Abstract
BACKGROUND
Uterine leiomyoma is the most common benign tumor in women and is thought to arise from the clonal expansion of a single myometrial smooth muscle cell transformed by a cellular insult. Leiomyomas cause a variety of symptoms, including abnormal uterine bleeding, pelvic pain, bladder or bowel dysfunction, and recurrent pregnancy loss, and are the most common indication for hysterectomy in the USA. A slow rate of cell proliferation, combined with the production of copious amounts of extracellular matrix, accounts for tumor expansion. A common salient feature of leiomyomas is their responsiveness to steroid hormones, thus providing an opportunity for intervention.
METHODS
A comprehensive search of PUBMED was conducted to identify peer-reviewed literature published since 1980 pertinent to the roles of steroid hormones and somatic stem cells in leiomyoma, including literature on therapeutics that target steroid hormone action in leiomyoma. Reviewed articles were restricted to English language only. Studies in both animals and humans were reviewed for the manuscript.
RESULTS
Estrogen stimulates the growth of leiomyomas, which are exposed to this hormone not only through ovarian steroidogenesis, but also through local conversion of androgens by aromatase within the tumors themselves. The primary action of estrogen, together with its receptor estrogen receptor α (ERα), is likely mediated via induction of progesterone receptor (PR) expression, thereby allowing leiomyoma responsiveness to progesterone. Progesterone has been shown to stimulate the growth of leiomyoma through a set of key genes that regulate both apoptosis and proliferation. Given these findings, aromatase inhibitors and antiprogestins have been developed for the treatment of leiomyoma, but neither treatment results in complete regression of leiomyoma, and tumors recur after treatment is stopped. Recently, distinct cell populations were discovered in leiomyomas; a small population showed stem-progenitor cell properties, and was found to be essential for ovarian steroid-dependent growth of leiomyomas. Interestingly, these stem-progenitor cells were deficient in ERα and PR and instead relied on the strikingly higher levels of these receptors in surrounding differentiated cells to mediate estrogen and progesterone action via paracrine signaling.
CONCLUSIONS
It has been well established that estrogen and progesterone are involved in the proliferation and maintenance of uterine leiomyoma, and the majority of medical treatments currently available for leiomyoma work by inhibiting steroid hormone production or action. A pitfall of these therapeutics is that they decrease leiomyoma size, but do not completely eradicate them, and tumors tend to regrow once treatment is stopped. The recent discovery of stem cells and their paracrine interactions with more differentiated cell populations within leiomyoma has the potential to provide the missing link between developing therapeutics that temper leiomyoma growth and those that eradicate them.
Keywords: leiomyoma, estrogen, progesterone, aromatase, stem cells
Introduction
Uterine leiomyomas represent the most common category of solid pelvic tumors in women, occurring in up to 80% of all women of reproductive age, with up to 30% of women experiencing severe enough symptoms to seek treatment (Cramer and Patel, 1990; Marshall et al., 1997; Myers et al., 2002; Parker, 2007). Leiomyomas can cause a range of symptoms, including abnormal uterine bleeding, pressure-related symptoms, recurrent pregnancy loss and infertility. Interestingly, leiomyoma cause many of these symptoms not only by virtue of the size and mass effect of the tumor itself, but also by modulating gene expression in the endometrium (Cakmak and Taylor, 2011; Sinclair et al., 2011). Women can have one or multiple leiomyomas, and they can achieve a wide range of sizes (Bulun, 2013). African-American women develop leiomyomas more frequently and at earlier ages than Caucasian women. Moreover, tumors in African-American women are more aggressive, as they present with larger leiomyomas and more significant symptoms than their Caucasian counterparts (Day Baird et al., 2003; Walker and Stewart, 2005). In addition to racial differences, leiomyomas show a high degree of heterogeneity in growth even within the same woman, and especially in women with multiple tumors (Peddada et al., 2008).
