Abstract
Purpose
Laparoscopic gastric wedge resection is a standard treatment for removing gastric submucosal tumors (SMTs). So far, however, there have been few reports of single-incision laparoscopic intragastric wedge resection. Our aim was to describe this procedure and our experience with it.
Methods
From January 2010 to December 2013, a total of 21 consecutive patients with gastric SMTs underwent single-incision intragastric resection at our institution. Their clinicopathologic data were analyzed retrospectively.
Results
The patients consisted of nine men and 12 women with a mean age of 51.9 ± 12.9 years (22-69 years). Their mean body mass index was 22.6 ± 2.0 kg/m2. Mean tumor size was 2.4 ± 0.7 cm, with the following anatomic distribution: esophagogastric junction in three patients, fundus in twelve, upper body in three, and lower body in two. Mean operating time was 68.6 ± 12.0 minutes. There were no conversions to open surgery and no major intraoperative complications. Time to resumption of water intake was 1.4 ± 0.5 days. Mean hospital stay was 4.9 ± 1.7 days. There were no recurrences or deaths during the mean 19-month follow-up.
Conclusion
Single-incision intragastric wedge resection is a feasible and safe procedure. It is especially efficient for treating small endophytic gastric SMTs located on the upper and mid portion of the stomach.
Keywords: Laparoscopy, Stomach neoplasm, Gastric mucosa, Gastrectomy, Gastrointestinal stromal tumors
INTRODUCTION
Because gastric submucosal tumors (SMTs) have a potential for malignancy, and it is difficult to obtain histological confirmation through a preoperative biopsy, surgical resection is their main treatment [1]. Local tumor resection with negative surgical margins has become accepted as standard treatment because of the rarity of lymph node metastasis with these lesions. Laparoscopic gastric wedge resection is commonly used for treating gastric SMTs, but operative methods have varied depending on the location and size of the lesion [2,3]. In the past, open gastrectomy was generally performed in patients with gastric SMT, but recent advances in surgical skills and instruments have led to important innovations in laparoscopy. Interest in minimally invasive surgery has increased, and many surgeons are attempting laparoscopic approaches [4,5]. Identifying the location of lesions during laparoscopy is problematic, however, unlike with open surgery where simple tactile sense can be used. This is especially true for small endophytic gastric SMTs, for which intraoperative esophagogastroduodenoscopy (EGD) is frequently required because of the difficulty of determining adequate surgical margins.
Conventional laparoscopic exogastric wedge resection has several disadvantages in that a large amount of stomach must be resected, including normal gastric tissue. Several intragastric studies to identify and resect these tumors under direct visualization for EGD or laparoscopy have been reported, but they have not been widely used. There are only rare reports of laparoscopic intragastric tumor resection through a single incision. Here, we review our experiences with single-incision intragastric resection of gastric SMTs. We describe the outcomes and some characteristic findings.
METHODS
Patients
This study was designed with single-arm, retrospective, case-series study. From January 2010 to December 2013, a total of 21 consecutive patients with gastric SMTs underwent single-incision intragastric resection in the Department of Surgery, Pusan National University Hospital. All patients were diagnosed with gastric SMTs based on preoperative endoscopic ultrasonography results. In each case, the location and type of tumor was confirmed with abdominal computed tomography. A single experienced surgeon skilled in laparoscopic gastric resection performed all the operations. The sex, age, hospital stay, comorbidities, body mass index (BMI), American Society of Anesthesiologists (ASA) score, tumor location, operating time, postoperative complications, and clinicopathologic data were analyzed. Variables were collected retrospectively and were reviewed with regard to the patients' demographics, perioperative data, clinicopathological data, and postoperative course. Cases that met any of the following criteria were excluded: (1) tumor size > 5 cm; (2) exophytic or dumbbell-type growth pattern; (3) tumor located in the lower third of the stomach including the angle.
