Abstract
Purpose
The standard treatment for primary localized gastric gastrointestinal stromal tumor (GIST) is surgical resection. The clinical behavior of gastric GIST after surgical resection is extremely variable. We conducted a multicenter, retrospective study of gastric GISTs patients who underwent curative surgical resection to evaluate clinical features and the prognosis of surgically treated gastric GISTs.
Methods
We performed a retrospective study on 406 consecutive patients who underwent curative resections for localized gastric GIST at four university hospitals in Daegu, Korea, between March 1998 and March 2012. The retrospectively collected medical records were reviewed with respect to clinical parameters including age, gender, tumor location, surgical approach, and recurrence.
Results
There were 406 patients: 157 males (38.7%) and 249 females (61.3%), with a mean age of 60.8 ± 10.8 (standard deviation) years. The mean tumor size was 4.9 cm (range, 0.3-29 cm). Curative surgical resection was performed in all patients without tumor rupture or spillage. Laparoscopic wedge resections were performed in 156 patients (38.4%) and open resections in 250 patients (61.6%). The tumor size of the laparoscopic wedge resection group was smaller than that of open resection group (3.45 cm vs. 5.46 cm; P < 0.001). There were 11 recurrent cases (2.7%). No recurrence was observed in patients who underwent laparoscopic wedge resections.
Conclusion
Gastric GISTs had a low recurrence rate after curative resection in our series. Laparoscopic gastric wedge resection is feasible for treating gastric GISTs in selected patients.
Keywords: Gastrointestinal stromal tumors, Stomach, Prognosis, Laparoscopy
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract [1]. It is well known that GISTs arise from the interstitial cells of Cajal or their common stem cell, and that activating mutations of the KIT or PDGFRA proto-oncogenes are related to the oncogenic development of GISTs [2,3].
The most common symptom of gastric GIST is bleeding, and other symptoms that may be present include dyspepsia, abdominal pain or discomfort, obstructive jaundice, and mass palpation. However, most patients are asymptomatic and are diagnosed incidentally during endoscopy [4].
GISTs represent less than 1% of all gastrointestinal (GI) tract malignancies. The most common location of GISTs is the stomach (70%), but they can occur in all parts of the GI tract, such as the small intestine (20%-30%), colon/rectum (10%), and rarely the omentum, esophagus, and appendix [5]. The annual incidence is 7-14 cases per million in Western countries. The median age is 63 years at the time of diagnosis, and a slight male predominance has been reported [4,6,7]. However, GISTs are reported at any age and their incidence varies according to race and country [4].
The standard treatment for primary localized GIST is surgical resection, to achieve tumor-free margins and an intact tumor capsule. The clinical behavior of GIST after surgical resection is extremely variable. Some tumors follow a benign, indolent course, but others show aggressive clinical courses despite adequate oncologic resection. The prognosis in reported series also varies widely [8,9,10].
Recently, gastric GISTs have attracted interest because of relative higher incidence than that of other GI tract organs, and advances in laparoscopic techniques, which have allowed the minimally invasive resection of tumors [9].
There have only been a few large-scale studies about clinical features and prognosis of surgically treated gastric GISTs. Here, we report a multicenter, retrospective study of 406 cases of gastric GIST patients who underwent curative surgical resection.
METHODS
Patients
This study was conducted at four tertiary referral university hospitals (Daegu Catholic University Medical Center, Kyungpook University Hospital, Keimyung University Dongsan Medical Center, and Yeungnam University Medical Center) in Daegu, Korea. We performed a retrospective study on 406 consecutive patients who underwent curative resection for localized gastric GIST between March 1998 and March 2012. No tumor rupture or spillage occurred during operation. All patients were confirmed as gastric GISTs by immunohistochemical staining for CD117(ckit), CD34, or both.
The retrospectively collected medical records were reviewed with respect to clinical parameters, including age, gender, tumor location, the surgical approach, pathological characteristics, and recurrence. Pathological parameters were assessed, including tumor size, mitotic count, cell type (pure spindle, mixed spindle and epithelioid, pure epithelioid), cellularity, and mucosal invasion. Tumor size was measured and the largest dimension was taken into account. The mitoses were counted on 50 high power fields (HPFs) (×400). Preoperative diagnostic studies included esophagogastroduodenoscopy and CT. Endoscopic ultrasonography was performed selectively to identify the nature and location of tumors. The study design was approved by the institutional review boards of each hospital.
