Abstract
BACKGROUND
Infections remain one of the leading causes of morbidity in pregnant women and newborns, with vaccine-preventable infections contributing significantly to the burden of disease. In the past decade, maternal vaccination has emerged as a promising public health strategy to prevent and combat maternal, fetal and neonatal infections. Despite a number of universally recommended maternal vaccines, the development and evaluation of safe and effective maternal vaccines and their wide acceptance are hampered by the lack of thorough understanding of the efficacy and safety in the pregnant women and the offspring.
METHODS
An outline was synthesized based on the current status and major gaps in the knowledge of maternal vaccination. A systematic literature search in PUBMED was undertaken using the key words in each section title of the outline to retrieve articles relevant to pregnancy. Articles cited were selected based on relevance and quality. On the basis of the reviewed information, a perspective on the future directions of maternal vaccination research was formulated.
RESULTS
Maternal vaccination can generate active immune protection in the mother and elicit systemic immunoglobulin G (IgG) and mucosal IgG, IgA and IgM responses to confer neonatal protection. The maternal immune system undergoes significant modulation during pregnancy, which influences responsiveness to vaccines. Significant gaps exist in our knowledge of the efficacy and safety of maternal vaccines, and no maternal vaccines against a large number of old and emerging pathogens are available. Public acceptance of maternal vaccination has been low.
CONCLUSIONS
To tackle the scientific challenges of maternal vaccination and to provide the public with informed vaccination choices, scientists and clinicians in different disciplines must work closely and have a mechanistic understanding of the systemic, reproductive and mammary mucosal immune responses to vaccines. The use of animal models should be coupled with human studies in an iterative manner for maternal vaccine experimentation, evaluation and optimization. Systems biology approaches should be adopted to improve the speed, accuracy and safety of maternal vaccine targeting.
Keywords: pregnancy, vaccine, immunology, antibody, animal model
Introduction
Immunization has played a crucial role in eliminating or reducing the occurrence of devastating infections worldwide (Roush et al., 2007; Andre et al., 2008). Maternal vaccination, a form of immunization for women of childbearing age before, during or after pregnancy, aims at protecting the mother against infections that may threaten healthy reproduction and allowing vaccine-induced maternal antibodies to be transferred via placenta to the fetus and in colostrum and breast milk to the infant for protection against diseases before routine childhood immunization can be initiated. The protection function of maternal vaccination in neonates was initially suggested by a correlation between a maternal deficiency of Group B streptococcus (GBS) anti-capsular antibodies and neonatal susceptibility to invasive GBS infection (Baker and Kasper, 1976). Because of the potential of protecting the mother and the fetus as well as the newborn and the advantage of circumventing the challenges of inducing efficient protective immunity in neonates, maternal vaccination has now emerged as a recommended public health approach against maternal and childhood infections.
In spite of the success of several maternal vaccines, many gaps exist in our knowledge of this promising public health strategy. All current maternal vaccine formulations were initially designed for and tested in non-pregnant populations, but the diverse immune modulations during pregnancy may cause pregnant women to respond sub-optimally or differently compared with non-pregnant populations. Efficacy is further affected by a plethora of other variables, such as the form, dose, route and timing of the vaccination. Very limited data exist on the effect in populations of high-risk pregnancies, such as recurrent miscarriage, pre-eclampsia, autoimmunity and immunodeficiency. Many recommended maternal vaccines are completely lacking in systematic surveillance data on their safety. A long list of pathogens have no available vaccines or vaccines that are contraindicated for pregnancy. By integrating the current status of major medical concerns over maternal vaccination, the recent advances in pregnancy-associated humoral immune modulation that may influence vaccine responsiveness and a discussion on the animal models for maternal vaccination development, this review aims to bridge the gaps in the literature, offer a mechanistic direction for maternal vaccine research and encourage basic, clinical and translational scientists to work together toward developing effective and safe maternal vaccines.
Methods
We first synthesized an outline of the review based on the current recommendations and major gaps in the knowledge of maternal vaccination. Following the outline, a systematic literature search was performed in PUBMED using the key words in each section title of the outline to retrieve articles relevant to pregnancy and published in English up to March 2014. The search was performed without limitations by species, but the species involved in the cited studies were indicated in the text when necessary. Relevant abstracts from recent scientific meetings were also included. Articles cited were selected based on relevance and quality as interpreted by all the authors. No quantitative or statistical analysis was performed. On the basis of the reviewed information and the recent progress in vaccinology and reproductive immunology, we formulated a perspective on the future directions of maternal vaccination research.
Rationales of maternal vaccination
Fetal and neonatal susceptibility to infections
A major rationale for vaccinating the mother during pregnancy is that neonates do not mount efficient protective immunity to many viral, bacterial and fungal pathogens and are prone to more severe or prolonged infections than adults (Silverstein, 1964; Darmstadt et al., 2011). The increased neonatal susceptibility to infections is more pronounced in infants born prematurely (Stoll et al., 2002; Stoll and Hansen, 2003). Therefore, by vaccinating the mother, humoral immunity can be passively transferred to the fetus and the newborn. Historically, the susceptibility of the fetus to infections was believed to be due to the immaturity of the fetal immune system (Billingham et al., 1953) and its tendency to mount tolerogenic responses to antigens (Silverstein, 1964). The heightened neonatal susceptibility was attributed to a less intact mucosal barrier and the lack of existing immunological memory as well as the immaturity of the neonatal immune system, being incapable of developing adult-like protective immune responses (Adkins et al., 2004; Levy, 2007). Lending credence to this historical notion, many quantitative and qualitative differences in both the innate and the adaptive components of fetal and neonatal immune systems from their adult counterparts were documented (Garcia et al., 2000; Adkins et al., 2004; Levy, 2007; Siegrist and Aspinall, 2009). Of note, fetal and neonatal T cells were found to deviate toward the development of regulatory T cell (Treg) or T helper type 2 (TH2) responses that are ineffective in protection against intracellular pathogens (Adkins et al., 2004; Michaelsson et al., 2006; Wang et al., 2010). The antibody responses to many encapsulated bacteria (such as Streptococcus pneumoniae, Haemophilus influenzae and Neisseria meningitidis), which are the leading causes of bacterial infections in infants, and their polysaccharide antigens are weak in early infancy. This is perhaps due to delayed formation of the splenic marginal zone (MZ) (MacLennan et al., 1985; Timens et al., 1989) that harbors MZ B cells producing polysaccharide-reactive antibodies (Cerutti et al., 2013) and reduced expression of activating receptors on neonatal B cells (Timens et al., 1989; Kaur et al., 2007; Kanswal et al., 2008).
Recent studies have argued against the fetal immune system being immature versions of the adult immune system (Mold et al., 2010). In addition, a series of studies have showed that the neonatal immune system can harbor considerable plasticity, and the intrinsic differences in neonatal immune cells from their adult counterparts can be overcome by appropriate manipulation of the neonatal immune environment to generate adult-like TH1, cytotoxic T lymphocyte (CTL) and humoral responses (Forsthuber et al., 1996; Ridge et al., 1996; Sarzotti et al., 1996; Hassett et al., 1997; Martinez et al., 1997; Bot et al., 1998; Brazolot Millan et al., 1998; Jakobsen et al., 1999; Franchini et al., 2001; Kovarik et al., 2001; Fadel et al., 2002; Wynn et al., 2008). These observations have engendered much effort in the design of vaccine formulations and protocols to stimulate neonatal immunity (Wood and Siegrist, 2011), although with limited success. Neonatal vaccination should be pursued but with caution. Many agents designed to break neonatal tolerance and induce vaccine responsiveness may trigger side effects, such as pathological inflammation or toxicity, which are deleterious to development (Kovarik et al., 2000). Furthermore, recent progress in our understanding of the immunologic challenges during prenatal life and the transition from fetal to neonatal life has revealed important physiologic significance to this attenuated perinatal immunity. The deviation toward an anti-inflammatory TH2 or Treg response during mid-to-late gestation may protect the fetus from preterm delivery or other unwanted pregnancy complications that could otherwise occur in a pro-inflammatory TH1 or TH17 milieu (Vitoratos et al., 2006; Ito et al., 2010), and compromised neonatal immunity may limit detrimental inflammation during mucosal colonization by commensal microbes shortly after birth (Lotz et al., 2006; Elahi et al., 2013). These potential hurdles to neonatal vaccination, coupled with the concern that infection can precede the development of a vaccine response, make maternal vaccination an appealing alternative strategy to induce immune protection in neonates.
Maternal susceptibility to infections
Similar to neonates, epidemiological data have shown that pregnant women have an increased incidence of and/or severity to a variety of infections, such as influenza, varicella, measles, severe acute respiratory syndrome, tuberculosis, listeriosis, pneumocystis, toxoplasmosis and malaria (Jamieson et al., 2006; Pazos et al., 2012a; Sappenfield et al., 2013). These observations have given rise to the theory that pregnancy represents an immunocompromised state associated with inefficient pathogen control. Further supporting this theory is the apparent immunological challenge women face during pregnancy, i.e. to be tolerant to the semi-allogeneic fetus, which requires maternal suppressive immune modulations. However, a careful examination of the epidemiological data suggests that the severity of infections varies at different stages of pregnancy. For example, the severity of Plasmodium falciparum malaria and of toxoplasmosis were found in some studies to be the highest during the first half of pregnancy and to decline gradually as pregnancy proceeded (Bray and Anderson, 1979; Jenum et al., 1998; Okoko et al., 2003), while women in the second and third trimesters were shown to have higher maternal and fetal mortality and morbidity from influenza A infection (Lindsay et al., 2006, Neuzil et al., 1998; Schanzer et al., 2007; Siston et al., 2010) and a higher incidence of Listeria monocytogenes (Gellin et al., 1991; Benshushan et al., 2002; Mylonakis et al., 2002). Such differences are likely to result from the distinct types of protective immunity required to control the various pathogens during acute or chronic infection and the unique immunological alterations occurring at different stages of pregnancy, both systemically and at the maternal-fetal interface. During early pregnancy when implantation and placentation take place, extensive tissue remodeling triggers a maternal local inflammatory immune reaction. During the second and third trimesters, the dramatic tissue remodeling subsides and rapid fetal growth occurs, which entails the mother and the developing conceptus co-existing peacefully in an anti-inflammatory environment in order to avoid fetal rejection. Toward the final phase of pregnancy when fetal development is complete, an inflammatory process takes places in the uterus to activate smooth muscle contraction and parturition ensues. Therefore, it would be conceivable that the higher severity of the mother and the fetus to certain placental parasitic infections, such as P. falciparum malaria and toxoplasmosis, during early pregnancy may reflect dominant local pro-inflammatory TH1 and TH17 immune responses that amplify collateral tissue damage (Fievet et al., 2001; Ge et al., 2008; Goldszmid and Trinchieri, 2012), while the higher severity to influenza A and L. monocytogenes during the second trimester may reflect diminished systemic and local TH1 immunity that is critical for protection (Barber et al., 2005). The complex spatial and temporal host-pathogen interaction during pregnancy dictates that the biology of the pathogen, the timing of vaccination as well as the effect of the vaccine on both maternal systemic and reproductive mucosal immune systems should be examined when designing maternal vaccine formulations and protocols that will be effective and safe for the mother and the fetus.
Principles of maternal vaccination
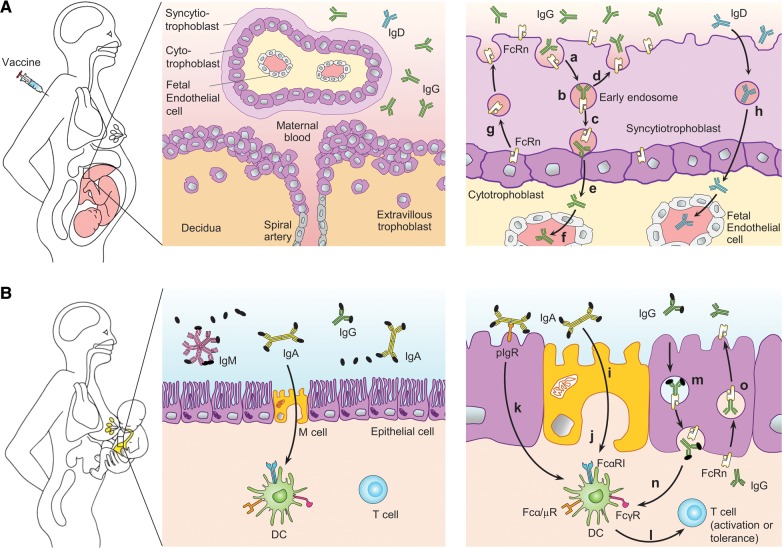
Maternal vaccination generates active innate, humoral and cell-mediated immune protection in the mother to increase resistance against infections and reduce the chance of vertical transmission of infections to the fetus (Fig. 1A, left). In addition, maternal vaccination elicits systemic immunoglobulin G (IgG) antibodies that can be transferred to the fetus via the placenta in humans (Fig. 1A, middle and right) and mucosal IgG, IgA and IgM antibodies that are secreted into the colostrum and milk and ingested by the newborn during breastfeeding (Fig. 1B) to confer immune protection. Species vary in the contribution each route makes to the transfer of immunity. In humans and mice, maternal antibodies can be transferred via both routes (Renegar, 2005).
Figure 1.