Surgical procedure
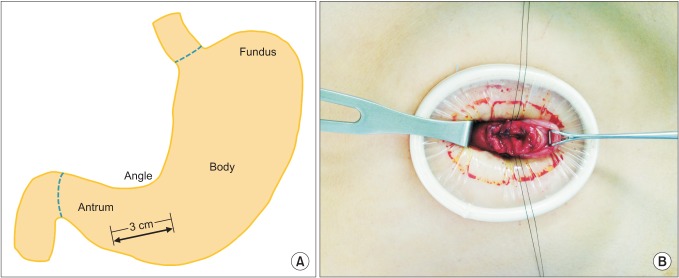
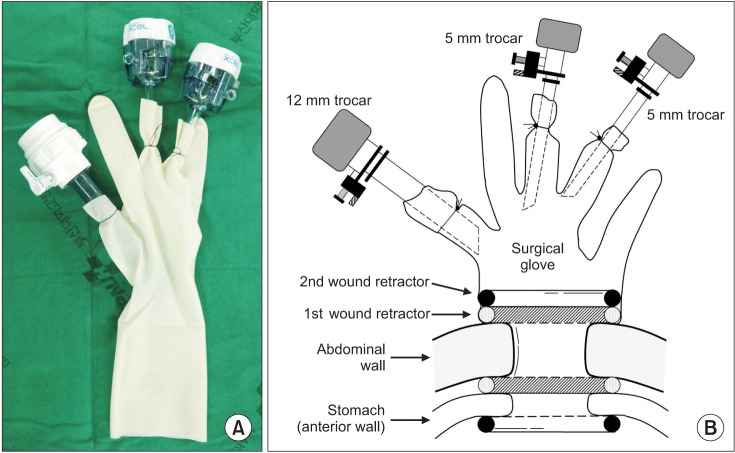
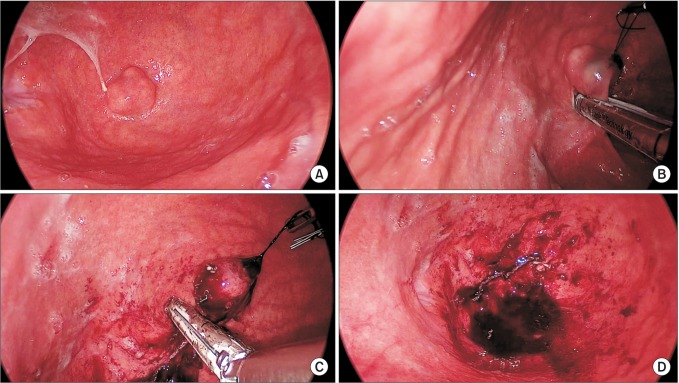
Under general anesthesia, the patient was placed in the supine position. The operator was on the right side of the patient. A split leg position was used for the first assistant to stand between the patient's legs. After making a vertical, approximately 3-cm umbilical incision with unwrinkling of the natural fold (Fig. 1), we applied a 6-cm diameter Alexis wound retractor (extra small; Applied Medical, Rancho Santa Margarita, CA, USA) to the umbilical incision. We identified the location of the stomach and made a 3-cm incision to the lower body, greater curvature side (Fig. 2A). A second Alexis wound retractor was applied to this gastrostomy. Because the stomach is mostly in an upward position from umbilicus, we incised it by pulling down on it, with the stomach being grasped with a Babcock forceps (Fig. 2B). After a surgical glove was applied to the second Alexis wound retractor, a 12-mm port and two 5-mm ports were inserted through first, third, and fourth fingers of the glove (Fig. 3). A direct gaseous inflation of the stomach was performed using a carbon dioxide pressure of up to 12 mmHg. Rigid laparoscopes of 5 mm, 30 cm, and 30o were used for the operation. The tumor was resected with a 45-mm laparoscopic linear stapler (Endo-GIA, Covidien, Elancourt, France) (Fig. 4A-C). We checked for any bleeding along the staple line. Active bleeding was controlled by electrocautery or metal clipping (Fig. 4D). The resected specimen was extracted through the umbilical incision. After removing the second Alexis wound retractor, the gastrostomy was closed with continuous absorbable monofilament suture material or with a 100-mm linear stapler (GIA, Covidien). We then removed the first Alexis wound retractor and closed the peritoneum, fascia, and skin layer by layer.