Follow-up clinical study
For the follow-up clinical studies, history taking, physical examination, chest x-ray, gastroduodenoscopy, and abdominal CT scans were carried out biannually until the second postoperative year; after that, annual follow-up was performed. If necessary, abdominal ultrasonography, chest CT scans, and PET scans were performed. Recurrences were investigated retrospectively using the medical records. The median follow-up period was 42.9 months (2-166 months).
Statistical analysis
Summaries of demographic and clinicopathological characteristics were performed using descriptive statistics. The values of means and standard deviations (SDs) are presented for quantitative variables and values of frequency and percent for qualitative variables. Comparisons between groups (open and laparoscopic) were performed using the chi-square test for qualitative variables and two-sample t-test with normality for quantitative variables. All tests were two-sided and P-values less than 0.05 were considered to indicate statistical significance. The IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA) was used for analyses.
RESULTS
Patient characteristics
The clinicopathological characteristics of all patients are shown in Table 1. There were 157 males (38.7%) and 249 females (61.3%), with a mean age of 60.8±10.8 (SD) years. Of the 406 tumors, 200 (49.3%), 122 (30.0%), and 84 (20.7%) were located in the upper, middle, and lower portion of the stomach, respectively. Immunohistochemistry showed that 380 tumors (96.6%) were positive for CD117 and 334 (82.3%) were positive for CD34. Tumor sizes were ≤ 2 cm in 51 patients (12.6%), 2 cm < tumor size ≤ 5 cm in 248 (61.1%), 5 cm < tumor size ≤ 10 cm in 79 (19.5%), and >10 cm in 28 (6.9%), with a mean size of 4.9 cm (range, 0.3-29.0 cm). Mitotic counts (per 50 HPF) were ≤ 5 in 306 patients (75.4%), 5 < mitotic count ≤ 10 in 91 (22.4%), and > 10 in 9 (2.2%).
Table 1.
Clinicopathological characteristics of 406 gastric GIST patients
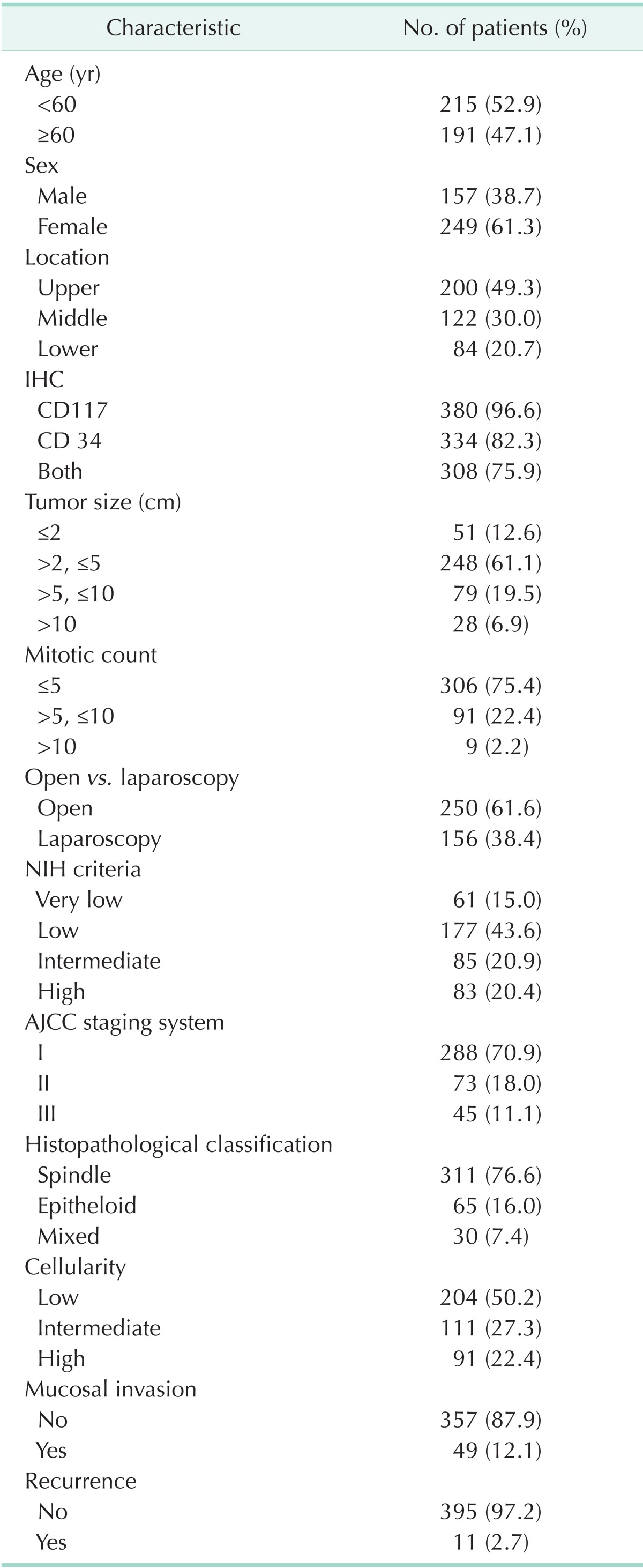
GIST, Gastrointestinal stromal tumor; IHC, Immuno-histo-chemistry; NIH, National Institute of Health; AJCC, American Joint Committee on Cancer, 7th ed.
