Abstract
To determine the influence of cell cycle-specific agents on primate hematopoiesis and fetal hemoglobin production, two juvenile cynomolgus monkeys (Macaca fascicularis) were repeatedly bled to maintain their hemoglobins at approximately 6.5 g/dl and fetal hemoglobin levels at 3-5%. Six separate 5-d courses of hydroxyurea at 100 mg/kg per d were then administered over the next 200 d while phlebotomy was continued. These courses of hydroxyurea progressively raised the fetal hemoglobin levels to 17 and 18%, respectively. The drug had very little effect on the frequency of immature erythroid progenitors (BFU-E) in the bone marrow, but caused a marked reduction in the frequency of later progenitors (CFU-E) and a transient fall in the reticulocyte count. Following the courses of hydroxyurea, the number of F cells and the fetal hemoglobin level fell to base line over a period of 4 wk. Two control animals which were not phlebotomized showed no detectable increase in F cells or fetal hemoglobin when treated with the same regimen of hydroxyurea. A 5-d course of 5-azacytidine at 8 mg/kg per d was then given to each of the phlebotomized animals. This produced a more profound, albeit transient, reticulocytopenia, a fall in the CFU-E/BFU-E ratio, and a prompt increase in the fetal hemoglobin to levels even higher than were seen following a single 5-d course of hydroxyurea at 100 mg/kg/d. Subsequently, the animals were given a single dose of vinblastine at 0.4 mg/kg which reduced reticulocytes and CFU-E to the same extent as hydroxyurea; however, vinblastine at this dose had no effect on hemoglobin F (HbF) production. In contrast, when vinblastine was administered to the phlebotomized monkeys as a 5-d course at 0.2 mg/kg/d, prolonged reticulocytopenia followed by dramatic F cell and HbF responses were seen. Combinations of single dose vinblastine and a 5-d course of hydroxyurea were subsequently administered using two different schedules. When the animals received vinblastine on the first day of a 5-d course of hydroxyurea, the