Abstract
The precise mechanism by which sickle erythrocytes (RBC) are removed from the circulation is controversial, although it is possible that enhanced recognition of these cells by circulating mononuclear phagocytes could contribute to this process. We investigated this possibility by interacting sickle cells with cultured human peripheral blood monocytes. Our results show that both irreversibly sickled cells (ISC) and deoxygenated reversibly sickled cells (RSC) had a higher avidity for adherence to monocytes than did oxygenated sickle and normal RBC. ISC were the most adherent cell type. Adherence of RSC to monocytes was found to be reversible; reoxygenation of deoxygenated RSC resulted in a significant decrease in RSC--monocyte adherence. Concomitant with alterations in sickle RBC adherence were alterations in the organization and bilayer distribution of membrane phospholipids in these cells. Specifically, enhanced adherence was associated with increased exposure of RBC membrane outer leaflet phosphatidylserine (PS) and phosphatidylethanolamine, whereas lack of adherence was associated with normal patterns of membrane phospholipid distribution. To investigate the possibility of whether the exposure of PS in the outer membrane leaflet of these cells might be responsible for their recognition by monocytes, the membranes of normal RBC were enriched with the fluorescent PS analogue 1-acyl-2[(N-4-nitro-benzo-2-oxa-1,3-diazole)aminocaproyl]-phosphatidy lse rine (NBD-PS) via transfer of the exogenous lipid from a population of donor phospholipid vesicles (liposomes). RBC enriched with NBD-PS exhibited enhanced adherence to monocytes, whereas adherence of RBC enriched with similar amounts of NBD-phosphatidylcholine (NBD-PC) was not increased. Furthermore, preincubation of monocytes with PS liposomes resulted in a approximately 60% inhibition of ISC adherence to monocytes, whereas no inhibition occurred when monocytes were preincubated with PC liposomes. These findings strongly suggest that erythrocyte surface PS may be a ligand recognized by receptors on human peripheral blood monocytes and that abnormal exposure of PS in the outer leaflet of the RBC membrane, as found in sickle RBC, might serve to trigger their recognition by circulating monocytes. Our results further suggest that abnormalities in the organization of erythrocyte membrane phospholipids may have significant pathophysiologic implications, possibly including shortened cell survival.
Full text
PDF



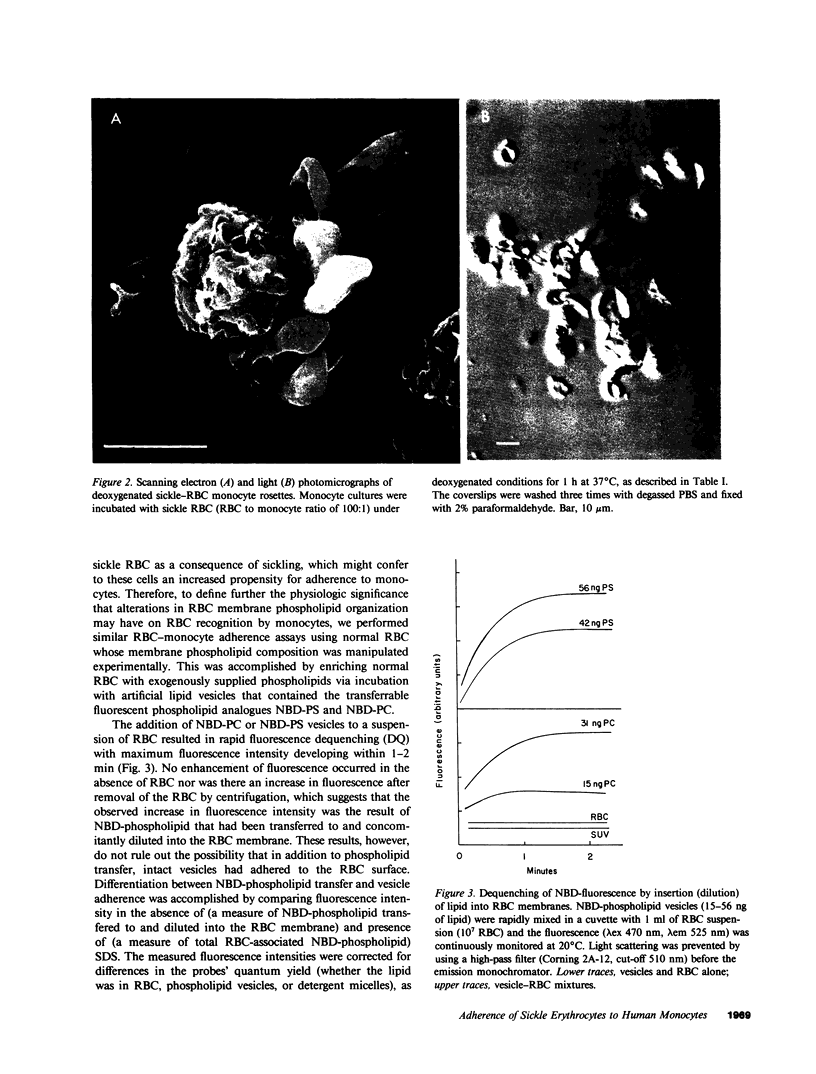



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson N., Lo Buglio A. F., Jandl J. H., Cotran R. S. The interaction between human monocytes and red cells. Binding characteristics. J Exp Med. 1970 Dec 1;132(6):1191–1206. doi: 10.1084/jem.132.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campwala H. Q., Desforges J. F. Membrane-bound hemichrome in density-separated cohorts of normal (AA) and sickled (SS) cells. J Lab Clin Med. 1982 Jan;99(1):25–28. [PubMed] [Google Scholar]
- Chien S., Usami S., Bertles J. F. Abnormal rheology of oxygenated blood in sickle cell anemia. J Clin Invest. 1970 Apr;49(4):623–634. doi: 10.1172/JCI106273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu D., Lubin B., Roelofsen B., van Deenen L. L. Sickled erythrocytes accelerate clotting in vitro: an effect of abnormal membrane lipid asymmetry. Blood. 1981 Aug;58(2):398–401. [PubMed] [Google Scholar]