Fig. 1.

Umbilical incision.
Fig. 2.
(A) Gastric incision after inserting first wound retractor. (B) Pulling the stomach down by grasping with Babcock forcep.
Fig. 3.
(A) Single port with glove, trocar, and wound retractor. (B) Illustration of single port application.
Fig. 4.
(A) Endophytic mass was located at the apex of the fundus of the stomach. (B) Laparoscopic linear stapler was applied to the tumor. Tagging sutures were applied to pull up the tumor. (C) Remaining mass was resected with the second laparoscopic linear stapler. This procedure can be performed more easily through proper articulation of the stapler and tumor traction. (D) Resection line is observed. Bleeding control can be performed with sutures or electrocautery.
RESULTS
Patient demographics
The patients consisted of nine men and 12 women with a mean age of 51.9 ± 12.9 years (22-69 years) and mean BMI of 22.6 ± 2.0 kg/m2. Comorbidities were as follows: hypertension in five, diabetes mellitus in three, asthma in one, ureteral stricture in one, old tuberculosis in one. The ASA scores were all <2.
Clinicopathologic data
The mean tumor size was 2.4 ± 0.7 cm. The anatomic distribution of the tumors were as follows: three were in the esophagogastric junction, Thirteen in the fundus, three in the upper body, and two in the lower body (Fig. 5). Sixteen of the tumors were diagnosed as gastrointestinal stromal tumors (GISTs). According to the US National Institutes of Health classification for risk of malignant potential, one was very low risk, three was low risk, seven were intermediate risk, and five were high risk. In addition, according to the American Joint Committee on Cancer (AJCC), four were classified as stage IA, one as stage IB, ten as stage II, and one as stage IIIB. Patient demographics and clinicopathologic data of each case are presented in Table 1.
Fig. 5.
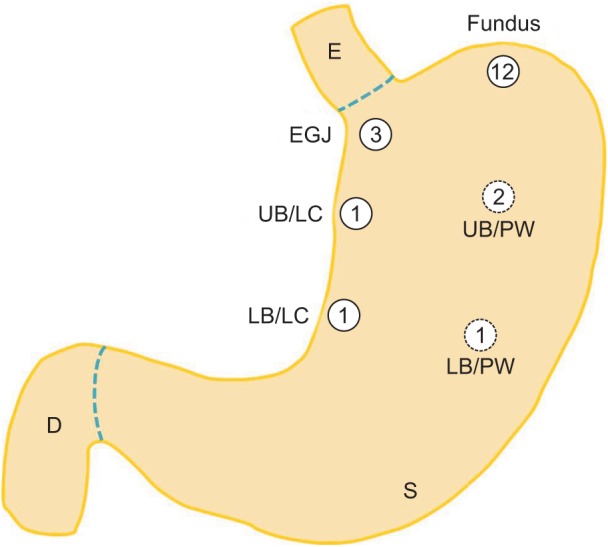
Distribution of submucosal tumors in various portions of the stomach. The number in each circle indicates the frequency. EGJ, esophagogastric junction; UB, upper body; LC, lesser curvature; PW, posterior wall; LB, lower body; E, esophagus; S, stomach; D, duodenum.
Table 1.