Despite the high prevalence of these tumors, relatively little is known about their specific etiology. X chromosome-linked clonality studies (using glucose-6-phosphate dehydrogenase) suggest that leiomyomas are monoclonal tumors derived from a single myocyte (Linder and Gartler, 1965; Townsend et al., 1970). The neoplastic transformation of a myocyte is likely due to some sort of cellular insult, although the exact etiology of this transforming event is currently unknown. Factors proposed to play a role in the conversion of a myocyte into a leiomyoma include genetic mutations, epigenetic aberrations and altered responses to hypoxia; however, the sequence of events in transformation and clonal expansion remains unclear (Bulun, 2013; Mehine et al., 2013; Tal and Segars, 2014). Regardless of the nature of the cellular insult, a common salient feature of leiomyomas is their responsiveness to steroid hormones, thus providing an opportunity for intervention. Estrogen and progesterone lead to tumor expansion by stimulating a modest rate of cellular proliferation and the production of copious amounts of extracellular matrix, which is predominantly collagen (Flake et al., 2013; Kim et al., 2013; Fig. 1). The excess collagen accumulation is also thought to contribute to the ultimate involution of leiomyomas (Flake et al., 2013).
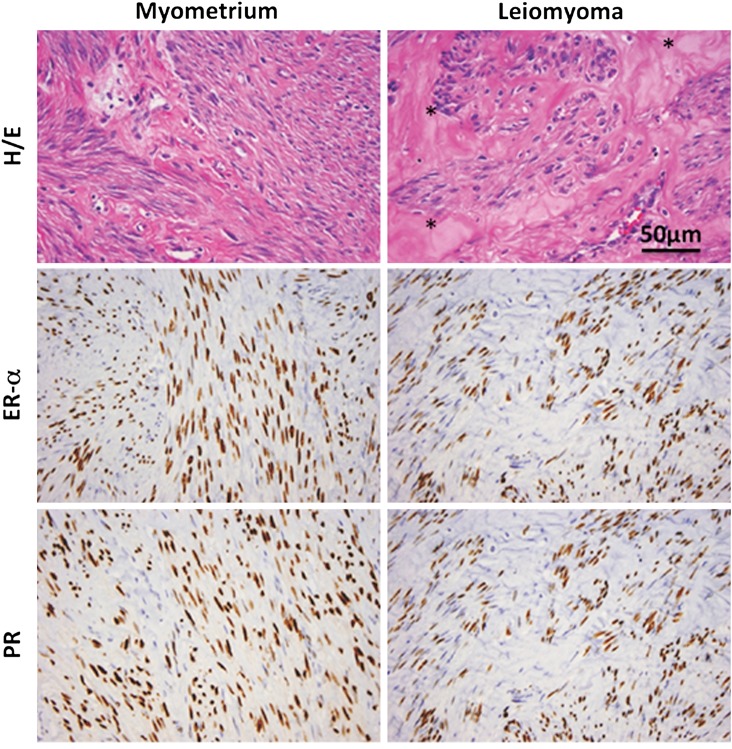
Figure 1.
Typical histology of human leiomyoma. Representative slides demonstrating the typical hematoxylin/eosin (H/E) slide and immunohistochemistry stains for estrogen receptor-α (ER-α) and PR in myometrium and leiomyoma; *denotes extracellular matrix (ECM) in the H/E-stained leiomyoma section. Note the expanded ECM leiomyoma tissue and increased nuclear size in leiomyoma smooth muscle cells. Immunoreactive ER and PR (brown stain) are localized to the nuclei of myometrial or leiomyoma smooth muscle cells. Images courtesy of Dr Jian-Jun Wei, Department of Pathology, Northwestern Memorial Hospital, Chicago, IL, USA. Scale bar represents 50 μm.
Over 200 000 surgical procedures are performed annually in the USA to remove or destroy uterine leiomyomas, with an estimated annual cost of $5.9–34.4 billion (Cardozo et al., 2012). Hysterectomy or myomectomy are still commonly utilized for leiomyoma treatment, however, more recently, less invasive procedures have been employed, such as uterine artery embolization and magnetic resonance guided focused ultrasound surgery (Al Hilli and Stewart, 2010; Freed and Spies, 2010). While GnRH agonists have traditionally been the mainstay of medical treatment for uterine leiomyoma, other classes of medications are being investigated with promising results, including aromatase inhibitors and selective progesterone receptor modulators (SPRMs) with primarily anti-progestogenic activity (Sabry and Al-Hendy, 2012a, b; Table I). Additionally, there is encouraging research on the role of supplements, such as vitamin D and green tea extract, as potential treatments for uterine fibroids (Halder et al., 2012, 2013; Sabry and Al-Hendy, 2012a, b; Roshdy et al., 2013). Medical treatment for uterine leiomyoma is ideally preferable to surgical management for many patients due to the inherent risks of surgery or desire to maintain future fertility. However, many of the medical options also have undesirable side effects, which often limits the beneficial duration of therapy. More importantly, leiomyoma growth also typically rebounds once treatment is stopped (Walker and Stewart, 2005). The recent discovery of a small population of stem-progenitor cells, important in leiomyoma pathophysiology, may provide the opportunity for new therapeutic targets (Mas et al., 2012; Ono et al., 2012). This review focuses on the role of aromatase, ovarian steroids and stem-progenitor cells in the pathogenesis of uterine leiomyoma, and discusses the therapeutic implications of the current state of knowledge of these pathways.
