Abstract
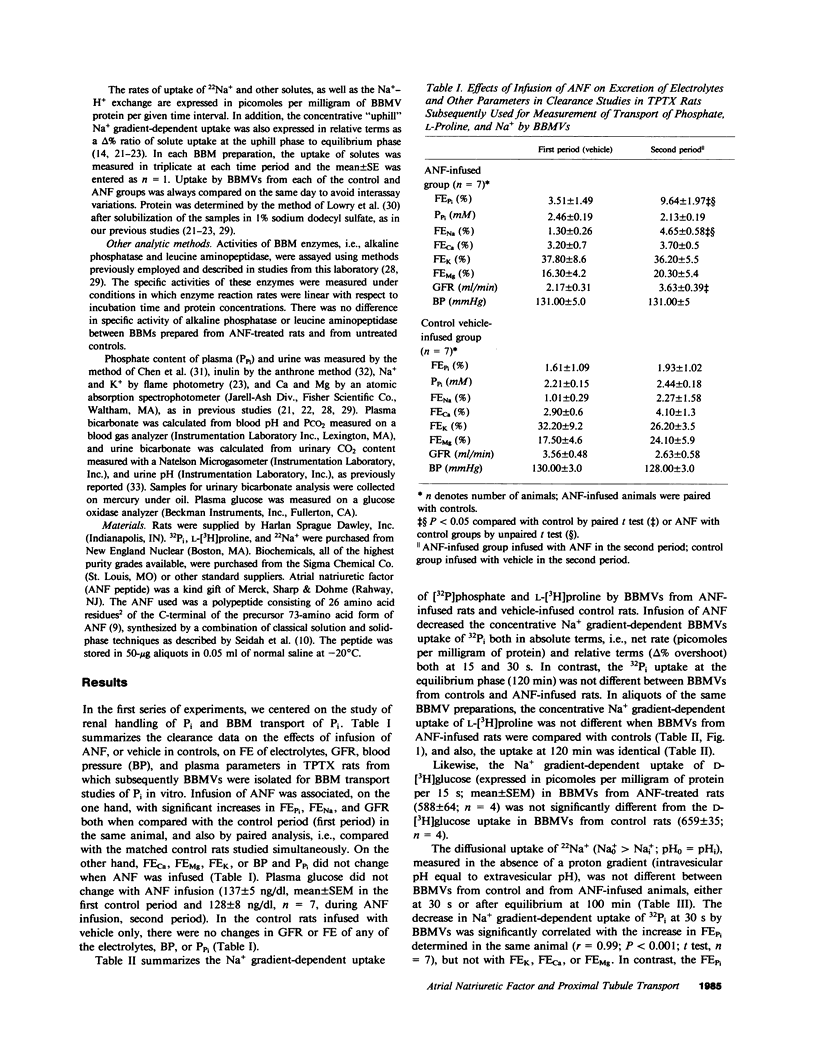
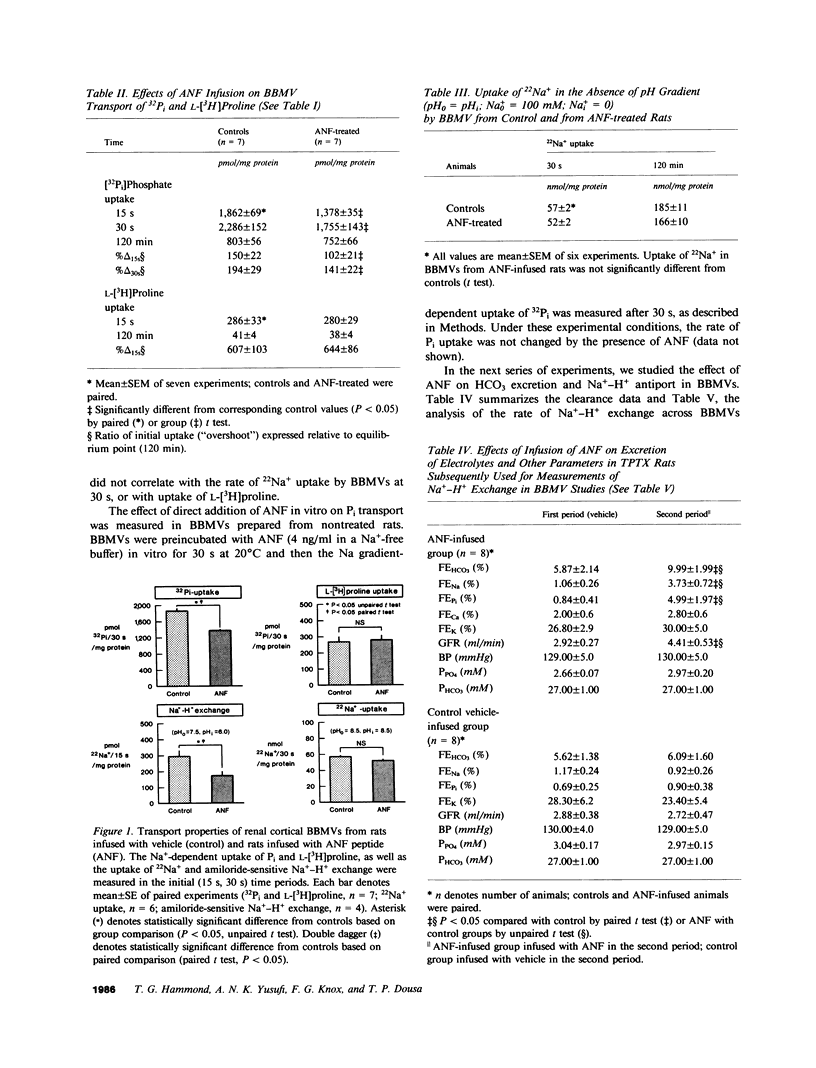
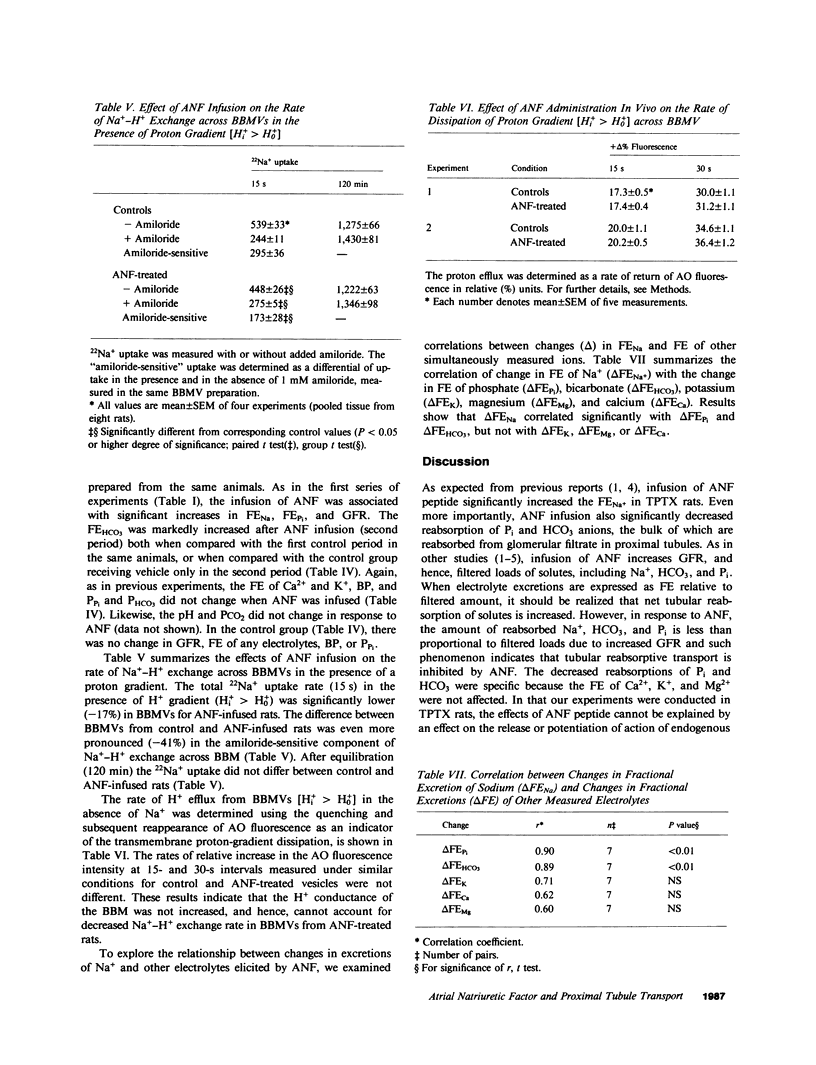
The newly discovered peptides extracted from cardiac atria, atrial natriuretic factors (ANFs), when administered parenterally cause renal hemodynamic changes and natriuresis. The nephron sites and cellular mechanism accounting for profound increase in Na+ excretion in response to ANFs are not yet clarified. In the present study we investigated whether synthetic ANF peptide alters the reabsorption of Na+ and reabsorption of solutes cotransported with Na+ in the proximal tubules of rats. Synthetic ANF peptide consisting of 26 amino acids, 4 micrograms/kg body wt/h, or vehicle in controls, was infused to surgically thyroparathyroidectomized anesthetized rats. After determination of the fractional excretion (FE) of electrolytes (Na+, K+, Pi, Ca2+, Mg2+, HCO3), the kidneys were removed and luminal brush border membrane vesicles (BBMVs) were prepared from renal cortex. Solute transport was measured in BBMVs by rapid filtration techniques. Infusion of ANF peptide increased FENa, FEPi, and FEHCO3; but FECa, FEK, and FEMg were not changed. The increase in FENa was significantly correlated, on the one hand, with increase of FEPi (r = 0.9, n = 7; P less than 0.01) and with increase of FEHCO3 (r = 0.89, n = 7; P less than 0.01). On the other hand, FENa did not correlate with FEK, FECa, or with FEMg. The Na+ gradient-dependent uptake of Pi by BBMVs prepared from renal cortex of rats receiving ANF infusion was significantly (P less than 0.05) decreased (-25%), whereas the Na+ gradient-dependent uptake of L-[3H]proline and of D-[3H]glucose or the diffusional uptake of 22Na+ were not changed. ANF-elicited change in FEPi showed a close inverse correlation with decrease of Na+-dependent Pi uptake by BBMVs isolated from infused rats (r = 0.99, n = 7; P less than 0.001). Direct addition of ANF to BBMVs in vitro did not change the Na+ gradient-dependent Pi uptake. In rats infused with ANF, the rate of amiloride-sensitive Na+-H+ exchange across the brush border membrane (BBM) was significantly (P less than 0.05) decreased (-40%), whereas the diffusional 22Na+ uptake (0.5 min) and the equilibrium (120 min) uptake of 22Na+ were