with those of B. sorokiniana. The combined gene and single gene analysis of ITS, GPDH and TEF markers of the ex-type of B. multiformis with an authentic culture of B. sorokiniana (CBS 120.24) suggests with great confidence that B. multiformis is a synonym of B. sorokiniana based on multigene phylogeny and morphological similarity.
Bipolaris stenospila (Drechsler) Shoemaker, Canad. J. Bot. 37: 884. 1959. Fig. 35.
Fig. 35.

Bipolaris stenospila (BPI 430476, BPI 430474, BPI 430476). A. Conidiophores and conidia on the host Saccharum officinarum. B, C. Conidiophores. D. Conidiophores. E–J. Conidia. Scale bars: A = 50 μm, B–J = 5 μm.
Basionym: Helminthosporium stenospilum Drechsler, Phytopathology 18: 136. 1928.
≡ Drechslera stenospila (Drechsler) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Type material: USA, Florida, Canal Point, on Saccharum officinarum, Dec. 1927, B.A. Bourne, deposited C. Drechsler, lectotype designated here BPI 430474 (Bipolaris stenospila) “MBT197974”; ibid., BPI 430493, paratype; on S. officinarum, 29 Jul. 1924, R.D. Rands, deposited by C. Drechsler, BPI 430481, paratype. Cuba, Central Cuba, on S. officinarum, 25 Mar. 1927, R.D. Rands, deposited by C. Drechsler, BPI 430479 (Helminthosporium stenospilum), paratype; ibid., BPI 430480, paratype.
Leaf spots on Saccharum sp.: Narrow brown stripes, brown linear streaks appearing first, later often becoming more extensive through enlargement or coalescence without conspicuous internal margins (modified from Drechsler 1928). Asexual morph on Saccharum officinarum: Conidiophores (90–)105–215(–255) × 6–8 μm (av. = 160, SD = 55, n = 15; av. = 7, SD = 1, n = 15), arising singly or in small groups, simple, septate, straight or flexuous, occasionally geniculate at upper part, usually with a swollen base, golden brown to dark brown. Conidiogenous nodes dark brown, distant. Conidia (43–)70–105(–120) × (9–)14–22 μm (av. = 86, SD = 17, n = 33; av. = 18, SD = 4, n = 30), curved, rarely straight, obclavate, dark olivaceous brown to golden brown, (3–)8(–12)-distoseptate. Hilum inconspicuous.
Hosts: On Saccharum officinarum (Poaceae). Also reported from: Brachiaria platyphylla, Cynodon dactylon, Imperata arundinacea, Pennisetum glaucum, Saccharum sp., Zea mays (Poaceae) (Farr & Rossman 2013).
Distribution: Cuba, Japan, Philippines, USA (FL, GA). Also reported from: Australia, Brazil, China, Cuba, Malaysia, South Africa, Thailand, Zambia (Drechsler 1928, Farr & Rossman 2013).
Additional material examined: Cuba, Central Baragua, on Saccharum officinarum, 11 Oct. 1930, J. A. Faris, BPI 430487. Japan, Kyoto, on undetermined substrate, 1977, T. Mitsuya, BPI 626686. Philippines, Manapla & Cadiz, Negros Occidental, on S. officinarum, 3 Jul. 1929, W. Dwight, BPI 430490. USA, Florida, Belle glade, on S. officinarum, 12 Nov. 1940, T. Bregger, BPI 430491; Florida, Belle Glade, on S. officinarum, 9 Apr. 1930, R.D. Rands, BPI 430486; Florida, Fort Pierce, on S. officinarum, 24 Aug. 1962, E.H. Todd, BPI 430471; ibid., BPI 430472; ibid., 2 Feb. 1962, BPI 430475; Florida, Lake Okeechobee, Ritta Plantation, on S. officinarum, 6 Jul. 1962, B.A. Bourne, BPI 430473; ibid., 15 Aug. 1962, BPI 430476; Florida, Canal Point, on S. officinarum, 26 Mar. 1959, E.H. Todd, BPI 430477; ibid., Feb. 1959, BPI 430478; Florida, on S. officinarum, Oct. 1931, R.D. Rands, BPI 430483; Florida, Felsmere, on S. officinarum, Nov. 1932, R.D. Rands, BPI 430484; ibid., 30 Jul. 1931, BPI 430485; Georgia, Cairo, on S. officinarum, 15 Sep. 1920, E.W. Brandes BPI 430494; Georgia, Cairo on S. officinarum, 9 Nov. 1930, R.D. Rand, BPI 430488; ibid., Oct. 1930, BPI 430489; Hawaii, Waialua, Oahu, on S. officinarum, D.W. Pierce, BPI 430482; ibid., BPI 430492. Unknown, on undetermined substrate, 2 Oct. 1962, J.A. Stevenson, BPI 626685 = ATCC 13447; ibid., BPI 626687.
Notes: According to the protologue, Helminthosporium stenospilum was found on Saccharum from Florida, Georgia and Cuba. No type specimen was designated. Based on examination of all the specimens listed in the protologue, we have chosen one of the specimens in BPI in good condition with disease symptoms to serve as the lectotype; the other specimens are considered paratypes. Bipolaris sacchari is commonly found associated with Sugar cane. This pathogen causes small, red spots that develop parallel to the mid-vein, appearing as “eye spots” with a straw-coloured centre and red halo. Bipolaris stenospila causes brown stripes with different symptoms on sugarcane. Conidia of B. stenospila are much darker and wider than the B. sacchari.
Matsumoto & Yamamoto (1936) introduced Cochliobolus stenospilus as the sexual morph of this species. There is an illustration in Matsumoto & Yamamoto (1936), but the species description is only in Japanese therefore it is not considered to be validly published.
Bipolaris triticicola Sivan., Trans. Brit. Mycol. Soc. 84: 410. 1985. Fig. 36.
Fig. 36.

Bipolaris triticicola (IMI 167363). A, B. Conidiophores. C–G. Conidia. Scale bars: A = 10 μm, B–G = 5 μm.
Type material: Nigeria, on Triticum sp., 8 Jul. 1972, O.H. Giha 1418, IMI 167363, holotype.
Asexual morph on Triticum sp.: Hyphae hyaline, septate, branched, smooth. Conidiophores (50–)87–270(–300) × (8–)10–12(–14) μm (av. = 177, SD = 90, n = 20; av.= 11, SD = 1, n = 20), arising singly or sometimes in small groups, branched, septate, straight or flexuous, geniculate at upper part, smooth, base swollen, brown to dark brown. Conidiogenous nodes dark brown. Conidia (53–)70–120(–180) × 18–22 μm (av. = 90, SD = 28, n = 50; av. = 20, SD = 2, n = 50), straight or curved, obclavate or ellipsoidal, distoseptate, pale brown to golden dark brown, sometimes olivaceous brown, end cells paler, (8–)9(–13)-distoseptate. Hilum truncate, slightly protruding, germinating from both ends. Conidial germination as described in Sivanesan (1987), first septum median, second septum delimits basal cell and third septum distal.
Cultural characters: Colonies grey to dark brown, velvety, effuse.
Hosts: Triticum sp. (Poaceae). Also reported from: Eleusine coracana (Poaceae). Also reported from non-Poaceae host: Musa sp. (Musaceae) (Sivanesan 1985, 1987).
Distribution: Nigeria. Also reported from: India, West Indies (Farr & Rossman 2013).
Notes: This species was described by Sivanesan (1985) on Triticum sp., who also reported that it occurred on Eleusine coracana and Musa sp. Since then, it has not been recorded and molecular data are not available. According to the conidial morphology and hilum structure, this species is accepted in the genus Bipolaris despite a lack of molecular data. A sexual morph is not recorded in association with this species.
Bipolaris urochloae (K.M. Putterill) Shoemaker, Canad. J. Bot. 37: 885. 1959.
Basionym: Helminthosporium urochloae K.M. Putterill, Bothalia 6: 366. 1954.
≡ Drechslera urochloae (K.M. Putterill) Subram. & B.L. Jain, Curr. Sci. 35: 355. 1966.
Type material: UK, Baberton, on Urochloa panicoides, Mar. 1932, V.A. Wager 26148, IMI 38028, holotype.
Leaf spots on Urochloa sp.: Brownish black irregular lesions with pale brown centre, 2 × 6 mm (Sivanesan 1987). Asexual morph on PDA: Conidiophores up to 300 μm long and 7–10 μm thick, pale to mid brown, single or in small groups, straight or flexuous, septate, smooth, sometimes with a swollen base. Conidiogenous nodes verrucose. Conidia 80–190 × 15–22 μm, mid to dark olivaceous brown, straight, slightly curved, flexuous, broadly fusoid to obclavate, 8–16-distoseptate. Septum ontogeny primary septum median or towards base.
Cultural characteristics: Colonies on PDA, greyish brown, effuse.
Hosts: Urochloa panicoides (Poaceae). Also reported from: Melinis minutiflora, Panicum laevifolium, P. maximum, Pennisetum americanum, P. glaucum, P. typhoides, Triticum aestivum, Urochloa helopus, U. mosambicensis, U. trichopus, Zea sp. (Poaceae). Also reported from non-Poaceae host: Dendrobium (Orchidaceae) (Sivanesan 1987, Farr & Rossman 2013).
Distribution: Australia, UK. Also reported from: Brazil, Ethiopia, India, Pakistan, South Africa, USA (HI), Zimbabwe.
Additional material examined: Australia, on Urochloa panicoides, J.L. Alcorn, culture ATCC 58317.
Notes: The species is characterised by larger conidia with up to 16 septa. In the phylogenetic analysis this species clusters as a distinct species.
Bipolaris victoriae (F. Meehan & H.C. Murphy) Shoemaker, Canad. J. Bot. 37: 882. 1959. Fig. 37.
Fig. 37.

Bipolaris victoriae (BPI 431485). A. Conidiophores and conidia on the host Avena sativa. B. Conidiophores and conidia. C–H. Conidiophores. I–Q. Conidia. Scale bars: A = 200 μm, B = 10 μm, C–Q = 5 μm.
Basionym: Helminthosporium victoriae F. Meehan & H.C. Murphy, Science, N.Y. 104: 413. 1946.
≡ Helminthosporium sativum var. victoriae (F. Meehan & H.C. Murphy) H.R. Rosen, Wiser & J.O. York, Beitr. Bau. Flecht. 533: 22. 1953. (fide Sivanesan 1987).
≡ Drechslera victoriae (F. Meehan & H.C. Murphy) Subram. & B.L. Jain, Curr. Sci. 35: 355. 1966.
= Cochliobolus victoriae R.R. Nelson, Phytopathology 50: 775. 1960. (fide Nelson 1960b).
Type material: USA, Iowa, Ames, Iowa Agricultural Experiment, on Avena sativa (cv. Boone), 25 Jul. 1946, M. Frances, BPI 431485 (Bipolaris victoriae), holotype; ibid., BPI 431486, isotype; on Avena sativa, Sep. 1964, R.R. Nelson, epitype designated here CBS H-12278 “MBT197975”; ibid., ex-type culture CBS 327.64; paired culture on Zea mays, R.R. Nelson, BPI (specimen cannot be located) (Cochliobolus victoriae), holotype; ibid., K, isotype, not seen.
Disease symptoms on Avena sp.: Infected seedlings necrotic at base, leaves striping or reddening, progressing upwards. Same symptoms observed on mature plants. Leaves and seedlings killed. Necrosis present at nodes and lower stems, roots rot, and stems break (modified from Meehan 1949, Sivanesan 1987). Asexual morph on PDA: Conidiophores (70–)100–250 × 6–10 μm (av. = 175, SD = 75, n = 20; av. = 8, SD = 6, n = 20), arising singly or in groups of few conidia, simple, septate, straight or flexuous, sometimes geniculate at upper part, smooth, pale to mid brown. Conidiogenous nodes dark brown, slightly verruculose, distinct. Conidia (25–)55–90(–110) × (10–)12–16(–19) μm (av. = 72, SD = 18, n = 66; av. = 14, SD = 2, n = 66), smooth, straight or curved, broadly fusiform or obclavate fusiform, widest near centre, tapering towards rounded ends, pale to mid brown, (4–)7(–11)-distoseptate. Hilum slightly protuberant, single germ tubes arising from each end. Sexual morph on Sach's agar: Ascomata 225–430 × 210–370 μm, black, ellipsoidal to globose. Setae brown produced over upper third of ascomata, conidiophores and conidia frequently developing on ascomata. Ostiolar beak 30–170 μm long, sub-conical to paraboloid, with a mass of hyaline cells frequently covering apex of beak. Pseudoparaphyses filamentous, hyaline. Asci 98–207 × 20–39 μm, arising from base of locule, developing among pseudoparaphyses, cylindrical to clavate, straight or slightly curved, with a short stipe, vestigial bitunicate, with 1–8 ascospores tightly coiled in a helix. Ascospores 147–302 × 6–13 μm filiform or flagelliform, somewhat tapered at extremities, hyaline, 5–9-septate after discharge, covered with a mucilaginous sheath, germinating from sides or ends (modified from Nelson 1960b).
Cultural characteristics: Colonies on PDA white and become pale greyish with maturity, rhizoid, effuse, velvety.
Hosts: Avena sativa. Also reported from: Agropyron cristatum, Agropyron sp., Chenopodium sp., Commelina benghalensis, Cymbopogon flexuosus, Dactylis glomerata, Digitaria ciliaris, Eleusine sp., Hordeum vulgare, Oryza sativa, Panicum maximum, Paspalum notatum, P. scrobiculatum, Phalaris arundinacea, Phleum pretense, Setaria sphacelata, S. verticillata, S. viridis, Setaria sp., Sorghum vulgare, Triticum sp., Zea mays (Poaceae) (Farr & Rossman 2013).
Distribution: Canada, USA (IA, LA). Also reported from: Australia, Brazil, India, Kenya, UK, Zambia, Zimbabwe (Farr & Rossman 2013).