Table I.
Medications that result in leiomyoma shrinkage and their effects in women.
| Class | Examples | Mechanism of action | Side effects | Pregnancy category | FDA approved for leiomyoma | Tumors recur after cessation | Comments |
|---|---|---|---|---|---|---|---|
| GnRH agonists | Leuprolide, Triptorelin | Abolishes GnRH pulsatility | Menopausal symptoms, bone loss | X | Yes, leuprolide for preoperative anemia only | Yes | Initial flare effect; Requires add-back therapy after 6 months |
| GnRH antagonists | Ganirelix, Elagolix | Competitive inhibition of GnRH | Menopausal symptoms, bone loss | X | No | Yes | Avoids flare effect of GnRH agonists; Elagolix is the only oral GnRH antagonist |
| SPRMs | Ulipristal, Mifepristone | Varied progesterone antagonism | Endometrial thickening/hyperplasia | X | No | Yes | |
| Aromatase inhibitors | Letrozole, Anastrozole | Competitive inhibition of the aromatase enzyme | Bone loss | D | No | Yes | Can cause follicular stimulation |
FDA, Food and Drug Administration.
Pregnancy categories are based on FDA classifications (A–D, X), D = established evidence of fetal risk, but potential benefits may outweigh risks in select patients; X = established evidence of fetal risk, with risks of use in pregnant women clearly outweighing the benefits.
Methods
A comprehensive search of PUBMED was conducted to identify peer-reviewed literature published since 1980 pertinent to the roles of steroid hormones and somatic stem cells in leiomyoma, including literature on therapeutics that target steroid hormone action in leiomyoma. The search included combinations of the following key words: leiomyoma, aromatase, aromatase inhibitors, anastrozole, letrozole, estrogen, estrogen receptor, progesterone, progesterone receptor, SPRMs, asoprisnil, mifepristone, ulipristal, telapristone, stem cells and progenitor cells. Reviewed articles were restricted to English language only. Studies in both animals and humans were reviewed for the manuscript. The reference lists of included articles were also reviewed to identify additional relevant studies.
Aromatase, estrogen and estrogen receptor α
The observation that leiomyomas occur primarily in women of reproductive age, grow during early pregnancy and regress during menopause supports the widely held view that ovarian steroids stimulate leiomyoma growth. Moreover, disruption of ovarian estrogen or progesterone production during treatment with GnRH agonist results in size reduction of leiomyomas, an effect that is reversed once treatment is stopped (West et al., 1987). Leiomyomas are exposed to estrogen not only through ovarian steroidogenesis, but also through local conversion of androgens by aromatase within the tumors themselves (Fig. 2; Bulun et al., 1994). Sumitani et al. (2000) showed that in cultured leiomyoma cells, the addition of androstenedione leads to production of estrone, which is then converted to the more potent estradiol (E2) by 17β-hydroxysteroid dehydrogenase (17β-HSD). Furthermore, the addition of androstenedione led to similar rates of cellular proliferation as the addition of E2, leading the authors to conclude that leiomyomas are capable of producing enough estrogen to sustain their own growth (Sumitani et al., 2000). The addition of aromatase inhibitors to the cultured leiomyoma cells decreased proliferation, further supporting that aromatase is the key enzyme mediating in situ estrogen production (Sumitani et al., 2000). Accordingly, leiomyomas have remarkably higher levels of aromatase and 17β-HSD type 1 compared with adjacent myometrium, which presumably leads to the higher tissue levels of estrogens observed in leiomyoma compared with the surrounding myometrium (Folkerd et al., 1984; Bulun et al., 1994; Sumitani et al., 2000; Shozu et al., 2004). Moreover, aromatase transcripts are not found in the myometrium of leiomyoma-free uteri (Bulun et al., 1994). Further evidence for the pathological role of aromatase is the quantifiable increase in aromatase expression found in the leiomyomas of African-American women, who typically have larger and greater numbers of leiomyomas, and the exhibited effectiveness of aromatase inhibitors in decreasing leiomyoma size clinically (Bulun, 2013).