Mechanisms of vaccine-induced maternal, fetal and neonatal immune protection. (A) Maternal vaccination induces innate, humoral and cell-mediated immunity that confers direct protection of the mother against infections (left panel). Vaccine-induced maternal IgG is also transferred to the fetus to confer systemic passive immunity (middle and right panels). Maternal IgG is endocytosed into villous syncytiotrophoblasts from the maternal surface (a) and binds to FcRn in the acidic environment of early endosomes (b). IgG-FcRn complexes are then either transcytosed to the fetal side of syncytiotrophoblasts (c) or recycled back to the maternal side (d). IgG dissociates from FcRn upon exposure to the neutral pH environment at the fetal side of syncytiotrophoblasts (e) and enters fetal circulation (f). FcRn on the fetal side of syncytiotrophoblasts can be retrieved back to the maternal side to participate in subsequent IgG transport (g). Maternal vaccine-induced IgD could cross trophoblasts and enter fetal circulation via an unknown mechanism (h). (B) Maternal vaccination-induced antibodies, including IgA, IgG, IgM and IgD, are also secreted into colostrum and milk. During breastfeeding, these antibodies are ingested by the neonate (left panel). IgA, IgG and IgM confer neonatal mucosal immune protection by binding to commensal and pathogenic microbes and their virulence factors to mediate immune exclusion and neutralization (middle panel). In addition, maternal IgA facilitates antigen sampling in the neonatal intestinal mucosa by crossing M cells via an unknown receptor (i) or apical-to-basolateral retro-transcytosis via polymeric Ig receptor (pIgR) (k). Besides delivering antigens to mucosal dendritic cells (DCs), IgA can interact with DCs via FcαRI, leading to either immunity against pathogenic microbes or tolerance to commensal microbes (l). IgA can also interact with Fcα/µR on DCs to mediate immune tolerance. Ingested maternal IgG can also cross epithelial cells via FcRn (m) through a mechanism similar to that in syncytiotrophoblasts. This pathway delivers antigens to, and regulates, DCs via activating or inhibitory FcγRs (n). Maternal IgG acquired during the perinatal period can be re-secreted by FcRn into the lumen to participate in mucosal immune defense (o).
Maternal immune protection
The argument for vaccination in pregnancy is not solely based on altruistic behavior on the part of the mother. As noted above, women who are pregnant remain at risk for a variety of vaccine-preventable diseases. These infectious processes result in identifiable morbidity and mortality in the mother, and the associated adverse host systemic responses can lead to disruptions in physiologic homeostasis thus compromising the co-existing fetus. Despite these rather obvious observations, few data exist examining the maternal and fetal benefits of vaccination. This is perhaps due to a general unwillingness to study the pregnant patients, which requires a reassessment of strategies (Brent, 2003; Healy, 2012). Most of the available literature on maternal immune protection by vaccination relates to influenza infection. A large cohort study demonstrated significant reduction in maternal flu-like disease in those vaccinated in pregnancy (Zaman et al., 2008). Of additional interest, the neonates from the pregnant mothers who were vaccinated also showed a significant reduction in influenza and flu-like respiratory disease after delivery.
Neonatal systemic immune protection
Significant placental transfer is found for maternal IgG. After endocytosis by placental syncytiotrophoblasts, maternal IgG binds to neonatal Fc receptor (FcRn) in the acidic environment of early endosomes. FcRn-IgG complexes are then transported to the fetal surface of the syncytiotrophoblasts, where the neutral pH promotes IgG dissociation. IgG subsequently passes through the villous stroma and fetal capillary endothelium and enters fetal circulation. The amount of IgG transferred is a function of maternal IgG concentration, IgG subclass, the level of FcRn expression on syncytiotrophoblasts and gestational age. Preferential transport was found for IgG1 and IgG4 over IgG3 and IgG2 (Costa-Carvalho et al., 1996). Vaccines that contain protein antigens, such as Tdap, elicit a predominantly IgG1 and IgG3 response, which is transferred more efficiently than polysaccharide vaccine antigens, which predominantly elicit an IgG2 response (van den Berg et al., 2010). IgG transfer can begin as early as 13 weeks of gestation and occurs as pregnancy proceeds, with the largest amount transferred in the third trimester (Saji et al., 1999). The fetal IgG concentration usually exceeds that in the maternal circulation at full term, consistent with placental IgG transfer as an active transport process. A sharp increase of maternal IgG in cord blood occurs after 36 weeks of gestation, and this has prompted the Advisory Committee on Immunization Practices (ACIP) to recommend that the optimal timing of Tdap vaccination is the third trimester, which would provide the highest concentration of maternal antibodies in the fetus at birth (Centers for Disease Control and Prevention, 2013b; Healy et al., 2013). However, a study on influenza vaccination found that first trimester vaccination could also improve fetal and neonatal outcomes by reducing the rate of stillbirth (Sheffield et al., 2012). Evidence supporting impaired placental IgG transfer in mothers infected with human immunodeficiency virus-1 (HIV-1) or malaria and in babies born at term with lower birthweight has been found (Wesumperuma et al., 1999; Okoko et al., 2002), highlighting the need for careful design and evaluation of maternal vaccines in mothers with existing infections or other underlying conditions.
Neonatal mucosal immunity and tolerance
Maternal non-specific and specific antibodies elicited by vaccination, including IgA, IgM and IgG, are secreted into colostrum and milk. After ingestion by the neonates during breastfeeding, they provide mucosal immune protection by inhibiting commensal and pathogen adhesion and invasion and by promoting exclusion and neutralization. Secretory IgA is the predominant antibody class in human colostrum and milk (Mickleson and Moriarty, 1982; Telemo and Hanson, 1996), while IgG is the most abundant antibody class in mouse milk (Ijaz et al., 1987). In the gut, ingested maternal IgA can undergo retrograde transport across M cells via an unknown receptor (Mantis et al., 2002) or across duodenal epithelial cells via the transferrin receptor (CD71) (Cerutti and Rescigno, 2008). Ingested maternal IgG can also undergo retrograde transport by FcRn expressed on the apical surface of intestinal epithelial cells (Israel et al., 1995). These mechanisms can promote the induction of immunity against luminal pathogens and tolerance to commensal microbes (Oda et al., 1983; Kohl and Loo, 1984; Heiman and Weisman, 1989; Yoshida et al., 2004, 2006; Favre et al., 2005). Intestinal FcRn can also mediate the resecretion of maternal IgG previously acquired via placental transfer during prenatal life and control luminal pathogens (Harris et al., 2006).
Developing a better understanding of IgD
Whereas much of the attention on maternal vaccination has been focused on vaccine-induced maternal antepartum IgG response and post-partum IgG, IgA and IgM responses in breast milk, IgD, an enigmatic member of the immunoglobulin family, has been left in oblivion. However, many features of IgD make it an appealing target of maternal vaccination. IgD is enriched in the upper respiratory mucosa, markedly increased in patients with selective IgA deficiency (Chen and Cerutti, 2010a) and contributes to immune defense against respiratory pathogens such as H. influenzae and Moraxella catarrhalis that are common neonatal infections (Chen et al., 2009). Maternal rubella-specific IgD persists longer than IgM and IgA after infection, and significant amounts of rubella-specific IgD can be transferred across the placenta during pregnancy, albeit through an unknown mechanism (Fig. 1A, right), allowing cord blood rubella-specific IgD levels to reach levels comparable to those in maternal blood (Salonen et al., 1985). IgD is also present in human amniotic fluid and is concentrated in milk (Cederqvist et al., 1978; Sewell et al., 1979; Steele and Leslie, 1985; Litwin et al., 1990), which may provide fetal and neonatal immune protection. Furthermore, secreted IgD exhibits extensive V(D)J gene somatic hypermutation and has a long, protruding, finger-like heavy chain complementarity determining region 3 (Koelsch et al., 2007), which may be key to the neutralization of highly conserved bacterial and viral epitopes with recessed topography (Saphire et al., 2001; Burton et al., 2005). IgD can also monitor the presence of systemic pathogens by activating the antimicrobial, antibody-inducing and pro-inflammatory functions of basophils (Chen et al., 2009). The production of IgD is positively regulated by TH2 cytokines (Levan-Petit et al., 1999), allowing IgD-inducing vaccines to be more compatible with pregnancy than vaccines whose induction and protection require a strong pro-inflammatory TH1 environment. Finally, IgD inhibits IgE-induced histamine release but not cytokine production by basophils (Cerutti and Chen, 2010) and thus may be targeted by maternal vaccination to control the rising rate childhood allergies without triggering adverse pregnancy outcomes associated with histamine, such as preterm labor, pre-eclampsia and spontaneous abortion (Bytautiene et al., 2004; Brew and Sullivan, 2006). However, IgD has been neglected for a long time, and there has been no study on the function of IgD in maternal, fetal or neonatal protection at the time of this review.
Current maternal vaccine recommendations, use and safety
General guidelines
All guidelines considered today for maternal vaccination during pregnancy in the USA are derived from the ACIP. Currently, the ACIP committee has found no evidence of risk to the fetus from maternal vaccination from dead, inactivated or toxoid sources (National Center for Immunization and Respiratory Diseases, 2011). For live vaccines, there have been few conclusive studies. As a result, attenuated viral or live bacterial vaccines are routinely avoided unless there is a high risk of exposure to disease in which the mother or child could be in danger.
Tdap, influenza and hepatitis
As of 2013, two vaccines, IIV for influenza and Tdap for diphtheria, tetanus and pertussis, are recommended by the ACIP to be administered to all women of reproductive age before, during or after pregnancy (National Center for Immunization and Respiratory Diseases, 2011) (Table I). Several other vaccines, including Hepatitis A and B and meningococcal, are recommended for women before, during or after pregnancy when risk factors exist (Centers for Disease Control and Prevention, 2013b).
Table I.
Current CDC recommendations of maternal vaccination.
| Vaccine | Type/form | Before pregnancy | During pregnancy | After pregnancy |
|---|---|---|---|---|
| Hepatitis A | Inactivated | Yes, if indicated | Yes, if indicated | Yes, if indicated |
| Hepatitis B | Inactivated | Yes, if indicated | Yes, if indicated | Yes, if indicated |
| HPV | Inactivated | No (under study) | No (under study) | Yes, if indicated (to 26 years of age) |
| Influenza | Inactivated | Yes | Yes | Yes |
| Live attenuated | Yes, if under 50 and healthy; avoid conception for 4 weeks | No | Yes, if under 50 and healthy; avoid conception for 4 weeks | |
| MMR | Live attenuated | Yes, if indicated; avoid conception for 4 weeks | No | Yes, if indicated. To be given immediately post-partum if susceptible to rubella |
| Meningococcal | Polysaccharide | Yes, if indicated | Yes, if indicated | Yes, if indicated |
| Conjugate | Yes, if indicated | Yes, if indicated | Yes, if indicated | |
| Tdap | Toxoid Inactivated |
Yes, if indicated | Yes, vaccinate during each pregnancy between 27–36 weeks of gestation | Yes, immediately post-partum if not given previously |
| Tetanus/diphtheria | Toxoid | Yes, if indicated | Yes, if indicated (Tdap preferred) | Yes, if indicated |
| Varicella | Live attenuated | Yes, if indicated; avoid conception for 4 weeks | No | Yes, give immediately post-partum if susceptible |
| Anthrax | Subunit | Yes, if indicated | No, unless risk of exposure is significant | No, unless risk of exposure is significant |
| BCG | Live attenuated | Yes, if indicated | No | No |
| Japanese Encephalitis | Inactivated | Yes, if indicated | Insufficient data for recommendation | Insufficient data for recommendation |
| MPSV4 | Polysaccharide | Yes | No, unless risk of exposure is significant | No, unless risk of exposure is significant |
| Rabies | Inactivated | Yes, if indicated | No, unless post-exposure | No, unless post-exposure |
| Typhoid | Live attenuated | Yes, if indicated | Insufficient data for recommendation | Insufficient data for recommendation |
| Smallpox | Live attenuated | Yes, if indicated | No, unless post-exposure | No, unless post-exposure |
| Yellow fever | Live attenuated | Yes, if indicated | No, unless risk of exposure is significant | No, unless risk of exposure is significant |
CDC, Centre for Disease Control and Prevention; HPV, human papillomavirus; MMR, Measles, mumps, rubella; BCG, baccilus (germ) of Calmette and Guerin; MPSV4, Meningococcal polysaccharide vaccine.
Sub-optimal vaccine usage in the obstetric population
Despite the recommendations and advocacy by many public health organizations worldwide, the concept of maternal vaccination has not been widely accepted by the general public or become a priority among medical professionals. For example, even with the encouraging data from post-licensure studies on maternal Tdap and influenza vaccinations suggesting that the perinatal use of these vaccines is safe and could be key to closing the gap between maternal immunologic protection and traditional immunization schedules (discussed below), the maternal influenza vaccination rate has been estimated to be only ∼50% in the USA (Centers for Disease Control and Prevention, 2013a). There are no retrospective studies on the rate of Tdap vaccination during pregnancy at the time of this review, and such information is even more sparse for other vaccines. Apart from the cultural, legal, educational and logistic barriers that are restricting the boarder usage of maternal vaccines, the lack of concrete scientific knowledge on the protective efficacy and adverse effects on the short- and long-term health status of various pregnant and pediatric populations, especially of the less studied vaccines, may have contributed to the underimmunization of pregnant women by medical professionals (Moffatt and McNally, 2013).
Concerns over maternal vaccination
Safety
Successful examples of maternal vaccines, such as influenza IIV, have shown efficacy in reducing maternal, fetal and neonatal morbidity due to infection or other pregnancy complications (Zaman et al., 2008; Omer et al., 2011; Steinhoff et al., 2012; Richards et al., 2013; El-Kady et al., 2014; Legge et al., 2014). Yet many concerns have been raised over maternal vaccination, which need to be addressed by further research. Apart from the ethical and legal issues (Riley and Minkoff, 2014), the potential short- and long-term deleterious effects of in utero exposure to maternal vaccines on the fetus and offspring are prominent concerns.