Additional specimens examined: Canada, Division of Forage Plants, Ottawa, Ontario, on Avena sativa, 11 Aug. 1947, R.J. Baylis, BPI 431481. USA, Florida, Quincy, on A. sativa, May 1937, T.R. Stanton & H.C. Murphy, BPI 431476; ibid., H.C. Murphy, BPI 431477; Florida, Belle Glade Experiment Station, Everglades, on A. sativa, May 1947, C.C. Seale, BPI 431484; ibid., A.G. Johnson, BPI 431479; Mississippi, Stoneville, on A. sativa, 23 May 1947, J. Neely, BPI 431480; South Carolina, Florence, on A. sativa, 19 May 1944, A.G. Johnson, BPI 431478; South Carolina, Hartsville, on A. sativa, 5 May 1947, H.H. McKikeny, BPI 431483; West Virginia, Morgantown, West Virginia University Agronomy Farm, on A. sativa, 15 Jun. 1953, E.S. Elliott, BPI 431482.
Notes: An epitype is designated for B. victoriae using an authentic culture collected by R.R. Nelson from the original host and location where the species was first described. The species was first reported as Helminthosporium victoriae by Meehan & Murphy (1946) as the cause of “Victoria blight” of oats. Bipolaris victoriae produces a host-selective toxin victorin, also known as HV-toxin. The structures of victorin B, C, D, E and another toxin produced by B. victoriae called “victoricine” have been determined (Wolpert et al. 1988). Bipolaris victoriae is phylogenetically closely related to B. microstegii and B. zeicola (Fig. 1). The interspecies fertility between B. victoriae and B. zeicola has previously been reported (Alcorn 1988). These two species cause destructive diseases on oat and maize, respectively. A genetic study done by Christiansen et al. (1998) using 44 isolates of B. victoriae determined that all of them contain only the MAT-2 locus, whereas B. zeicola isolates contain either one or both MAT-1 and MAT-2.
Bipolaris yamadai (Y. Nisik.) Shoemaker, Canad. J. Bot. 37: 884. 1959. Fig. 38.
Fig. 38.
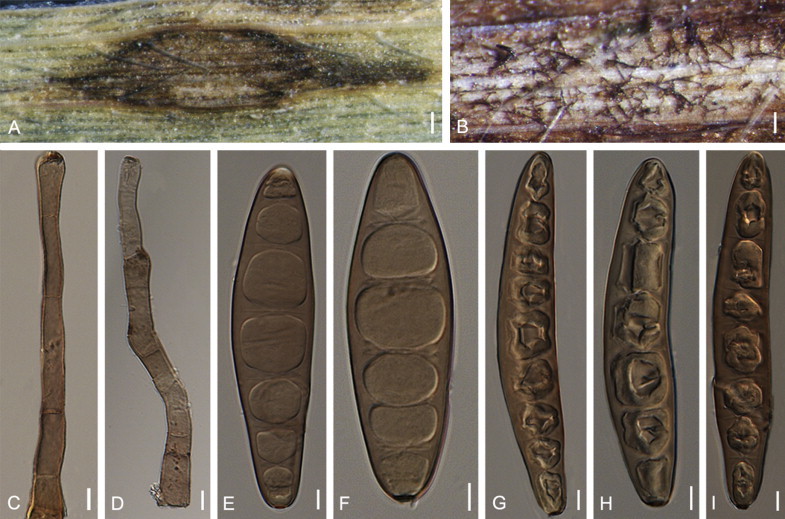
Bipolaris yamadae (BPI 431524). A. Leaf spots on Panicum capillare. B. Conidiophores and conidia on the leaf spot. C, D. Conidiophores. E–I. Conidia. Scale bars: A, B = 500 μm, C–I = 5 μm.
Basionym: Helminthosporium yamadai Y. Nisik., Rept. Ohara. Inst. Agr. Research 4:120. 1928.
≡ Drechslera yamadai (Y. Nisik.) Subram. & B.L. Jain, Curr. Sci. 35: 355. 1966.
Type material: Japan, on Panicum miliaceum, iconotype designated here Y. Nisikado (1928a), Rept. Ohara. Inst. Agr. Research 4: Plate XIII fig. 1 J.A. Stevenson Mycology Library, USDA-ARS, Beltsville, Maryland, USA (Helminthosporium yamadae) “MBT197976”; on Panicum miliaceum, deposited Nov. 1929, Y. Nisikado No. 273, epitype designated here CBS H-7221 (Bipolaris yamadae) “MBT197977”, ex-epitype culture CBS 202.29.
Leaf spots on Panicum sp.: ovoid, oblong, pale brown at margin and pale brown at centre, with an irregular concentric zone. Asexual morph on Panicum capillare: Conidiophores 40–165(–210) × 7–9 μm (av. = 102, SD = 63, n = 21; av. = 8, SD = 1, n = 21), arising from stromata or epidermal cells, arising singly or in groups of 3–4, straight, septate, straight or sometimes geniculate at upper part, smooth walled, olive brown to pale brown. Conidiogenous node slightly swollen, dark brown, sometimes slightly verruculose, basal cells slightly swollen. Conidia (60–)65–100(–120) × (12–)14–18 μm (av. = 84, SD = 17, n = 30; av. = 16, SD = 2, n = 30), smooth, straight or curved, cylindrical, fusiform, obclavate, sometimes obovoid, with rounded ends, pale brown to olive brown, (3–)7(–11)-distoseptate, Germination at both ends. Hilum 3–4 μm, non protuberant.
Hosts: Panicum capillare, P. miliaceum. Also reported from: Oryza sp., Panicum implicatum, P. maximum, Saccharum sp., Setaria plicata (Poaceae) (Farr & Rossman 2013).
Distribution: Cuba, Japan, USA (IA, ID, ND, WI). Also reported from: China, India (Farr & Rossman 2013).
Additional material examined: USA, Idaho, Pollock, on Panicum capillare, 21 Aug. 1941, G.W. Fischer, BPI 431529; Iowa, Ames, on P. capillare, 16 Aug. 1895, R. B. Carleton, BPI 431526; North Dakota, near Leonard, on P. capillare, 31 Aug. 1940, R. Sprague, BPI 431527; ibid., BPI 431528; Wisconsin, Waukesha Co., Eagleville, on P. capillare, 1 Sep. 1942, H.C. Greene, BPI 431524; Wisconsin, near Dane Co., Verona, on P. capillare, 25 Sep. 1962, H.C. Greene 2826, BPI 431525.
Notes: The specimen CBS H-7221 is designated here as an epitype of Helminthosporium yamadai, with an ex-epitype culture based on a collection deposited by Y. Nisikado in November 1929. Although this collection was made by the same author who described the species, a definitive date for this collection is unknown. These specimens were not mentioned in the protologue; therefore, an iconotype is used to interpret this taxon. Bipolaris maydis and B. heliconiae are closely related to B. yamadae in the phylogenetic analysis of the combined genes (Fig. 1). A sexual morph is not recorded in association with this species.
Bipolaris zeae Sivan., Trans. Brit. Mycol. Soc. 84: 418. 1985. Fig. 39.
Fig. 39.

Bipolaris zeae (IMI 202085, AR 3795). A. Conidiophore. B–F. Conidia. G. Conidia and conidiophores on a sterilised toothpick. H–J. Conidiophores. K–M. Conidia. Scale bars: A–F, H–M = 5 μm, G = 200 μm.
= Cochliobolus zeae H.S. Chang, Bot. Bull. Acad. Sin., Taipei 33: 175. 1992. (fide Chang 1992).
Type material: Australia, on Zea mays, 19 Jan. 1975, P.E. Mayers 20425b, IMI 202085 (Bipolaris zeae), holotype. Taiwan, on Pennisetum clandestinum, 14 Aug. 1987, H.S. Chang, by pairing cultures ASIB 02; ibid., IMI 350958, isotype, not seen.
Asexual morph on PDA: Conidiophores (80–)100–350(–370) × 6–8 μm (av. = 225, SD = 125, n = 30; av. = 7, SD = 1, n = 30), usually arising singly or sometimes in small groups, branched, septate, cylindrical, straight or flexuous, geniculate at upper part, smooth, dark brown. Conidiogenous nodes dark brown, surface smooth to slightly roughened. Conidia (30–)40–80(–120) × 12–18(–21) μm (av. = 59, SD = 20, n = 100; av. = 15, SD = 3, n = 100), straight to slightly curved, smooth, obclavate to fusiform, hyaline to olivaceous brown when immature and brown to reddish brown when mature, end cells normally paler than middle cells, sometimes on PDA “Y” shaped conidia develop, (6–)9(–12)-distoseptate, end cells often cut off by a thick dark septum. Hilum truncate, slightly protruding, 3–5 μm. Septum ontogeny first septum median, second delimits basal cells and third delimits distal cell. Sexual morph on Sach's agar: Ascomata 390–510 × 340–440 μm, globose, black with a well-defined ostiolar neck. Asci 150–210 × 18–20 μm, thin-walled, cylindrical to broadly clavate, sessile or with a pedicel, 4–8 ascospores helically packed in an ascus. Ascospores 260 × 6–8 μm, filiform, hyaline, 6–8-septate. Pseudoparaphyses hyaline, filiform, septate.
Cultural characteristics: Colonies on PDA dark brown to black, velvety, effuse, zonate, sporulating at black outside margin. Hyphae hyaline to pale brown, septate, branched, smooth to verruculose, 5–7 μm wide.
Hosts: Panicum virgatum, Pennisetum clandestinum, Sorghum bicolor, Triticum vulgare, Zea mays (Poaceae). Also reported from: Alloteropsis semialata, Brachiaria decumbens, Cenchrus ciliaris, Cynodon dactylon, Dactylis sp., Imperata cylindrica var. major, Paspalum sp., Pennisetum americanum, P. glaucum, P. typhoides, Sorghum halepense. Also reported from non-Poaceae host: Acer truncatum (Sapindaceae).
Distribution: Australia, Canada, Japan, Taiwan, USA (ND). Also reported from: Argentina, Brazil, China, Colombia, India, Iran (Farr & Rossman 2013).
Additional material examined: Canada, Ontario, on Triticum aestivum, 1989, R.M. Clear, DAOM 211267. Japan, Tochigi, on Sorghum bicolor, N. Nishihara, culture AR 5181. USA, North Dakota, on Panicum virgatum, 1999, J. Krupinsky, dried culture specimen BPI 842260, culture AR 3795.
Notes: Although occurring on a broad range of grass hosts, Bipolaris zeae is not known to be a serious pathogen. It shows a close relationship to B. microstegii, B. victoriae, and B. zeicola in the phylogenetic tree (Fig. 1). The sexual morph was obtained by pairing B. zeae isolates from Pennisetum clandestinum in Taiwan (Chang 1992). In the present study, we report B. zeae on Sorghum bicolor from Japan for the first time.
Bipolaris zeicola (G.L. Stout) Shoemaker, Canad. J. Bot. 37: 885. 1959. Fig. 40.
Fig. 40.

Bipolaris zeicola (BPI 626376, BPI 626668, FIP 532). A, B. Ascomata. C–E. Asci. F. Ascospores. G. Conidiophores and conidia on Zea mays. H–J. Conidiophores and conidia. I–M. Conidia. Scale bars: A = 100 μm, B = 50 μm, C–F = 20 μm, G = 100 μm, H–n = 5 μm.
Basionym: Helminthosporium zeicola G.L. Stout, Mycologia 22: 273. 1930.
≡ Drechslera zeicola (G.L. Stout) Subram. & B.L. Jain, Curr. Sci. 35: 355. 1966.
= Helminthosporium carbonum Ullstrup, Phytopathology 34: 219. 1944. (fide Shoemaker 1959).
≡ Drechslera carbonum (Ullstrup) Sivan., Bitunicate Ascomycetes and their Anamorphs (Vaduz): 369. 1984.
= Cochliobolus carbonum R.R. Nelson, Phytopathology 49: 809. 1959. (fide Sivanesan 1987).
Type material: USA, Illinos, Dixon Lee Co., on Zea mays, 26 Sep. 1926, Nat. Hist. Surv. Acc. No 19884 (Bipolaris zeicola), holotype; Ohio, on leaf of Z. mays, R. Hite, epitype designated here BPI 892947 (Bipolaris zeicola) “MBT198049”; ibid., ex-epitype culture FIP 532; culture grown on Z. mays, May 1959, BPI 626376 (Cochliobolus carbornum), holotype; Indiana, swaddling on culms of Z. mays, Aug. 1958, A.J. Ullstrup, BPI 626668 (Helminthosporium carbornum), holotype.
Leaf spots on Zea mays: Oval to circular or sometimes irregular, straw-coloured or chocolate brown spots with light to purple margin. Asexual morph on PDA: Conidiophores (80–)100–240(–270) × 6–8 μm (av. = 170, SD = 70, n = 20; av. = 7, SD = 1, n = 20), arising singly or in small groups, usually simple, occasionally branched, septate, straight or flexuous, geniculate at upper part. Conidiogenous nodes verruculose, mid brown to dark brown. Conidia dark brown to dark reddish brown, concolorous or end cells paler than middle cells. Conidia (45–)65–90(–105) × (10–)15–19(–22) μm (av. = 76, SD = 12, n = 102; av. = 17, SD = 2, n = 102), usually curved, sometimes straight, ellipsoid, widest at middle, tapering towards rounded ends, (6–)7(–12)-distoseptate. Hilum inconspicuous. Sexual morph on Sach's agar: Ascomata (302–)340–500(–550) × (233–)290–485(–500) μm (av. = 420, SD = 80, n = 15; av. = 388, SD = 96, n = 15), black, globose, elliptical, setae on upper part, wall mixed with conidia and conidiophores. Ostiolar beak 50–200 μm long (av. = 138, n = 10), well defined, sub-conical. Pseudoparaphyses filiform, hyaline, septate, branched. Asci (135–)145–200(–255) × (15–)17–19(–22) μm (av. = 172, SD = 26, n = 30; av. = 18, SD = 1, n = 30), cylindrical to clavate, bitunicate, short stalked, straight or slightly curved, 1–8-spored. Ascospores 150–300 × 6–9 μm (av. = 9), hyaline, filiform or flagelliform, tapering towards ends, distinctly coiled inside ascus, 5–9-septate.