We classified the patients by risk group according to both of the National Institute of Health (NIH) criteria and the American Joint Committee on Cancer (AJCC) staging system. According to the NIH criteria, 15.0% of patients were classified in the very low risk group, 43.6% were in low risk group, 20.9% were intermediate risk, and 20.4% were high risk. According to AJCC staging, 70.9% of patients were stage I, 18.0% were stage II, and 11.1% were stage III.
Operative method
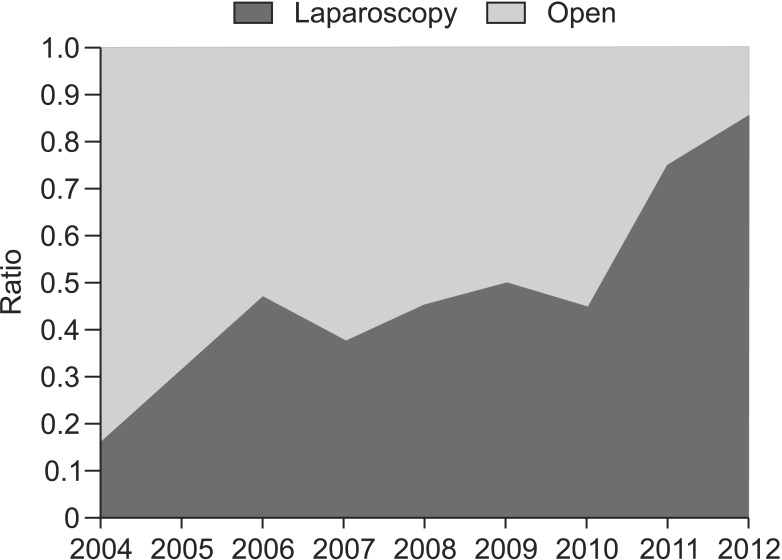
Complete surgical resection was performed for all 406 patients with tumor-free resection margins. No intraoperative tumor rupture or spillage event occurred. Laparoscopic wedge resections were performed for 156 patients (38.4%) and conventional open surgical resections for 250 patients (61.6%). The laparoscopic wedge resection was first performed in 2004, and the proportion of the laparoscopic approach increased annually, reaching 85% in 2012 (Fig. 1). A comparison of the characteristics of open resection group and laparoscopic resection group is shown in Table 2. Compared with open resection group, patients who underwent laparoscopic resection had smaller tumors (3.45 cm vs. 5.46 cm, P < 0.001). There was no significant difference in tumor location or mitotic count. Recurrence occurred in 11 patients who underwent open resection, while no recurrence was observed in laparoscopic resections (P = 0.008).
Fig. 1.
Yearly changes in the proportion of open resection and laparoscopic resection for gastric gastrointestinal stromal tumors (GISTs). There showed an increasing tendency to perform laparoscopic resections for gastric GISTs.
Table 2.