Prior to the recommended use on pregnant women, both IIV and Tdap vaccines were extensively studied in non-pregnant populations. However, the renewed ACIP recommendation of Tdap vaccination in every pregnancy, as mentioned earlier, has spurred increased interest in post-licensure studies to examine the effects that Tdap may have on pregnancy outcomes. It was recently reported that no negative consequences of administration to infants, regardless of the timing of vaccination in pregnancy, was found (Shakib et al., 2013), and maternal administration of Tdap correlated with a higher level of neonatal Pertussis-specific antibodies between birth and the first vaccine dose (Hardy-Fairbanks et al., 2013). In the case of influenza, the long-standing observation of its heightened severity on the mother and the fetus from across the world (Callaghan et al., 2010; Liu et al., 2011; Beigi, 2012; Hansen et al., 2012; Soydinc et al., 2012; Beau et al., 2014) and the dramatic disease morbidity and mortality in pregnant women during the 2009 H1N1 pandemic (Creanga et al., 2010) have underscored the importance of maternal vaccination and promoted the ACIP recommendation. Several wide-ranging surveillance studies in North America, Europe, Asia, Australia and Latin America all found no evidence to suggest that the IIV vaccine posed significant risk to either the mother or the fetus (Lim et al., 2010; Moro et al., 2011; Omon et al., 2011; Fell et al., 2012; Mackenzie et al., 2012; Oppermann et al., 2012; Pasternak et al., 2012; Carcione et al., 2013; Conlin et al., 2013; Irving et al., 2013; Louik et al., 2013; Nazareth et al., 2013; Nordin et al., 2013). In terms of hepatitis vaccination, there appears to be little or no data evaluating the effectiveness of the inactivated hepatitis vaccines in perinatal contexts. Centers for Disease Control and Prevention (CDC) guidelines recommend usage only if ‘other high-risk conditions or indications are present’ (National Center for Immunization and Respiratory Diseases, 2011). Examination of the Vaccine Adverse Event Reporting System (VAERS) over a 13-year period between 1996 and 2013 found no adverse events correlated with either hepatitis A or hepatitis B vaccines. In fact, several studies have pointed out that both hepatitis A (Moro et al., 2014) and hepatitis B (Ayoola and Johnson, 1987; Gupta and Ratho, 2003; Moro et al., 2014) vaccines are safe to administer, and also in the case of hepatitis B, that the vaccine clearly imparted high levels of immunogenicity to both the mother and fetus (Gupta and Ratho, 2003). Of note, the Hepatitis B vaccine series is recommended by the ACIP to be started on all neonates before hospital discharge.
For the other maternal vaccines routinely recommended in the CDC guidelines, there remains a significant gap in our knowledge of their short- and long-term safety. Studies on inadvertent pneumococcal polysaccharide, rubella or yellow fever vaccination cases found no significant maternal or fetal risk (Nasidi et al., 1993; Centers for Disease Control and Prevention, 1997; Castillo-Solorzano et al., 2011). However, follow-up requires both voluntary reporting and retrospective analysis involving significant speculation. Virtually nothing is known regarding a wide category of other vaccines that are considered ‘non-routine’, i.e. against possible biological agents, such as anthrax, or diseases which are exceedingly uncommon in the developed world, such as typhus or smallpox. In most cases, the available recommendations rely heavily on a theoretical benefit-to-risk ratio (Centers for Disease Control and Prevention, 2013b). Finally, questions continue to surface regarding the safety of vaccine components (i.e. thimerosal), long-term childhood neurodevelopmental conditions (i.e. autism) and venues to seek relief in the event of an identifiable vaccine-related injury. Despite reassurances from agencies, such as the Institute of Medicine (IOM), CDC and American Congress of Obstetricians and Gynecologists (ACOG), based on expanding reports of both short and long-term vaccine safety, the responsibility lies with the scientific community to continue a vigilant watch through basic research efforts and post-marketing surveillance systems (Poland, 2011).
Interference with infant response to vaccination
Another major concern surrounding maternal vaccination stems from the long-standing observation that the presence of maternal antibodies in the infant is able to interfere with the infant's humoral immune response to vaccines both systemically and at mucosal districts (Burstyn et al., 1983; Enriquez-Rincon and Klaus, 1984; Claesson et al., 1989; Daum et al., 1991; Booy et al., 1992; Sarvas et al., 1992; Yamazaki et al., 1994; Englund et al., 1995; Troisi et al., 1997; Dagan et al., 2000; Kanra et al., 2000; Crowe et al., 2001; Getahun and Heyman, 2009), although cell-mediated immune responses are not affected (Martinez et al., 1997, 1999; Siegrist et al., 1998a, b). The inhibitory effect on infant response to vaccination has been, however, highly variable among different vaccines and even different studies of the same vaccine (Siegrist, 2003).
Many mechanisms of how maternal antibodies may inhibit infant humoral immune response to vaccines (Table II) can be postulated, some of which are based on the understanding of the immunosuppressive mechanism of passive intravenous immunoglobulins (IVIGs) (Schwab and Nimmerjahn, 2013). However, studies on how maternal antibodies may actually interfere with vaccine-induced humoral immunity in infants are needed, as maternal antibodies differ from IVIGs in quantity, structure, composition and function, such as half-life, glycosylation pattern, isotype and affinity for antigens and Fc receptors, and may interfere with the infant immune response via distinct mechanisms from those used by IVIGs. For example, the significant increase in the production of maternal asymmetric IgG with an extra carbohydrate moiety in one of the F(ab’) domains during pregnancy (Gutierrez et al., 2005) may allow such IgG molecules to uniquely function as univalent blocking antibodies against vaccine antigens differently from IVIGs in infants (Pasetti et al., 1997). Since maternal antibodies decline in the infant, interference of the infant humoral immunity to vaccination was found to mainly impact primary immunization in early infancy but not subsequent boosting (Glezen, 2003). However, this should not be a reason to dismiss maternal immunization, as a reduced antibody titer after infant vaccination may be acceptable if the high morbidity and mortality can be mitigated in the first months of life by maternal vaccination. Indeed, studies in mice show that maternal antibodies can promote immune maturation in the offspring (Malanchere et al., 1997; Fink et al., 2008). The pros and cons of maternal vaccination on the immune responses to any given infant vaccination protocol should therefore be evaluated individually.
Table II.
Postulated mechanisms of maternal antibody-mediated inhibition of infant humoral immune response to vaccination.
| Mechanism |
Supporting references | |
|---|---|---|
| F(ab’)2-dependent | Clearance of vaccine antigens by maternal IgG via opsonization and subsequent FcγR-mediated phagocytosis | Getahun and Heyman (2009) |
| Neutralization of live viral vaccine epitopes by maternal IgG | Albrecht et al. (1977) and Naniche (2009) | |
| Inhibition of infant B cell recognition of vaccine epitopes by maternal IgG via antigenic masking | Wiersma et al. (1989), Jelonek et al. (1996), Nohynek et al. (1999) and Getahun and Heyman (2009) | |
| Fc-dependent | Clearance of vaccine antigens by maternal IgG via FcγR-mediated phagocytosis after antigen opsonization | Getahun and Heyman (2009) |
| Inhibition of infant B cell activation, survival and antibody production by maternal IgG via the inhibitory receptor FcγRIIB | Victor et al. (2010) and Kim et al. (2011) | |
| Inhibition of infant antigen-presenting cells by maternal IgG via the inhibitory receptor Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Non-integrin (DC-SIGN), also called CD209 | Anthony et al. (2008) | |
| Saturation of infant endothelial or myeloid FcRn by maternal IgG and acceleration of catabolism of vaccine-induced infant IgG | Vieira and Rajewsky (1988), Junghans and Anderson (1996), Hansen and Balthasar (2002) and Li et al. (2005) | |
| Inhibition of infant dendritic cells (DCs) by ingested and absorbed maternal IgA via FcαRI | Pasquier et al. (2005) and Kanamaru et al. (2008) | |
| Inhibition of infant B cells and follicular DCs and macrophages by ingested and absorbed maternal IgA via Fcα/µR | Honda et al. (2009) | |
Humoral immune modulations in pregnancy that influence vaccine efficacy and safety
All of the current maternal vaccination formulations are initially designed for and tested in the non-pregnant population. However, substantial immune modulations take place both systemically and in the reproductive mucosa during different stages of pregnancy, highlighting the distinct possibility of sub-optimal or qualitatively different vaccine responses in pregnant women. Research is thus needed to elucidate pregnancy-associated immune alterations in both normal and complicated pregnancies that can influence vaccine responses. The various pregnancy-associated changes in the T, natural killer, myeloid, cytokine and chemokine compartments have been discussed in several excellent reviews (Moffett and Loke, 2006; Mor and Cardenas, 2010, Chen et al., 2012; Pazos et al., 2012a; Erlebacher, 2013). As B cells are the final effectors of humoral immunity, we focus on the modulations in the B cell compartment and their potential influence on vaccine-induced antibody response.
The central and peripheral B cell compartments undergo quantitative changes during pregnancy, with a contraction of peripheral B cell numbers (Fig. 2). Initial studies in mice showed a profound reduction of B cell precursors in the bone marrow from early pregnancy, which was likely mediated by estrogen (Medina et al., 1993, 2000). Consistently, the overall antibody titers to influenza infection are lower in pregnant mice (Medina and Kincade, 1994; Smithson et al., 1998; Chan et al., 2010). Similar changes have also been found in humans by many studies (Christiansen et al., 1976; Moore et al., 1983; Valdimarsson et al., 1983; Iwatani et al., 1988; Watanabe et al., 1997; Mahmoud et al., 2001a). Of note, steroid hormones regulate humoral immunity at multiple stages of B cell development. For example, the very early precursors of pro-B cells are particularly sensitive to negative regulation by estrogen (Kincade et al., 2000; Medina et al., 2001), allowing estrogen to control the size of the B cell pool, while estrogen has an opposite effect at later stages of B cell development, where it promotes B cell maturation and antibody production (Verthelyi and Ahmed, 1998; Grimaldi et al., 2002, 2006). Consequently, normal or even elevated percentages of peripheral mature B cells are found during pregnancy in mice (Medina et al., 1993). Estrogen has also been shown to be able to expand MZ B cells and follicular B cells in mice (Grimaldi et al., 2001, 2006).
Figure 2.
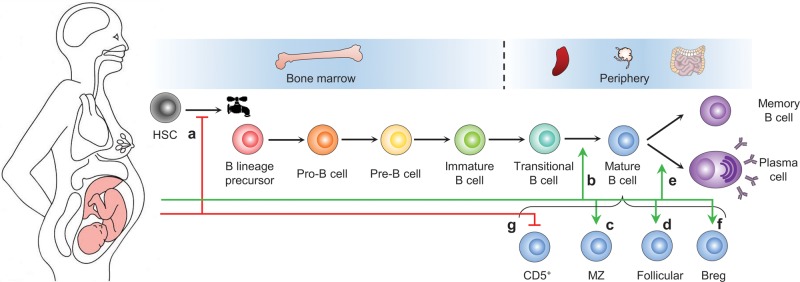
Pregnancy-associated humoral immune alterations that can influence vaccine responses. Pregnancy is accompanied by a marked suppression of the generation of B lineage precursors from hematopoietic stem cells (HSCs) in the bone marrow (a), leading to a reduction of the size of the B cell pool. This suppression of B lymphopoiesis is likely mediated by steroid hormones, such as estrogen. However, estrogen has an opposite effect at later stages of B cell differentiation by promoting the maturation of immature B cells (b) and the generation of marginal zone (MZ) B cells (c) and follicular B cells (d). The combined effect is a reduction of the percentages of immature and transitional B cells in the reduced B cell pool. Estrogen has also been shown to promote the development of plasma cells (e), whereas estrogen and human chorionic gonadotrophin (hCG) have been shown to promote the development of regulatory B cells (Bregs) (f). In normal pregnancy, peripheral CD5+ B cells, which have been implicated in adverse pregnancy outcomes, are suppressed by a mechanism that is not well-known (g). This mechanism and the proper negative selection of autoreactive B cells in the bone marrow are critical to avoid the stimulation of potentially pathogenic B cells by estrogen during pregnancy. Of note, the identity of human B-1 cells is under contentious debate, and the developmental relationship between human Bregs, MZ B cells and B-1 cells is unclear.
Coupled with such stage-specific regulation of B cells by estrogen, are the mechanisms that remove or reduce potentially pathogenic B cells during normal pregnancy. The percentage of circulating CD5+ B cells (Bhat et al., 1995), a population enriched with autoreactivity and partially overlapping with the human B-1 cells (Griffin et al., 2011) recently postulated to be implicated in adverse pregnancy outcomes, such as recurrent pregnancy loss, pre-eclampsia and preterm birth (Kwak et al., 1995; Beer et al., 1996; Roberts et al., 1996; Mahmoud et al., 2001b; Tamiolakis et al., 2001; Darmochwal-Kolarz et al., 2002; Jensen et al., 2012; Wang et al., 2013), is reduced in healthy human pregnancy. Paternal antigen-specific maternal B cells are also suppressed (Ait-Azzouzene et al., 1998, 2001). In addition, estrogen can induce regulatory B cells (Bregs) that express interleukin-10 and programmed death ligand-1 (Bodhankar et al., 2011), which have been implicated with protective functions in pregnancy (Rolle et al., 2013; Wang et al., 2013). The mechanisms to remove or reduce auto- and allo-reactive B cells are critical, because if they fail, estrogen would stimulate the production of pathogenic antibodies by these B cells. Together with the predominant autoantibody production by human early immature B cells (Wardemann et al., 2003), mouse and human data suggest a selective down-regulation of pathogenic B cells in normal pregnancy. This notion has important implications for the development of maternal vaccines, which should leverage the antibody-promoting function of steroid hormones, such as estrogen, and at the same time target the B cell populations in pregnant women to generate high levels of class-switched IgG while avoiding triggering B cells that can mount detrimental autoimmune or alloimmune reactions. This entails a thorough understanding of the type of B cells in pregnancy that are responsible for the production of protective antibodies in response to maternal vaccines, as well as the previous or current autoimmune diagnosis of the pregnant women to be vaccinated. The answer to this question is also relevant to the efficiency of placental antibody transfer to and persistence in the fetus, as different B cell populations can undergo class switching in response to different antigens, preferentially to specific IgG subclasses that vary in their binding affinities to FcRn (Costa-Carvalho et al., 1996) and in vivo half-life (Morell et al., 1970; Stapleton et al., 2011). The analysis of safe and effective examples of maternal vaccines, including Tdap and IIV, in terms of the maternal B cell populations targeted and the composition of maternal antibodies produced, will offer clues to the answer of the above question.