Cultural characteristics: Colonies on PDA, whitish grey when young, becoming blackish grey when mature, effuse, velvety, entire or irregular margin. Hyphae hyaline to dark brown, septate, branched.
Hosts: Bouteloua curtipendula, Eragrostis cilianensis, Zea mays. Also reported from: Arundo donax, Brachiaria foliosa, Chloris gayana, C. verticillata, Cynodon dactylon (Poaceae). Also reported from non-Poaceae host: Coffea arabica (Rubiaceae) (Farr & Rossman 2013).
Distribution: USA (IL, OH, VA). Also reported from: Australia, Brazil, Canada, China, Denmark, Egypt, Japan, New Zealand, Zimbabwe (Farr & Rossman 2013).
Additional material examined: Japan, Kyoto, substrate undetermined, 1977, T. Mitsuya, BPI 626667. USA, Virginia, Montgomery Co., Blacksburg, on Arundo donax, 7 Oct. 2007, C.W. Roane, BPI 880376B; Virginia, Montgomery Co., side of N.S. Rwy off Rt. 660 near underpass, on Bouteloua curtipendula, 8 Sep. 2005, C.W. Roane, BPI 880317C; Virginia, Montgomery Co., Blacksburg. Roane garden, on Eragrostis cilianensis, 11 Sep. 2006, C.W. Roane, BPI 880335C; Nebraska, on Sorghum sp., D. Funnell-Harris, culture AR 5166; ibid., culture AR 5168.
Notes: Bipolaris victoriae is epitypified here from Zea mays in USA, the host from which this species was originally isolated. Three pathogenic races of this fungus were identified according to the symptoms produced on maize. Among the pathogenic races, Race 1 produces HC toxin and causes severe damage on maize leaves whereas Race 2 does not produce HC toxins. Race 3 also causes severe damage on rice as well as maize (Xiao et al. 1992). Race 3 is known to produce a complex of toxins designated as BZR-toxin. BZR-toxins exhibited a rice-specific phytotoxicity causing leaf chlorosis (Xiao et al. 1992). The genes responsible for host-specific toxins have been widely studied in B. zeicola. The production of host-selective Helminthosporium carbonum toxin (HC-toxin) is controlled by a single gene locus Tox2 (Bronson 1991). An epitype culture for Cochliobolus carbornum was proposed by A.J. Ullstrup and it is deposited as DAOM 600061; however, our sequence data revealed that the culture is an Alternaria sp. It could probably be contaminated and therefore is not useful to represent the taxon.
| A key to species in the genus Bipolaris | ||
| (1) | Conidia slightly echinulate | B. hadrotrichoides |
| Conidia smooth or slightly verruculose | 2 | |
| (2) | Conidia often or sometimes rostrate | 3 |
| Conidia fusoid, ellipsoidal, obclavate, ellipsoidal obclavate or cylindrical | 4 | |
| (3) | Conidia 10–11 μm at the widest part, 2–7-distoseptate | B. eragrostiellae |
| Conidia 14–18 μm at the widest part, 7–10-distoseptate | B. gossypina | |
| (4) | End cells sometimes swollen to produce a thin, globose vesicle where germ tubes originate | 5 |
| Germ tubes originating from both or one end cells without forming a vesicle | 6 | |
| (5) | Conidia hyaline when immature, turning olivaceous green, then brown or golden brown when mature, 40–80 × 12–18 μm | B. cynodontis |
| Conidia pale brown to dark brown, 65–105 × 15–20 μm | B. leersiae | |
| (6) | Conidia less than 100 μm long | 7 |
| Conidia longer than 100 μm | 8 | |
| (7) | Septa equal or less than 5 | B. obclavata |
| Septa usually more than 5 | 9 | |
| (8) | Conidia equal or greater than 150 μm | 20 |
| Conidia less than 150 μm | 21 | |
| (9) | Sexual morph produced on Sach’s agar medium + rice/wheat straw; conidia hyaline, pale brown or reddish brown | 18 |
| Sexual morph not reported; conidia olivaceous brown to dark golden-brown, greyish brown or brown | 10 | |
| (10) | Producing secondary conidiophores in culture | 11 |
| Producing only primary conidiophores in culture | 12 | |
| (11) | Conidiophores up to 750 μm long, 7–9-distoseptate | B. microstegii |
| Conidiophores 690 μm or less, 3–7-distoseptate | B. cookei | |
| (12) | Conidia up to 60 μm long | 13 |
| Conidia 60 μm or longer | 14 | |
| (13) | Conidia less or equal to 40 μm long | B. colocasiae |
| Conidia longer than 40 μm | 15 | |
| (14) | Conidiophores up to 300 μm long | 16 |
| Conidiophores longer than 300 μm | B. salkadehensis | |
| (15) | Conidia 35–50 × 10–14 μm, up to 6-distoseptate; causing leaf spots on Muhlenbergia wrightii | B. arizonica |
| Conidia 50–60 × 13–15 μm, up to 7-distoseptate; on Euchlaenae mexicana | B. euchlaenae | |
| (16) | Conidiophores branched, arranged in dense groups | B. eragrostidis |
| Conidiophores arising singly or in small groups | 17 | |
| (17) | Conidia 55–90 × 12–14 μm, usually curved, 5–9-distoseptate | B. sacchari |
| Conidia 35–50 × 14–20 μm, usually straight, 4–7-septate | B. coffeana | |
| (18) | Conidia concolorous | 19 |
| In mature conidia end cells paler than the middle cells | B. bicolor | |
| (19) | Conidia 65–90 μm long and 13–20 μm wide | B. chloridis |
| Conidia 60–75 μm long and 14–16 μm wide | B. peregianensis | |
| (20) | Conidia distinctly curved, often C-shaped, occasionally horseshoe-shaped, more than 250 μm long | B. pluriseptata |
| Conidia straight or curved, less than 250 μm long | 22 | |
| (21) | Conidia wider or equal to 21 μm | 28 |
| Conidial length less than 21 μm | 29 | |
| (22) | Conidia usually greater or equal to 20 μm wide | 23 |
| Conidia less than 20 μm wide | B. salviniae | |
| (23) | Conidiophores longer than 350 μm | 24 |
| Conidiophores shorter than 350 μm | 25 | |
| (24) | Reported on Poaceae | 26 |
| Reported on non-poaceous hosts | 27 | |
| (25) | Conidia 80–150 × 15–19 μm, pale brown to dark olivaceous brown; sexual morph can be formed on WA + wheat straw | B. microlaenae |
| Conidiophores 70–120 × 18–22 μm, brown to dark brown; sexual morph not formed | B. triticicola | |
| (26) | Conidia 68–108 × 14–20 μm, brown when mature | B. oryzae |
| Conidia 90–140 × 16–20 μm, olivaceous green when mature | B. panici-miliacei | |
| (27) | Conidia 100–150 μm long, 8–13-distoseptate | B. incurvata |
| Conidia 65–150 μm long, 7–10-distoseptate | B. heliconiae | |
| (28) | Conidia end cells usually paler than the middle cells | 39 |
| Conidia end cells concolorous | 40 | |
| (29) | Conidia reddish brown | 30 |
| Conidia pale brown, olivaceous brown, golden brown | 31 | |
| (30) | Conidia clavate to fusoid | B. clavata |
| Conidia ellipsoid | B. zeicola | |
| (31) | On Euphorbiaceae | B. euphorbiae |
| On Poaceae | 32 | |
| (32) | Conidial end cells paler than the middle cells | 33 |
| Conidia concolorous | 34 | |
| (33) | Conidiophores up to 300 μm long, conidia pale, mid olivaceous or golden olivaceous | 35 |
| Conidiophores up to 150 μm long, yellowish brown to dark olivaceous brown | 36 | |
| (34) | Conidiophores more than 350 μm long | B. maydis |
| Conidiophores less than 350 μm long | 37 | |
| (35) | Conidia ellipsoidal, obclavate, to obclavate-ellipsoidal | B. drechsleri |
| Conidia fusoid to obclavate fusoid | 46 | |
| (36) | Conidia 70–110 × 9.5–17.5 μm | B. poae-pratensis |
| Conidia 40–108 × 13–18 μm | B. mediocris | |
| (37) | Conidiophores verrucose | 38 |
| Conidiophores smooth | B. setariae | |
| (38) | Conidia 55–90 × 12–16 μm; sexual morph observed on Sach’s agar + rice/wheat straw | B. victoriae |
| Conidia 65–100 × 14–18 μm | B. yamadae | |
| (39) | On Costaceae; conidia 70–105 × 14–22 μm, 7–10-septate | B. costina |
| On Poaceae; conidia (30–) 40–80 (–120) × 12–18(–21) μm, 6–12-septate | B. zeae | |
| (40) | Conidial width greater than or equal to 25 μm | 41 |
| Conidial width less than 25 μm | 42 | |
| (41) | Conidiophores longer than or equal to 250 μm | 43 |
| Conidiophores usually less than 250 μm long | B. musae-sapientium | |
| (42) | On Hevea brasiliensis | B. heveae |
| On Poaceae | 44 | |
| (43) | On Croton sp.; conidia (51–)60–110(–138) × (14–)20–25(–32) μm | B. crotonis |
| On Poaceae; conidia (31–)40–72(–100) × 15–25(–27) μm | B. sorokiniana | |
| (44) | Conidia usually curved, rarely straight, (43–)70–105(–120) × (9–)14–22 μm | B. stenospila |
| Conidia straight or curved | 45 | |
| (45) | Conidia 80–190 × 15–22 μm, 8–16-distoseptate | B. urochloae |
| Conidia (41–)60–110(–138) × (9–)13–19(–24) μm, 4–12-distoseptate | B. halepensis | |
| (46) | 4–9-distoseptate (mostly 8) | B. secalis |
| 8–13-distoseptate (mostly 10) | B. luttrellii | |
Excluded species and novel combinations
Johnalcornia aberrans (Alcorn) Y.P. Tan & R.G. Shivas, Australas. Pl. Pathol. 2014 (in press).
Basionym: Bipolaris aberrans Alcorn, Mycotaxon 39: 364. 1990.
= Cochliobolus aberrans Alcorn, Mycotaxon 39: 362. 1990.
Type material: Australia, Queensland, on Eragrostis paviflora, 22 Mar. 1988, J.L. Alcorn, BRIP 16281 (Bipolaris aberrans), holotype, not seen; ibid., IMI 335210 (Bipolaris aberrans), isotype; ibid., ex-isotype culture CBS 510.91.
Notes: In the single-gene analysis of the ITS, GPDH and TEF loci, the ex-isotype culture CBS 510.91 of B. aberrans clustered separately from other species of Bipolaris and Curvularia. Recently, a monotypic genus, Johnalcornia, was introduced to accommodate this species (Tan et al. 2014). Johnalcornia differs from Bipolaris and Curvularia in that the second conidial septum forms distally, delimiting the apical cell. In Bipolaris and Curvularia, the second conidial septum delimits the basal cell (Alcorn 1990).
Curvularia australis (Alcorn) Y.P. Tan & R.G. Shivas, Australas. Pl. Pathol. 2014. (in press)
≡ Bipolaris australis Alcorn, Mycotaxon 15: 38. 1982.
Type material: Australia, Queensland, on Sporobolus caroli, 12 May 1977, J.L. Alcorn 77134, BRIP 12521, holotype, not seen; ibid., IMI 261917, isotype; ibid., DAOM 38000, isotype; on S. mitchellii, 12 May 1977, J.L. Alcorn 77139, BRIP 12525, paratype.
Notes: The phylogenetic sequence analysis of the ex-holotype culture (Tan et al. 2014) and an ex-paratype culture (Berbee et al. 1999) has shown that this species groups in Curvularia (Fig. 2).
Drechslera brizae (Y. Nisik.) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Basionym: Helminthosporium brizae Y. Nisik., Rept. Ohara. Inst. Agr. Research 4: 121. 1928.
≡ Bipolaris brizae (Y. Nisik.) Shoemaker, Canad. J. Bot. 37: 882. 1959.
Type material: Japan, Okayama, on Briza minor, iconotype designated here Spec. Rept. Ohara. Inst. Agr. Research 4: Plate XV, fig. 2, J.A. Stevenson Mycology Library, USDA-ARS, Beltsville, Maryland, USA “MBT197979”; on B. minor, Nov. 1929, Y. Nisikado, epitype designated here CBS H-7218 (Drechslera brizae) “MBT197978”, ex-epitype culture CBS 190.29.
Notes: An epitype for Drechslera brizae is designated here using a specimen and culture isolated by the original author from the same host and location. The ex-epitype culture of B. brizae (CBS 190.29) was included in the single locus and combined phylogenetic analyses of ITS, GPDH and LSU sequences for selected Bipolaris, Curvularia and Drechslera species. These phylogenetic trees confirmed that this species does not cluster with either Bipolaris or Curvularia but with Drechslera. Strain CBS 190.29 clustered close to D. biseptata and D. dematioidea. Therefore this species is placed in the genus Drechslera. All listed Bipolaris species were synonymised with Drechslera by Subramanian & Jain (1966). However, these name changes were not adopted by later authors. Based on the phylogenetic data, we accept the name Drechslera brizae (Y. Nisik.) Subramanian & Jain. Conidia of B. brizae are typically straight and have a distinctive hilum (Nisikado 1928a), which are characteristic of the genus Drechslera.
Curvularia buchloës (Lefebvre & A.G. Johnson) Manamgoda, Rossman & K.D Hyde, comb. nov. MycoBank: MB809648.
Basionym: Helminthosporium buchloës Lefebvre & Aar.G. Johnson, Mycologia 41: 204. 1949.
≡ Bipolaris buchloës (Lefebvre & Aar.G. Johnson) Shoemaker, Canad. J. Bot. 37: 882. 1959.