F cell response was double that seen following hydroxyurea treatment alone. In contrast, when vinblastine was administered on the final day of hydroxyurea treatment, the magnitude of the F cell response was the same as that which occurred following hydroxyurea treatment alone, but the onset of the rise was delayed for 4 d and HbF/F cell response was much higher. These results establish several important features of the fetal hemoglobin response to cytotoxic agents in the primate model. The response requires accelerated erythropoiesis and is preceded by transient reticulocytopenia. The response is produced by S phase- and M phase-specific agents when given in sufficient doses and at appropriate schedules. Passage of erythrocyte progenitors through M phase appears to be necessary for expression of the effect produced by S phase agents. The fetal hemoglobin response induced by cytotoxic drug administration occurs during the recovery of erythropoiesis following marrow suppression.
Full text
PDF


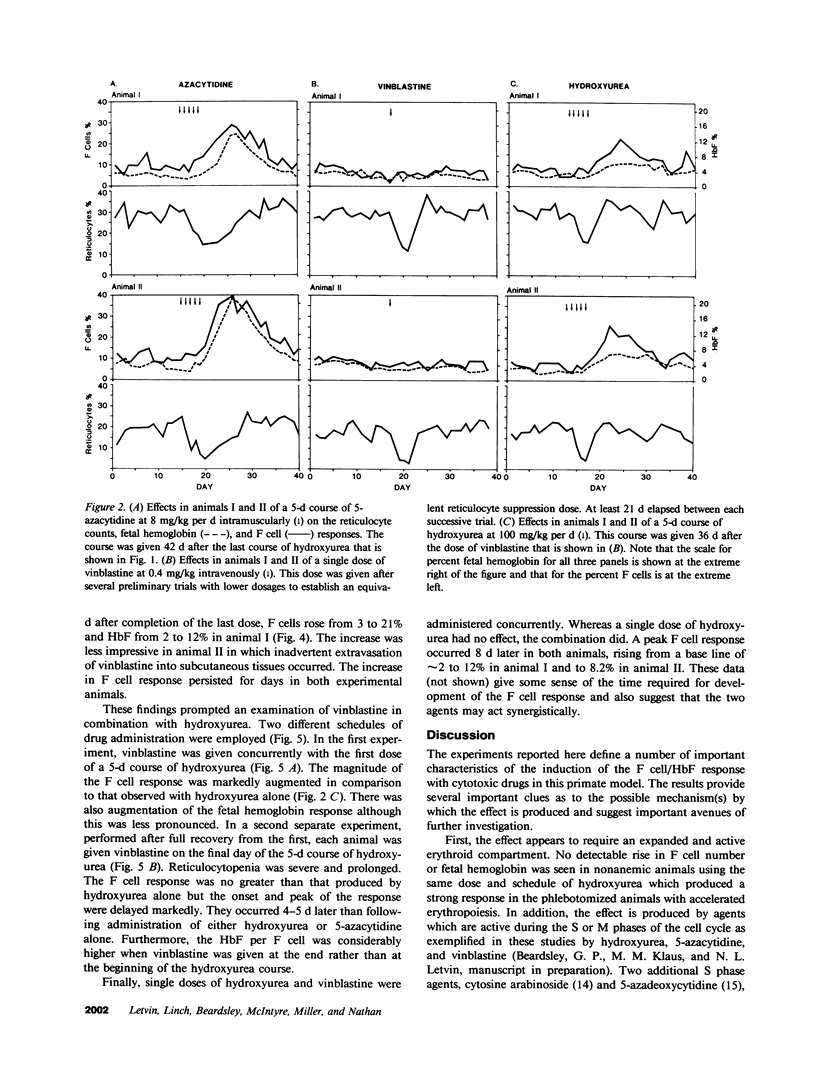
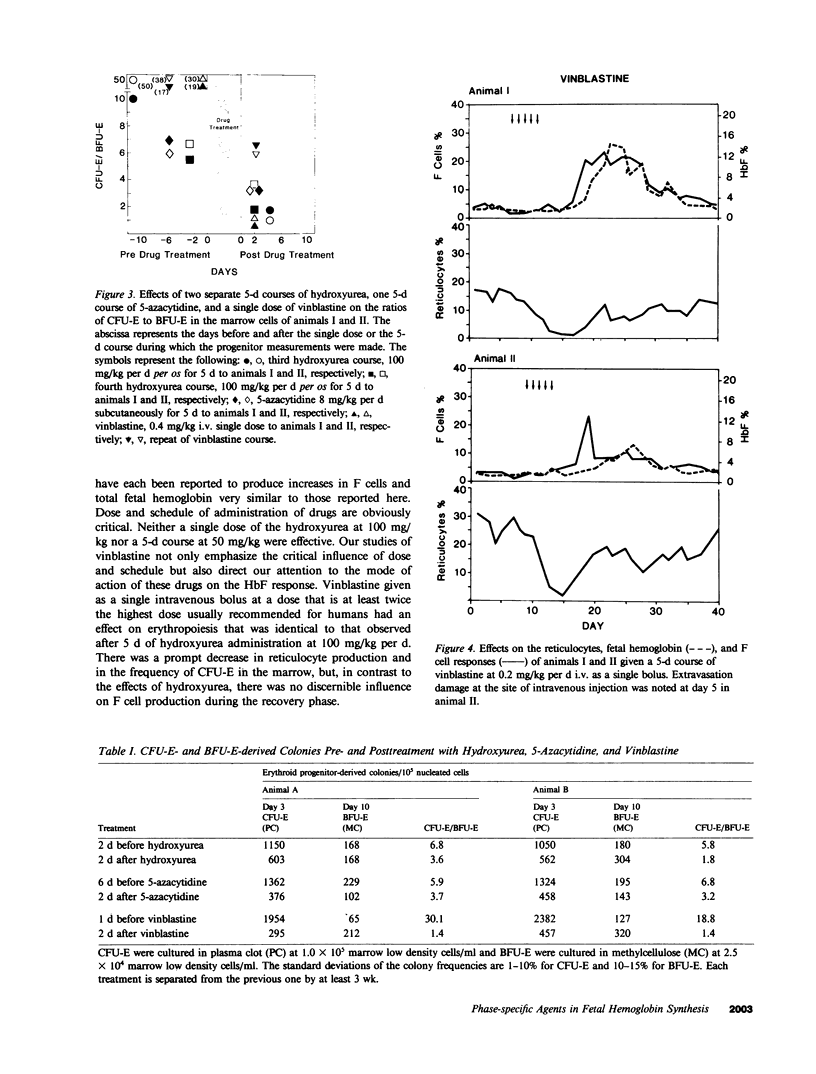
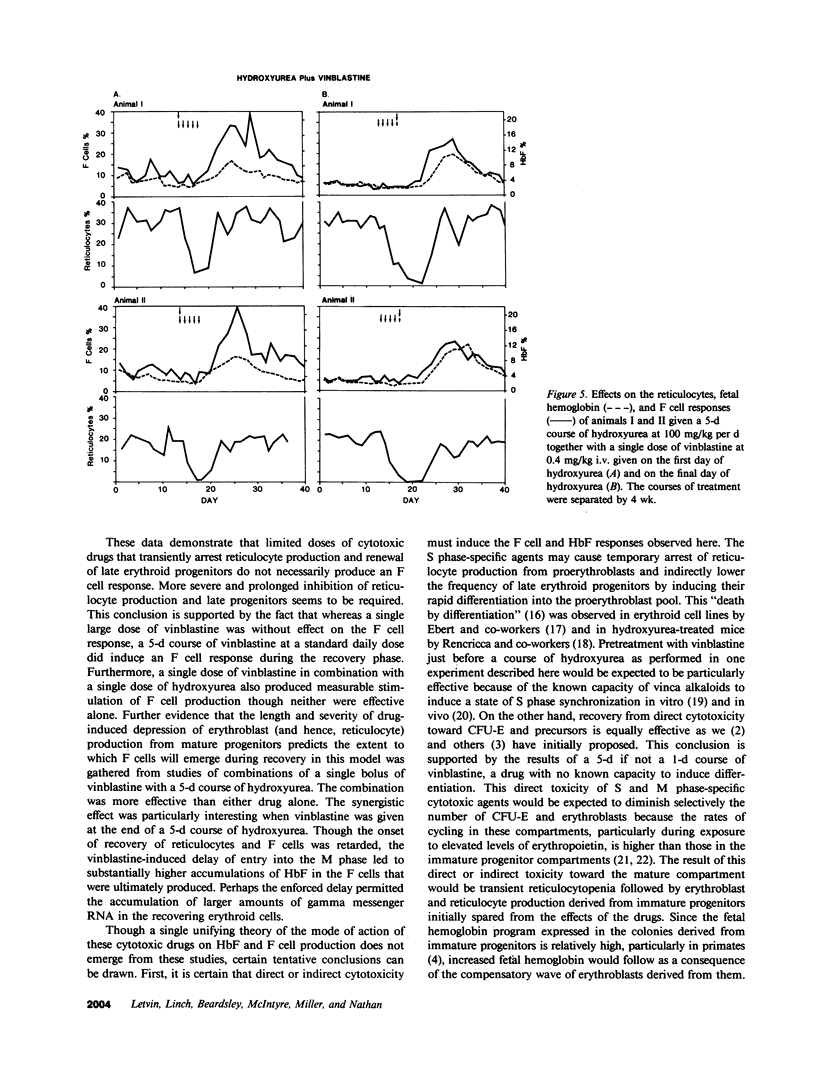

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charache S., Dover G., Smith K., Talbot C. C., Jr, Moyer M., Boyer S. Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the gamma-delta-beta-globin gene complex. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4842–4846. doi: 10.1073/pnas.80.15.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone J., Heller P., Adams J. G. Hemopoietic stress and fetal hemoglobin synthesis: comparative studies in vivo and in vitro. Blood. 1979 Nov;54(5):1176–1181. [PubMed] [Google Scholar]
- DeSimone J., Heller P., Hall L., Zwiers D. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4428–4431. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone J., Heller P., Schimenti J. C., Duncan C. H. Fetal hemoglobin production in adult baboons by 5-azacytidine or by phenylhydrazine-induced hemolysis is associated with hypomethylation of globin gene DNA. Prog Clin Biol Res. 1983;134:489–500. [PubMed] [Google Scholar]