not changed. The inhibition of Na+-H+ exchange after ANF was likely due to alteration of the BBM antiporter itself, in that the H+ conductance of BBMVs was not increased. We conclude that synthetic ANF (a) decreases tubular Na+ reabsorption linked to reabsorption of HCO3 in proximal tubules, and (b) inhibits proximal tubular reabsorption of Pi coupled to Na+ reabsorption, independent of secretion and/or action of parathyroid hormone or calcitonin. These ANF effects are associated with inhibition of Na+-Pi synport and of Na+-H+ antiport in luminal BBMs. Our findings document that inhibition of Na+-coupled transport processes in proximal tubules is an integral part of the renal response to ANF.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus Z. S., Wasserstein A., Goldfarb S. PTH, calcitonin, cyclic nucleotides and the kidney. Annu Rev Physiol. 1981;43:583–595. doi: 10.1146/annurev.ph.43.030181.003055. [DOI] [PubMed] [Google Scholar]
- Atarashi K., Mulrow P. J., Franco-Saenz R., Snajdar R., Rapp J. Inhibition of aldosterone production by an atrial extract. Science. 1984 Jun 1;224(4652):992–994. doi: 10.1126/science.6326267. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs J. P., Steipe B., Schubert G., Schnermann J. Micropuncture studies of the renal effects of atrial natriuretic substance. Pflugers Arch. 1982 Dec;395(4):271–276. doi: 10.1007/BF00580789. [DOI] [PubMed] [Google Scholar]
- Burnett J. C., Jr, Granger J. P., Opgenorth T. J. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol. 1984 Nov;247(5 Pt 2):F863–F866. doi: 10.1152/ajprenal.1984.247.5.F863. [DOI] [PubMed] [Google Scholar]
- Chantrelle B., Cogan M. G., Rector F. C., Jr Evidence for coupled sodium/hydrogen exchange in the rat superficial proximal convoluted tubule. Pflugers Arch. 1982 Nov 11;395(3):186–189. doi: 10.1007/BF00584807. [DOI] [PubMed] [Google Scholar]
- Cohn D. E., Hruska K. A., Klahr S., Hammerman M. R. Increased Na+-H+ exchange in brush border vesicles from dogs with renal failure. Am J Physiol. 1982 Sep;243(3):F293–F299. doi: 10.1152/ajprenal.1982.243.3.F293. [DOI] [PubMed] [Google Scholar]
- Cohn D. E., Klahr S., Hammerman M. R. Metabolic acidosis and parathyroidectomy increase Na+-H+ exchange in brush border vesicles. Am J Physiol. 1983 Aug;245(2):F217–F222. doi: 10.1152/ajprenal.1983.245.2.F217. [DOI] [PubMed] [Google Scholar]
- Dousa T. P., Kempson S. A. Regulation of renal brush border membrane transport of phosphate. Miner Electrolyte Metab. 1982 Mar;7(3):113–121. [PubMed] [Google Scholar]
- Espinosa R. E., Keller M. J., Yusufi A. N., Dousa T. P. Effect of thyroxine administration on phosphate transport across renal cortical brush border membrane. Am J Physiol. 1984 Feb;246(2 Pt 2):F133–F139. doi: 10.1152/ajprenal.1984.246.2.F133. [DOI] [PubMed] [Google Scholar]
- Evers C., Murer H., Kinne R. Effect of parathyrin on the transport properties of isolated renal brush-border vesicles. Biochem J. 1978 Apr 15;172(1):49–56. doi: 10.1042/bj1720049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Flynn T. G., de Bold M. L., de Bold A. J. The amino acid sequence of an atrial peptide with potent diuretic and natriuretic properties. Biochem Biophys Res Commun. 1983 Dec 28;117(3):859–865. doi: 10.1016/0006-291x(83)91675-3. [DOI] [PubMed] [Google Scholar]
- Freiberg J. M., Kinsella J., Sacktor B. Glucocorticoids increase the Na+-H+ exchange and decrease the Na+ gradient-dependent phosphate-uptake systems in renal brush border membrane vesicles. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4932–4936. doi: 10.1073/pnas.79.16.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J. J., Edwards R. M. Natriuretic hormones: at last, bottled in bond? J Lab Clin Med. 1984 Mar;103(3):333–336. [PubMed] [Google Scholar]
- Hruska K. A., Klahr S., Hammerman M. R. Decreased luminal membrane transport of phosphate in chronic renal failure. Am J Physiol. 1982 Jan;242(1):F17–F22. doi: 10.1152/ajprenal.1982.242.1.F17. [DOI] [PubMed] [Google Scholar]
- Kempson S. A., Berndt T. J., Turner S. T., Zimmerman D., Knox F., Dousa T. P. Relationship between renal phosphate reabsorption and renal brush-border membrane transport. Am J Physiol. 1983 Feb;244(2):R216–R223. doi: 10.1152/ajpregu.1983.244.2.R216. [DOI] [PubMed] [Google Scholar]
- Kempson S. A., Colon-Otero G., Ou S. Y., Turner S. T., Dousa T. P. Possible role of nicotinamide adenine dinucleotide as an intracellular regulator of renal transport of phosphate in the rat. J Clin Invest. 1981 May;67(5):1347–1360. doi: 10.1172/JCI110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempson S. A., Shah S. V., Werness P. G., Berndt T., Lee P. H., Smith L. H., Knox F. G., Dousa T. P. Renal brush border membrane adaptation to phosphorus deprivation: effects of fasting versus low-phosphorus diet. Kidney Int. 1980 Jul;18(1):36–47. doi: 10.1038/ki.1980.108. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1981 Oct;241(4):F374–F379. doi: 10.1152/ajprenal.1981.241.4.F374. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Properties of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1980 Jun;238(6):F461–F469. doi: 10.1152/ajprenal.1980.238.6.F461. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Michael U. F., Kelley J., Vaamonde C. A. Impaired renal bicarbonate reabsorption in the hypothyroid rat. Am J Physiol. 1979 Jun;236(6):F536–F540. doi: 10.1152/ajprenal.1979.236.6.F536. [DOI] [PubMed] [Google Scholar]
- Murer H., Kinne R. The use of isolated membrane vesicles to study epithelial transport processes. J Membr Biol. 1980 Jul 15;55(2):81–95. doi: 10.1007/BF01871151. [DOI] [PubMed] [Google Scholar]
- NATELSON S. Routine use of ultramicro methods in the clinical laboratory; estimation of sodium, potassium, chloride, protein, hematocrit value, sugar, urea and nonprotein nitrogen in fingertip blood; construction of ultramicro pipets; a practical microgasometer for estimation of carbon dioxide. Am J Clin Pathol. 1951 Dec;21(12):1153–1172. [PubMed] [Google Scholar]
- Oshima T., Currie M. G., Geller D. M., Needleman P. An atrial peptide is a potent renal vasodilator substance. Circ Res. 1984 May;54(5):612–616. doi: 10.1161/01.res.54.5.612. [DOI] [PubMed] [Google Scholar]
- Pamnani M. B., Clough D. L., Chen J. S., Link W. T., Haddy F. J. Effects of rat atrial extract on sodium transport and blood pressure in the rat. Proc Soc Exp Biol Med. 1984 Jun;176(2):123–131. doi: 10.3181/00379727-176-41851. [DOI] [PubMed] [Google Scholar]
- Pollock D. M., Mullins M. M., Banks R. O. Failure of atrial myocardial extract to inhibit renal Na+, K+-ATPase. Ren Physiol. 1983;6(6):295–299. doi: 10.1159/000172915. [DOI] [PubMed] [Google Scholar]
- Reenstra W. W., Warnock D. G., Yee V. J., Forte J. G. Proton gradients in renal cortex brush-border membrane vesicles. Demonstration of a rheogenic proton flux with acridine orange. J Biol Chem. 1981 Nov 25;256(22):11663–11666. [PubMed] [Google Scholar]
- Sabolić I., Burckhardt G. Effect of the preparation method on Na+-H+ exchange and ion permeabilities in rat renal brush-border membranes. Biochim Biophys Acta. 1984 May 16;772(2):140–148. doi: 10.1016/0005-2736(84)90037-3. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Lazure C., Chrétien M., Thibault G., Garcia R., Cantin M., Genest J., Nutt R. F., Brady S. F., Lyle T. A. Amino acid sequence of homologous rat atrial peptides: natriuretic activity of native and synthetic forms. Proc Natl Acad Sci U S A. 1984 May;81(9):2640–2644. doi: 10.1073/pnas.81.9.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg H., Cupples W. A., de Bold A. J., Veress A. T. Intrarenal localization of the natriuretic effect of cardiac atrial extract. Can J Physiol Pharmacol. 1982 Sep;60(9):1149–1152. doi: 10.1139/y82-166. [DOI] [PubMed] [Google Scholar]
- Thibault G., Garcia R., Seidah N. G., Lazure C., Cantin M., Chrétien M., Genest J. Purification of three rat atrial natriuretic factors and their amino acid composition. FEBS Lett. 1983 Dec 12;164(2):286–290. doi: 10.1016/0014-5793(83)80303-2. [DOI] [PubMed] [Google Scholar]
- Tsai C. J., Ives H. E., Alpern R. J., Yee V. J., Warnock D. G., Rector F. C., Jr Increased Vmax for Na+/H+ antiporter activity in proximal tubule brush border vesicles from rabbits with metabolic acidosis. Am J Physiol. 1984 Aug;247(2 Pt 2):F339–F343. doi: 10.1152/ajprenal.1984.247.2.F339. [DOI] [PubMed] [Google Scholar]
- Turner S. T., Kiebzak G. M., Dousa T. P. Mechanism of glucocorticoid effect on renal transport of phosphate. Am J Physiol. 1982 Nov;243(5):C227–C236. doi: 10.1152/ajpcell.1982.243.5.C227. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Klöss S. Transport of inorganic and organic substances in the renal proximal tubule. Klin Wochenschr. 1982 Oct 1;60(19):1165–1172. doi: 10.1007/BF01716718. [DOI] [PubMed] [Google Scholar]
- Yusufi A. N., Murayama N., Keller M. J., Dousa T. P. Modulatory effect of thyroid hormones on uptake of phosphate and other solutes across luminal brush border membrane of kidney cortex. Endocrinology. 1985 Jun;116(6):2438–2449. doi: 10.1210/endo-116-6-2438. [DOI] [PubMed] [Google Scholar]