≡ Drechslera buchloës (Lefebvre & Aar.G. Johnson) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Type material: USA, Kansas, Hayes on Buchloe dactyloides, 18 Jun. 1942, C.L. Lefebvre, BPI 428770, holotype, ex-type culture CBS 246.49; Nebraska, Lincoln, on B. dactyloides, 12 Sep. 1940, R. Sprague, BPI 428763, paratype.
Additional material examined: USA, Kansas, on Buchloe dactyloides, 4 Jul. 1958, Karmer & Duffield, BPI 428764; Kansas, Latham, Butler, on B. dactyloides, 26 Jul. 1957, C.T. Rogerson, BPI 428765; Kansas, Manhattan, Riley Co. Kansas, on B. dactyloides, 22 Jun. 1955, C.T. Rogerson, BPI 428766; Kansas, Rooks Co., on B. dactyloides, 1895, E. Bartholomew, BPI 428767; Texas, College Station, on B. dactyloides, 26 May 1946, C.L. Lefebvre, BPI 428768; Kansas, Rooks Co., on B. dactyloides, 24 Jul. 1895, E. Bartholomew, BPI 428769.
Notes: This species usually produces short, straight or curved conidia, (28–)50–60(–63) × 8–10 μm. According to the phylogenetic analysis, Bipolaris buchloës (CBS 246.49) appears to belong in the genus Curvularia. Based on the phylogenetic and morphological evidence, this species is placed in the genus Curvularia (Fig. 2).
Drechslera catenaria (Drechsler) S. Ito (as “catenarium”), Proc. Imper. Acad. Tokyo 6: 355. 1930.
Basionym: Helminthosporium catenarium Drechsler, J. Agric. Res. 24: 627. 1923.
≡ Bipolaris catenaria (Drechsler) Somal, Indian J. Mycol. Pl. Pathol. 4: 160. 1975. (1974)
Type material: USA, New York, Douglaston, on Cinna arundinacea, 26 Sep. 1920, C. Drechsler, BPI 428835, holotype.
Notes: This species was originally described as Helminthosporium catenarium. Ito (1930) placed the species in the genus Drechslera. When Shoemaker (1959) described the genus Bipolaris, this species was not included. Later this species was placed in Bipolaris, but this was not accepted by Sivanesan (1987) who regarded it as Drechslera catenaria. Conidia of D. catenaria are obclavate cylindrical, with a well-defined intrahilar cavity and darkly pigmented distinct hilum. This hilum structure is a characteristic of Drechslera. Based on the current morphological evidence, we accept this species in Drechslera in agreement with Ito (1930).
Curvularia crustacea (Henn.) Y.P. Tan & R.G. Shivas, Australas. Pl. Pathol. 2014. (in press)
Basionym: Helminthosporium crustaceum Henn, Hedwigia 41: 147. 1902.
≡ Bipolaris crustacea (Henn.) Alcorn, Mycotaxon 15: 27. 1982.
Notes: According to the protologue, this species was isolated from Java without an indication of where the type specimen was deposited. Shoemaker (1959) synonymised this species with B. ravenelii, which should be placed in Curvularia according to the phylogeny in Manamgoda et al. (2012). An authentic culture provided by J.L. Alcorn was sequenced by Goh et al. (1998). According to those sequence data B. crustacea did not cluster in Bipolaris but in the genus Curvularia, close to C. ravenelii (Fig. 2). Therefore, this species is excluded from the genus Bipolaris and the combination made by Tan et al. (2014) is adopted.
Curvularia ryleyi (Alcorn) Y.P. Tan & R.G. Shivas, Australas. Pl. Pathol. 2014. (in press)
Replaced synonym: Bipolaris cylindrica Alcorn, Mycotaxon 15: 42. 1982.
Type material: Australia, New South Wales, Yetman, on inflorescence of Sporobolus scraber, 12 May 1977, J.L. Alcorn, 77154, BRIP 12554, holotype, not seen; ibid., IMI 261918, isotype; ex-type culture CBS 349.90.
Notes: The type culture (CBS 349.90) was sequenced. According the phylogenetic placement of the ITS and GPDH sequences, this species groups within Curvularia (Fig. 2). Tan et al. (2014) introduced a novel epithet in order to prevent creating a homonym of Curvularia cylindrica M. Zhang & T.Y. Zhang (2005).
Curvularia dactyloctenii (Alcorn) Y.P. Tan & R.G. Shivas, Australas. Pl. Pathol. 2014. (in press)
Basionym: Bipolaris dactyloctenii Alcorn, Mycotaxon 15: 3. 1982.
= Cochliobolus dactyloctenii Alcorn, Mycotaxon 15: 3. 1982.
Type material: Australia, Queensland, Goondiwindi, on Dactyloctenium radulans, 15 Mar. 1979, J.L. Alcorn, BRIP 12846, holotype (Bipolaris dactyloctenii), not seen; ibid., DAR 35055, IMI 264353, isotypes; from a paired crossing of an isolate from Melinis minutiflora with one from D. radulans, May 1979, J.L. Alcorn, BRIP 13498 (Cochliobolus dactyloctenii), holotype.
Notes: Conidia of B. dactyloctenii are straight and short, 35–43 × 6–8 μm. Phylogenetic sequence analysis of the ex-holotype culture of Bipolaris dactyloctenii (Tan et al. 2014) and an ex-type culture of Cochliobolus dactyloctenii (BRIP 12913 = 7938-9) (Berbee et al. 1999) has shown that this species grouped in Curvularia.
Curvularia homomorpha (Luttr. & Rogerson) Y.P. Tan & R.G. Shivas, Australas. Pl. Pathol. 2014. (in press)
Basionym: Helminthosporium homomorphum Luttr. & Rogerson (as “homomorphus”), Mycologia 51: 195. 1959.
≡ Bipolaris homomorpha (Luttr. &Rogerson) Subram. ex Alcorn (as “homomorphus”), Mycotaxon 16: 374. 1983.
Type material: USA, Kansas, Kansas State College, cultured on Hordeum vulgare, 11 Jun. 1957, E.S. Luttrell & C.T. Rogerson 6002, BPI 626670, holotype; ibid., ex iso-type culture DAOM 63822 (= Luttrell 6002).
Notes: The conidia of B. homomorpha are short and straight (25–42 × 10–13 μm), resembling a species of Curvularia. According to single- and combined (ITS and GPDH) gene phylogenetic analyses, this species is placed between Bipolaris and Curvularia and therefore the accurate generic placement is problematic. Similar results were obtained by previous authors (Berbee et al. 1999, Manamgoda et al. 2012). Tan et al. (2014) placed this species in the genus Curvularia based on the sequences of a different ex-isotype culture (BRIP 59391). However, due to the confusion of placement of this isolate as analysed by Berbee et al. (1999) and Manamgoda et al. (2012), we refrain from including this species in this study. Instead, an additional phylogenetic tree with B. homomorpha was deposited in TreeBASE (16356) to indicate the placement of this species.
Curvularia neoindica (J.N. Rai, Wadhwani & J.P. Tewari) Manamgoda, Rossman & K.D. Hyde, comb. et nom. nov. MycoBank: MB810140.
Replaced synonym: Bipolaris indica J.N. Rai, Wadhwani & J.P. Tewari, Sydowia 23: 8. 1970. [1969].
≡ Drechslera indica (J.N. Rai, Wadhwani & J.P. Tewari) Mouch., Revue Mycol. (Paris) 38: 106. 1975. [1974].
Type material: India, Lucknow, on Brassica nigra, 26 Oct. 1967, J.N. Rai 3, IMI 129790, holotype.
Notes: Based on the examination of the type specimen (IMI 129790), the conidia of B. indica are smaller and wider, (27)35–55(–65) × (17–)19–25(–27) μm (av. = 45, SD = 10, n = 30; av. = 22, SD = 3, n = 30) than species of Bipolaris and have a distinct protuberant hilum. Since sequences from the the ex-type are not available, ITS and GPDH sequences of an authenticated strain (BRIP 17439), provided by Berbee et al. (1999), confirmed the placement of this species within Curvularia (Fig. 2). A nomenclatural novelty is introduced here to avoid creating a homonym with Curvularia indica Subram., Proc. natn. Acad. Sci. India, Sect. B, Biol. Sci. 38 (Sec. B): 34 (1953). Curvularia neoindica and C. indica differ morphologically as the latter species have smaller conidia (24–47 × 8–16 μm); also C. neoindica is 3–6-distoseptate whereas C. indica is 3-distoseptate (Subramanium 1953).
Curvularia kusanoi (Y. Nisik.) Manamgoda, Rossman & K.D. Hyde, comb. nov. MycoBank: MB809649.
Basionym: Ophiobolus kusanoi Y. Nisik., J. Jap. Bot. 4: 108. 1928.
≡ Cochliobolus kusanoi (Y. Nisik.) Drechsler ex Dastur, Indian J. Agric. Res. 12: 733. 1942.
= Helminthosporium kusanoi Y. Nisik., Ber. Ohara Inst. landw. Forsch. 4: 122 (1929).
≡ Drechslera kusanoi (Y. Nisik.) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
≡ Bipolaris kusanoi (Y. Nisik.) Shoemaker, Canad. J. Bot. 37: 883. 1959.
Type material: Japan, iconotype designated here Y. Nisikado (1928b). J. Jap. Bot. 4: 108. Plates XI–XV, J.A. Stevenson Mycology Library, USDA-ARS, Beltsville, Maryland, USA “MBT198401”; on Eragrostis major, 1929, Y. Nisikado, epitype designated here CBS H-7029 “MBT198402”, ex-epitype culture CBS 137.29.
Notes: The ex-epitype culture (CBS 137.29), which was isolated and deposited by the original author Y. Nisikado, was used in the phylogenetic analysis. The phylogenetic position of B. kusanoi is confirmed within the genus Curvularia (Fig. 2), and therefore the name is placed in this genus.
Curvularia miyakei (Y. Nisik.) Manamgoda, Rossman & K.D. Hyde, comb. nov. MycoBank: MB809653.
Basionym: Helminthosporium miyakei Y. Nisik., Ber. Ohara Inst. landw. Forsch. 4: 122. 1929.
≡ Bipolaris miyakei (Y. Nisik.) Shoemaker, Canad. J. Bot. 37: 883. 1959.
Type material: Japan, on Eragrostis pilosa, iconotype designated here Y. Nisikado (1929), Ber. Ohara Inst. landw. Forsch. 4: 122 (1929): Plate XVII figs 2 and 3 J.A. Stevenson Mycology Library, USDA-ARS, Beltsville, Maryland, USA “MBT197980”; ibid., ex-syntype culture CBS 197.29.
Notes: Ex-syntype culture (CBS 197.29) was used in the phylogenetic analysis and placement of this species in the genus Curvularia was confirmed. This species resembles C. ravenelii based on the symptoms produced on Eragrostis and the mode of branching of conidiophores but differs in the size of conidia (Nisikado 1929). Curvularia ravenelii and C. miyakei are phylogentically distinct (Fig. 2).
Curvularia neergaardii (Danquah) Y.P. Tan & R.G. Shivas, Australas. Pl. Pathol. 2014. (in press)
≡ Drechslera neergaardii Danquah, Trans. Brit. mycol. Soc. 64: 545. 1975.
≡ Bipolaris neergaardii (Danquah) Alcorn, Mycotaxon 17: 68. 1983.
= Cochliobolus neergaardii Alcorn, Mycotaxon 39: 385. 1990. (fide Alcorn 1990).
Type material: Australia, paired cultures on Dactyloctenium aegyptium leaves, 10 Aug. 1988, J.L. Alcorn, BRIP 16385, holotype; ibid., IMI 335219, isotype.
Additional material examined: Chile, on desert soil, collected by E. Piontelli, identified by J.L. Alcorn, DAOM 228085.
Notes: In the sexual morph of this species, ascospores are loosely coiled or parallel. In other Bipolaris species ascospores are tightly coiled within the asci. Conidia are shorter compared to other Bipolaris species (19–30 × 15–18 μm) and mostly with three septa. An authentic culture verified by J.L. Alcorn (DAOM 228085) confirmed the placement of this species in the genus Curvularia (Fig. 2). Tan et al. (2014) also sequenced an ex-isotype culture (BRIP 12919) from seed of Oryza sativa, and placed this species in Curvularia as accepted here.
Curvularia nicotiae (Mouch.) Y.P. Tan & R.G. Shivas, Australas. Pl. Pathol. 2014. (in press)
Basionym: Drechslera nicotiae Mouch., Revue Mycol. (Paris) 38: 108. 1975. [1974]
≡ Bipolaris nicotiae (Mouch.) Alcorn, Mycotaxon 17: 68. 1983.
Type material: Algeria, on desert soil, 1974, J. Mouchacca, CBS H-07030, isotype, ex-isotype culture CBS 655.74.
Notes: The ex-isotype culture CBS 655.74 was used to confirm the placement of this species within the genus Curvularia (Fig. 2). Bipolaris nicotiniae is closely related to B. neergardii in accordance with the phylogenetic analysis and both of these species cluster in Curvularia. We accept the synonymy of Tan et al. (2014).
Curvularia nodulosa (Sacc.) Manamgoda, Rossman & K.D. Hyde, comb. nov. MycoBank: MB809652.
Basionym: Helminthosporium nodulosum Berk. & M.A. Curtis ex Sacc., Syll. Fung. 4: 421. 1886.
≡ Helminthosporium nodosum Berk. & M.A. Curtis [as “Helmisporium”], in Berkeley, Grevillea 3: 102. 1875. non Wallroth, Flor, Crypt, Germ 2: 165. 1833.]
≡ Bipolaris nodulosa (Sacc.) Shoemaker, Canad. J. Bot. 37: 883. 1959.
≡ Drechslera nodulosa (Sacc.) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
= Cochliobolus nodulosus Luttr., Phytopathology 47: 547. 1957.
= Helminthosporium leucostylum Drechsler, J. Res. 24: 711. 1923. (fide Sivanesan 1987).
≡ Bipolaris leucostyla (Drechsler) Shoemaker, Canad. J. Bot. 37: 883. 1959.
≡ Drechslera leucostyla (Drechsler) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Type material: USA, on an unknown substrate, 26 Sep. 1967, E.S. Luttrell, BPI 626677 (Cochliobolus nodulosus), holotype.