Comparison of open resection group and laparoscopic resection group for patients of gastric GISTs
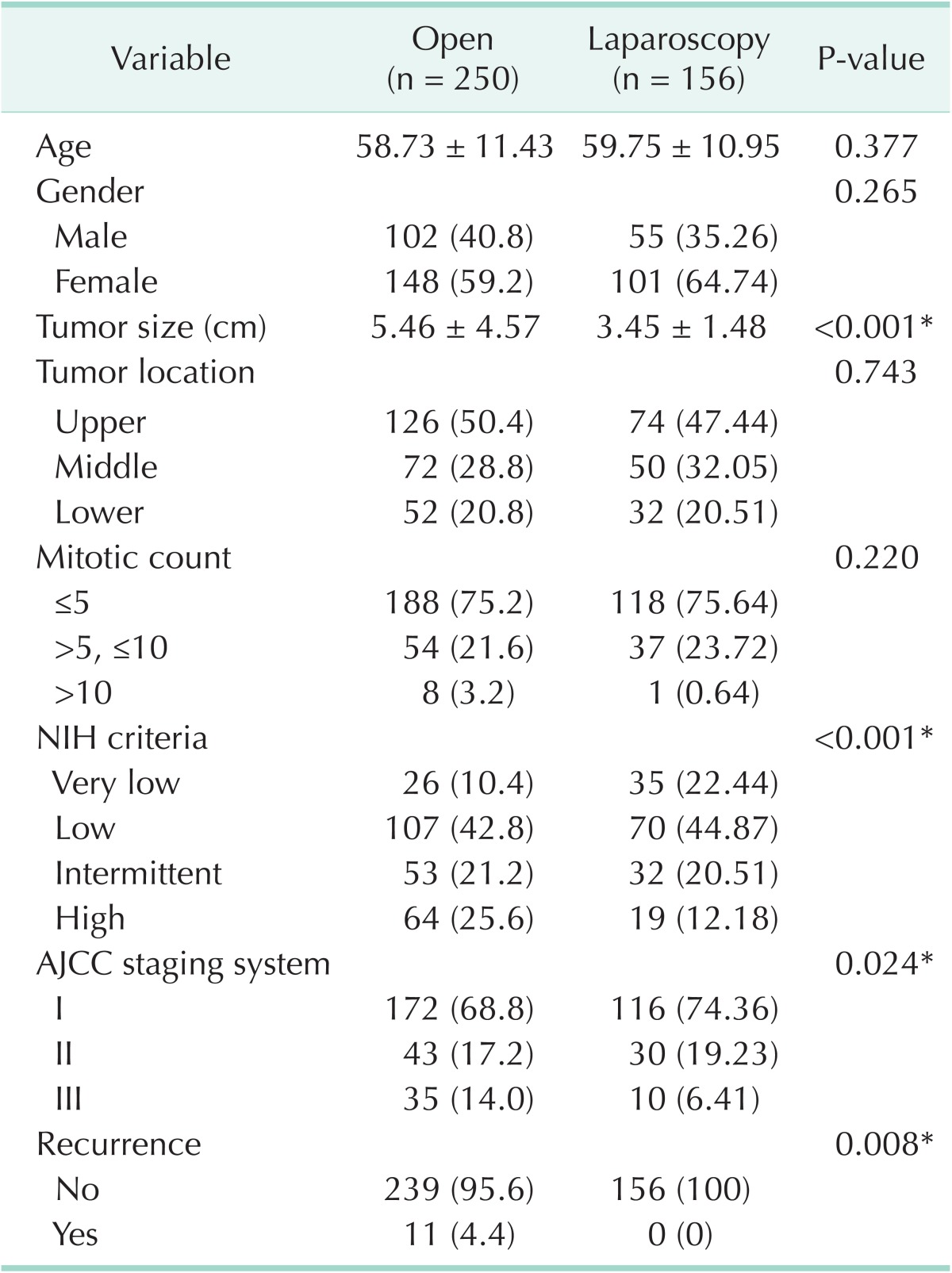
GIST, Gastrointestinal stromal tumor; NIH, National Institute of Health; AJCC, American Joint Committee on Cancer, 7th ed.
Values are presented as mean ± standard deviation or number (%).
*Statistically significant with P < 0.05.
Recurrence
The median follow-up period was 42.9 months (2-166 months) and there were 11 recurrence patients (2.7%). Clinicopathological characteristics of the recurrent cases after curative resection are shown in Table 3. Nine of the eleven recurrent cases were in the high-risk group according to the NIH criteria. Recurrence sites were the liver in nine cases, and peritoneal recurrences were observed in two. All recurrent patients were treated with imatinib mesylate (Gleevec).
Table 3.