Figure 2.
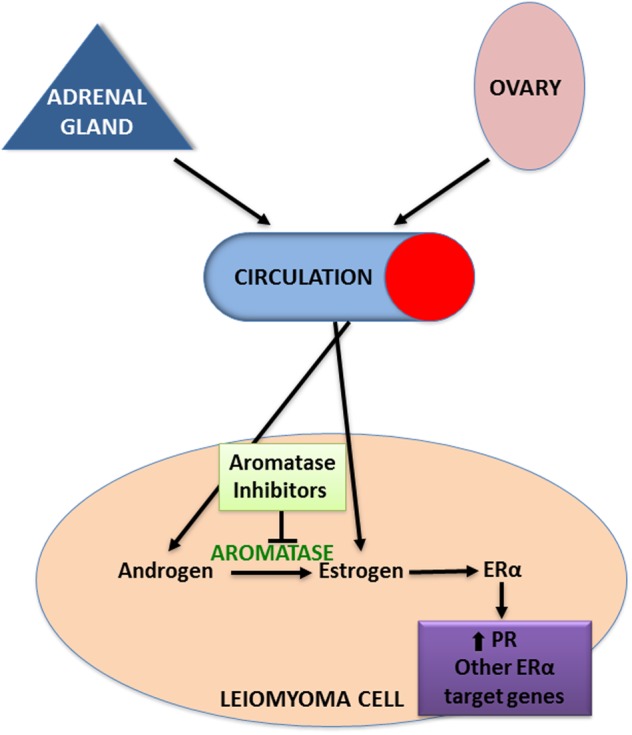
Role of estrogen in leiomyoma pathogenesis. Although leiomyomas are exposed to circulating estrogens from the ovary, it appears that substantial amounts of estrogen are also produced in situ via aromatization of androgens from the adrenal gland and ovary. The biologically active estrogen, estradiol, acts primarily through ERα to induce transcription of genes involved in proliferation and ECM formation, but its principal function is up-regulation of PR expression, thereby increasing leiomyoma responsiveness to progesterone. Aromatase inhibitors effectively block the in situ production of estradiol, thus decreasing leiomyoma responsiveness to both estrogen and progesterone signaling.
Aromatase is a member of the cytochrome P450 family and is encoded by the gene CYP19A1. CYP19A1 expression is sophisticatedly regulated through multiple tissue- and cell-specific promoters and transcription factors (Bulun et al., 1994; Imir et al., 2007; Ishikawa et al., 2008). The primary aromatase promoters utilized by leiomyoma cells are remarkably similar to breast cancer cells, being predominantly the proximal promoters I.3/II, which are activated by prostaglandin E2 or cAMP analogs (Imir et al., 2007). However, in Asian women, aromatase expression is primarily regulated by the distal promoter I.4, which is activated by a glucocorticoid and a class I cytokine (Shozu et al., 2002; Imir et al., 2007). Although the molecular mechanisms underlying regulation of aromatase have not been completely elucidated, Ishikawa et al. (2008) reported that the transcription factor CCAAT/enhancer-binding protein β is a key inducer of aromatase expression via regulating its proximal promoter I.3/II region. Further investigation into these molecular mechanisms may help guide the development of new therapeutics that could lead to leiomyoma-specific aromatase inhibition (Ishikawa et al., 2008) and a resultant decrease in action of locally produced estrogen.