Additional material examined: USA, on Eleusine indica, Mar. 1958, E.S. Luttrell, CBS 160.58 (authentic strain).
Notes: An authentic culture (CBS 160.58), which was isolated and deposited by E.S. Lutrrell, was used to confirm the placement of this species within the genus Curvularia (Fig. 2) and the nomenclatural changes are made. Curvularia nodulosa is phylogenetically close to C. kusanoi.
Curvularia papendorfii Aa, Persoonia 5: 45. 1967.
≡ Drechslera papendorfii (Aa) M.B. Ellis, Dematiaceous Hyphomycetes (Kew): 413. 1971.
≡ Bipolaris papendorfii (Aa) Alcorn, Mycotaxon 17: 68. 1983.
[= Curvularia siddiquii S.I. Ahmed & M. Qureshi (as “siddiqui”), Pakist. J. scient. ind. Res. 3: 177. 1960. (nom. inval., fide Sivanesan 1987)]
Type material: South Africa, Transvaal, Potchefstroom, on leaf litter, 1967, M.C. Papendorf, IMI 136484, holotype, ex-type culture CBS 308.67.
Notes: This species was originally described as a Curvularia species, which is confirmed by our phylogenetic analysis (Fig. 2). The conidial morphology is also similar to Curvularia; the conidia are shorter than most species of Bipolaris and typically curved, not more than 3-distoseptate with the second cell being broadest. Based on the single and combined phylogenetic analyses (Fig. 2), this species belongs in the genus Curvularia; therefore the name in Curvularia is accepted.
Curvularia portulacae (Rader) Y.P. Tan & R.G. Shivas, Australas. Pl. Pathol. 2014. (in press)
Basionym: Helminthosporium portulaceae Rader, Mycologia 40: 344. 1948.
≡ Drechslera portulaceae (Rader) de Hoog & Oorschot, Proc. K. Ned. Akad. Wet., Ser. C, Biol. Med. Sci. 86: 59. 1983.
≡ Bipolaris portulaceae (Rader) Alcorn, Mycotaxon 41: 330. 1991.
Type material: USA, New York, Watkins Glen, on Portulaca oleracea, Aug. 1945, W.E. Rader, CUP 37970, holotype; ibid., CBS H-7033, isotype, ex-type culture CBS 239.48.
Notes: The phylogenetic placement of the ex-type culture (CBS 239.48) of Bipolaris portulaceae confirmed that this species belongs in the genus Curvularia (Fig. 2), supporting the synonymy by Tan et al. (2014). However, the species has very long, cylindrical spores as well as small spores (28–185 × 9–16 μm) and thereby conidial morphology is somewhat different compared to other species in the genus Curvularia.
[Bipolaris stipae (Trab.) Gornostaĭ, in Azbukina et al. (eds), Vodorosli, GribyiMkhiDal'negoVostoka [Algae, Fungi and Mosses of the Soviet Far-East] (Vladivostok): 79. 1978].
Note: The basionym, Helminthosporium stipae Trab., was not indicated in this transfer, making the name, Bipolaris stipae, nomenclaturally invalid.
Curvularia sesuvii (Jing Z. Zhang) Manamgoda, Rossman & K.D. Hyde, comb. nov. MycoBank: MB809655.
Basionym: Bipolaris sesuvii Jing Z. Zhang, Mycotaxon 109: 292. 2009.
Type material: China, Zanjinag, on Sesuvium portulacastrum, 20 Aug. 2006, J.Z. Zhang, HMAS 63207, holotype, ex-type culture Bp Zj 01.
Notes: The ITS sequence from the ex-type culture was analysed together with Bipolaris and Curvularia species. According to the phylogeny, this species clustered within the genus Curvularia (Fig. 2) thus the name is placed in the genus Curvularia. The species is closely related to Curvularia neoindica.
Curvularia subpapendorfii (Mouch.) Manamgoda, Rossman & K.D. Hyde, comb. nov. MycoBank: MB809654.
Basionym: Drechslera subpapendorfii Mouch., Revue Mycol., Paris 38: 107. 1973.
≡ Bipolaris subpapendorfii (Mouch.) Alcorn, Mycotaxon 17: 69. 1983.
Type material: Egypt, iconotype designated here Mouch. (1973) Revue Mycol. (Paris) 38: 105, fig. 2 “MBT198292”; New Valley Region, on desert soil, 1974, J. Mouchacca, lectotype designated here MFLU 14-0336; ibid., ex-type culture CBS 656.74 ”MBT198455”.
Notes: Only a type culture is listed in the protologue but without a type specimen. Therefore a dried specimen of the ex-type culture was deposited as a lectotype in this study. This species produces stromata in culture and conidia are 2–3-distoseptate, short, 14–30 μm, larger at the second septum and curved. According to these morphological data this species belongs to the genus Curvularia and the phylogenetic placement of ex-type culture CBS 656.74 confirmed the placement within Curvularia (Fig. 2).
[Bipolaris triticalis Sisterna, Pl. Path. 38: 98. 1989.]
Note: The species is not validly published as a type specimen is not indicated in the publication.
[Bipolaris vassilevae Gornostaĭ, in Azbukina et al. (eds), Vodorosli, Griby i Mkhi Dal'nego Vostoka [Algae, Fungi and Mosses of the Soviet Far-East] (Vladivostok): 80. 1978].
Note: The name is nomenclaturally invalid according to Art. 39.1 (Melbourne).
Doubtful species
Bipolaris cactivora (Petr.) Alcorn, Mycotaxon 17: 67. 1983.
Basionym: Helminthosporium cactivorum Petr., Gartenbauwissenschaft 5: 226. 1931.
≡ Drechslera cactivora (Petr.) M.B. Ellis, Dematiaceous Hyphomycetes (Kew): 432. 1971.
Notes: The type specimen of this species could not be located. There is an illustration and a description of this fungus in Ellis (1971). Conidia are short, straight, 30–65 μm long and 2–4-distoseptate (Ellis 1971). This species resembles Curvularia hawaiiensis, which was recently transferred from Bipolaris to Curvularia, but the latter species differs in having smaller conidia (20–40 μm). Generic placement of this species is doubtful, as there are no molecular data available.
Bipolaris eleusines J.H. Peng & J.Y. Lu (as “eleusinea”), J. Nanjing Agric. Univ. 12: 47. 1989.
Notes: A morphological description for this species could not be located and molecular data are not available. It is not recorded in the literature after it was originally described. The type specimen is listed in Index Fungorum as NAUPP 3-32. The species is considered to be doubtful.
Bipolaris flagelloidea (G.F. Atk.) Shoemaker, Canad. J. Bot. 37: 883. 1959.
Basionym: Helminthosporium flagelloideum G.F. Atk., Bull. Cornell Univ. 3: 47. 1897.
≡ Alternaria flagelloidea (G.F. Atk.) Luttr., Mycologia 47: 270. 1955.
Notes: In the protologue the species is described as having a long, hyaline, slender flagellum as in Cercospora crassa. Such a structure is not reported for any Bipolaris species. Luttrell (1955) observed a few conidiophores and conidia on the type specimen (CUP, not seen) and described and illustrated the type specimen. The conidia have few longitudinal septa, a character not found in Bipolaris species. This species probably belongs in the genus Alternaria (Luttrell 1955).
Bipolaris fusca Y.L. Jiang & T.Y. Zhang, Mycotaxon 104: 135. 2008.
Type material: China, in soil, 26 Jun. 2008, Y.L. Jinag, HSAUP 069079, holotype.
Notes: This species was isolated from soil in China. The two other Bipolaris isolates found from soil are B. nicotiae and B. subpapendorfii, which do not belong in the genus Bipolaris according to molecular data. However, B. fusca has a large number of pseudosepta (up to 11) and produces short, straight conidia, 31–67 × 11–20 μm (Jiang & Zhang 2008). There are no molecular data to confirm the phylogenetic placement of this species.
Bipolaris glycines (S. Naray. & Durairaj) B.A. Khasanov (as “glycinii”), Opredelitel' Gribov-Vozbuditeleĭ 'Gel'mintosporiozov' Rasteniĭ iz Rodov Bipolaris, Drechslera, Exserohilum (Tashkent): 53. 1992.
Basionym: Drechslera glycines S. Naray. & Durairaj [as “glycine”], Madras Agric. J. 58: 712. 1971.
Type material: India, Coimbatore, on the leaf of Glycine max, P. Narayanasamy & P. Durairaj, holotype Agr. College and Res. Institute, Coimbatore-3.
Notes: Bipolaris glycines is similar to the other species occurring on Glycine, B. sorokiniana, in conidial dimensions. However, the type specimen is not available to study, and no molecular data are available. Therefore this species is retained as doubtful.
Bipolaris israeli Steiman, Guiraud, Seigle-Mur. & Sage, Syst. Appl. Microbiol. 19: 183. 1996.
Type material: Israel, Judean desert, isolated from salty soil, Aug. 1990, CMPG 1021, not seen, holotype.
Notes: This species was isolated from soil in Israel. Bipolaris israeli has short, straight, somewhat curved conidia that resemble Curvularia species. We were unable to obtain molecular data for this species to confirm the accurate generic placement.
Bipolaris palousensis R. Sprague (as “palousense”), Res. Stud. Washington State Univ. 29: 77. 1961.
Type material: USA, Washington, on Juncus ensifolius, 18 May 1948, R. Sprague, WSP 46818, holotype; ibid., CSN 3925.
Additional material examined: USA, Oregon, on J. ensifolius, 22 May 1957, R. Sprague, WSP 42983.
Notes: This is the only Bipolaris species reported from Juncus ensifolius (Juncaceae). The type specimen (WSP 46818) has few conidia and is not in good condition. Some conidia and conidiophores were observed on the slide with the specimen by J.L. Alcorn. According to the characters observed from the slide, the conidia are cylindrical with non-protruding hilum, rounded, with a well-defined intrahilar cavity. Based on the hilum morphology this could be a Drechslera species. However, due to lack of good type and molecular data, we retain the species as doubtful.
Bipolaris micropus (Drechsler) Shoemaker, Canad. J. Bot. 37: 884. 1959.
Basionym: Helminthosporium micropus Drechsler, J. Agric. Res. 24: 722. 1923.
= Drechslera micropus (Drechsler) Subram. & B.L. Jain (as “micropa”), Curr. Sci. 35: 354. 1966.
= Helminthosporium leptochloae Y. Nisik. & C. Miyake, Ber. Ohara. Inst. Forsch. Japan 2: 483. 1924. (fide Sivanesan 1987).
Type material: USA, Florida, Wauchula, on Paspalum boscianum, 2 May 1921, C. Drechsler, BPI 429621 (Helminthosporium micropus), syntype; ibid., BPI 429620, syntype; ibid., BPI 429615, syntype.
Additional material examined: USA, South Carolina, Charleston, on P. boscianum, 23 Jun. 1932, C. Drechsler, BPI 429617; ibid., BPI 429618.
Notes: A subconical basal cell with a short protuberant hilum distinguishes this species (Drechsler 1923, Sivanesan 1987). Such a feature has not been recorded in any other Bipolaris species. Sivanesan (1987) listed Helminthosporium leptochloae as synonym of B. micropus based on the similar phenotypic characters. The primary septum in the conidia is formed near the base as in Exserohilum species. Based on the morphological characters, this species probably belongs in Exserohilum. Molecular data are needed to confirm this placement.
Bipolaris prieskaensis W.Q. Chen & W.J. Swart, Mycotaxon 76: 150. 2000.
Type material: South Africa, Northern Cape Province, Prieska, on debris of Pistacia vera, 15 Mar. 1998, T.D Nieuwoudt, PREM 56306, holotype, ex-type culture DN 123.
Notes: This species has smaller conidia (30–40 × 16–20 μm) than other Bipolaris species. No DNA sequence data are available from the type material. A putative ITS sequence for this species available in GenBank (JQ517482) grouped within Curvularia.
Bipolaris siliculosa (P. Crouan & H. Crouan) Shoemaker, Canad. J. Bot. 37: 884. 1959.
Basionym: Helminthosporium siliculosum P. Crouan & H. Crouan, Florule Finistère (Paris): 33. 1867.
≡ Drechslera siliculosa (P. Crouan & H. Crouan) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Notes: Subramanian & Jain (1966) placed this species in the genus Drechslera along with other Bipolaris species. There is little information available for this species and the type specimen could not be located. Therefore, placement of this species is doubtful.
Bipolaris triticigrani (A.P. Misra & R.A. Singh) Sivan., Mycol. Pap. 158: 100. 1987.
Basionym: Drechslera triticigrani A.P. Misra & R.A. Singh, Sydowia 32: 187. 1980. [1979]
Notes: This species was first introduced as a species of Drechslera, and Sivanesan (1987) placed it in Bipolaris. The type specimen deposited in IMI is lost (Sivanesan 1987). According to the protologue, conidia germinate from the polar cells and also one or two central cells. This germination pattern is different from the genus Bipolaris where the germ tube originates from one or both polar cells (Alcorn 1988). Germination from central cells is a characteristic of the genus Drechslera. Although this species probably belongs in the genus Drechslera, molecular data are needed to confirm the placement.
Bipolaris tropicalis Sivan., Trans. Brit. mycol. Soc. 84: 411. 1985.
Notes: This species produces stromata in culture, which is a characteristic of many Curvularia species. The conidia are 35–48 × 14–16 μm, short, straight or curved, and usually 3-distoseptate. Based on these morphological characters, the species probably belongs to the genus Curvularia. Molecular data are needed to confirm this placement.
Bipolaris xanthosomatis Huguenin (as “xanthosomae”), Bull. trimest. Soc. mycol. Fr. 81: 698. 1966. [1965]
Notes: There is little information available for this species and the type specimen could not be located. The species has not been recorded since it was first described, and molecular data are not available.