Patient demographics and clinicophathologic data (all patients)
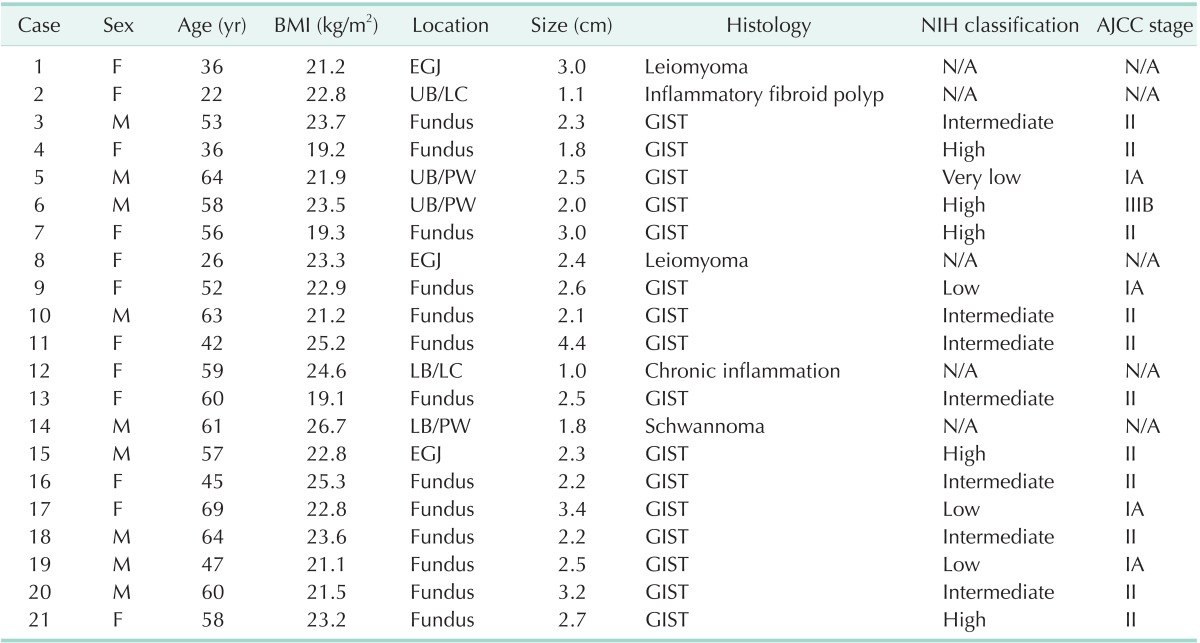
BMI, body mass index; NIH, National Institute of Health; AJCC, American Joint Committee on Cancer; EGJ, esophagogastric junction; N/A, not applicable; UB, upper body; LC, lesser curvature; PW, posterior wall; LB, lower body; GIST, gastrointestinal stromal tumor.
Perioperative data
The mean operating time was 68.6 ± 12.0 minutes. There were no conversions to open surgery or major intraoperative complications. The mean time to resumption of water intake was 1.4 ± 0.5 days. The mean hospital length of stay was 4.9 ± 1.7 days. Postoperative intragastric bleeding occurred in two patients, who underwent endoscopy and were each given a blood transfusion. There were no recurrences or deaths during the mean 19-month follow-up period (Table 2).
Table 2.
Perioperative data
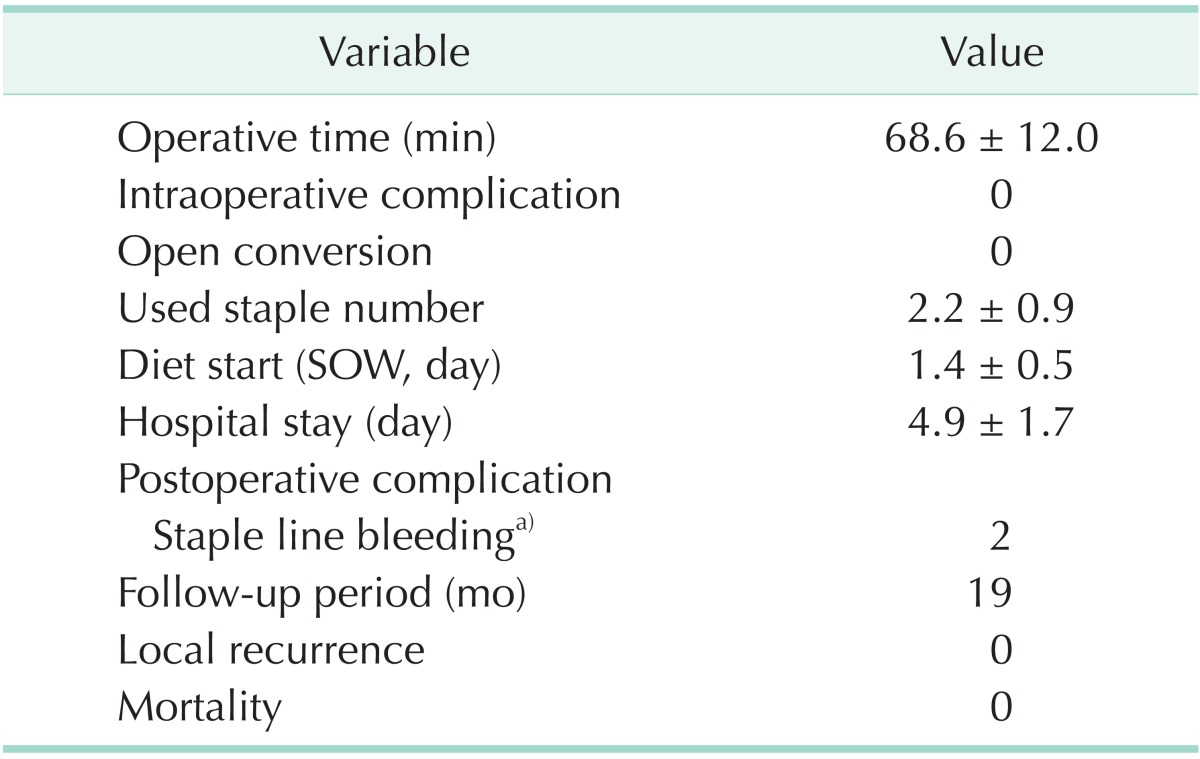
Values are presented as mean±standard deviation or number.
SOW, sips of water.
a)All cases were given a transfusion.
DISCUSSION
Gastric SMTs account for about 2% of all neoplasms of the stomach. They arise from the submucosa or muscle propria layer of the gastric wall [6]. Gastric SMTs are classified as myogenic tumors (e.g., leiomyoma, leiomyosarcoma), neurogenic tumors (e.g., schwannomas, neurofibromas), or GISTs, the latter being most common [7]. Because of less invasiveness and the rarity of regional lymph node metastases, lymph node dissection is unnecessary. Thus, local resection with negative margins is recommended as appropriate treatment [8].
Although conventionally open laparotomy has been performed as standard treatment for gastric SMTs, recent improvements in laparoscopic surgical skills and instruments have allowed expansion of the surgical options [4,5]. De Vogelaere et al. [8] reported that laparoscopic surgery was feasible for gastrointestinal tumors > 2 cm. In a large-scale study, Novitsky et al. [9] demonstrated that the laparoscopic approach produced good surgical results and a long-term disease-free survival of 92% in patients with variously sized tumors (1.0-8.5 cm).