Recurrences in patient underwent surgical resection for gastric GISTs
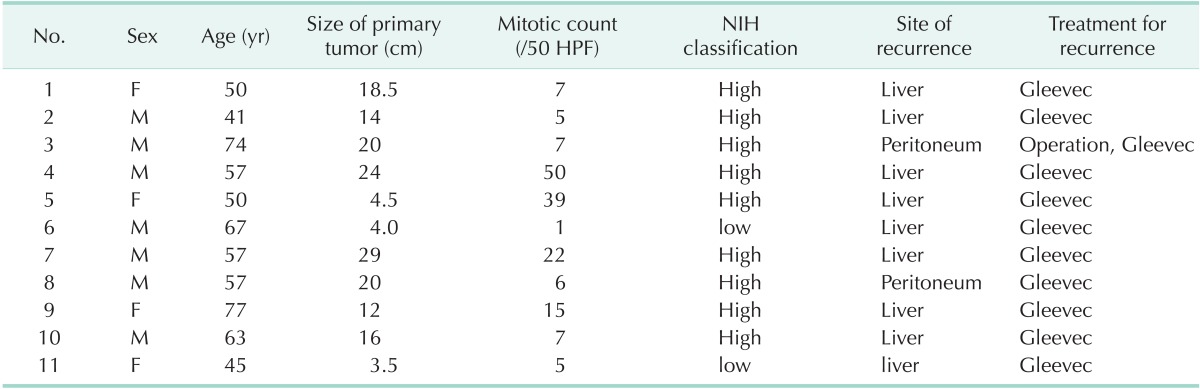
GIST, gastrointestinal stromal tumor; HPF, high power field; NIH, National Institute of Health.
DISCUSSION
We analyzed 406 consecutive patients retrospectively who had undergone resection of gastric GISTs at four centers between March 1998 and March 2012. In previous studies by Miettinen et al. [4] and Tran et al. [7], median age of GIST patients at the diagnosis was 63 and there was a slight male predominance. However, our study showed a female predominance, with mean age of 60.8 years. Several studies in Korean populations have reported female predominance [11,12]. The stomach is the most common site of GISTs, 52%-60% of cases, with the proximal stomach involved in about two-thirds of those patients [13,14]. Similarly, in our study, the upper third was the most common site, involved in 49.3% of patients.
A consensus conference held at the National Institutes of Health in 2001 provided the first practical scheme for the assessment of risk in the clinical course of GISTs. The risk categorization was based on an evaluation of the size and mitotic count (per 50 HPF) of tumors [2]. The NIH criteria have been used widely for risk assessment of GISTs in the clinical field. In 2010, the AJCC TNM staging system included GISTs in an attempt to assist in the management and surveillance of GISTs [15]. Distributions of the risk groups according to both NIH criteria and AJCC 7th staging in our study were similar to previous large-scale studies of gastric GISTs [16,17].
The primary goal of treatment for localized gastric GISTs is surgical resection with negative margins. In the past, it was said that a 1- to 2-cm margin was necessary for adequate resection [4,18]. However, recent studies suggested that extensive resection to obtain wide margin was not necessary and did not improve outcome [14,19]. Thus, it is now accepted that the surgical goal for GIST is complete resection with a negative margin only. Lymphadenectomy is also not indicated generally because of the rarity of lymphatic spread of GISTs [9,10]. For this reason, the laparoscopic wedge resection for gastric GISTs is now used more frequently with the advantages of a small incision, less pain, and oncologic safety [19,20,21].
In Europe, the recommendations of the GIST consensus conference in 2004 were that the laparoscopic approach should be limited to small GISTs (<2 cm) [1] and the Japanese guidelines suggested that the laparoscopic approach was acceptable for lesions smaller than 5 cm and only when performed by skilled surgeons [22]. However, no absolute indication for laparoscopic surgery for GISTs has yet been established. In our study, laparoscopic wedge resections were performed in 156/406 patients (38.4%). We first performed laparoscopic wedge resections in 2004 and the proportion increased annually (Fig. 1). The median tumor size of the patients who underwent laparoscopic wedge resection was 3.45 cm (0.4-8.5 cm), which was significantly smaller than that of open surgery, 5.46 cm (0.3-29 cm). Comparing open and laparoscopic surgery groups, the risk group distribution was also significantly different, but there was no significant difference in mitotic count. Although no definite indication or guideline for laparoscopic surgery was used in our study, tumor size may be a factor that affects the decisions of surgeons as to whether to perform open or laparoscopic surgery.