Discussion
In this study, the genus Bipolaris was re-assessed using morphological and molecular data for taxonomic clarification of species concepts. The phylogeny of the genus Bipolaris (Fig. 1) is inferred from DNA sequence data and a phylogenetic tree is provided to distinguish it from its sister genus Curvularia (Fig. 2). The addition of new isolates updates the analysis of Manamgoda et al. (2012) (Fig. 2) that included only eight species of Bipolaris. In the current study the number of species is increased to 29 with several new epi- and neotypes designated here. We have observed that a number of species previously regarded as Bipolaris cluster in Curvularia or Drechslera based on the ex-type isolates included in the phylogenetic analysis (Fig. 2) and taxonomic refinements are done accordingly.
The distinction of the two sister genera Bipolaris and Curvularia was first presented by Berbee et al. (1999) based on molecular data and later re-defined by phylogenetic analyses of ITS, GPDH, TEF and LSU sequences in Manamgoda et al. (2012). Several species with a previously uncertain generic placement, such as B. crustacea, B. kusanoi, B. neergaardii, B. nicotiniae, B. nodulosa, B. papendorfii, B. portulaceae, B. ravenellii, B. sesuvum and B. subpapendorfii, are now placed in Curvularia based on phylogenetic analyses (Manamgoda et al. 2012, Tan et al. 2014). Most of these species produce short, curved curvularia-like conidia except B. portulaceae and B. sesuvum, which produce longer, straight conidia. Both of these species show high intra-species variability with a wide range of conidial dimensions. However, we have observed some separation of these two species from the major clade of Curvularia in the LSU analysis (tree not shown). Although LSU has been primarily used to distinguish genera within Pleosporales (Hyde et al. 2013), caution is warranted in the generic delimitation of closely related genera Bipolaris-Curvularia-Porocercospora-Johnalcornia.
The genus Bipolaris was historically characterised by brown conidiophores and conidia that are fusoid, straight or curved, and germinating from one germ tube at each end. Some Bipolaris species having short, curved conidia with hyaline apical cells are morphologically similar to Curvularia. Those Bipolaris species are morphologically distinguished from Curvularia based on slight differences in the median cells of the conidia. The median cells of conidia of Bipolaris are of nearly equal width, while those of Curvularia have enlarged darkened median cells that results in curvature (Shoemaker 1959). Interspecific and infraspecific variation was observed in the degree of swelling in the median cell of Curvularia (Sivanesan 1987). Some Curvularia species produce black or dark brown, cylindrical, thick hyphal masses in culture, but such formation cannot be found in Bipolaris. Most Bipolaris species have longer conidia than Curvularia and are straight or curved, with the curvature continuous throughout the spore. On the other hand, conidia of Curvularia can be straight or curved and, when curved, the conidia have intermediate cells inordinately enlarged and this contributes to their curvature (Manamgoda et al. 2012). However, there are exceptional cases in morphology in both Bipolaris and Curvularia. For example B. chloridis, which clusters with the type species B. maydis, is reported to produce conidia as short as 42 μm (Sivanesan 1987). In such exceptional cases it is better to rely on molecular data for identification of species. Therefore it has been difficult to distinguish species of Bipolaris and Curvularia solely based on conidial morphology (Sivanesan 1987).
The single and combined analyses of ITS, GPDH and TEF are able to reliably separate the genera Bipolaris and Curvularia. In the genus Curvularia, some species are found with relatively short, straight or curved conidia. Several species of Curvularia are known to be human pathogens. Curvularia brachyspora, C. geniculata, C. inaequalis and C. senegalensis have been reported to cause keratitis, sinusitis, cutaneous and subcutaneous infections, peritonitis, onychomycosis, endocarditis, endophthalmitis, cerebral phaeohyphomycosis, and allergic bronchopulmonary as well as disseminated disease (da Cunha et al. 2013). After the re-circumscription of these two genera, all clinically relevant pathogens previously included in Bipolaris are now placed in Curvularia (da Cunha et al. 2013).
Species remaining in Bipolaris produce multi-septate, usually more than 4-distoseptate, straight, curved or fusiform conidia. The curvature is not only evident in the median cells but throughout the conidium. Some species of Curvularia produce stromata in culture, a feature not associated with species of Bipolaris. The sexual morphs of Bipolaris and Curvularia are not found in nature but sometimes induced under laboratory conditions. There are no consistent distinguishing morphological differences recorded between the sexual morphs of these two genera. However, in most species of Bipolaris the ascospores are tightly coiled throughout the asci whereas in most species in Curvularia the ascospores are loosely coiled or partially coiled in the asci (Tsuda et al. 1977).
The monotypic genus Porocercospora is a recently described genus introduced by Amaradasa et al. (2014), placed between the genera Bipolaris and Curvularia in their phylogenetic analysis. The genus is typified by Porocercospora seminalis, based on Cercospora seminalis, the cause of buffalo grass false smut known in USA. The combined analysis of ITS, LSU and RPB2 phylogeny revealed that this genus is phylogenetically close to, but distinct from, Bipolaris and Curvularia. Porocercospora is morphologically distinct from Bipolaris in having densely aggregated conidiophores arising from brown stroma with characteristic conidial morphology. The cylindrical or subcylindrical conidia generally have a sub-obtuse apex and obconically truncate base with a distinct thickened and brown hilum (Amaradasa et al. 2014).
In our study the phylogenetic species recognition in the genus Bipolaris was accomplished by the application of GCPSR. Each of the single-locus trees and the combined analysis were compared in order to determine the species limits. The GPDH phylogenetic tree closely resembles the combined phylogenetic tree as it resolves most species with high bootstrap support. One exception is B. sacchari and B. peregianensis, which have similar GPDH sequences and thus they cluster together. However, these two species can be separated using ITS and TEF sequence data (trees not shown). As a single marker either GPDH or ITS can resolve most of the species and GPDH was determined to be the best single locus for this. In the analyses of single gene regions, ITS and GPDH, the isolates of B. cookei clustered within the genus with high bootstrap support, having a minor variation in the TEF gene. The LSU analysis of Bipolaris and Curvularia (tree not shown here) could not resolve most species level relationships of Bipolaris and therefore it is not useful for the species level phylogenetic reconstruction.
Although biological species recognition has been used in previous studies of Bipolaris, its use is complicated by the lack of sexually produced spores. Many of the cross mating experiments in laboratory conditions were unsuccessful. In most species of Bipolaris a sexual morph has not been recorded, neither in nature nor in culture. In addition complete or partial hybridisation has been reported between Bipolaris species (Alcorn 1988), but these species can be differentiated using morphology and GCPSR (Manamgoda et al. 2012). Conidiophores and conidial measurements show a large range of variability with high standard deviations and measurements that overlap between species. Many Bipolaris species have overlapping characters; therefore, the use of morphology to identify the species is limited. Also morphological diversity within a species is high, with a broad range of conidial dimensions. Therefore species described solely based on morphological data are often doubtful. Several such species were synonymised when the molecular phylogenetic data were applied in our study. On the other hand, some species have unique characteristics. For example B. hadrotrichoides has large, echinulate conidia, while B. pluriseptata has large, distinctly curved, “C”-shaped conidia. Interspecific compatibility has been observed between some taxa of Bipolaris. Although these taxa have retained their potential mating compatibility, they are considered as distinct phylogenetic species based on our phylogenetic analysis. For example Bipolaris zeicola and B. victoriae have overlapping conidial dimensions and successful hybridisation leading to ascospore production has been reported (Nelson 1960a, b). A similar situation is reported for B. maydis and B. oryzae (Alcorn 1983a). However, these species are phylogenetically distinct pathogens that cause different diseases on different host plants.
The ecology and the host range of many Bipolaris species are poorly known. Only a few important pathogens on high value crops are well studied with respect to their biology, infection and populations. Species of Bipolaris occur mainly on grass hosts but have also been reported from non-grass hosts (Shimizu et al. 1998, Tsukiboshi et al. 2005, Manamgoda et al. 2011). Most species are opportunistic pathogens on grasses and some of the species occur on a wide range of hosts. For example, the important and highly virulent plant pathogens, B. maydis, B. sacchari, B. sorokiniana, B. victoriae and B. zeicola are reported on many crops other than their original hosts (Manamgoda et al. 2011, 2012, Farr & Rossman 2013). Their ability to cause devastating diseases can be influenced by the environmental conditions and the abiotic stresses on plants (Krupinsky et al. 2004, Fajolu et al. 2013). Warm and humid environments are always favourable for the pathogens on seasonal grasses and crops (Carissimi et al. 2010, Eisa et al. 2013). Most of the earlier identifications of species of Bipolaris based on morphology and used to determine host ranges must be re-evaluated with the application of recent molecular data. Some Bipolaris species such as B. cynodontis, B. oryzae, and B. sorokiniana have been confirmed from a broad host range (Fig. 1) while a few species of Bipolaris are found only on a single host in this study. For instance, B. clavata is known only on Dactyloctenium radulans (Poaceae), B. microstegii has only been reported on Microstegium vimineum (Poaceae), B. gossypina has only been reported from Gossipyum species and B. heveae has only been reported from Hevea brasiliensis (Euphorbiaceae). Most other species of Bipolaris known on a single host are only known from the type specimen and a few additional reports. Extensive sampling and accumulation of molecular data will improve the understanding of host range and ecological significance. In general, most of the species can be found in both temperate and tropical regions of the world. The modern monograph of Bipolaris provided in this study will be a resource for plant pathologists, plant quarantine officials and taxonomists for identification of species as well as to access the knowledge on biology, ecology, and geographic distribution.
Acknowledgements
Kevin D. Hyde thanks the Chinese Academy of Sciences, project number 2013T2S0030, for the award of Visiting Professorship for Senior International Scientists at Kunming Institute of Botany. The Molecular Biology Center in Germplasm Bank of Wild Species at Kunming Institute of Botany is thanked for the help with molecular work. The Humidtropics, a CGIAR Research Program that aims to develop new opportunities for improved livelihoods in a sustainable environment, is thanked for partially funding this work. This work was completed at the Systematic Mycology and Microbiology Laboratory (SMML), Agricultural Research Service, United States Department of Agriculture in Beltsville, MD, USA, under the direction of co-authors Lisa Castlebury and Amy Rossman. D.S.M. is grateful for the assistance of the U.S. Forest Service (USDA) in sponsoring a visiting studentship at SMML. We thank Gillian Turgeon, Stephen Rehner, Megan Romberg, William L. Bruckart, Nathan Kleczewski, Mary Barkworth (USA), Lewis Mejia (Panama), N. Nishihara, T. Tsukiboshi, (Japan) and for the fresh collections and isolates provided. Roger Shivas and Yu Pei Tan are thanked for sharing some sequences and helpful comments. The assistance of Shannon Dominick in accessing specimens at BPI and obtaining specimen loans from other herbaria is greatly appreciated. The curators and managers of BRIP, CUP, K, PREM and WSP are thanked for the loan of specimens. Dhanushka Udayanga is thanked for the comments to improve the manuscript. D.S.M. thanks all the collegues in MFLU for their various assistance. Cai Lei, Conrad Schoch and Eric McKenzie are thanked for their advice on the project. Andrew Minnis, Christian Feuillet and John Wiersema are appreciated for comments on nomenclatural clarifications. Technical support for this project was provided by Tunesha Phipps (USDA-ARS), whose assistance is greatly appreciated.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer.