The product of aromatase, estrogen, up-regulates the expression of several genes thought to play a role in leiomyoma pathogenesis, including multiple growth factors, collagens, and the estrogen and progesterone receptors (ER, PR; Fig. 2; Andersen et al., 1995; Li and McLachlan, 2001; Maruo et al., 2004). Estrogen acts primarily via two nuclear receptors, ERα and ERβ, which are expressed in both the myometrium and in leiomyoma where they uniquely coordinate gene transcription (Andersen and Barbieri, 1995; Pedeutour et al., 1998; Benassayag et al., 1999). Some studies have reported increased ER expression in leiomyoma compared with the surrounding myometrium, whereas other studies report no such difference (Wilson et al., 1980; Tamaya et al., 1985; Jakimiuk et al., 2004). Although the exact roles and interplay of ERα and ERβ have not been fully elucidated in the pathogenesis of leiomyoma, it is thought that ERβ may regulate the transcriptional activity of ERα, which is the stronger activator of transcription of the two (Jakimiuk et al., 2004). Additionally, several studies have suggested that polymorphisms of the ERα gene may increase susceptibility to leiomyoma (Al-Hendy and Salama, 2006; Feng et al., 2013). In addition to activation from estrogen binding, ERα is also activated through its phosphorylation by the mitogen-activated protein kinase (MAPK) pathway and possibly via other kinases (Hermon et al., 2008). Based on these findings, Hermon et al. (2008) hypothesized that estrogen-bound ERα induces growth factor expression, which can then stimulate the MAPK pathway and further activate ERα via phosphorylation in an autocrine fashion.
Although estrogen was traditionally thought of as the primary stimulus of leiomyoma growth, clinical studies, as well as a xenograft mouse model, have demonstrated that progesterone is necessary for estrogen-related leiomyoma growth, suggesting that estrogen alone is necessary, but not sufficient for proliferation (Lamminen et al., 1992; Ishikawa et al., 2008). Ishikawa et al. (2010) showed that estrogen/ERα regulates expression of PR and that estrogen alone is not a mitogen in vivo. Moreover, Hassan et al. (2007) reported that disruption of the estrogen signaling pathway by transfecting leiomyoma cells with an ER mutant that suppresses the activity of wild-type ER diminishes both ER- and PR-gene expression. These findings suggest a more permissive role for estrogen, acting via induction of PR expression, and thereby allowing leiomyoma responsiveness to progesterone (Ishikawa et al., 2010; Bulun, 2013).
Progesterone and progesterone receptor
Progesterone is a steroid hormone essential for coordinating normal mammalian female reproductive physiology (Lydon et al., 1995; Graham and Clarke, 1997; Lee et al., 2006). The physiologic actions of progesterone are mediated by interaction with PR, a member of the nuclear hormone superfamily of ligand-activated transcription factors (Mangelsdorf et al., 1995; Robinson-Rechavi et al., 2003). There are two predominant PR isoforms, designated PR-A and PR-B, which are transcribed from the same gene by two distinct promoters, with the only difference being that human PR-B is larger by an additional 164 amino acids at the amino terminus (Lessey et al., 1983; Kastner et al., 1990; Gronemeyer et al., 1991). As a result, PR-A and PR-B may have distinct transcriptional activities (Tung et al., 1993, 2006; Vegeto et al., 1993; McDonnell et al., 1994; Tetel et al., 1999; Edwards, 2000).
Evidence from clinical and experimental studies indicates that progesterone and PR play key roles in uterine leiomyoma growth and development (Cermik et al., 2002; Fig. 3). Several studies have reported increased expression of both PR-A and PR-B in leiomyoma tissue compared with adjacent normal myometrial tissue (Fig. 1; Brandon et al., 1993; Englund et al., 1998; Nisolle et al., 1999). Interestingly, Ishikawa et al. demonstrated that PR mRNA levels were significantly higher in leiomyomas in Japanese women compared with African-American or Caucasian women (Ishikawa et al., 2009). Studies in mice with selective ablation of PR isoforms revealed that PR-A is necessary for ovulation and modulates the anti-proliferative effects of progesterone in the uterus, and PR-B is required for normal mammary gland development and function (Mulac-Jericevic et al., 2000, 2003). Little is known about the specific roles of each PR isoform in uterine leiomyoma.
Figure 3.