Laparoscopic gastric wedge resection has been recommended as effective, safe treatment of gastric SMTs, although the surgical approach varied according to the growth type and the location of the tumor [2]. Especially in the case of endophytic gastric SMT, it is not easy to determine an adequate resection margin because of the difficulty of confirming the tumor's location. When an endophytic gastric SMT is located on the cardia or prepylorus, partial or total gastrectomy might be needed because complications such as stricture may occur after gastric wedge resection. In such cases, an additional procedure to create an anastomosis might be required to maintain the continuity of the gastric route. Also, when the endophytic gastric SMT is located on the posterior wall, extended dissection may be necessary to obtain a good operative view. Conventional exogastric wedge resection in these patients requires massive resection of normal gastric tissue [3]. Sasaki et al. [2] noted the need for tailored gastric resections based on the varying tumor characteristics of SMTs.
Song et al. [10] and Huguet et al. [11] suggested a transgastric approach as an alternative method for these endophytic tumors. It is novel technique that can preserve normal gastric tissue with a safe resection margin and can identify the tumor location under a direct laparoscopic view. However, the procedure requires incision of the stomach, which can give rise to contamination by gastric juice or tumor dissemination into the peritoneal cavity. Also, intraoperative EGD is often required to confirm the exact location of the gastrostomy.