Animal models for maternal vaccination
General guidelines
Epidemiological studies of pregnant women exposed to vaccines have proved to be a useful source of information for the efficacy and safety of these vaccines, but animal models are required to dissect the mechanism of vaccine-induced protection, side effects and to develop new maternal vaccines. Historically, the development and testing of maternal vaccines has critically relied on animal models, which have served at least two purposes. They are used to understand the in vivo mechanism of pathogenesis and the protective immunity required to control and eradicate the pathogen. Once a lead vaccine candidate is identified, animal models are used to evaluate its safety, immunogenicity, pharmacokinetics and efficacy. Many species, including mouse (Oda et al., 1983; Paoletti et al., 2000; Abram et al., 2003; Chan et al., 2010; Rahman et al., 2010; Monney et al., 2012; Pazos et al., 2012a, b), rat (Heiman and Weisman, 1989, 1990; Zenner et al., 1993; Hernando-Insua et al., 2010; Kim et al., 2011), hamster (Freyre et al., 2012), guinea pig (Harrison et al., 1995; Bourne et al., 2001; Schleiss et al., 2007, 2013, 2014; Leviton et al., 2013), rabbit (Wessels et al., 1990, 1993; Barrow, 2012; Barrow and Allais, 2013), sheep (Perez-Sancho et al., 2014), pig (Elahi et al., 2006) and non-human primates (Paoletti et al., 2000; Barry et al., 2006; Warfel et al., 2014), have been used.
In addition to cost and the availability of reagents, various experimental species differ from humans in immune regulation, susceptibility to the pathogen, pathogenesis of infection, length of gestation, placenta physiology and the relative contribution of placental (or yolk sac) antibody transfer versus post-natal transfer of milk antibodies via milk (Table III). Certain species, such as ruminants, horses and pigs, have no or little placental transfer of maternal antibodies to the fetus (Tizzard, 1987), and intestinal absorption occurs for only the first 1–2 days after birth (Tizzard, 1987; Yoshida et al., 2004, 2006; Zaman et al., 2008), which makes these species unsuitable for testing the function of maternal vaccination in fetal or neonatal immune protection.
Table III.
Comparison of human and the common animal models for maternal vaccination research.
| Features | Human | Mouse | Rat | Guinea pig | Rabbit | Rhesus monkey | Pig |
|---|---|---|---|---|---|---|---|
| Gestational length | 270–280 days | 20 days | 22 days | 59–72 days | 32 days | 164 days | 115 days |
| Placenta morphology | Hemochorial, discoid, villi | Hemochorial, discoid, labyrinth | Hemochorial, discoid, labyrinth | Hemochorial, discoid, labyrinth | Hemochorial, discoid, labyrinth | Hemochorial, bidiscoid, villi | Epitheliochorial, diffuse, folded |
| Source of progesterone | Corpus luteum, then placenta and fetal membrane | Corpus luteum | Corpus luteum | Corpus luteum | Corpus luteum | Corpus luteum, then placenta | Corpus luteum |
| Progesterone withdrawal in parturition | No* | Yes | Yes | No* | Yes | No* | Yes |
| Prenatal transfer of IgG | Placenta, FcRn | Inverted yolk sac, FcRn | Inverted yolk sac, FcRn | Inverted yolk sac, fetal gut, FcRn | Inverted yolk sac, FcRn | Placenta, FcRn | No transfer |
| Post-natal transfer of IgG | Gut (1–2 days after birth), FcRn | Proximal small intestine, FcRn | Proximal small intestine, FcRn | No significant transfer | No significant transfer | Gut (1–2 days after birth), FcRn | Gut (2–3 days after birth), FcRn |
*Functional progesterone withdrawal may occur via the expression of inhibitory progesterone receptors in parturition.
The World Health Organization has recommended general guidelines to assess the potential adverse effects of in utero exposure to maternal vaccines using animal models (World Health Organization, 2003). The animal is usually exposed to the vaccine from implantation to the completion of pregnancy via a route similar to that used clinically. For the species with a relative short gestation period, when compared with the time required to develop a vaccine response, vaccination before mating is necessary to allow the fetus to be exposed to full vaccine-induced response. The maximal human dose is recommended for the animal as a starting point. However, if toxicity is observed or if the large administration volume in not feasible for a smaller animal, a mg/kg dose that is higher than the human dose and immunogenic in the animal should be used. The titers of vaccine-induced antibodies in maternal, cord and fetal blood should be determined to verify fetal exposure. Multiple doses may be required depending on the nature of the vaccine formulation and response. Booster immunizations during pregnancy may be necessary to maintain high antibody titers throughout the gestation period so that the embryo is exposed to both the maximal maternal immune response and the complete components of the vaccine formulation. Fetal viability, resorption, abortion, weight and morphology should be determined. In addition, pups should be monitored until weaning for growth, weight gain and viability, whereas the mother should be monitored for nursing activity.
The lesson from mice
Mice have been extensively used for maternal vaccination studies, including influenza (Chan et al., 2010; Pazos et al., 2012a, b), pertussis (Oda et al., 1983; Quinello et al., 2010) and GBS (Lagergard et al., 1990; Wessels et al., 1990, 1993; Madoff et al., 1992; Paoletti et al., 2000). In the case of influenza, the effect of infection on maternal immunity and pregnancy outcome are largely conserved. Infection results in more severe morbidity and mortality in pregnant mice and adversely impacts litter size and health (Siem et al., 1960; Mackenzie et al., 1977; Williams and Mackenzie, 1977; Chan et al., 2010). Pregnant mice also have altered or delayed cytokine production similar to that in pregnant women (Chan et al., 2010), which was likely mediated by estrogen (Pazos et al., 2012b). For pertussis, placental and post-natal transfer of maternal antibodies confers neonatal protection similarly to that in humans, although substantially greater protection has been found to be transferred via milk post-natally (Oda et al., 1983; Quinello et al., 2010). For GBS, the murine model of maternal vaccination followed by neonatal challenge has been used to study both maternal immunogenicity and the efficacy of neonatal protection (Madoff et al., 1992; Rodewald et al., 1992; Paoletti et al., 1994). Preclinical evaluation of maternal GBS glycoconjugate vaccines has largely relied on mouse models. Female mice are vaccinated before pregnancy and their offspring are challenged with viable GBS (Madoff et al., 1994; Paoletti et al., 1994). An immunogenic GBS glycoconjugate vaccine, but not capsular polysaccharides, has been shown to confer protection of most of the pups. Mice have also been used to test the therapeutic efficacy of GBS glycoconjugate vaccine-induced passive immunity in human antisera (Paoletti et al., 1997).
Nonetheless, mice differ from us in many key features pertaining to pregnancy, including gestational length, placentation and endocrinology (Table III), as well as a myriad of other differences in the immune system (Mestas and Hughes, 2004). Various strains of mice also exhibit subtle or even substantial differences in the susceptibility to certain pathogens (Johnson, 2012), which, conceivably, reflects the intrinsic differences in their immune systems. Therefore, the use of mouse models to research maternal vaccination is not expected to completely replicate human physiology, but should be coupled with human studies in an iterative manner, whereby hypotheses drawn based on the observations in humans are tested in mouse models under controlled conditions with detailed sample and data collection, which in turn refines the hypotheses to be further validated in additional human studies (Bonney, 2013). Only by adopting such an iterative approach that mirrors the cycle of vaccine development (Trautmann and Sekaly, 2011) can animal and human studies synergize to make existing maternal vaccines more effective and safer and to facilitate the development of new vaccines.
Conclusions and future directions
Maternal vaccination has emerged as a promising public health approach to prevent or combat maternal and neonatal morbidity. Considerable achievements have been made in the past decade, with a number of vaccines being universally recommended for pregnancy. However, the public acceptance of maternal vaccination has been low in many countries. Besides the ethical, legal and socioeconomic restraints, significant gaps exist in our knowledge of the efficacy and safety of maternal vaccines in pregnant women and those susceptible to high-risk pregnancies, and no maternal vaccines against a large number of old and emerging pathogens are available. To tackle these scientific challenges and provide the public with informed choices in vaccination, obstetricians, gynecologists, reproductive biologists and immunologists must transcend the traditional disciplinary barriers and work in concert, to be guided by a mechanistic understanding of the maternal, fetal and neonatal immunologic responses to vaccines. Our shallow overview of the various topics in this review is precisely intended for such a purpose.
Can we be faster and more effective?
The empirical quest of maternal vaccines has largely relied on a reductionist approach of hypothesis creation followed by experimental validation in animal models and clinical trials. This approach can be time consuming, not allowing the rapid development of new vaccines, especially in case of an emerging pandemic. Neither does it offer a systemic view of the complex behavior of the maternal immune system after vaccination. Recently studies have highlighted the power of reverse vaccinology for systematic and improved antigen discovery (Sette and Rappuoli, 2010) and systems vaccinology to profile vaccine response (Pulendran et al., 2010) and even to predict vaccine efficacy (Querec et al., 2009; Nakaya et al., 2011; Li et al., 2014). We believe the application of such approaches at all stages of maternal vaccination research, from animal experimentation to human trials and evaluation, will dramatically improve the speed, accuracy and safety of maternal vaccine targeting. Lastly, as the efficacy of maternal vaccines also significantly relies on the secretion of antibodies at the maternal–fetal interface and in the mammary gland, a thorough understanding of the unique mechanisms of mucosal immune regulation and the microbiota influence (Brandtzaeg, 2010, Chen and Cerutti, 2010b) as well as the incorporation of mucosal immune assessment into maternal vaccine experimentation and evaluation protocols are required.
Authors' roles
A.N.F. and B.L.U. reviewed the literature and wrote the manuscript; B.G. revised the manuscript; K.C. conceived the study, reviewed the literature, and wrote and revised the manuscript.
Funding
The authors' research is supported by the US National Institutes of Health (U01 AI95776 Young Investigator Award), American Congress of Obstetricians and Gynecologists (Merck Industrial Research Award), Burroughs Wellcome Fund (Preterm Birth Research Award), Wayne State University Perinatal Initiative (176512), Wayne State University Office of the Vice President for Research and Barbara Ann Karmanos Cancer Institute to K.C.
Conflict of interest
None declared.
Acknowledgements
We apologize to those authors whose work we could not cite due to space limitations.
References
- Abram M, Schluter D, Vuckovic D, Wraber B, Doric M, Deckert M. Murine model of pregnancy-associated Listeria monocytogenes infection. FEMS Immunol Med Microbiol. 2003;35:177–182. doi: 10.1016/S0928-8244(02)00449-2. [DOI] [PubMed] [Google Scholar]
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- Ait-Azzouzene D, Gendron MC, Houdayer M, Langkopf A, Burki K, Nemazee D, Kanellopoulos-Langevin C. Maternal B lymphocytes specific for paternal histocompatibility antigens are partially deleted during pregnancy. J Immunol. 1998;161:2677–2683. [PubMed] [Google Scholar]
- Ait-Azzouzene D, Caucheteux S, Tchang F, Wantyghem J, Moutier R, Langkopf A, Gendron MC, Kanellopoulos-Langevin C. Transgenic major histocompatibility complex class I antigen expressed in mouse trophoblast affects maternal immature B cells. Biol Reprod. 2001;65:337–344. doi: 10.1095/biolreprod65.2.337. [DOI] [PubMed] [Google Scholar]
- Albrecht P, Ennis FA, Saltzman EJ, Krugman S. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J Pediatr. 1977;91:715–718. doi: 10.1016/s0022-3476(77)81021-4. [DOI] [PubMed] [Google Scholar]
- Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, Lee BW, Lolekha S, Peltola H, Ruff TA, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 2008;86:81–160. doi: 10.2471/BLT.07.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoola EA, Johnson AO. Hepatitis B vaccine in pregnancy: immunogenicity, safety and transfer of antibodies to infants. Int J Gynaecol Obstet. 1987;25:297–301. doi: 10.1016/0020-7292(87)90289-x. [DOI] [PubMed] [Google Scholar]