References
- Ahmadpour A., Heidarian Z., Donyadoost-Chelan M. A new species of Bipolaris from Iran. Mycotaxon. 2012;120:301–307. [Google Scholar]
- Aiguo L., Chenghe Z. The developing trend of the predominant population of Bipolaris maydis race O and the disease resistance monitoring of the maize cultivars. Journal of Agricultural University of Hebei. 1997;20:35–38. [Google Scholar]
- Alcorn J.L. Two new Cochliobolus species. Transactions of the British Mycological Society. 1978;70:61–65. [Google Scholar]
- Alcorn J.L. New Cochliobolus and Bipolaris species. Mycotaxon. 1982;15:1–19. [Google Scholar]
- Alcorn J.L. On the genera Cochliobolus and Pseudocochliobolus. Mycotaxon. 1983;16:353–379. [Google Scholar]
- Alcorn J.L. Generic concepts of Drechslera, Bipolaris and Exserohilum. Mycotaxon. 1983;17:1–86. [Google Scholar]
- Alcorn J.L. The taxonomy of “Helminthosporium” species. Annual Review of Phytopathology. 1988;26:37–56. [Google Scholar]
- Alcorn J.L. Additions to Cochliobolus, Bipolaris and Curvularia. Mycotaxon. 1990;39:361–392. [Google Scholar]
- Alcorn J.L. New combinations and synonymy in Bipolaris and Curvularia, and a new species of Exserohilum. Mycotaxon. 1991;41:329–343. [Google Scholar]
- Amaradasa B.S., Madrid H., Groenewald J.Z. Porocercospora seminalis gen. et comb. nov., the causal organism of buffalograss false smut. Mycologia. 2014;106:163–172. doi: 10.3852/13-147. [DOI] [PubMed] [Google Scholar]
- Badu-Apraku B., Fakorede M.A.B., Lum A.F. Improvement of yield and other traits of extra-early maize under stress and non-stress environments. Agronomy Journal. 2009;101:381–389. [Google Scholar]
- Berbee M.L., Pirseyedi M., Hubbard S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia. 1999;91:964–977. [Google Scholar]
- Berbee M.L., Carmean D.A., Winka K. Ribosomal DNA and resolution of branching order among the ascomycota: how many nucleotides are enough? Molecular Phylogenetics and Evolution. 2000;17:337–344. doi: 10.1006/mpev.2000.0835. [DOI] [PubMed] [Google Scholar]
- Breda de Haan J van. Oogvlekken-ziekte door Cercospora sacchari n. sp. Mededeelingen van het Proefstation voor Suikerriet in West-Java. 1892;3:15–21. [Google Scholar]
- Bernard C. Helminthosporium incurvatum. Bulletin du Département de l'agriculture aux Indes Néerlandaises. 1906;2(31):1906. [Google Scholar]
- Boedijn K.B. Ueber einige phragmosporen Dematiazeen. Annales du Jardin Botanique de Buitenzorg. 1933;13:123. [Google Scholar]
- Bronson C.R. The genetics of phytotoxin production by plant pathogenic fungi. Experientia. 1991;47:771–776. [Google Scholar]
- Butler E.J., Khan A.H. Some new sugarcane diseases. Memoirs of the Department of Agriculture in India, Botanical Series. 1913;6:181–208. [Google Scholar]
- Cai L., Giraud T., Zhang N. The evolution of species concepts and species recognition criteria in plant pathogenic fungi. Fungal Diversity. 2011;50:121–133. [Google Scholar]
- Carissimi M., Giraudo M.S., Germani J.C. Antifungal activity of Bacillus sp. E164 against Bipolaris sorokiniana. Biociências. 2010;17:48–58. [Google Scholar]
- Carson M.L. Aggressiveness and perennation of isolates of Cochliobolus heterostrophus from North Carolina. Plant Disease. 1998;82:1043–1047. doi: 10.1094/PDIS.1998.82.9.1043. [DOI] [PubMed] [Google Scholar]
- Chang H.S. Cochliobolus zeae sp. nov., the teleomorph of Bipolaris zeae. Botanical Bulletin of Academia Sinica. 1992;33:175–177. [Google Scholar]
- Cholil A., Hoog GS de. Variability in Drechslera oryzae. Transactions of the British Mycological Society. 1982;79:491–496. [Google Scholar]
- Christensen J.J. Physiologic specialization and parasitism of Helminthosporium sativum. Technical Bulletin of University of Minnesota Agricultural Experiment Station. 1926;37:1–101. [Google Scholar]
- Christiansen S.K., Wirsel S., Yun S.H. The two Cochliobolus mating type genes are conserved among species but one of them is missing in C. victoriae. Mycological Research. 1998;102:919–929. [Google Scholar]
- Condon B.J., Leng Y., Wu D. Comparative genome structure, secondary metabolite, and effectors coding capacity across Cochliobolus pathogens. PLoS Genetics. 2013;9:e1003233. doi: 10.1371/journal.pgen.1003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke M.C. Ravenel's American Fungi. Grevillia. 1878;6:129–146. [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Crous P.W., Shivas R.G., Wingfield M.J. Fungal Planet description sheets: 128–153. Persoonia. 2012;29:146–201. doi: 10.3767/003158512X661589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Guarro J. Fungal Planet description sheets: 154–213. Persoonia. 2013;31:188–296. doi: 10.3767/003158513X675925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cunha K.C., Sutton D.A., Fothergill A.W. In vitro antifungal susceptibility and molecular identity of 99 clinical isolates of the opportunistic fungal genus Curvularia. Diagnostic Microbiology and Infectious Disease. 2013;76:168–174. doi: 10.1016/j.diagmicrobio.2013.02.034. [DOI] [PubMed] [Google Scholar]
- Datnoff L.E., Rutherford B.A. Effects of silicon on leaf spot and melting out in bermuda grass. Golf Course Management. 2004;5:89–92. [Google Scholar]
- Deng H., Zhang T.Y. Taxonomic studies of Bipolaris buchloes. Mycosystema. 2002;21:327–333. [Google Scholar]
- Dettman J.R., Jacobson D.J., Taylor J.W. A multilocus genealogical approach to phylogenetic species recognition in the model eukaryote Neurospora. Evolution. 2003;57:2703–2720. doi: 10.1111/j.0014-3820.2003.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Doidge E.M. The South African fungi and lichens to the end of 1945. Bothalia. 1950;5:1–1094. [Google Scholar]
- Drechsler C. Some graminicolous species of Helminthosporium. Journal of Agricultural Research. 1923;24:641–739. [Google Scholar]
- Drechsler C. A species of Helminthosporium distinct from Helminthosporium sacchari, causing brown stripe of sugar cane. Phytopathology. 1928;18:135–136. [Google Scholar]
- Drechsler C. Phytopathological and taxonomical aspects of Ophilobolus, Pyrenophora, Helminthosporium and a new genus Cochliobolus. Phytopathology. 1934;24:953–981. [Google Scholar]
- Duveiller E., Gilchrist L.I. 1994. Production constraints due to Bipolaris sorokiniana in wheat: current situation and future prospects; pp. 343–345. Proceedings of the CIMMYT/UNDP workshop, Nashipur (Dinajpur), Bangladesh, February 1994. [Google Scholar]
- Duveiller E., Garcia-Altamirano I. Pathogenicity of Bipolaris sorokiniana isolates from wheat roots, leaves and grains in Mexico. Plant Pathology. 2000;49:235–242. [Google Scholar]
- Eisa M., Chand R., Joshi A.K. Biochemical and histochemical traits: a promising way to screen resistance against spot blotch (Bipolaris sorokiniana) of wheat. European Journal of Plant Pathology. 2013;137:805–820. [Google Scholar]
- Ellis M.B. Commonwealth Mycological Institute; Kew, UK: 1971. Dematiaceous Hyphomycetes. [Google Scholar]
- Fajolu O.L., Wadl P.A., Vu A.L. Development and characterization of simple sequence repeats for Bipolaris sorokiniana and cross transferability to related species. Mycologia. 2013;105:1164–1173. doi: 10.3852/12-210. [DOI] [PubMed] [Google Scholar]
- Faris J.A. Three Helminthosporium diseases of sugar cane. Phytopathology. 1928;18:753–774. [Google Scholar]
- Farr D.F., Rossman A.Y. ARS, USDA; 2013. Fungal databases, systematic mycology and microbiology laboratory.http://nt.ars-grin.gov/fungaldatabases [Downloaded 31 December 2013] [Google Scholar]
- Fetch T.G., Jr., Steffenson B.J. Identification of Cochliobolus sativus isolates expressing differential virulence on two-row barley genotypes from North Dakota. Canadian Journal of Plant Pathology. 1994;16:202–206. [Google Scholar]
- Goh T.-K., Hyde K.D., Lee D.K.L. Generic distinction in the Helminthosporium-complex based on restriction analysis of the nuclear ribosomal RNA gene. Fungal Diversity. 1998;1:85–107. [Google Scholar]
- Gray S.F. vol. 1. Baldwin, Cradock, and Joy; London, UK: 1821. (A natural arrangement of British plants). [Google Scholar]
- Guseva N.N., Levitin M.M., Mikhailova L.A. Genetic mechanisms of variability in phytopathogenic fungi. Acta Phytopathologica Academiae Scientiarum Hungaricae. 1979;14:441–447. [Google Scholar]
- Hagan A. 2005. Leaf spot and rust diseases of turf grasses. Alabama Cooperative Extension System. [Downloaded 3 February 2014]. http://www.aces.edu/pubs/docs/A/ANR-0621/ANR-0621.pdf. [Google Scholar]
- Hawksworth D.L. A new dawn for the naming of fungi: impacts of decisions made in Melbourne in July 2011 on the future publication and regulation of fungal names. IMA Fungus. 2011;2:155–162. doi: 10.5598/imafungus.2011.02.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Hyde K.D., Abd-Elsalam K., Cai L. Morphology: still essential in a molecular world. Mycotaxon. 2010;114:439–451. [Google Scholar]
- Hyde K.D., Jones E.B.G., Liu J.K. Families of Dothideomycetes. Fungal Diversity. 2013;63:1–313. [Google Scholar]
- Inagaki Y.S., Noutoshi Y., Fujita K. Infection-inhibition activity of avenacin saponins against the fungal pathogens Blumeria graminis f. sp. hordei, Bipolaris oryzae, and Magnaporthe oryzae. Journal of General Plant Pathology. 2013;79:69–73. [Google Scholar]
- Ito S., Kuribayashi K. Production of the ascigerous stage in culture of Helminthosporium oryzae. Annals of the Phytopathological Society of Japan. 1927;2:1–8. [Google Scholar]
- Ito S. On some new ascigerous stages of the species of Helminthosporium parasitic on cereals. Supplement of Proceedings of the Imperial Academy of Japan. 1930;6:352–355. [Google Scholar]
- Jiang Y.L., Zhang T.Y. New species of Bipolaris, Scolecobasidium and Torula from soil. Mycotaxon. 2008;104:135–140. [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger W. Magdeburg and Wien; Germany: 1899. Das Zuckerrohr und seine Kultur [Sugarcane and its cultivation] [Google Scholar]
- Krupinsky J.M., Berdahl J.D., Schoch C.L. Leaf spot on switch grass (Panicum virgatum), symptoms of a new disease caused by Bipolaris oryzae. Canadian Journal of Plant Pathology. 2004;26:371–378. [Google Scholar]
- Kumar J., Schäfer P., Hückelhoven R. Bipolaris sorokiniana, a cereal pathogen of global concern: cytological and molecular approaches towards better control. Molecular Plant Pathology. 2002;3:185–195. doi: 10.1046/j.1364-3703.2002.00120.x. [DOI] [PubMed] [Google Scholar]
- Lau K., Sheridan J.E. Mycoflora of rice (Oryza sativa L.) seed in New Zealand. New Zealand Journal of Agriculture Research. 1975;18:237–250. [Google Scholar]
- Lefebvre C.L., Sherwin S.H. An undescribed species of Helminthosporium on sudan grass and Sorghum. Mycologia. 1949;40:708–716. [PubMed] [Google Scholar]
- Lenne J.M. World list of fungal diseases of tropical pasture species. Phytopathological Papers. 1990;31:1–162. [Google Scholar]
- Leonard K.J., Suggs E.G. Setosphaeria prolata, the ascigerous state of Exserohilum prolatum. Mycologia. 1974;66:197–281. [Google Scholar]
- Leonard K.J. Virulence, temperature optima, and competitive abilities of iso lines of races T and O of Bipolaris maydis. Phytopathology. 1977;67:1273–1279. [Google Scholar]
- Lev S., Sharon A., Hadar R. A mitogen-activated protein kinase of the corn leaf pathogen Cochliobolus heterostrophus is involved in conidiation, appressorium formation, and pathogenicity: diverse roles for mitogenactivated protein kinase homologs in foliar pathogens. Proceedings of National Academy of Sciences, USA. 1999;96:13542–13547. doi: 10.1073/pnas.96.23.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind J. Nordisk Publishers; Copenhagen, Denmark: 1913. Danish fungi. [Google Scholar]
- Link J.H.F. Observationes in ordines plantarum naturales. Dissertatio Ima Gesellschaft Naturforschender Freunde zu Berlin, Magazine. 1809;3:3–42. [Google Scholar]
- Link J.H.F. Species Hyphomycetum et Gymnomycetum. In: Linne C., editor. Species Plantarum. G.C. Nauk; Germany: 1824. pp. 1–33. [Google Scholar]
- Lorang J.M., Sweat T.A., Wolpert T.J. Plant disease susceptibility conferred by a “resistance” gene. Proceedings of the National Academy of Sciences, USA. 2007;104:14861–14866. doi: 10.1073/pnas.0702572104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell E.S. A taxonomic revision of Helminthosporium sativum and related species. American Journal of Botany. 1955;42:57–68. [Google Scholar]
- Luttrell E.S. Taxonomic criteria in Helminthosporium. Mycologia. 1963;55:643–674. [Google Scholar]
- Luttrell E.S. Curvularia coicis and the nodulosa group of Bipolaris. Mycologia. 1969;61:1031–1034. [PubMed] [Google Scholar]
- Luttrell E.S. Correlations between conidial and ascigerous state characters in Pyrenophora, Cochliobolus and Setosphaeria. Revue de Mycologie (Paris) 1977;41:271–279. [Google Scholar]
- Luttrell E.S. Biosystematics in Agriculture, Beltsville Symposia in Agricultural Research. 2nd edn. Allanheld Osmon & Co.; USA: 1978. Biosystematics of Helminthosporium: Impact on agriculture; pp. 193–209. [Google Scholar]
- Madrid H., da Cunha KC., Gené J. Novel Curvularia species from clinical specimens. Persoonia. 2014;33:48–60. doi: 10.3767/003158514X683538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manamgoda D.S., Cai L., Bahkali A.H. Cochliobolus: an overview and current status of species. Fungal Diversity. 2011;51:3–42. [Google Scholar]
- Manamgoda D.S., Cai L., McKenzie E.H.C. A phylogenetic and taxonomic re-evaluation of the Bipolaris – Cochliobolus – Curvularia complex. Fungal Diversity. 2012;56:131–144. [Google Scholar]
- Marignoni G.B. Manifattura nazionale etichette; Italy: 1909. Micromiceti di Schio – Prima Contribuzione alla flora micologica della provincia di vicenza Schio. [Google Scholar]
- Matsumoto T., Yamamoto W. On the perfect and imperfect stages of the fungi causing sugar cane diseases. Journal of Plant Protection – Tokyo. 1936;23:9–14. [Google Scholar]