The intragastric approach we have described has several advantages over the transgastric approach. Although the tumor is small, our method facilitates exact, minimum gastric resection because the operator can readily identify the tumor's location. Also, the risk of intra-abdominal tumor seeding is relatively low because peritoneal spillage of gastric contents rarely occurs during the operation.
Various methods of intragastric wedge resection have been reported. Tagaya et al. [12] introduced an intragastric approach during which the stomach is inflated by airflow via oral endoscopy. After elevating the gastric anterior wall using a double straight needle device, two or three laparoscopic ports were placed in the stomach. Intragastric resection was then performed under endoscopic view with endoscopic linear staplers. Specimens were retrieved through the oral route. Li et al. [13] reported a similar intragastric approach. They inserted two 5-mm trocars and a 12-mm trocar into the stomach. Then, without intraoperative EGD, they resected the tumor through the laparoscopic view. Retrieved tissues were extracted through the 12-mm trocar. Privette et al. [3] and Sahm et al. [14] also reported a hybrid operation to resect the tumor after inserting a trocar into the stomach under an endoscopic view.
In the present study, we performed intragastric resection of the endophytic mass located on the upper and mid portion of the stomach through a single umbilical hole without multiple gastric entry holes. The gastrostomy can be easily extended using the wound retractor. Thus, the resected specimen was extracted more safely and easily than when using other intragastric approaches in which the resected specimen was removed via the oral route or through a 12-mm laparoscopic trocar.
Recently, laparoscopic and endoscopic cooperative surgery (LECS) has been accepted as a good treatment choice for gastric SMTs. This technique was also introduced by Tsujimoto et al. [15] as a hybrid operation using endoscopic submucosal dissection and laparoscopic resection. It is obvious that LECS has several advantages in terms of exact identification of the tumor location and facilitating the minimum gastric resection. But LECS needs an experienced endoscopist and takes longer operative time (157.0 ± 68.4 minutes) than our intragastric approach.
We could perform meticulous resection without intraoperative EGD under the good laparoscopic view, preserving normal gastric tissue like LECS. This simplicity-avoiding intraoperative EGD and closing the single gastrostomy without additional trocars-helps decrease the operating time. Also, the small skin incision provides a better cosmetic effect by maintaining the natural fold of the umbilicus.
The mean operating time was 68.6 ± 12.0 minutes, which is not significantly different from that required for our open gastric wedge resection, reported elsewhere (62.7 ± 14.0 minutes) [16]. It is also acceptable result compared to other conventional exogastric wedge resection. In an analysis of the operating time for the individual cases, we were unable to confirm a significant correlation of the surgeon's experience with the length of the operation, which ranged between approximately 40 and 80 minutes. This finding indicates that our intragastric resection is an accessible technique that can be performed without a learning curve if the operator has some experience with laparoscopic surgery.
The pathology reports showed that the tumors were GISTs in sixteen cases, leiomyomas in two, schwannoma in one and others in two. There were no recurrences during the mean 19-month follow-up period. However, long-term follow-up is needed to confirm disease progress because the patient data did include some high-risk tumors according to the National Institute of Health classification and stage IIIB tumors according to AJCC staging.
Postoperative intragastric bleeding occurred in two patients, who recovered after preventive blood transfusion with no other specific intervention. Lesser and greater curvature of stomach have high vascularity which can lead to postoperative bleeding, however, the all bleeding complications in our study occurred on fundus during the early operative period. Although postoperative intragastric bleeding may be often minor complication not to progress to fatal course, if it is concerned, hemostasis with electrocautery, metal clipping or suture on resection line is enough to prevent them. Actually, there was no further bleeding complication after we performed such meticulous manipulations.