- Baker CJ, Kasper DL. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976;294:753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- Barber EM, Fazzari M, Pollard JW. Th1 cytokines are essential for placental immunity to Listeria monocytogenes. Infect Immun. 2005;73:6322–6331. doi: 10.1128/IAI.73.10.6322-6331.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow P. Developmental and reproductive toxicity testing of vaccines. J Pharmacol Toxicol Methods. 2012;65:58–63. doi: 10.1016/j.vascn.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Barrow PC, Allais L. Developmental toxicity testing of vaccines. Methods Mol Biol. 2013;947:81–89. doi: 10.1007/978-1-62703-131-8_7. [DOI] [PubMed] [Google Scholar]
- Barry PA, Lockridge KM, Salamat S, Tinling SP, Yue Y, Zhou SS, Gospe SM, Jr, Britt WJ, Tarantal AF. Nonhuman primate models of intrauterine cytomegalovirus infection. ILAR J. 2006;47:49–64. doi: 10.1093/ilar.47.1.49. [DOI] [PubMed] [Google Scholar]
- Beau AB, Hurault-Delarue C, Vidal S, Guitard C, Vayssiere C, Petiot D, Montastruc JL, Damase-Michel C, Lacroix I. Pandemic A/H1N1 influenza vaccination during pregnancy: a comparative study using the EFEMERIS database. Vaccine. 2014;32:1254–1258. doi: 10.1016/j.vaccine.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Beer AE, Kwak JY, Ruiz JE. Immunophenotypic profiles of peripheral blood lymphocytes in women with recurrent pregnancy losses and in infertile women with multiple failed in vitro fertilization cycles. Am J Reprod Immunol. 1996;35:376–382. doi: 10.1111/j.1600-0897.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- Beigi RH. Influenza during pregnancy: a cause of serious infection in obstetrics. Clin Obstet Gynecol. 2012;55:914–926. doi: 10.1097/GRF.0b013e31827146bd. [DOI] [PubMed] [Google Scholar]
- Benshushan A, Tsafrir A, Arbel R, Rahav G, Ariel I, Rojansky N. Listeria infection during pregnancy: a 10 year experience. Isr Med Assoc J. 2002;4:776–780. [PubMed] [Google Scholar]
- Bhat NM, Mithal A, Bieber MM, Herzenberg LA, Teng NN. Human CD5+ B lymphocytes (B-1 cells) decrease in peripheral blood during pregnancy. J Reprod Immunol. 1995;28:53–60. doi: 10.1016/0165-0378(94)00907-o. [DOI] [PubMed] [Google Scholar]
- Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Bodhankar S, Wang C, Vandenbark AA, Offner H. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. Eur J Immunol. 2011;41:1165–1175. doi: 10.1002/eji.201040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney EA. Demystifying animal models of adverse pregnancy outcomes: touching bench and bedside. Am J Reprod Immunol. 2013;69:567–584. doi: 10.1111/aji.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booy R, Aitken SJ, Taylor S, Tudor-Williams G, Macfarlane JA, Moxon ER, Ashworth LA, Mayon-White RT, Griffiths H, Chapel HM. Immunogenicity of combined diphtheria, tetanus, and pertussis vaccine given at 2, 3, and 4 months versus 3, 5, and 9 months of age. Lancet. 1992;339:507–510. doi: 10.1016/0140-6736(92)90336-2. [DOI] [PubMed] [Google Scholar]
- Bot A, Bot S, Bona C. Enhanced protection against influenza virus of mice immunized as newborns with a mixture of plasmids expressing hemagglutinin and nucleoprotein. Vaccine. 1998;16:1675–1682. doi: 10.1016/s0264-410x(98)00054-1. [DOI] [PubMed] [Google Scholar]
- Bourne N, Schleiss MR, Bravo FJ, Bernstein DI. Preconception immunization with a cytomegalovirus (CMV) glycoprotein vaccine improves pregnancy outcome in a guinea pig model of congenital CMV infection. J Infect Dis. 2001;183:59–64. doi: 10.1086/317654. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr. 2010;156:S8–15. doi: 10.1016/j.jpeds.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Bray RS, Anderson MJ. Falciparum malaria and pregnancy. Trans R Soc Trop Med Hyg. 1979;73:427–431. doi: 10.1016/0035-9203(79)90170-6. [DOI] [PubMed] [Google Scholar]
- Brazolot Millan CL, Weeratna R, Krieg AM, Siegrist CA, Davis HL. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc Natl Acad Sci USA. 1998;95:15553–15558. doi: 10.1073/pnas.95.26.15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent RL. Immunization of pregnant women: reproductive, medical and societal risks. Vaccine. 2003;21:3413–3421. doi: 10.1016/s0264-410x(03)00396-7. [DOI] [PubMed] [Google Scholar]
- Brew O, Sullivan MH. The links between maternal histamine levels and complications of human pregnancy. J Reprod Immunol. 2006;72:94–107. doi: 10.1016/j.jri.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Burstyn DG, Baraff LJ, Peppler MS, Leake RD, St Geme J, Jr, Manclark CR. Serological response to filamentous hemagglutinin and lymphocytosis-promoting toxin of Bordetella pertussis. Infect Immun. 1983;41:1150–1156. doi: 10.1128/iai.41.3.1150-1156.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Stanfield RL, Wilson IA. Antibody vs. HIV in a clash of evolutionary titans. Proc Natl Acad Sci USA. 2005;102:14943–14948. doi: 10.1073/pnas.0505126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bytautiene E, Romero R, Vedernikov YP, El-Zeky F, Saade GR, Garfield RE. Induction of premature labor and delivery by allergic reaction and prevention by histamine H1 receptor antagonist. Am J Obstet Gynecol. 2004;191:1356–1361. doi: 10.1016/j.ajog.2004.06.092. [DOI] [PubMed] [Google Scholar]
- Callaghan WM, Chu SY, Jamieson DJ. Deaths from seasonal influenza among pregnant women in the United States, 1998–2005. Obstet Gynecol. 2010;115:919–923. doi: 10.1097/AOG.0b013e3181d99d85. [DOI] [PubMed] [Google Scholar]
- Carcione D, Blyth CC, Richmond PC, Mak DB, Effler PV. Safety surveillance of influenza vaccine in pregnant women. Aust N Z J Obstet Gynaecol. 2013;53:98–99. doi: 10.1111/ajo.12034. [DOI] [PubMed] [Google Scholar]
- Castillo-Solorzano C, Reef SE, Morice A, Vascones N, Chevez AE, Castalia-Soares R, Torres C, Vizzotti C, Ruiz Matus C. Rubella vaccination of unknowingly pregnant women during mass campaigns for rubella and congenital rubella syndrome elimination, the Americas 2001–2008. J Infect Dis. 2011;204(Suppl 2):S713–S717. doi: 10.1093/infdis/jir489. [DOI] [PubMed] [Google Scholar]
- Cederqvist LL, Ewool LC, Bonsnes RW, Litwin SD. Detectability and pattern of immunoglobulins in normal amniotic fluid throughout gestation. Am J Obstet Gynecol. 1978;130:220–224. doi: 10.1016/0002-9378(78)90370-8. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1997;46:1–24. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Influenza vaccination coverage among pregnant women—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep. 2013a;62:787–792. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013b;62:131–135. [PMC free article] [PubMed] [Google Scholar]
- Cerutti A, Chen K. USA: Cornell University; 2010. Methods for treating IgE-mediated disorder. In Office USPaT (ed) [Google Scholar]
- Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KH, Zhang AJ, To KK, Chan CC, Poon VK, Guo K, Ng F, Zhang QW, Leung VH, Cheung AN, et al. Wild type and mutant 2009 pandemic influenza A (H1N1) viruses cause more severe disease and higher mortality in pregnant BALB/c mice. PLoS One. 2010;5:e13757. doi: 10.1371/journal.pone.0013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Cerutti A. New insights into the enigma of immunoglobulin D. Immunol Rev. 2010a;237:160–179. doi: 10.1111/j.1600-065X.2010.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Cerutti A. Vaccination strategies to promote mucosal antibody responses. Immunity. 2010b;33:479–491. doi: 10.1016/j.immuni.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, Edholm ES, Santini PA, Rath P, Chiu A, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Liu YL, Sytwu HK. Immunologic regulation in pregnancy: from mechanism to therapeutic strategy for immunomodulation. Clin Dev Immunol. 2012;2012:258391. doi: 10.1155/2012/258391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen JS, Andersen AR, Osther K, Peitersen B, Bach-Mortensen N, Lebech PE. The relationship between pregnancy, HCS and B lymphocytes. Acta Pathol Microbiol Scand C. 1976;84:313–318. doi: 10.1111/j.1699-0463.1976.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Claesson BA, Schneerson R, Robbins JB, Johansson J, Lagergard T, Taranger J, Bryla D, Levi L, Cramton T, Trollfors B. Protective levels of serum antibodies stimulated in infants by two injections of Haemophilus influenzae type b capsular polysaccharide-tetanus toxoid conjugate. J Pediatr. 1989;114:97–100. doi: 10.1016/s0022-3476(89)80611-0. [DOI] [PubMed] [Google Scholar]
- Conlin AM, Bukowinski AT, Sevick CJ, DeScisciolo C, Crum-Cianflone NF. Safety of the pandemic H1N1 influenza vaccine among pregnant U.S. military women and their newborns. Obstet Gynecol. 2013;121:511–518. doi: 10.1097/AOG.0b013e318280d64e. [DOI] [PubMed] [Google Scholar]
- Costa-Carvalho BT, Vieria HM, Dimantas RB, Arslanian C, Naspitz CK, Sole D, Carneiro-Sampaio MM. Transfer of IgG subclasses across placenta in term and preterm newborns. Braz J Med Biol Res. 1996;29:201–204. [PubMed] [Google Scholar]
- Creanga AA, Johnson TF, Graitcer SB, Hartman LK, Al-Samarrai T, Schwarz AG, Chu SY, Sackoff JE, Jamieson DJ, Fine AD, et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115:717–726. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- Crowe JE, Jr, Firestone CY, Murphy BR. Passively acquired antibodies suppress humoral but not cell-mediated immunity in mice immunized with live attenuated respiratory syncytial virus vaccines. J Immunol. 2001;167:3910–3918. doi: 10.4049/jimmunol.167.7.3910. [DOI] [PubMed] [Google Scholar]
- Dagan R, Amir J, Mijalovsky A, Kalmanovitch I, Bar-Yochai A, Thoelen S, Safary A, Ashkenazi S. Immunization against hepatitis A in the first year of life: priming despite the presence of maternal antibody. Pediatr Infect Dis J. 2000;19:1045–1052. doi: 10.1097/00006454-200011000-00004. [DOI] [PubMed] [Google Scholar]
- Darmochwal-Kolarz D, Leszczynska-Gorzelak B, Rolinski J, Oleszczuk J. The immunophenotype of patients with recurrent pregnancy loss. Eur J Obstet Gynecol Reprod Biol. 2002;103:53–57. doi: 10.1016/s0301-2115(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Darmstadt GL, Zaidi AKM, Stoll BJ. Neonatal infections: a global perspective. In: Remington JS, Klein JO, Wilson CB, Nizet V, Maldonado YA, editors. Infectious Diseases of the Fetus and Newborn Infant. Philadelphia, PA, USA: Saunders/Elsevier; 2011. pp. 24–51. [Google Scholar]
- Daum RS, Siber GR, Ballanco GA, Sood SK. Serum anticapsular antibody response in the first week after immunization of adults and infants with the Haemophilus influenzae type b-Neisseria meningitidis outer membrane protein complex conjugate vaccine. J Infect Dis. 1991;164:1154–1159. doi: 10.1093/infdis/164.6.1154. [DOI] [PubMed] [Google Scholar]
- Elahi S, Buchanan RM, Babiuk LA, Gerdts V. Maternal immunity provides protection against pertussis in newborn piglets. Infect Immun. 2006;74:2619–2627. doi: 10.1128/IAI.74.5.2619-2627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kady D, Strassberg ER, Khan M, Yens D. Does influenza vaccination in pregnancy reduce the risk of preeclampsia? Obstet Gynecol. 2014;123(Suppl 1):48S–49S. [Google Scholar]
- Englund JA, Anderson EL, Reed GF, Decker MD, Edwards KM, Pichichero ME, Steinhoff MC, Rennels MB, Deforest A, Meade BD. The effect of maternal antibody on the serologic response and the incidence of adverse reactions after primary immunization with acellular and whole-cell pertussis vaccines combined with diphtheria and tetanus toxoids. Pediatrics. 1995;96:580–584. [PubMed] [Google Scholar]
- Enriquez-Rincon F, Klaus GG. Differing effects of monoclonal anti-hapten antibodies on humoral responses to soluble or particulate antigens. Immunology. 1984;52:129–136. [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- Fadel SA, Ozaki DA, Sarzotti M. Enhanced type 1 immunity after secondary viral challenge in mice primed as neonates. J Immunol. 2002;169:3293–3300. doi: 10.4049/jimmunol.169.6.3293. [DOI] [PubMed] [Google Scholar]
- Favre L, Spertini F, Corthesy B. Secretory IgA possesses intrinsic modulatory properties stimulating mucosal and systemic immune responses. J Immunol. 2005;175:2793–2800. doi: 10.4049/jimmunol.175.5.2793. [DOI] [PubMed] [Google Scholar]
- Fell DB, Sprague AE, Liu N, Yasseen AS, III, Wen SW, Smith G, Walker MC, Better Outcomes R, Network O. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health. 2012;102:e33–e40. doi: 10.2105/AJPH.2011.300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fievet N, Moussa M, Tami G, Maubert B, Cot M, Deloron P, Chaouat G. Plasmodium falciparum induces a Th1/Th2 disequilibrium, favoring the Th1-type pathway, in the human placenta. J Infect Dis. 2001;183:1530–1534. doi: 10.1086/320201. [DOI] [PubMed] [Google Scholar]
- Fink K, Zellweger R, Weber J, Manjarrez-Orduno N, Holdener M, Senn BM, Hengartner H, Zinkernagel RM, Macpherson AJ. Long-term maternal imprinting of the specific B cell repertoire by maternal antibodies. Eur J Immunol. 2008;38:90–101. doi: 10.1002/eji.200737872. [DOI] [PubMed] [Google Scholar]
- Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- Franchini M, Abril C, Schwerdel C, Ruedl C, Ackermann M, Suter M. Protective T-cell-based immunity induced in neonatal mice by a single replicative cycle of herpes simplex virus. J Virol. 2001;75:83–89. doi: 10.1128/JVI.75.1.83-89.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyre A, Araujo FA, Fialho CG, Bigatti LE, Falcon JD. Protection in a hamster model of congenital toxoplasmosis. Vet Parasitol. 2012;183:359–363. doi: 10.1016/j.vetpar.2011.07.039. [DOI] [PubMed] [Google Scholar]
- Garcia AM, Fadel SA, Cao S, Sarzotti M. T cell immunity in neonates. Immunol Res. 2000;22:177–190. doi: 10.1385/IR:22:2-3:177. [DOI] [PubMed] [Google Scholar]