- Meehan F., Murphy H.C. A new Helminthosporium blight of oats. American Association for the Advancement of Science. 1946;104:412–414. [PubMed] [Google Scholar]
- Meehan E.J. Variation of monomer pressure with degree of conversion in emulsion polymerization of butadiene and of butadiene-styrene. Journal of the American Chemical Society. 1949;71:628–633. [Google Scholar]
- Mehta Y.R., Angra D.C. Somaclonal variation for disease resistance in wheat and production of dihaploids through wheat x maize hybrids. Genetics and Molecular Biology. 2000;23:617–622. [Google Scholar]
- Mena-Portales J., Heredia G., Mercado-Sierra A. Especies de Bipolaris y Curvularia halladas sobre hojas de Quercus y Liquidambar en el estado de Veracruz, México. Revista Mexicana de Micología. 1995;11:109–121. [Google Scholar]
- Miller M.A., Pfeiffer W., Schwartz T. 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees; pp. 1–8. Proceedings of the Gateway Computing Environments Workshop (GCE), November 14, 2010, New Orleans, Louisiana. [Google Scholar]
- Misra A.P. Variability, physiologic specialization and genetics of pathogenicity in graminicolous Helminthosporia affecting cereal crops. Indian Phytopathology. 1979;32:1–22. [Google Scholar]
- Muchovej J.J., Carvalho A.O. A new combination for Helminthosporium euphorbiae. Mycotaxon. 1989;35:159–162. [Google Scholar]
- Müller M.V.G., Germani J.C., Sand ST van der. The use of RAPD to characterize Bipolaris sorokiniana isolates. Genetics and Molecular Research. 2005;4:642–652. [PubMed] [Google Scholar]
- Nakajima H., Isomi K., Hamasaki T. Sorokinianin: a novel phytotoxin produced by the phytopathogenic fungus Bipolaris sorokiniana. Tetrahedron Letters. 1994;35:9597–9600. [Google Scholar]
- Nelson R.R. Cochliobolus intermedius, the perfect stage of Curvularia intermedia. Mycologia. 1960;52:775–778. [Google Scholar]
- Nelson R.R. Cochliobolus victoriae, the perfect stage of Helminthosporium victoriae. Phytopathology. 1960;50:774–775. [Google Scholar]
- Nelson R.R. The perfect stage of Helminthosporium cynodontis. Mycologia. 1964;56:64–69. [Google Scholar]
- Nisikado Y. Studies on Helminthosporium diseases of Graminae in Japan (in Japanese) Reports of the Ohara Institute of Agricultural Research. 1928;4:1–384. [Google Scholar]
- Nisikado T. Leaf Blight of Eragrostis major Host. caused by Ophiobolus kusanoi n. sp., the ascigerous stage of a Helminthosporium. Japanese Journal of Botany. 1928;4:99–112. [Google Scholar]
- Nisikado Y. Studies on the Helminthosporium diseases of Gramineae in Japan. Berichte Des Ohara Institute Landwirtschaftliche Forschungen. 1929;4:111–126. [Google Scholar]
- Nizam S., Verma S., Singh K. High reliability transformation of the wheat pathogen Bipolaris sorokiniana using Agrobacterium tumefaciens. Journal of Microbiological Methods. 2012;88:386–392. doi: 10.1016/j.mimet.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Nylander J.A.A. Evolutionary Biology Centre, Uppsala University; Sweden: 2004. MrModeltest v2. Program distributed by the author. [Google Scholar]
- Ohm R.A., Feau N., Henrissat B. Diverse lifestyles and strategies of plant pathogenesis encoded in the genomes of eighteen Dothideomycetes fungi. PLoS Pathogens. 2012;8:e1003037. doi: 10.1371/journal.ppat.1003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou S.H. 2nd edn. CAB International; UK: 1985. Rice diseases. [Google Scholar]
- Page R.D.M. Treeview: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences. 1996;12 doi: 10.1093/bioinformatics/12.4.357. 357–35. [DOI] [PubMed] [Google Scholar]
- Panchi Z.C.L.A.L., Xiaoqing Z. Identification of resistance of corn hybrids and self-lines to six corn diseases. Acta Agriculturae Boreali-Sinica. 1993 1993–3. [Google Scholar]
- Paul R., Parbery D.G. The perfect stage of Helminthosporium bicolor. Transactions of the British Mycological Society. 1966;49:385–386. [Google Scholar]
- Peng J.H., Lu J.Y. Bipolaris eleusinea. Journal of Nanjing Agricultural University. 1989;12(4):47. [Google Scholar]
- Persoon C.H. Helminthosporium. Mycologia Europea. 1822;1:1–56. [Google Scholar]
- Preston D.A. Host index of Oklahoma plant diseases. Oklahoma Agricultural Colleage. Agricultural Experimental Station Technical Bulletin. 1945:1–168. T-21. [Google Scholar]
- Putterill K.M. Some graminicolous species of Helminthosporium and Curvularia occurring in South Africa. Bothalia. 1954;6:347–378. [Google Scholar]
- Rambaut A., Drummond A. Institute of Evolutionary Biology, University of Edinburgh; UK: 2008. FigTree: tree figure drawing tool, version 1.2.2. [Google Scholar]
- Rayner R.W. Commonwealth Mycological Institute; UK: 1970. A mycological colour chart. [Google Scholar]
- Rehner S. 2001. Primers for elongation factor 1-α (EF1-α)http://ocid.nacse.org/research/deephyphae/EF1primer.pdf [Downloaded 23 February 2012] [Google Scholar]
- Richardson M.J. 4th edn. International Seed Testing Association; Zurich, Switzerland: 1990. An annotated list of seed-borne diseases. [Google Scholar]
- Rossman A.Y., Palm-Hernández M.E. Systematics of plant pathogenic fungi: why it matters. Plant Disease. 2008;92:1376–1386. doi: 10.1094/PDIS-92-10-1376. [DOI] [PubMed] [Google Scholar]
- Rossman A.Y., Manamgoda D.S., Hyde K.D. Proposal to conserve the name Bipolaris against Cochliobolus (Ascomycota: Pleosporales: Pleosporaceae) Taxon. 2013;62:1331–1332. [Google Scholar]
- Rossman A.Y., Manamgoda D.S., Hyde K.D. Proposal to conserve the name Helminthosporium maydis Y. Nisik. & C. Miyake (Bipolaris maydis) against H. maydis Brond. and Ophiobolus heterostrophus (Ascomycota: Pleosporales: Pleosporaceae) Taxon. 2013;62:1332–1333. [Google Scholar]
- Saccardo P.A. Sylloge Hyphomycetum. Sylloge Fungorum. 1886;4:1–807. [Google Scholar]
- Sawada K. Disease of crops in Formosa II, disease of Italian millet. Department of Agriculture Bulletin Formosa. 1912;64:15–19. [Google Scholar]
- Scheffer R.P. Cambridge University Press; Cambridge, UK: 1997. The nature of disease in plants. [Google Scholar]
- Schweinitz LD de. Synopsis Fungorum in America Boreali media degentium. Transactions of the American Philosophical Society Of Philadelfia New Series. 1832;4:131–141. [Google Scholar]
- Shimizu K., Tanaka C., Peng Y.L. Phylogeny of Bipolaris inferred from nucleotide sequences of Brn1, a reductase gene involved in melanin biosynthesis. Journal of General and Applied Microbiology. 1998;44:251–258. doi: 10.2323/jgam.44.251. [DOI] [PubMed] [Google Scholar]
- Shoemaker R.A. Nomenclature of Drechslera and Bipolaris, grass parasites segregated from Helminthosporium. Canadian Journal of Botany. 1959;37:879–887. [Google Scholar]
- Sisterna M.N. Two new species of Bipolaris. Plant Pathology. 1989;38:98–100. [Google Scholar]
- Sivanesan A. New species of Bipolaris. Transactions of the British Mycological Society. 1985;84:403–421. [Google Scholar]
- Sivanesan A. Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Mycological Papers. 1987;158:1–261. [Google Scholar]
- Sorokin N. Ueber einige Krankheiten der Kulturpflanzen im süd-ussurischen Gebiet. Arbeiten der Naturforscher-Gesellschaft bei der Universität Kasan. 1890;22:3–32. [Google Scholar]
- Sprague R. Some Leaf spot Fungi on Western Gramineae: XIII. Mycologia. 1960;52:357–377. [Google Scholar]
- Stamatakis A., Hoover P., Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Subramanian C.V. Fungi imperfecti from Madaras-V Curvularia. Proceedings of the National Academic Science India Section B, Biological Sciences. 1953;38:27–39. [Google Scholar]
- Subramanian C.V., Jain B.L. A revision of some graminicolous Helminthosporia. Current Science. 1966;35:352–355. [Google Scholar]
- Subramanian C.V. Indian Council of Agricultural Research Publishers; India: 1971. Hyphomycetes. An account of Indian species, except Cercosporae. [Google Scholar]
- Subramanian C.V., Bhat V.R. Taxonomy of Drechslera oryzae (Breda de Haan) Subramanian & Jain. A reappraisal. Proceedings of the International Symposium on Taxonomy of Fungi (1973) 1978;1:136–148. [Google Scholar]
- Sugawara F., Strobel G., Fisher L.E. Bipolaroxin, a selective phytotoxin produced by Bipolaris cynodontis. Proceedings of the National Academy of Sciences USA. 1985;82:8291–8294. doi: 10.1073/pnas.82.24.8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Sakuno E., Ishihara A. Conversions of deoxyradicinin to radicinin and of radicinin to 3-epi-radicinin in the phytopathogenic fungus Bipolaris coicis. Phytochemistry. 2012;75:14–20. doi: 10.1016/j.phytochem.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Swofford D. Sinauer Associates; Sunderland, Massachusetts, USA: 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. [Google Scholar]
- Talbot P.H.B. On the genus Helminthosporium sensu lato. Australasian Plant Pathology Society Newsletter. 1973;2:3–7. [Google Scholar]
- Tamura K., Peterson D., Peterson N. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.P., Madrid H., Crous P.W. Johnalcornia gen. et. comb. nov., and nine new combinations in Curvularia based on molecular phylogenetic analysis. Australasian Plant Pathology. 2014 (in press) [Google Scholar]
- Tandon M.P., Bhargava V. Drechslera colocaseae – a new species. Current Science. 1980;49:75–76. [Google Scholar]
- Taylor J.W., Jacobson D.J., Kroken S. Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology. 2000;31:21–32. doi: 10.1006/fgbi.2000.1228. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Ueyama A., Nishihara N. Pseudocochliobolus nisikadoi, the perfect state of Helminthosporium coicis. Mycologia. 1977;69:1109–1120. [Google Scholar]
- Tsuda M., Ueyama A. Two new Pseudocochliobolus and a new species of Curvularia. Transactions of the Mycological Society of Japan. 1985;26:321–330. [Google Scholar]
- Tsukiboshi T., Chung W.H., Yoshida S. Cochliobolus heveicola sp. nov. (Bipolaris heveae) causes brown stripe of bermuda grass and Zoysia grass. Mycoscience. 2005;46:17–21. [Google Scholar]
- Tucker C.M. Helminthosporium gossypii. Journal of Agricultural Research. 1926;32:391–395. [Google Scholar]
- Turgeon B.G., Baker S.E. Genetic and genomic dissection of the Cochliobolus heterostrophus Tox1 locus controlling biosynthesis of the polyketide virulence factor T-toxin. Advances in Genetics. 2007;57:219–261. doi: 10.1016/S0065-2660(06)57006-3. [DOI] [PubMed] [Google Scholar]
- Udayanga D., Castlebury L.A., Rossman A.Y. Species limits in Diaporthe: molecular re-assessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia. 2014;32:83–101. doi: 10.3767/003158514X679984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullstrup A.J. The impacts of the southern corn leaf blight epidemics of 1970–1971. Annual Reviews of Phytopathology. 1972;10:37–50. [Google Scholar]
- Valjavec-Gratian M., Steffenson B.J. Genetics of virulence in Cochliobolus sativus and resistance in barley. Phytopathology. 1997;87:1140–1143. doi: 10.1094/PHYTO.1997.87.11.1140. [DOI] [PubMed] [Google Scholar]
- Valjavec-Gratian M., Steffenson B.J. Pathotypes of Cochliobolus sativus on barley in North Dakota. Plant Disease. 1997;81:1275–1278. doi: 10.1094/PDIS.1997.81.11.1275. [DOI] [PubMed] [Google Scholar]
- Wakker J.H., Went F.A.F.C. Overzicht van de Ziekten van het suikerriet op Java. Mededeelingen van het Proefstation Oost-Java (New Series) 1899;22:11. [Google Scholar]
- Wang Z.Y., Xie S.N., Wang Y. First report of Bipolaris peregianensis causing leaf spot of Cynodon dactylon in China. Plant Disease. 2012;96 doi: 10.1094/PDIS-12-11-1066-PDN. 917–917. [DOI] [PubMed] [Google Scholar]
- Wei J.K., Liu K.M., Chen J.P. Pathological and physiological identification of race C of Bipolaris maydis in China. Phytopathology. 1988;78:550–554. [Google Scholar]
- Wolpert T.J., Macko V., Acklin W. Molecular features affecting the biological activity of the host-selective toxins from Cochliobolus victoriae. Plant Physiology. 1988;88:37–41. doi: 10.1104/pp.88.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W.S. Sorghum disease in Taiwan and characterization and control of its seed borne pathogens. Bulletin of Plant Protection in Taiwan. 1983;25:1–13. [Google Scholar]
- Xiao J.Z., Tsuge T., Doke N. Further evaluation of the significance of BZR-toxin produced by Bipolaris zeicola race 3 in pathogenesis on rice and maize plants. Physiological and Molecular Plant Pathology. 1992;40:359–370. [Google Scholar]
- Yaqoob M., Mann R.A., Iqbal S.M. 2012. Screening of some exotic rice germ plasm against brown spot (Bipolaris oryzae) disease under rainfed conditions. Proceedings of the National Seminar on Role of Agronomy in National Food Security held on January 7 2012, Pakistan: 90. [Google Scholar]
- Yassin M.A., El-Samawaty A.R., Bahkali A. Mycotoxin-producing fungi occurring in sorghum grains from Saudi Arabia. Fungal Diversity. 2010;44:45–52. [Google Scholar]
- Zhang J.Z., Li M.J. A new species of Bipolaris from the halophyte Sesuvium portulacastrum in Guangdong province, China. Mycotaxon. 2009;109:289–300. [Google Scholar]
- Zhang Y., Crous P.W., Schoch C.L. Pleosporales. Fungal Diversity. 2012;53:1–221. doi: 10.1007/s13225-011-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Rossman A.Y., Seifert K. 2013. Impacts of the international code of nomenclature for algae, fungi and plants (Melbourne Code) on the scientific names of plant pathogenic fungi. Online APSnet Feature. [Downloaded 23 December 2013]. http://www.apsnet.org/publications/apsnetfeatures/Pages/Melbourne.asppx. [Google Scholar]
- Zillinsky F.J. CIMMYT; Mexico: 1983. Common diseases of small grain cereals: a guide to identification. [Google Scholar]
- Zummo N., Gourley L.M. Occurrence of target leafspot (Bipolaris sorghicola) on Sorghum in Mississippi. Plant Disease. 1987;71:1045. [Google Scholar]