Although there has been no prospective randomized trial comparing results of laparoscopic and open surgery, several studies have reported the feasibility and oncologic safety of laparoscopic surgery for GISTs [12,19]. In our study there was no recurrence in patients who underwent laparoscopic wedge resection, whereas there were 11 recurrences with open surgery. We also suggest that laparoscopic wedge resection is feasible to treat gastric GISTs for selected patients. However the difference of recurrence rates between open resection and laparoscopic resection groups does not represent oncological safety or superiority of laparoscopic resection. Because tumor size of laparoscopic resection group was significant smaller than open resection group, lower recurrence rate of laparoscopic resection group might be affected by smaller tumor size.
The recurrence rates of gastric GISTs after surgery in previous studies ranged from 17% to 24% [23,24]. In this study, although most of our patients who underwent surgical resection were at very low or low risk group, according to the NIH criteria (238/406, 58.6%), we had a lower recurrence rate (11/406, 2.7%) in our series than those in other Western reports [23,24]. Tumor recurrence tends to be intra-abdominal and it usually occurs in the liver, the peritoneum, or both [9], similar to our results (Table 3). In this study, the most common site of recurrence was the liver (9 patients, 81.8%) followed by the peritoneum (2 patients, 18.2%). In recurrent gastric GISTs, some reports demonstrated that a combination of surgery and targeted therapy may reduce the development of recurrence or decrease the risk of disease progression [5,25]. In this study, imatinib mesylate was administrated to all patients with recurrence, 8/11 (72.7%) of whom showed partial responses to treatment.
Unfortunately, in this study, due to limitations of retrospective study and the small number of recurrence events, we could not assess risk factors affecting recurrence. It is difficult to compare results between previous studies directly, because of wide variety of patient characteristics among the studies. We think possible reasons for the discrepancies in prognosis between prior studies and ours include that we conducted this study only for gastric GISTs, which have a more favorable prognosis than that of intestinal GISTs. There was no intraoperative rupture or spillage of tumors. Also, previous studies included a greater number of large tumors than ours [4,13,14,26].
Recently, with advances in operative methods and early diagnoses, several studies have reported better oncological outcome of gastric GIST undergoing curative resection, especially in Korea and Japan. There are high incidences of gastric cancer in Korea and Japan, so there may be more of a possibility of the early detection of gastric GISTs because diagnostic studies, such as gastroduodenoscopy, are performed more often for the diagnosis and screening of gastric cancer. Furthermore, most gastric GISTs patients in these countries may undergo surgery by a surgeon who specializes in gastric cancer surgery. In Korea, Yang et al. [27] reported that 6 of 105 gastric GIST patients (5.7%) who underwent curative resections had recurrences. Kim et al. [12] also studied gastric GIST patients who underwent curative resection; there were 5 recurrences (4.8%) after surgery. In Japan, Honda et al. [28] reported that only 1 recurrence (1.3%) developed in 78 patients who underwent laparoscopic surgery for gastric GISTs.
In conclusion, there was a lower recurrence rate of gastric GISTs after curative resection in our series than in other Western reports. Laparoscopic gastric wedge resection is feasible to treat gastric GISTs in selected patients and further efforts are necessary to conduct a prospective randomized study to prove the oncological safety and establish a clear indication of this procedure.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566–578. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 3.Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 5.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era: a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 7.Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100:162–168. doi: 10.1111/j.1572-0241.2005.40709.x. [DOI] [PubMed] [Google Scholar]
- 8.Pierie JP, Choudry U, Muzikansky A, Yeap BY, Souba WW, Ott MJ. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg. 2001;136:383–389. doi: 10.1001/archsurg.136.4.383. [DOI] [PubMed] [Google Scholar]