- Ge YY, Zhang L, Zhang G, Wu JP, Tan MJ, Hu E, Liang YJ, Wang Y. In pregnant mice, the infection of Toxoplasma gondii causes the decrease of CD4+CD25+-regulatory T cells. Parasite Immunol. 2008;30:471–481. doi: 10.1111/j.1365-3024.2008.01044.x. [DOI] [PubMed] [Google Scholar]
- Gellin BG, Broome CV, Bibb WF, Weaver RE, Gaventa S, Mascola L. The epidemiology of listeriosis in the United States—1986. Listeriosis Study Group. Am J Epidemiol. 1991;133:392–401. doi: 10.1093/oxfordjournals.aje.a115893. [DOI] [PubMed] [Google Scholar]
- Getahun A, Heyman B. Studies on the mechanism by which antigen-specific IgG suppresses primary antibody responses: evidence for epitope masking and decreased localization of antigen in the spleen. Scand J Immunol. 2009;70:277–287. doi: 10.1111/j.1365-3083.2009.02298.x. [DOI] [PubMed] [Google Scholar]
- Glezen WP. Effect of maternal antibodies on the infant immune response. Vaccine. 2003;21:3389–3392. doi: 10.1016/s0264-410x(03)00339-6. [DOI] [PubMed] [Google Scholar]
- Goldszmid RS, Trinchieri G. The price of immunity. Nat Immunol. 2012;13:932–938. doi: 10.1038/ni.2422. [DOI] [PubMed] [Google Scholar]
- Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J Immunol. 2001;167:1886–1890. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. 2002;109:1625–1633. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi CM, Jeganathan V, Diamond B. Hormonal regulation of B cell development: 17 beta-estradiol impairs negative selection of high-affinity DNA-reactive B cells at more than one developmental checkpoint. J Immunol. 2006;176:2703–2710. doi: 10.4049/jimmunol.176.5.2703. [DOI] [PubMed] [Google Scholar]
- Gupta I, Ratho RK. Immunogenicity and safety of two schedules of Hepatitis B vaccination during pregnancy. J Obstet Gynaecol Res. 2003;29:84–86. doi: 10.1046/j.1341-8076.2002.00076.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez G, Gentile T, Miranda S, Margni RA. Asymmetric antibodies: a protective arm in pregnancy. Chem Immunol Allergy. 2005;89:158–168. doi: 10.1159/000087964. [DOI] [PubMed] [Google Scholar]
- Hansen RJ, Balthasar JP. Intravenous immunoglobulin mediates an increase in anti-platelet antibody clearance via the FcRn receptor. Thromb Haemost. 2002;88:898–899. [PubMed] [Google Scholar]
- Hansen C, Desai S, Bredfeldt C, Cheetham C, Gallagher M, Li DK, Raebel MA, Riedlinger K, Shay DK, Thompson M, et al. A large, population-based study of 2009 pandemic Influenza A virus subtype H1N1 infection diagnosis during pregnancy and outcomes for mothers and neonates. J Infect Dis. 2012;206:1260–1268. doi: 10.1093/infdis/jis488. [DOI] [PubMed] [Google Scholar]
- Hardy-Fairbanks AJ, Pan SJ, Decker MD, Johnson DR, Greenberg DP, Kirkland KB, Talbot EA, Bernstein HH. Immune responses in infants whose mothers received Tdap vaccine during pregnancy. Pediatr Infect Dis J. 2013;32:1257–1260. doi: 10.1097/INF.0b013e3182a09b6a. [DOI] [PubMed] [Google Scholar]
- Harris NL, Spoerri I, Schopfer JF, Nembrini C, Merky P, Massacand J, Urban JF, Jr, Lamarre A, Burki K, Odermatt B, et al. Mechanisms of neonatal mucosal antibody protection. J Immunol. 2006;177:6256–6262. doi: 10.4049/jimmunol.177.9.6256. [DOI] [PubMed] [Google Scholar]
- Harrison CJ, Britt WJ, Chapman NM, Mullican J, Tracy S. Reduced congenital cytomegalovirus (CMV) infection after maternal immunization with a guinea pig CMV glycoprotein before gestational primary CMV infection in the guinea pig model. J Infect Dis. 1995;172:1212–1220. doi: 10.1093/infdis/172.5.1212. [DOI] [PubMed] [Google Scholar]
- Hassett DE, Zhang J, Whitton JL. Neonatal DNA immunization with a plasmid encoding an internal viral protein is effective in the presence of maternal antibodies and protects against subsequent viral challenge. J Virol. 1997;71:7881–7888. doi: 10.1128/jvi.71.10.7881-7888.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy CM. Vaccines in pregnant women and research initiatives. Clin Obstet Gynecol. 2012;55:474–486. doi: 10.1097/GRF.0b013e31824f3acb. [DOI] [PubMed] [Google Scholar]
- Healy CM, Rench MA, Baker CJ. Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants. Clin Infect Dis. 2013;56:539–544. doi: 10.1093/cid/cis923. [DOI] [PubMed] [Google Scholar]
- Heiman HS, Weisman LE. Transplacental or enteral transfer of maternal immunization-induced antibody protects suckling rats from type III group B streptococcal infection. Pediatr Res. 1989;26:629–632. doi: 10.1203/00006450-198912000-00023. [DOI] [PubMed] [Google Scholar]
- Heiman HS, Weisman LE. The opsonic antibody response of female rats to type III group B streptococcal immunization: a model for maternal immunity. Vet Immunol Immunopathol. 1990;24:79–89. doi: 10.1016/0165-2427(90)90079-8. [DOI] [PubMed] [Google Scholar]
- Hernando-Insua A, Rodriguez JM, Elias F, Flo J, Lopez R, Franco R, Lago N, Zorzopulos J, Montaner AD. A high dose of IMT504, the PyNTTTTGT prototype immunostimulatory oligonucleotide, does not alter embryonic development in rats. Oligonucleotides. 2010;20:33–36. doi: 10.1089/oli.2009.0206. [DOI] [PubMed] [Google Scholar]
- Honda S, Kurita N, Miyamoto A, Cho Y, Usui K, Takeshita K, Takahashi S, Yasui T, Kikutani H, Kinoshita T, et al. Enhanced humoral immune responses against T-independent antigens in Fc alpha/muR-deficient mice. Proc Natl Acad Sci USA. 2009;106:11230–11235. doi: 10.1073/pnas.0809917106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz MK, Sabara MI, Frenchick PJ, Babiuk LA. Effect of different routes of immunization with bovine rotavirus on lactogenic antibody response in mice. Antiviral Res. 1987;8:283–297. doi: 10.1016/S0166-3542(87)80006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving SA, Kieke BA, Donahue JG, Mascola MA, Baggs J, DeStefano F, Cheetham TC, Jackson LA, Naleway AL, Glanz JM, et al. Trivalent inactivated influenza vaccine and spontaneous abortion. Obstet Gynecol. 2013;121:159–165. doi: 10.1097/aog.0b013e318279f56f. [DOI] [PubMed] [Google Scholar]
- Israel EJ, Patel VK, Taylor SF, Marshak-Rothstein A, Simister NE. Requirement for a beta 2-microglobulin-associated Fc receptor for acquisition of maternal IgG by fetal and neonatal mice. J Immunol. 1995;154:6246–6251. [PubMed] [Google Scholar]
- Ito M, Nakashima A, Hidaka T, Okabe M, Bac ND, Ina S, Yoneda S, Shiozaki A, Sumi S, Tsuneyama K, et al. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol. 2010;84:75–85. doi: 10.1016/j.jri.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Iwatani Y, Amino N, Tachi J, Kimura M, Ura I, Mori M, Miyai K, Nasu M, Tanizawa O. Changes of lymphocyte subsets in normal pregnant and postpartum women: postpartum increase in NK/K (Leu 7) cells. Am J Reprod Immunol Microbiol. 1988;18:52–55. doi: 10.1111/j.1600-0897.1988.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Jakobsen H, Saeland E, Gizurarson S, Schulz D, Jonsdottir I. Intranasal immunization with pneumococcal polysaccharide conjugate vaccines protects mice against invasive pneumococcal infections. Infect Immun. 1999;67:4128–4133. doi: 10.1128/iai.67.8.4128-4133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis. 2006;12:1638–1643. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelonek MT, Maskrey JL, Steimer KS, Potts BJ, Higgins KW, Keller MA. Maternal monoclonal antibody to the V3 loop alters specificity of the response to a human immunodeficiency virus vaccine. J Infect Dis. 1996;174:866–869. doi: 10.1093/infdis/174.4.866. [DOI] [PubMed] [Google Scholar]
- Jensen F, Wallukat G, Herse F, Budner O, El-Mousleh T, Costa SD, Dechend R, Zenclussen AC. CD19+CD5+ cells as indicators of preeclampsia. Hypertension. 2012;59:861–868. doi: 10.1161/HYPERTENSIONAHA.111.188276. [DOI] [PubMed] [Google Scholar]
- Jenum PA, Stray-Pedersen B, Melby KK, Kapperud G, Whitelaw A, Eskild A, Eng J. Incidence of Toxoplasma gondii infection in 35,940 pregnant women in Norway and pregnancy outcome for infected women. J Clin Microbiol. 1998;36:2900–2906. doi: 10.1128/jcm.36.10.2900-2906.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. Laboratory mice and rats. Mater Methods. 2012;2:113. [Google Scholar]
- Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamaru Y, Pfirsch S, Aloulou M, Vrtovsnik F, Essig M, Loirat C, Deschenes G, Guerin-Marchand C, Blank U, Monteiro RC. Inhibitory ITAM signaling by Fc alpha RI-FcR gamma chain controls multiple activating responses and prevents renal inflammation. J Immunol. 2008;180:2669–2678. doi: 10.4049/jimmunol.180.4.2669. [DOI] [PubMed] [Google Scholar]
- Kanra G, Yalcin SS, Ceyhan M, Yurdakok K. Clinical trial to evaluate immunogenicity and safety of inactivated hepatitis A vaccination starting at 2-month-old children. Turk J Pediatr. 2000;42:105–108. [PubMed] [Google Scholar]
- Kanswal S, Katsenelson N, Selvapandiyan A, Bram RJ, Akkoyunlu M. Deficient TACI expression on B lymphocytes of newborn mice leads to defective Ig secretion in response to BAFF or APRIL. J Immunol. 2008;181:976–990. doi: 10.4049/jimmunol.181.2.976. [DOI] [PubMed] [Google Scholar]
- Kaur K, Chowdhury S, Greenspan NS, Schreiber JR. Decreased expression of tumor necrosis factor family receptors involved in humoral immune responses in preterm neonates. Blood. 2007;110:2948–2954. doi: 10.1182/blood-2007-01-069245. [DOI] [PubMed] [Google Scholar]
- Kim D, Huey D, Oglesbee M, Niewiesk S. Insights into the regulatory mechanism controlling the inhibition of vaccine-induced seroconversion by maternal antibodies. Blood. 2011;117:6143–6151. doi: 10.1182/blood-2010-11-320317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade PW, Medina KL, Payne KJ, Rossi MI, Tudor KS, Yamashita Y, Kouro T. Early B-lymphocyte precursors and their regulation by sex steroids. Immunol Rev. 2000;175:128–137. [PubMed] [Google Scholar]
- Koelsch K, Zheng NY, Zhang Q, Duty A, Helms C, Mathias MD, Jared M, Smith K, Capra JD, Wilson PC. Mature B cells class switched to IgD are autoreactive in healthy individuals. J Clin Invest. 2007;117:1558–1565. doi: 10.1172/JCI27628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S, Loo LS. The relative role of transplacental and milk immune transfer in protection against lethal neonatal herpes simplex virus infection in mice. J Infect Dis. 1984;149:38–42. doi: 10.1093/infdis/149.1.38. [DOI] [PubMed] [Google Scholar]
- Kovarik J, Martinez X, Pihlgren M, Bozzotti P, Tao MH, Kipps TJ, Wild TF, Lambert PH, Siegrist CA. Limitations of in vivo IL-12 supplementation strategies to induce Th1 early life responses to model viral and bacterial vaccine antigens. Virology. 2000;268:122–131. doi: 10.1006/viro.1999.0159. [DOI] [PubMed] [Google Scholar]
- Kovarik J, Gaillard M, Martinez X, Bozzotti P, Lambert PH, Wild TF, Siegrist CA. Induction of adult-like antibody, Th1, and CTL responses to measles hemagglutinin by early life murine immunization with an attenuated vaccinia-derived NYVAC(K1L) viral vector. Virology. 2001;285:12–20. doi: 10.1006/viro.2001.0945. [DOI] [PubMed] [Google Scholar]
- Kwak JY, Beaman KD, Gilman-Sachs A, Ruiz JE, Schewitz D, Beer AE. Up-regulated expression of CD56+, CD56+/CD16+, and CD19+ cells in peripheral blood lymphocytes in pregnant women with recurrent pregnancy losses. Am J Reprod Immunol. 1995;34:93–99. doi: 10.1111/j.1600-0897.1995.tb00924.x. [DOI] [PubMed] [Google Scholar]
- Lagergard T, Shiloach J, Robbins JB, Schneerson R. Synthesis and immunological properties of conjugates composed of group B streptococcus type III capsular polysaccharide covalently bound to tetanus toxoid. Infect Immun. 1990;58:687–694. doi: 10.1128/iai.58.3.687-694.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge A, Dodds L, MacDonald NE, Scott J, McNeil S. Rates and determinants of seasonal influenza vaccination in pregnancy and association with neonatal outcomes. CMAJ. 2014;186:E157–E164. doi: 10.1503/cmaj.130499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levan-Petit I, Lelievre E, Barra A, Limosin A, Gombert B, Preud'homme JL, Lecron JC. T(h)2 cytokine dependence of IgD production by normal human B cells. Int Immunol. 1999;11:1819–1828. doi: 10.1093/intimm/11.11.1819. [DOI] [PubMed] [Google Scholar]
- Leviton MP, Lacayo JC, Choi KY, Hernandez-Alvarado N, Wey A, Schleiss MR. An attenuated cytomegalovirus vaccine with a deletion of a viral chemokine gene is protective against congenital CMV transmission in a guinea pig model. Clin Dev Immunol. 2013;2013:906948. doi: 10.1155/2013/906948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- Li N, Zhao M, Hilario-Vargas J, Prisayanh P, Warren S, Diaz LA, Roopenian DC, Liu Z. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest. 2005;115:3440–3450. doi: 10.1172/JCI24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Rouphael N, Duraisingham S, Romero-Steiner S, Presnell S, Davis C, Schmidt DS, Johnson SE, Milton A, Rajam G, et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol. 2014;15:195–204. doi: 10.1038/ni.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ML, Chong CY, Tee WS, Lim WY, Chee JJ. Influenza A/H1N1 (2009) infection in pregnancy--an Asian perspective. BJOG. 2010;117:551–556. doi: 10.1111/j.1471-0528.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Lindsay L, Jackson LA, Savitz DA, Weber DJ, Koch GG, Kong L, Guess HA. Community influenza activity and risk of acute influenza-like illness episodes among healthy unvaccinated pregnant and postpartum women. Am J Epidemiol. 2006;163:838–848. doi: 10.1093/aje/kwj095. [DOI] [PubMed] [Google Scholar]