Although there was some conflict between laparoscopic instruments in the narrow operative field-a characteristic of single-port surgery-we were able to perform singleport laparoscopic surgery comfortably without additional instruments. If forceps that enabled articulation were available, we could perform an easier, safer operation. As it is easy to damage the gastric mucosa by the forceps because of the narrow operative field, cautious manipulation is required under a direct visual field.
A mean of 2.2 ± 0.9 laparoscopic linear staplers were used, indicating little difference from the number used during conventional exogastric wedge resection. If tumor traction was easy or the tumor base was not wide, we could resect the tumor without difficulty using one or two laparoscopic linear staplers. If not, we needed more.
The single-port method in this study did not require pneumoperitoneum to explore the abdominal cavity because of direct gastric inflation with carbon dioxide gas. Tagaya et al. [17] introduced a jejunal clamping method with bowel clamp forceps and inserted a balloon catheter orally into the duodenum to prevent gaseous distension of the intestine during an intragastric approach. Our patients did not experience any complications related to postoperative intestinal gas after pneumoperitoneum of 12 mmHg. If this is a concern, it may be sufficient to insert a Levin tube for a day after the operation.
The study has some limitations. First, there were no large endophytic gastric SMTs. The mean tumor size was 2.4 cm (range, 1.0-4.4 cm). Large tumors were excluded from this study because of the difficulty of manipulating them and the risk of tumor rupture. In the Japanese Clinical Practice Guideline, gastric SMTs < 5 cm are included in the indications for laparoscopic surgery [18]. Hence, we used the cutoff of tumors ≥ 5 cm because we had no experience with them regarding intragastric resection. Although excellent longterm survivals are reported for patients with relatively large gastric SMTs, there is still a lack of long-term survival reported after intragastric resection. Thus, a judicious approach was needed. The second limitation is the location of the tumor. Our procedure was valuable for resecting endophytic tumors located on the esophagogastric junction, gastric fundus, and body.
The cases in which surgery was performed on the angle and antrum of the stomach (not included in this study) posed several tiresome problems, such as extreme narrowing of the operative field or limited articulation of the linear stapler caused by the gastric structure. These restrictions made the procedure more difficult. Therefore, we think that a different approach should be attempted for lesions distal to the gastric angle. Also, as the gastrostomy is made on the gastric anterior wall, all laparoscopic instruments were controlled in the direction from the anterior wall to the posterior wall of the stomach. Therefore, this procedure would not be appropriate for an anterior tumor that is not high-lying. We think that the eversion technique, suggested by Hyung et al. [19], is more effective for these tumors.
Single-incision intragastric resection is feasible and safe procedure. It is especially efficient for removing small endophytic gastric SMTs located in the upper and mid portion of the stomach. Despite the small number of cases and lack of long-term follow-up data in our study, this procedure may be one of good treatment option for gastric SMTs, we think.
ACKNOWLEDGEMENTS
This study was supported by a grant (0920050) from the National R&D Program for Cancer Control, Ministry for Health, Welfare, and Family Affairs, Republic of Korea.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Berindoague R, Targarona EM, Feliu X, Artigas V, Balague C, Aldeano A, et al. Laparoscopic resection of clinically suspected gastric stromal tumors. Surg Innov. 2006;13:231–237. doi: 10.1177/1553350606295960. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki A, Koeda K, Obuchi T, Nakajima J, Nishizuka S, Terashima M, et al. Tailored laparoscopic resection for suspected gastric gastrointestinal stromal tumors. Surgery. 2010;147:516–520. doi: 10.1016/j.surg.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 3.Privette A, McCahill L, Borrazzo E, Single RM, Zubarik R. Laparoscopic approaches to resection of suspected gastric gastrointestinal stromal tumors based on tumor location. Surg Endosc. 2008;22:487–494. doi: 10.1007/s00464-007-9493-4. [DOI] [PubMed] [Google Scholar]