- 9.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan I, You YN, Shyyan R, Dozois EJ, Smyrk TC, Okuno SH, et al. Surgically managed gastrointestinal stromal tumors: a comparative and prognostic analysis. Ann Surg Oncol. 2008;15:52–59. doi: 10.1245/s10434-007-9633-z. [DOI] [PubMed] [Google Scholar]
- 11.Cho MY, Sohn JH, Kim JM, Kim KM, Park YS, Kim WH, et al. Current trends in the epidemiological and pathological characteristics of gastrointestinal stromal tumors in Korea, 2003-2004. J Korean Med Sci. 2010;25:853–862. doi: 10.3346/jkms.2010.25.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KH, Kim MC, Jung GJ, Kim SJ, Jang JS, Kwon HC. Long term survival results for gastric GIST: is laparoscopic surgery for large gastric GIST feasible? World J Surg Oncol. 2012;10:230. doi: 10.1186/1477-7819-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews BD, Joels CS, Kercher KW, Heniford BT. Gastrointestinal stromal tumors of the stomach. Minerva Chir. 2004;59:219–231. [PubMed] [Google Scholar]
- 14.Das A, Wilson R, Biankin AV, Merrett ND. Surgical therapy for gastrointestinal stromal tumours of the upper gastrointestinal tract. J Gastrointest Surg. 2009;13:1220–1225. doi: 10.1007/s11605-009-0885-8. [DOI] [PubMed] [Google Scholar]
- 15.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 16.Kim KM, Kang DW, Moon WS, Park JB, Park CK, Sohn JH, et al. Gastrointestinal stromal tumors in Koreans: it\'s incidence and the clinical, pathologic and immunohistochemical findings. J Korean Med Sci. 2005;20:977–984. doi: 10.3346/jkms.2005.20.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutkowski P, Wozniak A, Debiec-Rychter M, Kakol M, Dziewirski W, Zdzienicki M, et al. Clinical utility of the new American Joint Committee on Cancer staging system for gastrointestinal stromal tumors: current overall survival after primary tumor resection. Cancer. 2011;117:4916–4924. doi: 10.1002/cncr.26079. [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto Y, Nakanishi Y, Yoshimura K, Shimoda T. Clinicopathologic study of primary malignant gastrointestinal stromal tumor of the stomach, with special reference to prognostic factors: analysis of results in 140 surgically resected patients. Gastric Cancer. 2003;6:39–48. doi: 10.1007/s101200300005. [DOI] [PubMed] [Google Scholar]
- 19.Privette A, McCahill L, Borrazzo E, Single RM, Zubarik R. Laparoscopic approaches to resection of suspected gastric gastrointestinal stromal tumors based on tumor location. Surg Endosc. 2008;22:487–494. doi: 10.1007/s00464-007-9493-4. [DOI] [PubMed] [Google Scholar]
- 20.Matthews BD, Walsh RM, Kercher KW, Sing RF, Pratt BL, Answini GA, et al. Laparoscopic vs open resection of gastric stromal tumors. Surg Endosc. 2002;16:803–807. doi: 10.1007/s00464-001-8319-z. [DOI] [PubMed] [Google Scholar]
- 21.Rosen MJ, Heniford BT. Endoluminal gastric surgery: the modern era of minimally invasive surgery. Surg Clin North Am. 2005;85:989–1007. doi: 10.1016/j.suc.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008;13:416–430. doi: 10.1007/s10147-008-0798-7. [DOI] [PubMed] [Google Scholar]
- 23.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 24.Iesalnieks I, Rummele P, Dietmaier W, Jantsch T, Zulke C, Schlitt HJ, et al. Factors associated with disease progression in patients with gastrointestinal stromal tumors in the pre-imatinib era. Am J Clin Pathol. 2005;124:740–748. doi: 10.1309/AKK3-VFF6-10CW-M566. [DOI] [PubMed] [Google Scholar]
- 25.van der Zwan SM, DeMatteo RP. Gastrointestinal stromal tumor: 5 years later. Cancer. 2005;104:1781–1788. doi: 10.1002/cncr.21419. [DOI] [PubMed] [Google Scholar]
- 26.Bucher P, Egger JF, Gervaz P, Ris F, Weintraub D, Villiger P, et al. An audit of surgical management of gastrointestinal stromal tumours (GIST) Eur J Surg Oncol. 2006;32:310–314. doi: 10.1016/j.ejso.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Yang HK, Park do J, Lee HJ, Kim HH, Kim WH, Lee KU. Clinicopathologic characteristics of gastrointestinal stromal tumor of the stomach. Hepatogastroenterology. 2008;55:1925–1930. [PubMed] [Google Scholar]
- 28.Honda M, Hiki N, Nunobe S, Ohashi M, Kiyokawa T, Sano T, et al. Long-term and surgical outcomes of laparoscopic surgery for gastric gastrointestinal stromal tumors. Surg Endosc. 2014;28:2317–2322. doi: 10.1007/s00464-014-3459-0. [DOI] [PubMed] [Google Scholar]