- Litwin SD, Zehr BD, Insel RA. Selective concentration of IgD class-specific antibodies in human milk. Clin Exp Immunol. 1990;80:263–267. doi: 10.1111/j.1365-2249.1990.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li Q, Cui H, Liu C. Severe and critical cases of H1N1 influenza in pregnancy: a Chinese perspective. J Postgrad Med. 2011;57:298–301. doi: 10.4103/0022-3859.90079. [DOI] [PubMed] [Google Scholar]
- Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louik C, Ahrens K, Kerr S, Pyo J, Chambers C, Jones KL, Schatz M, Mitchell AA. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: exposure prevalence, preterm delivery, and specific birth defects. Vaccine. 2013;31:5033–5040. doi: 10.1016/j.vaccine.2013.08.096. [DOI] [PubMed] [Google Scholar]
- Mackenzie JS, Williams K, Papadimitriou J. Influenza A virus and its influence on the outcome of pregnancy in the mouse. Dev Biol Stand. 1977;39:489–496. [PubMed] [Google Scholar]
- Mackenzie IS, MacDonald TM, Shakir S, Dryburgh M, Mantay BJ, McDonnell P, Layton D. Influenza H1N1 (swine flu) vaccination: a safety surveillance feasibility study using self-reporting of serious adverse events and pregnancy outcomes. Br J Clin Pharmacol. 2012;73:801–811. doi: 10.1111/j.1365-2125.2011.04142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan IC, Bazin H, Chassoux D, Gray D, Lortan J. Comparative analysis of the development of B cells in marginal zones and follicles. Adv Exp Med Biol. 1985;186:139–144. doi: 10.1007/978-1-4613-2463-8_17. [DOI] [PubMed] [Google Scholar]
- Madoff LC, Michel JL, Gong EW, Rodewald AK, Kasper DL. Protection of neonatal mice from group B streptococcal infection by maternal immunization with beta C protein. Infect Immun. 1992;60:4989–4994. doi: 10.1128/iai.60.12.4989-4994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madoff LC, Paoletti LC, Tai JY, Kasper DL. Maternal immunization of mice with group B streptococcal type III polysaccharide-beta C protein conjugate elicits protective antibody to multiple serotypes. J Clin Invest. 1994;94:286–292. doi: 10.1172/JCI117319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud F, Abul H, Omu A, Al-Rayes S, Haines D, Whaley K. Pregnancy-associated changes in peripheral blood lymphocyte subpopulations in normal Kuwaiti women. Gynecol Obstet Invest. 2001a;52:232–236. doi: 10.1159/000052981. [DOI] [PubMed] [Google Scholar]
- Mahmoud F, Diejomaoh M, Omu AE, Abul H, Haines D. Lymphocyte subpopulation frequency and presence of anti-cardiolipin and anti-nuclear antibodies in peripheral blood of Kuwaiti women experiencing recurrent pregnancy loss. J Obstet Gynaecol. 2001b;21:587–590. doi: 10.1080/01443610120087805. [DOI] [PubMed] [Google Scholar]
- Malanchere E, Huetz F, Coutinho A. Maternal IgG stimulates B lineage cell development in the progeny. Eur J Immunol. 1997;27:788–793. doi: 10.1002/eji.1830270330. [DOI] [PubMed] [Google Scholar]
- Mantis NJ, Cheung MC, Chintalacharuvu KR, Rey J, Corthesy B, Neutra MR. Selective adherence of IgA to murine Peyer's patch M cells: evidence for a novel IgA receptor. J Immunol. 2002;169:1844–1851. doi: 10.4049/jimmunol.169.4.1844. [DOI] [PubMed] [Google Scholar]
- Martinez X, Brandt C, Saddallah F, Tougne C, Barrios C, Wild F, Dougan G, Lambert PH, Siegrist CA. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc Natl Acad Sci USA. 1997;94:8726–8731. doi: 10.1073/pnas.94.16.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez X, Li X, Kovarik J, Klein M, Lambert PH, Siegrist CA. Combining DNA and protein vaccines for early life immunization against respiratory syncytial virus in mice. Eur J Immunol. 1999;29:3390–3400. doi: 10.1002/(SICI)1521-4141(199910)29:10<3390::AID-IMMU3390>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Medina KL, Kincade PW. Pregnancy-related steroids are potential negative regulators of B lymphopoiesis. Proc Natl Acad Sci USA. 1994;91:5382–5386. doi: 10.1073/pnas.91.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Smithson G, Kincade PW. Suppression of B lymphopoiesis during normal pregnancy. J Exp Med. 1993;178:1507–1515. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Strasser A, Kincade PW. Estrogen influences the differentiation, proliferation, and survival of early B-lineage precursors. Blood. 2000;95:2059–2067. [PubMed] [Google Scholar]
- Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Michaelsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176:5741–5748. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- Mickleson KN, Moriarty KM. Immunoglobulin levels in human colostrum and milk. J Pediatr Gastroenterol Nutr. 1982;1:381–384. doi: 10.1097/00005176-198201030-00018. [DOI] [PubMed] [Google Scholar]
- Moffatt K, McNally C. Vaccine refusal: perspectives from pediatrics. In: Chatterjee A, editor. Vaccinophobia and Vaccine Controversies of the 21st Century. New York: Springer Science+Business Media; 2013. pp. 97–118. [Google Scholar]
- Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monney T, Debache K, Grandgirard D, Leib SL, Hemphill A. Vaccination with the recombinant chimeric antigen recNcMIC3-1-R induces a non-protective Th2-type immune response in the pregnant mouse model for N. caninum infection. Vaccine. 2012;30:6588–6594. doi: 10.1016/j.vaccine.2012.08.024. [DOI] [PubMed] [Google Scholar]
- Moore MP, Carter NP, Redman CW. Lymphocyte subsets defined by monoclonal antibodies in human pregnancy. Am J Reprod Immunol. 1983;3:161–164. doi: 10.1111/j.1600-0897.1983.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell A, Terry WD, Waldmann TA. Metabolic properties of IgG subclasses in man. J Clin Invest. 1970;49:673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro PL, Broder K, Zheteyeva Y, Revzina N, Tepper N, Kissin D, Barash F, Arana J, Brantley MD, Ding H, et al. Adverse events following administration to pregnant women of influenza A (H1N1) 2009 monovalent vaccine reported to the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol. 2011;205:473. doi: 10.1016/j.ajog.2011.06.047. e471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro PL, Museru OI, Niu M, Lewis P, Broder K. Reports to the Vaccine Adverse Event Reporting System after hepatitis A and hepatitis AB vaccines in pregnant women. Am J Obstet Gynecol. 2014;210:561.e1–e6. doi: 10.1016/j.ajog.2013.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonakis E, Paliou M, Hohmann EL, Calderwood SB, Wing EJ. Listeriosis during pregnancy: a case series and review of 222 cases. Medicine (Baltimore) 2002;81:260–269. doi: 10.1097/00005792-200207000-00002. [DOI] [PubMed] [Google Scholar]
- Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naniche D. Human immunology of measles virus infection. Curr Top Microbiol Immunol. 2009;330:151–171. doi: 10.1007/978-3-540-70617-5_8. [DOI] [PubMed] [Google Scholar]
- Nasidi A, Monath TP, Vandenberg J, Tomori O, Calisher CH, Hurtgen X, Munube GR, Sorungbe AO, Okafor GC, Wali S. Yellow fever vaccination and pregnancy: a four-year prospective study. Trans R Soc Trop Med Hyg. 1993;87:337–339. doi: 10.1016/0035-9203(93)90156-k. [DOI] [PubMed] [Google Scholar]
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60:1–64. [PubMed] [Google Scholar]
- Nazareth I, Tavares F, Rosillon D, Haguinet F, Bauchau V. Safety of AS03-adjuvanted split-virion H1N1 (2009) pandemic influenza vaccine: a prospective cohort study. BMJ Open. 2013;3:e001912. doi: 10.1136/bmjopen-2012-001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148:1094–1102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- Nohynek H, Gustafsson L, Capeding MR, Kayhty H, Olander RM, Pascualk L, Ruutu P. Effect of transplacentally acquired tetanus antibodies on the antibody responses to Haemophilus influenzae type b-tetanus toxoid conjugate and tetanus toxoid vaccines in Filipino infants. Pediatr Infect Dis J. 1999;18:25–30. doi: 10.1097/00006454-199901000-00008. [DOI] [PubMed] [Google Scholar]
- Nordin JD, Kharbanda EO, Benitez GV, Nichol K, Lipkind H, Naleway A, Lee GM, Hambidge S, Shi W, Olsen A. Maternal safety of trivalent inactivated influenza vaccine in pregnant women. Obstet Gynecol. 2013;121:519–525. doi: 10.1097/AOG.0b013e3182831b83. [DOI] [PubMed] [Google Scholar]
- Oda M, Izumiya K, Sato Y, Hirayama M. Transplacental and transcolostral immunity to pertussis in a mouse model using acellular pertussis vaccine. J Infect Dis. 1983;148:138–145. doi: 10.1093/infdis/148.1.138. [DOI] [PubMed] [Google Scholar]
- Okoko BJ, Wesumperuma HL, Fern J, Yamuah LK, Hart CA. The transplacental transfer of IgG subclasses: influence of prematurity and low birthweight in the Gambian population. Ann Trop Paediatr. 2002;22:325–332. doi: 10.1179/027249302125001985. [DOI] [PubMed] [Google Scholar]
- Okoko BJ, Enwere G, Ota MO. The epidemiology and consequences of maternal malaria: a review of immunological basis. Acta Trop. 2003;87:193–205. doi: 10.1016/s0001-706x(03)00097-4. [DOI] [PubMed] [Google Scholar]
- Omer SB, Goodman D, Steinhoff MC, Rochat R, Klugman KP, Stoll BJ, Ramakrishnan U. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med. 2011;8:e1000441. doi: 10.1371/journal.pmed.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omon E, Damase-Michel C, Hurault-Delarue C, Lacroix I, Montastruc JL, Oustric S, Escourrou B. Non-adjuvanted 2009 influenza A (H1N1)v vaccine in pregnant women: the results of a French prospective descriptive study. Vaccine. 2011;29:9649–9654. doi: 10.1016/j.vaccine.2011.10.034. [DOI] [PubMed] [Google Scholar]
- Oppermann M, Fritzsche J, Weber-Schoendorfer C, Keller-Stanislawski B, Allignol A, Meister R, Schaefer C. A(H1N1)v2009: a controlled observational prospective cohort study on vaccine safety in pregnancy. Vaccine. 2012;30:4445–4452. doi: 10.1016/j.vaccine.2012.04.081. [DOI] [PubMed] [Google Scholar]
- Paoletti LC, Wessels MR, Rodewald AK, Shroff AA, Jennings HJ, Kasper DL. Neonatal mouse protection against infection with multiple group B streptococcal (GBS) serotypes by maternal immunization with a tetravalent GBS polysaccharide-tetanus toxoid conjugate vaccine. Infect Immun. 1994;62:3236–3243. doi: 10.1128/iai.62.8.3236-3243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti LC, Pinel J, Rodewald AK, Kasper DL. Therapeutic potential of human antisera to group B streptococcal glycoconjugate vaccines in neonatal mice. J Infect Dis. 1997;175:1237–1239. doi: 10.1086/593678. [DOI] [PubMed] [Google Scholar]
- Paoletti LC, Pinel J, Kennedy RC, Kasper DL. Maternal antibody transfer in baboons and mice vaccinated with a group B streptococcal polysaccharide conjugate. J Infect Dis. 2000;181:653–658. doi: 10.1086/315285. [DOI] [PubMed] [Google Scholar]
- Pasetti M, Eriksson P, Dokmetjian J, Brero ML, Ferrero F, Manghi M. Modulation of the immune response against tetanus and diphtheria during pregnancy and its influence in the protection of the new-born. Pediatr Res. 1997;42:923. [Google Scholar]
- Pasquier B, Launay P, Kanamaru Y, Moura IC, Pfirsch S, Ruffie C, Henin D, Benhamou M, Pretolani M, Blank U, et al. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Pasternak B, Svanstrom H, Molgaard-Nielsen D, Krause TG, Emborg HD, Melbye M, Hviid A. Vaccination against pandemic A/H1N1 2009 influenza in pregnancy and risk of fetal death: cohort study in Denmark. BMJ. 2012;344:e2794. doi: 10.1136/bmj.e2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos M, Sperling RS, Moran TM, Kraus TA. The influence of pregnancy on systemic immunity. Immunol Res. 2012a;54:254–261. doi: 10.1007/s12026-012-8303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos MA, Kraus TA, Munoz-Fontela C, Moran TM. Estrogen mediates innate and adaptive immune alterations to influenza infection in pregnant mice. PLoS One. 2012b;7:e40502. doi: 10.1371/journal.pone.0040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Sancho M, Adone R, Garcia-Seco T, Tarantino M, Diez-Guerrier A, Drumo R, Francia M, Dominguez L, Pasquali P, Alvarez J. Evaluation of the immunogenicity and safety of Brucella melitensis B115 vaccination in pregnant sheep. Vaccine. 2014;32:1877–1881. doi: 10.1016/j.vaccine.2014.01.070. [DOI] [PubMed] [Google Scholar]
- Poland GA. MMR vaccine and autism: vaccine nihilism and postmodern science. Mayo Clin Proc. 2011;86:869–871. doi: 10.4065/mcp.2011.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity. 2010;33:516–529. doi: 10.1016/j.immuni.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinello C, Quintilio W, Carneiro-Sampaio M, Palmeira P. Passive acquisition of protective antibodies reactive with Bordetella pertussis in newborns via placental transfer and breast-feeding. Scand J Immunol. 2010;72:66–73. doi: 10.1111/j.1365-3083.2010.02410.x. [DOI] [PubMed] [Google Scholar]
- Rahman MJ, Degano IR, Singh M, Fernandez C. Influence of maternal gestational treatment with mycobacterial antigens on postnatal immunity in an experimental murine model. PLoS One. 2010;5:e9699. doi: 10.1371/journal.pone.0009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renegar KB. Passive immunization: systemic and mucosal. In: Mestecky J, Lamm ME, McGhee JR, Bienenstock J, Mayer L, Strober W, editors. Mucosal Immunol. Burlington, MA, USA: Elsevier Academic Press; 2005. pp. 841–851. [Google Scholar]
- Richards JL, Hansen C, Bredfeldt C, Bednarczyk RA, Steinhoff MC, Adjaye-Gbewonyo D, Ault K, Gallagher M, Orenstein W, Davis RL, et al. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clin Infect Dis. 2013;56:1216–1222. doi: 10.1093/cid/cit045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- Riley LE, Minkoff H. Ethical issues with vaccination for the obstetrician-gynecologist. Obstet Gynecol. 2014;124:889. doi: 10.1097/AOG.0000000000000198. [DOI] [PubMed] [Google Scholar]