- Fukuda M., Fukuda M. N., Hakomori S., Papayannopoulou T. Anomalous cell surface structure of sickle cell anemia erythrocytes as demonstrated by cell surface labeling and endo-beta-galactosidase treatment. J Supramol Struct Cell Biochem. 1981;17(3):289–297. doi: 10.1002/jsscb.380170309. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Miller W. J. Phagocytosis of sickle erythrocytes: immunologic and oxidative determinants of hemolytic anemia. Blood. 1984 Sep;64(3):733–741. [PubMed] [Google Scholar]
- Hebbel R. P., Yamada O., Moldow C. F., Jacob H. S., White J. G., Eaton J. W. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest. 1980 Jan;65(1):154–160. doi: 10.1172/JCI109646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R., Rubin R., Wise G., Warren R. Adhesion of normal and sickle erythrocytes to endothelial monolayer cultures. Blood. 1979 Oct;54(4):872–876. [PubMed] [Google Scholar]
- Kleinerman E. S., Schroit A. J., Fogler W. E., Fidler I. J. Tumoricidal activity of human monocytes activated in vitro by free and liposome-encapsulated human lymphokines. J Clin Invest. 1983 Jul;72(1):304–315. doi: 10.1172/JCI110970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin B., Chiu D., Bastacky J., Roelofsen B., Van Deenen L. L. Abnormalities in membrane phospholipid organization in sickled erythrocytes. J Clin Invest. 1981 Jun;67(6):1643–1649. doi: 10.1172/JCI110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Karnovsky M. J. Irreversible deformation of the spectrin-actin lattice in irreversibly sickled cells. J Clin Invest. 1976 Oct;58(4):955–963. doi: 10.1172/JCI108549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti G. V., Cattieu K. Asymmetric metabolism of phosphatidylethanolamine in the human red cell membrane. J Biol Chem. 1982 Jan 10;257(1):245–248. [PubMed] [Google Scholar]
- McCurdy P. R. 32-DFP and 51-Cr for measurement of red cell life span in abnormal hemoglobin syndromes. Blood. 1969 Feb;33(2):214–224. [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Palek J., Thomae M., Ozog D. Red cell calcium content and transmembrane calcium movements in sickle cell anemia. J Lab Clin Med. 1977 Jun;89(6):1365–1374. [PubMed] [Google Scholar]
- Petz L. D., Yam P., Wilkinson L., Garratty G., Lubin B., Mentzer W. Increased IgG molecules bound to the surface of red blood cells of patients with sickle cell anemia. Blood. 1984 Jul;64(1):301–304. [PubMed] [Google Scholar]
- Raz A., Bucana C., Fogler W. E., Poste G., Fidler I. J. Biochemical, morphological, and ultrastructural studies on the uptake of liposomes by murine macrophages. Cancer Res. 1981 Feb;41(2):487–494. [PubMed] [Google Scholar]
- Schroit A. J., Fidler I. J. Effects of liposome structure and lipid composition on the activation of the tumoricidal properties of macrophages by liposomes containing muramyl dipeptide. Cancer Res. 1982 Jan;42(1):161–167. [PubMed] [Google Scholar]
- Schwartz R. S., Düzgünes N., Chiu D. T., Lubin B. Interaction of phosphatidylserine-phosphatidylcholine liposomes with sickle erythrocytes. Evidence for altered membrane surface properties. J Clin Invest. 1983 Jun;71(6):1570–1580. doi: 10.1172/JCI110913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S. D., Hanahan D. J. Identification of domains of phosphatidylcholine in human erythrocyte plasma membranes. Differential action of acidic and basic phospholipases A2 from Agkistrodon halys blomhoffii. J Biol Chem. 1982 Mar 25;257(6):2908–2911. [PubMed] [Google Scholar]
- Tanaka Y., Schroit A. J. Insertion of fluorescent phosphatidylserine into the plasma membrane of red blood cells. Recognition by autologous macrophages. J Biol Chem. 1983 Sep 25;258(18):11335–11343. [PubMed] [Google Scholar]