- Ebert P. S., Wars I., Buell D. N. Erythroid differentiation in cultured Friend leukemia cells treated with metabolic inhibitors. Cancer Res. 1976 May;36(5):1809–1813. [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Iscove N. N. The role of erythropoietin in regulation of population size and cell cycling of early and late erythroid precursors in mouse bone marrow. Cell Tissue Kinet. 1977 Jul;10(4):323–334. doi: 10.1111/j.1365-2184.1977.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Krishan A. Time-lapse and ultrastructure studies on the reversal of mitotic arrest induced by vinblastine sulfate in Earle's L cells. J Natl Cancer Inst. 1968 Aug;41(2):581–595. [PubMed] [Google Scholar]
- Letvin N. L., Linch D. C., Beardsley G. P., McIntyre K. W., Nathan D. G. Augmentation of fetal-hemoglobin production in anemic monkeys by hydroxyurea. N Engl J Med. 1984 Apr 5;310(14):869–873. doi: 10.1056/NEJM198404053101401. [DOI] [PubMed] [Google Scholar]
- Ley T. J., DeSimone J., Anagnou N. P., Keller G. H., Humphries R. K., Turner P. H., Young N. S., Keller P., Nienhuis A. W. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982 Dec 9;307(24):1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- Macklis R. M., Javid J., Lipton J. M., Kudisch M., Pettis P. K., Nathan D. G. Synthesis of hemoglobin F in adult simian erythroid progenitor-derived colonies. J Clin Invest. 1982 Oct;70(4):752–761. doi: 10.1172/JCI110671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani B. D., Schimke R. T. Gene amplification in a single cell cycle in Chinese hamster ovary cells. J Biol Chem. 1984 Feb 10;259(3):1901–1910. [PubMed] [Google Scholar]
- Nathan D. G., Alter B. P. F-cell regulation. Ann N Y Acad Sci. 1980;344:219–232. doi: 10.1111/j.1749-6632.1980.tb33663.x. [DOI] [PubMed] [Google Scholar]