- Roberts J, Jenkins C, Wilson R, Pearson C, Franklin IA, MacLean MA, McKillop JH, Walker JJ. Recurrent miscarriage is associated with increased numbers of CD5/20 positive lymphocytes and an increased incidence of thyroid antibodies. Eur J Endocrinol. 1996;134:84–86. doi: 10.1530/eje.0.1340084. [DOI] [PubMed] [Google Scholar]
- Rodewald AK, Onderdonk AB, Warren HB, Kasper DL. Neonatal mouse model of group B streptococcal infection. J Infect Dis. 1992;166:635–639. doi: 10.1093/infdis/166.3.635. [DOI] [PubMed] [Google Scholar]
- Rolle L, Memarzadeh Tehran M, Morell-Garcia A, Raeva Y, Schumacher A, Hartig R, Costa SD, Jensen F, Zenclussen AC. Cutting edge: IL-10-producing regulatory B cells in early human pregnancy. Am J Reprod Immunol. 2013;70:448–453. doi: 10.1111/aji.12157. [DOI] [PubMed] [Google Scholar]
- Roush SW, Murphy TV Vaccine-Preventable Disease Table Working Group. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA. 2007;298:2155–2163. doi: 10.1001/jama.298.18.2155. [DOI] [PubMed] [Google Scholar]
- Saji F, Samejima Y, Kamiura S, Koyama M. Dynamics of immunoglobulins at the feto-maternal interface. Rev Reprod. 1999;4:81–89. doi: 10.1530/ror.0.0040081. [DOI] [PubMed] [Google Scholar]
- Salonen EM, Hovi T, Meurman O, Vesikari T, Vaheri A. Kinetics of specific IgA, IgD, IgE, IgG, and IgM antibody responses in rubella. J Med Virol. 1985;16:1–9. doi: 10.1002/jmv.1890160102. [DOI] [PubMed] [Google Scholar]
- Saphire EO, Parren PW, Pantophlet R, Zwick MB, Morris GM, Rudd PM, Dwek RA, Stanfield RL, Burton DR, Wilson IA. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science. 2001;293:1155–1159. doi: 10.1126/science.1061692. [DOI] [PubMed] [Google Scholar]
- Sappenfield E, Jamieson DJ, Kourtis AP. Pregnancy and susceptibility to infectious diseases. Infect Dis Obstet Gynecol. 2013;2013:752852. doi: 10.1155/2013/752852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvas H, Kurikka S, Seppala IJ, Makela PH, Makela O. Maternal antibodies partly inhibit an active antibody response to routine tetanus toxoid immunization in infants. J Infect Dis. 1992;165:977–979. doi: 10.1093/infdis/165.5.977. [DOI] [PubMed] [Google Scholar]
- Sarzotti M, Robbins DS, Hoffman PM. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science. 1996;271:1726–1728. doi: 10.1126/science.271.5256.1726. [DOI] [PubMed] [Google Scholar]
- Schanzer DL, Langley JM, Tam TW. Influenza-attributed hospitalization rates among pregnant women in Canada 1994–2000. J Obstet Gynaecol Can. 2007;29:622–629. doi: 10.1016/s1701-2163(16)32559-2. [DOI] [PubMed] [Google Scholar]
- Schleiss MR, Lacayo JC, Belkaid Y, McGregor A, Stroup G, Rayner J, Alterson K, Chulay JD, Smith JF. Preconceptual administration of an alphavirus replicon UL83 (pp65 homolog) vaccine induces humoral and cellular immunity and improves pregnancy outcome in the guinea pig model of congenital cytomegalovirus infection. J Infect Dis. 2007;195:789–798. doi: 10.1086/511982. [DOI] [PubMed] [Google Scholar]
- Schleiss MR, Buus R, Choi KY, McGregor A. An attenuated CMV vaccine with a deletion in tegument protein GP83 (pp65 Homolog) protects against placental infection and improves pregnancy outcome in a guinea pig challenge model. Future Virol. 2013;8:1151–1160. doi: 10.2217/fvl.13.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiss MR, Choi KY, Anderson J, Mash JG, Wettendorff M, Mossman S, Van Damme M. Glycoprotein B (gB) vaccines adjuvanted with AS01 or AS02 protect female guinea pigs against cytomegalovirus (CMV) viremia and offspring mortality in a CMV-challenge model. Vaccine. 2014;32:2756–62. doi: 10.1016/j.vaccine.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol. 2013;13:176–189. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- Sette A, Rappuoli R. Reverse vaccinology: developing vaccines in the era of genomics. Immunity. 2010;33:530–541. doi: 10.1016/j.immuni.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell HF, Matthews JB, Flack V, Jefferis R. Human immunoglobulin D in colostrum, saliva and amniotic fluid. Clin Exp Immunol. 1979;36:183–188. [PMC free article] [PubMed] [Google Scholar]
- Shakib JH, Korgenski K, Sheng X, Varner MW, Pavia AT, Byington CL. Tetanus, diphtheria, acellular pertussis vaccine during pregnancy: pregnancy and infant health outcomes. J Pediatr. 2013;163:1422–1426. doi: 10.1016/j.jpeds.2013.06.021. e1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JS, Greer LG, Rogers VL, Roberts SW, Lytle H, McIntire DD, Wendel GD., Jr Effect of influenza vaccination in the first trimester of pregnancy. Obstet Gynecol. 2012;120:532–537. doi: 10.1097/AOG.0b013e318263a278. [DOI] [PubMed] [Google Scholar]
- Siegrist CA. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine. 2003;21:3406–3412. doi: 10.1016/s0264-410x(03)00342-6. [DOI] [PubMed] [Google Scholar]
- Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- Siegrist CA, Barrios C, Martinez X, Brandt C, Berney M, Cordova M, Kovarik J, Lambert PH. Influence of maternal antibodies on vaccine responses: inhibition of antibody but not T cell responses allows successful early prime-boost strategies in mice. Eur J Immunol. 1998a;28:4138–4148. doi: 10.1002/(SICI)1521-4141(199812)28:12<4138::AID-IMMU4138>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Siegrist CA, Cordova M, Brandt C, Barrios C, Berney M, Tougne C, Kovarik J, Lambert PH. Determinants of infant responses to vaccines in presence of maternal antibodies. Vaccine. 1998b;16:1409–1414. doi: 10.1016/s0264-410x(98)00100-5. [DOI] [PubMed] [Google Scholar]
- Siem RA, Ly H, Imagawa DT, Adams JM. Influenza virus infections in pregnant mice. J Neuropathol Exp Neurol. 1960;19:125–129. doi: 10.1097/00005072-196001000-00013. [DOI] [PubMed] [Google Scholar]
- Silverstein AM. Ontogeny of the immune response. Science. 1964;144:1423–1428. doi: 10.1126/science.144.3625.1423. [DOI] [PubMed] [Google Scholar]
- Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Louie J, Doyle TJ, Crockett M, Lynfield R, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J Immunol. 1998;161:27–34. [PubMed] [Google Scholar]
- Soydinc HE, Celen MK, Yildiz B, Sak ME, Evsen MS, Gul T. Pregnancy and H1N1 infection in Southeast Turkey. J Infect Dev Ctries. 2012;6:644–649. doi: 10.3855/jidc.1956. [DOI] [PubMed] [Google Scholar]
- Stapleton NM, Andersen JT, Stemerding AM, Bjarnarson SP, Verheul RC, Gerritsen J, Zhao Y, Kleijer M, Sandlie I, de Haas M, et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat Commun. 2011;2:599. doi: 10.1038/ncomms1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele MG, Leslie GA. Immunoglobulin D in rat serum, saliva and milk. Immunology. 1985;55:571–577. [PMC free article] [PubMed] [Google Scholar]
- Steinhoff MC, Omer SB, Roy E, El Arifeen S, Raqib R, Dodd C, Breiman RF, Zaman K. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. CMAJ. 2012;184:645–653. doi: 10.1503/cmaj.110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003;27:293–301. doi: 10.1016/s0146-0005(03)00046-6. [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- Tamiolakis D, Anastasiadis P, Hatzimichael A, Liberis B, Karamanidis D, Kotini A, Petrakis G, Romanidis K, Papadopoulos N. Spontaneous abortions with increased CD5 positive cells in the placental tissue during the first trimester of gestation. Clin Exp Obstet Gynecol. 2001;28:261–265. [PubMed] [Google Scholar]
- Telemo E, Hanson LA. Antibodies in milk. J Mammary Gland Biol Neoplasia. 1996;1:243–249. doi: 10.1007/BF02018077. [DOI] [PubMed] [Google Scholar]
- Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J Immunol. 1989;143:3200–3206. [PubMed] [Google Scholar]
- Tizzard I. Immunity in the fetus and newborn. In: Tizzard I, editor. Veterinary Immunology. Philadelphia: WB Saunders; 1987. pp. 171–184. [Google Scholar]
- Trautmann L, Sekaly RP. Solving vaccine mysteries: a systems biology perspective. Nat Immunol. 2011;12:729–731. doi: 10.1038/ni.2078. [DOI] [PubMed] [Google Scholar]
- Troisi CL, Hollinger FB, Krause DS, Pickering LK. Immunization of seronegative infants with hepatitis A vaccine (HAVRIX; SKB): a comparative study of two dosing schedules. Vaccine. 1997;15:1613–1617. doi: 10.1016/s0264-410x(97)00199-0. [DOI] [PubMed] [Google Scholar]
- Valdimarsson H, Mulholland C, Fridriksdottir V, Coleman DV. A longitudinal study of leucocyte blood counts and lymphocyte responses in pregnancy: a marked early increase of monocyte-lymphocyte ratio. Clin Exp Immunol. 1983;53:437–443. [PMC free article] [PubMed] [Google Scholar]
- van den Berg JP, Westerbeek EA, Berbers GA, van Gageldonk PG, van der Klis FR, van Elburg RM. Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, Haemophilus influenzae type b, and Neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatr Infect Dis J. 2010;29:801–805. doi: 10.1097/inf.0b013e3181dc4f77. [DOI] [PubMed] [Google Scholar]
- Verthelyi DI, Ahmed SA. Estrogen increases the number of plasma cells and enhances their autoantibody production in nonautoimmune C57BL/6 mice. Cell Immunol. 1998;189:125–134. doi: 10.1006/cimm.1998.1372. [DOI] [PubMed] [Google Scholar]
- Victor JR, Muniz BP, Fusaro AE, de Brito CA, Taniguchi EF, Duarte AJ, Sato MN. Maternal immunization with ovalbumin prevents neonatal allergy development and up-regulates inhibitory receptor Fc gamma RIIB expression on B cells. BMC Immunol. 2010;11:11. doi: 10.1186/1471-2172-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988;18:313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- Vitoratos N, Papadias C, Economou E, Makrakis E, Panoulis C, Creatsas G. Elevated circulating IL-1beta and TNF-alpha, and unaltered IL-6 in first-trimester pregnancies complicated by threatened abortion with an adverse outcome. Mediators Inflamm. 2006;2006:30485. doi: 10.1155/MI/2006/30485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Miyahara Y, Guo Z, Khattar M, Stepkowski SM, Chen W. ‘Default’ generation of neonatal regulatory T cells. J Immunol. 2010;185:71–78. doi: 10.4049/jimmunol.0903806. [DOI] [PubMed] [Google Scholar]
- Wang Z-Y, Mathiarasu A, Hong P, Xu Y, Li A, Bluth M, Lum L, Huang B, Romero R, Cerutti A, et al. Defective induction of regulatory B cells by decidua stromal cells underlies the pathogenesis of preterm birth. J Immunol. 2013;190 171.119. [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Warfel JM, Papin JF, Wolf RF, Zimmerman LI, Merkel TJ. Maternal and neonatal vaccination protects newborn baboons from pertussis infection. J Infect Dis. 2014 doi: 10.1093/infdis/jiu090. doi:10.1093/infdis/jiu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Iwatani Y, Kaneda T, Hidaka Y, Mitsuda N, Morimoto Y, Amino N. Changes in T, B, and NK lymphocyte subsets during and after normal pregnancy. Am J Reprod Immunol. 1997;37:368–377. doi: 10.1111/j.1600-0897.1997.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Wessels MR, Paoletti LC, Kasper DL, DiFabio JL, Michon F, Holme K, Jennings HJ. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J Clin Invest. 1990;86:1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels MR, Paoletti LC, Rodewald AK, Michon F, DiFabio J, Jennings HJ, Kasper DL. Stimulation of protective antibodies against type Ia and Ib group B streptococci by a type Ia polysaccharide-tetanus toxoid conjugate vaccine. Infect Immun. 1993;61:4760–4766. doi: 10.1128/iai.61.11.4760-4766.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesumperuma HL, Perera AJ, Pharoah PO, Hart CA. The influence of prematurity and low birthweight on transplacental antibody transfer in Sri Lanka. Ann Trop Med Parasitol. 1999;93:169–177. doi: 10.1080/00034989958654. [DOI] [PubMed] [Google Scholar]
- Wiersma EJ, Coulie PG, Heyman B. Dual immunoregulatory effects of monoclonal IgG-antibodies: suppression and enhancement of the antibody response. Scand J Immunol. 1989;29:439–448. doi: 10.1111/j.1365-3083.1989.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Williams K, Mackenzie JS. Influenza infections during pregnancy in the mouse. J Hyg (Lond) 1977;79:249–257. doi: 10.1017/s0022172400053067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood N, Siegrist CA. Neonatal immunization: where do we stand? Curr Opin Infect Dis. 2011;24:190–195. doi: 10.1097/QCO.0b013e328345d563. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Geneva, Switzerland: World Health Organisation; 2003. p. 31. WHO Guidelines on nonclinical evaluation of vaccines 54th meeting of the WHO Expert Committee on Biological Standardization. [Google Scholar]
- Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, Ungaro R, Levy O, Moldawer LL. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H, Tsutsumi H, Matsuda K, Nagai K, Ogra PL, Chiba S. Effect of maternal antibody on IgA antibody response in nasopharyngeal secretion in infants and children during primary respiratory syncytial virus infection. J Gen Virol. 1994;75(Pt 8):2115–2119. doi: 10.1099/0022-1317-75-8-2115. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006;116:2142–2151. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- Zenner L, Darcy F, Cesbron-Delauw MF, Capron A. Rat model of congenital toxoplasmosis: rate of transmission of three Toxoplasma gondii strains to fetuses and protective effect of a chronic infection. Infect Immun. 1993;61:360–363. doi: 10.1128/iai.61.1.360-363.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]