- 4.Matthews BD, Walsh RM, Kercher KW, Sing RF, Pratt BL, Answini GA, et al. Laparoscopic vs open resection of gastric stromal tumors. Surg Endosc. 2002;16:803–807. doi: 10.1007/s00464-001-8319-z. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa K, Inomata M, Etoh T, Shiromizu A, Shiraishi N, Arita T, et al. Long-term outcome of laparoscopic wedge resection for gastric submucosal tumor compared with open wedge resection. Surg Laparosc Endosc Percutan Tech. 2006;16:82–85. doi: 10.1097/00129689-200604000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566–578. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 7.Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293–1301. doi: 10.14670/HH-15.1293. [DOI] [PubMed] [Google Scholar]
- 8.De Vogelaere K, Van Loo I, Peters O, Hoorens A, Haentjens P, Delvaux G. Laparoscopic resection of gastric gastrointestinal stromal tumors (GIST) is safe and effective, irrespective of tumor size. Surg Endosc. 2012;26:2339–2345. doi: 10.1007/s00464-012-2186-7. [DOI] [PubMed] [Google Scholar]
- 9.Novitsky YW, Kercher KW, Sing RF, Heniford BT. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. 2006;243:738–745. doi: 10.1097/01.sla.0000219739.11758.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song KY, Kim SN, Park CH. Tailored-approach of laparoscopic wedge resection for treatment of submucosal tumor near the esophagogastric junction. Surg Endosc. 2007;21:2272–2276. doi: 10.1007/s00464-007-9369-7. [DOI] [PubMed] [Google Scholar]
- 11.Huguet KL, Rush RM, Jr, Tessier DJ, Schlinkert RT, Hinder RA, Grinberg GG, et al. Laparoscopic gastric gastrointestinal stromal tumor resection: the mayo clinic experience. Arch Surg. 2008;143:587–590. doi: 10.1001/archsurg.143.6.587. [DOI] [PubMed] [Google Scholar]
- 12.Tagaya N, Mikami H, Kubota K. Laparoscopic resection of gastrointestinal mesenchymal tumors located in the upper stomach. Surg Endosc. 2004;18:1469–1474. doi: 10.1007/s00464-004-8800-6. [DOI] [PubMed] [Google Scholar]
- 13.Li VK, Hung WK, Chung CK, Ying MW, Lam BY, Kan DM, et al. Laparoscopic intragastric approach for stromal tumours located at the posterior gastric wall. Asian J Surg. 2008;31:6–10. doi: 10.1016/S1015-9584(08)60047-0. [DOI] [PubMed] [Google Scholar]
- 14.Sahm M, Pross M, Lippert H. Intraluminal resection of gastric tumors using intragastric trocar technique. Surg Laparosc Endosc Percutan Tech. 2011;21:e169–e172. doi: 10.1097/SLE.0b013e318221749c. [DOI] [PubMed] [Google Scholar]
- 15.Tsujimoto H, Yaguchi Y, Kumano I, Takahata R, Ono S, Hase K. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J Surg. 2012;36:327–330. doi: 10.1007/s00268-011-1387-x. [DOI] [PubMed] [Google Scholar]
- 16.Choi CI, Kim DH, Lee SH, Kim JH, Hwang SH, Jeon TY, et al. The comparison between laparoscopic wedge resection and open wedge resection for gastric gastrointestinal stromal tumors. J Korean Soc Endosc Laparosc Surg. 2010;13:6–10. [Google Scholar]
- 17.Tagaya N, Mikami H, Kogure H, Kubota K, Hosoya Y, Nagai H. Laparoscopic intragastric stapled resection of gastric submucosal tumors located near the esophagogastric junction. Surg Endosc. 2002;16:177–179. doi: 10.1007/s004640080158. [DOI] [PubMed] [Google Scholar]
- 18.Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008;13:416–430. doi: 10.1007/s10147-008-0798-7. [DOI] [PubMed] [Google Scholar]
- 19.Hyung WJ, Lim JS, Cheong JH, Kim J, Choi SH, Noh SH. Laparoscopic resection of a huge intraluminal gastric submucosal tumor located in the anterior wall: eversion method. J Surg Oncol. 2005;89:95–98. doi: 10.1002/jso.20195. [DOI] [PubMed] [Google Scholar]