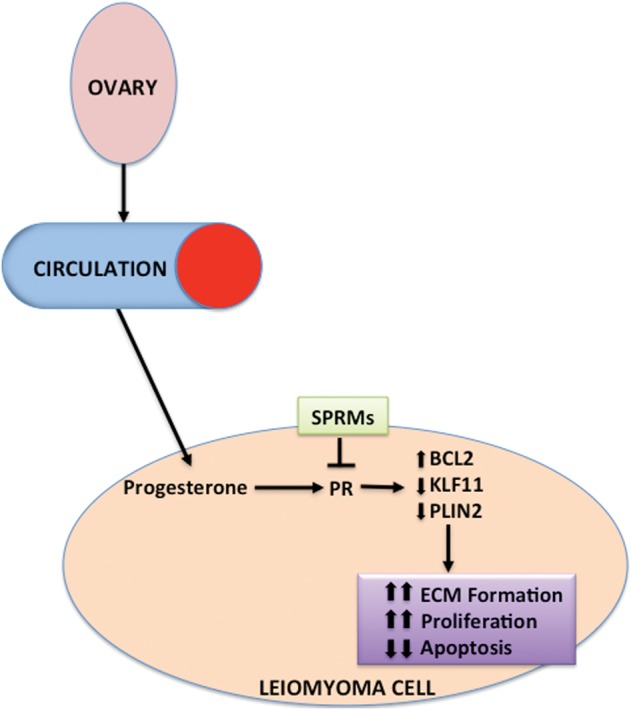
Role of progesterone in leiomyoma pathogenesis. Ovarian progesterone acts via PR to regulate transcription of key genes for apoptosis, proliferation and ECM formation. Effective SPRMs act as progesterone antagonists at PR and block transcription of these genes.
In vivo, the proliferation marker proliferating cell nuclear antigen and mitotic counts are highest in leiomyoma tissue during the luteal/secretory phase (Kawaguchi et al., 1989; Lamminen et al., 1992). Clinical studies indicate that leiomyoma proliferative activity in post-menopausal women increases significantly with combined estrogen plus progestin replacement but not with estrogen replacement alone (Lamminen et al., 1992). Most importantly, in an in vivo human leiomyoma xenograft model where human leiomyoma cells dissociated from fibroid tissues were grafted underneath the renal capsules of immunodeficient mice, progesterone and its receptor directly stimulated tumor growth, whereas the key action of estrogen and its receptor was to maintain PR expression in leiomyoma tissue (Ishikawa et al., 2010). These data suggested that progesterone might be the primary hormone driving the growth of uterine leiomyoma. In this model, estrogen plus progesterone not only stimulated leiomyoma cell proliferation, but also extracellular matrix formation, which was abolished by co-treatment with the progesterone antagonist mifepristone (RU-486; Ishikawa et al., 2010).
Using the same in vivo human leiomyoma xenograft model, Qiang et al. (2014) recently demonstrated that estrogen plus progesterone induces extracellular matrix production via down-regulation of miR-29b. Using microarray-based global micro RNA expression analysis, we and others have discovered that miR-29b expression was reduced in leiomyoma tissues compared with adjacent normal myometrium tissues (Wang et al., 2007; Marsh et al., 2008). Although in vitro culture significantly alters gene expression profiles in leiomyoma smooth muscle cells (Zaitseva et al., 2006), mir-29b levels are consistently lower in cultured leiomyoma cells than in myometrial cells, similar to in vivo tissue (Qiang et al., 2014). Consistent with previous reports that miR-29b binds to the 3′ untranslated region of mRNAs of multiple collagen genes and represses their expression, increasing mir-29b levels in leiomyoma cells using mir-29b lentivirus proportionally reduces the collagen 1a1 level (Qiang et al., 2014). This observation was further confirmed in vivo using a xenograft model (Qiang et al., 2014). Leiomyoma cells transduced with mir-29b lentiviral vector or control vector were embedded in collagen and grafted under the kidney capsule in immunodeficient mice, which were ovariectomized and supplemented with estrogen plus progesterone (Qiang et al., 2014). Although grafts containing control vector-transduced cells gave rise to typical solid leiomyoma tumors underneath the kidney capsule, leiomyoma grafts with ectopic mir-29b expression failed to form solid tumors in the presence of estrogen plus progesterone (Qiang et al., 2014), supporting the hypothesis that dysregulation of mir-29b plays an important role in tissue fibrosis and tumor formation (van Rooij et al., 2008; Cushing et al., 2011; Qin et al., 2011; Roderburg et al., 2011; Zhou et al., 2011).
Simultaneously, immunofluorescence analysis demonstrated that protein levels of collagen 1a1 and 3a1 were significantly reduced in the mir-29b restoration group. