Abstract
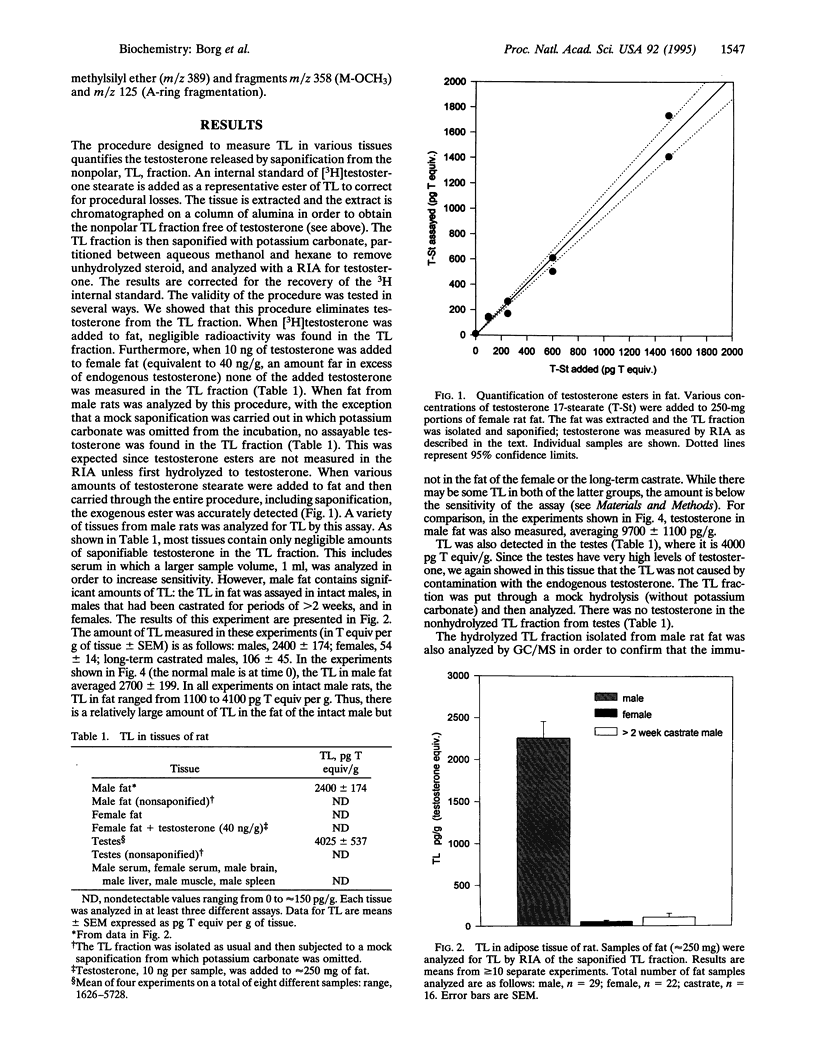
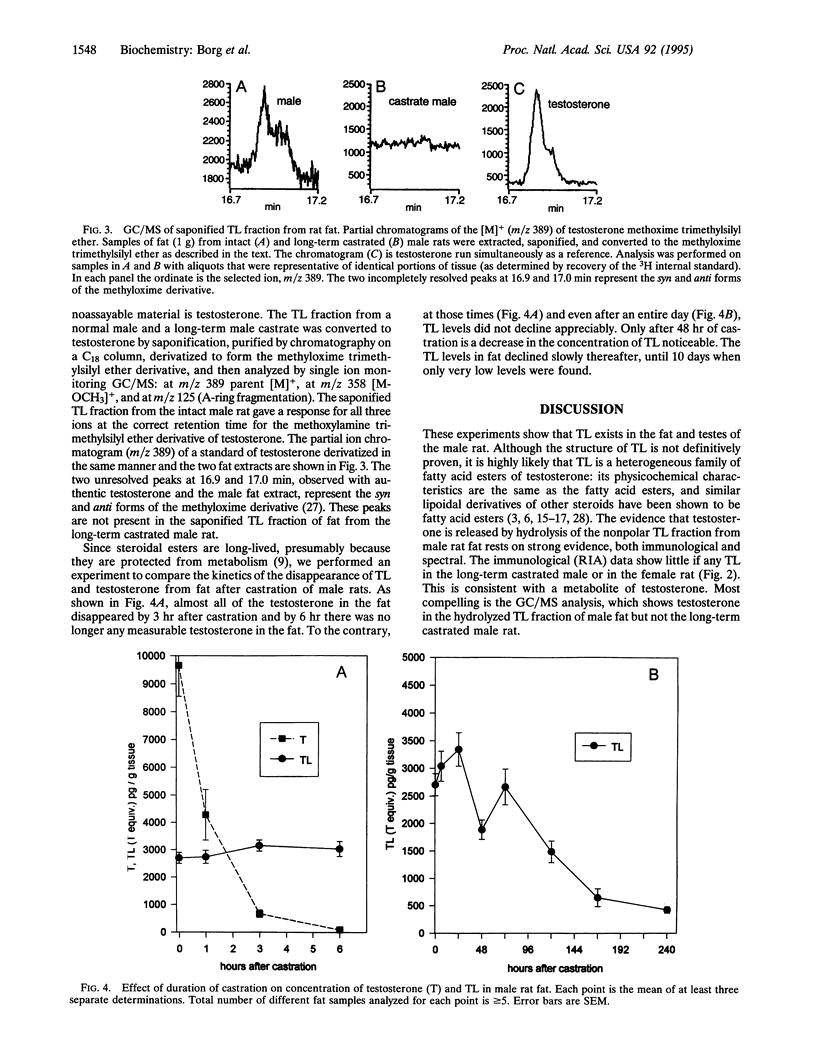
Over the past decade it has become increasingly clear that steroid hormones are enzymatically esterified with fatty acids. These steroidal esters are the natural analogs of synthetic esters that are used therapeutically. One such family of pharmacological steroids is the synthetic alkyl esters of testosterone, androgens with great hormonal potency. We have investigated whether testosterone esters exist naturally by using the rat as a model. Most tissues of male rats, including blood, have very little if any ester (quantified by immunoassay as a nonpolar saponifiable metabolite), but fat and testes have sizable quantities, approximately 3 ng of testosterone equivalents per g of tissue. Testosterone in fat averages 9 ng/g. The fat from female rats and long-term (> 2 weeks) castrated males has no detectable testosterone ester. The presence of testosterone esters was confirmed by GC/MS, which clearly showed the presence of testosterone in the hydrolyzed ester fraction of fat from intact males but not long-term castrates. Upon castration, testosterone levels in the fat completely disappear within 6 hr. To the contrary, it is not until 48 hr after castration that a measurable fall in the testosterone ester fraction was observed; even after 10 days a small amount of ester is still present in the fat. These experiments demonstrate the existence of a previously unknown androgen with a potentially important physiological impact; testosterone esters, natural analogs of potent therapeutic agents, occur in the fat where they can serve as a reservoir of preformed androgen to stimulate neighboring target tissues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addo S. B., Diamond E., Hollander V. P. Non-polar extracts of serum from males contain covert radioimmunoassayable testosterone. Steroids. 1989 Sep;54(3):257–269. doi: 10.1016/0039-128x(89)90001-9. [DOI] [PubMed] [Google Scholar]
- Addo S. B., Holland J. F., Kirschenbaum A., Mandeli J., Hollander V. P. Serum of patients with prostatic cancer or benign prostatic hypertrophy contains nonpolar testosterone. Steroids. 1990 Nov;55(11):491–494. doi: 10.1016/0039-128x(90)90086-q. [DOI] [PubMed] [Google Scholar]
- Albert D. H., Ponticorvo L., Lieberman S. Identification of fatty acid esters of pregnenolone and allopregnanolone from bovine corpora lutea. J Biol Chem. 1980 Nov 25;255(22):10618–10623. [PubMed] [Google Scholar]
- Bélanger B., Caron S., Bélanger A., Dupont A. Steroid fatty acid esters in adrenals and plasma: effects of ACTH. J Endocrinol. 1990 Dec;127(3):505–511. doi: 10.1677/joe.0.1270505. [DOI] [PubMed] [Google Scholar]
- Hochberg R. B., Bandy L., Ponticorvo L., Lieberman S. Detection in bovine adrenal cortex of a lipoidal substance that yields pregnenolone upon treatment with alkali. Proc Natl Acad Sci U S A. 1977 Mar;74(3):941–945. doi: 10.1073/pnas.74.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg R. B., Pahuja S. L., Zielinski J. E., Larner J. M. Steroidal fatty acid esters. J Steroid Biochem Mol Biol. 1991;40(4-6):577–585. doi: 10.1016/0960-0760(91)90279-e. [DOI] [PubMed] [Google Scholar]
- Hochberg R., Bandy L., Ponticorvo L., Welch M., Lieberman S. Naturally occurring lipoidal derivatives of 3 beta-hydroxy-5-pregnen-20-one; 3 beta,17 alpha-dihydroxy-5-pregnen-20-one and 3 beta-hydroxy-5-androsten-17-one. J Steroid Biochem. 1979 Oct;11(4):1333–1340. doi: 10.1016/0022-4731(79)90103-1. [DOI] [PubMed] [Google Scholar]
- Holinka C. F., Hata H., Kuramoto H., Gurpide E. Effects of steroid hormones and antisteroids on alkaline phosphatase activity in human endometrial cancer cells (Ishikawa line). Cancer Res. 1986 Jun;46(6):2771–2774. [PubMed] [Google Scholar]
- Janocko L., Larner J. M., Hochberg R. B. The interaction of C-17 esters of estradiol with the estrogen receptor. Endocrinology. 1984 Apr;114(4):1180–1186. doi: 10.1210/endo-114-4-1180. [DOI] [PubMed] [Google Scholar]
- Jones D. L., James V. H. The identification, quantification and possible origin of non-polar conjugates in human plasma. J Steroid Biochem. 1985 Feb;22(2):243–247. doi: 10.1016/0022-4731(85)90119-0. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y. Fatty acid esters of testosterone in rat brain: identification, distribution, and some properties of enzymes which synthesize and hydrolyze the esters. Arch Biochem Biophys. 1973 Nov;159(1):528–542. doi: 10.1016/0003-9861(73)90485-2. [DOI] [PubMed] [Google Scholar]
- Larner J. M., Hochberg R. B. The clearance and metabolism of estradiol and estradiol-17-esters in the rat. Endocrinology. 1985 Sep;117(3):1209–1214. doi: 10.1210/endo-117-3-1209. [DOI] [PubMed] [Google Scholar]
- Larner J. M., MacLusky N. J., Hochberg R. B. The naturally occurring C-17 fatty acid esters of estradiol are long-acting estrogens. J Steroid Biochem. 1985 Mar;22(3):407–413. doi: 10.1016/0022-4731(85)90446-7. [DOI] [PubMed] [Google Scholar]
- Larner J. M., Pahuja S. L., Brown V. M., Hochberg R. B. Aromatase and testosterone fatty acid esters: the search for a cryptic biosynthetic pathway to estradiol esters. Steroids. 1992 Oct;57(10):475–479. doi: 10.1016/0039-128x(92)90040-g. [DOI] [PubMed] [Google Scholar]
- Larner J. M., Pahuja S. L., Shackleton C. H., McMurray W. J., Giordano G., Hochberg R. B. The isolation and characterization of estradiol-fatty acid esters in human ovarian follicular fluid. Identification of an endogenous long-lived and potent family of estrogens. J Biol Chem. 1993 Jul 5;268(19):13893–13899. [PubMed] [Google Scholar]
- Larner J. M., Shackleton C. H., Roitman E., Schwartz P. E., Hochberg R. B. Measurement of estradiol-17-fatty acid esters in human tissues. J Clin Endocrinol Metab. 1992 Jul;75(1):195–200. doi: 10.1210/jcem.75.1.1619010. [DOI] [PubMed] [Google Scholar]
- Littlefield B. A., Gurpide E., Markiewicz L., McKinley B., Hochberg R. B. A simple and sensitive microtiter plate estrogen bioassay based on stimulation of alkaline phosphatase in Ishikawa cells: estrogenic action of delta 5 adrenal steroids. Endocrinology. 1990 Dec;127(6):2757–2762. doi: 10.1210/endo-127-6-2757. [DOI] [PubMed] [Google Scholar]
- Mellon-Nussbaum S. H., Ponticorvo L., Schatz F., Hochberg R. B. Estradiol fatty acid esters. The isolation and identification of the lipoidal derivative of estradiol synthesized in the bovine uterus. J Biol Chem. 1982 May 25;257(10):5678–5684. [PubMed] [Google Scholar]
- Mellon-Nussbaum S., Ponticorvo L., Lieberman S. Characterization of the lipoidal derivatives of pregnenolone prepared by incubation of the steroid with adrenal mitochondria. J Biol Chem. 1979 Dec 25;254(24):12500–12505. [PubMed] [Google Scholar]
- Osawa Y., Spaeth D. G. Estrogen biosynthesis. Stereospecific distribution of tritium in testosterone-1 alpha,2 alpha-t2. Biochemistry. 1971 Jan 5;10(1):66–71. doi: 10.1021/bi00777a011. [DOI] [PubMed] [Google Scholar]
- Poulin R., Poirier D., Thériault C., Couture J., Bélanger A., Labrie F. Wide spectrum of steroids serving as substrates for the formation of lipoidal derivatives in ZR-75-1 human breast cancer cells. J Steroid Biochem. 1990 Feb;35(2):237–247. doi: 10.1016/0022-4731(90)90280-6. [DOI] [PubMed] [Google Scholar]
- Raju U., Levitz M., Banerjee S., Bencsath F. A., Field F. H. Androsterone long chain fatty acid esters in human breast cyst fluid. J Clin Endocrinol Metab. 1985 May;60(5):940–946. doi: 10.1210/jcem-60-5-940. [DOI] [PubMed] [Google Scholar]
- Roy R., Bélanger A. Formation of lipoidal steroids in follicular fluid. J Steroid Biochem. 1989 Aug;33(2):257–262. doi: 10.1016/0022-4731(89)90302-6. [DOI] [PubMed] [Google Scholar]
- Roy R., Bélanger A. Presence of fatty acid esters of pregnenolone in follicular fluid from women undergoing follicle stimulation. Steroids. 1989 Oct;54(4):385–400. doi: 10.1016/0039-128x(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Schatz F., Hochberg R. B. Lipoidal derivative of estradiol: the biosynthesis of a nonpolar estrogen metabolite. Endocrinology. 1981 Sep;109(3):697–703. doi: 10.1210/endo-109-3-697. [DOI] [PubMed] [Google Scholar]
- WEICHSELBAUM T. E., MARGRAF H. W. Isolation and identification of adrenocortical steroids in human peripheral plasma. I. A naturally occurring C21 steroid acetate (11-dehydrocorticosterone acetate) and "free" tetrahydrocortisone in normal plasma. J Clin Endocrinol Metab. 1960 Oct;20:1341–1350. doi: 10.1210/jcem-20-10-1341. [DOI] [PubMed] [Google Scholar]
- Zielinski J. E., Pahuja S. L., Larner J. M., Hochberg R. B. Estrogenic action of estriol fatty acid esters. J Steroid Biochem Mol Biol. 1991 Apr;38(4):399–405. doi: 10.1016/0960-0760(91)90327-2. [DOI] [PubMed] [Google Scholar]