- Nathan D. G., Clarke B. J., Hillman D. G., Alter B. P., Housman D. E. Erythroid precursors in congenital hypoplastic (Diamond-Blackfan) anemia. J Clin Invest. 1978 Feb;61(2):489–498. doi: 10.1172/JCI108960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D. G. Regulation of erythropoiesis and hemoglobin F production. Prog Clin Biol Res. 1983;134:365–378. [PubMed] [Google Scholar]
- Papayannopoulou T., Torrealba de Ron A., Veith R., Knitter G., Stamatoyannopoulos G. Arabinosylcytosine induces fetal hemoglobin in baboons by perturbing erythroid cell differentiation kinetics. Science. 1984 May 11;224(4649):617–619. doi: 10.1126/science.6200940. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S. E., Tolmach L. J. Selecting synchronous populations of mammalian cells. Nature. 1967 Jan 14;213(5072):139–142. doi: 10.1038/213139a0. [DOI] [PubMed] [Google Scholar]
- Platt O. S., Orkin S. H., Dover G., Beardsley G. P., Miller B., Nathan D. G. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J Clin Invest. 1984 Aug;74(2):652–656. doi: 10.1172/JCI111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rencricca N. J., Morse B. S., Monette F. C., Howard D., Stohlman F., Jr Hydroxyurea-induced erythroid differentiation. Proc Soc Exp Biol Med. 1975 Sep;149(4):1052–1054. doi: 10.3181/00379727-149-38956. [DOI] [PubMed] [Google Scholar]
- Till J. E. Stem cells in differentiation and neoplasia. J Cell Physiol Suppl. 1982;1:3–11. doi: 10.1002/jcp.1041130405. [DOI] [PubMed] [Google Scholar]
- Torrealba-de Ron A. T., Papayannopoulou T., Knapp M. S., Fu M. F., Knitter G., Stamatoyannopoulos G. Perturbations in the erythroid marrow progenitor cell pools may play a role in the augmentation of HbF by 5-azacytidine. Blood. 1984 Jan;63(1):201–210. [PubMed] [Google Scholar]
- Udupa K. B., Reissmann K. R. In vivo erythropoietin requirements of regenerating erythroid progenitors (BFU-e, CFU-e) in bone marrow of mice. Blood. 1979 Jun;53(6):1164–1171. [PubMed] [Google Scholar]
- Vadlamudi S., Goldin A. Influence of mitotic cycle inhibitors on the antileukemic activity of cytosine arabinoside (NSC-63878) in mice bearing leukemia L1210. Cancer Chemother Rep. 1971 Dec;55(5):547–555. [PubMed] [Google Scholar]




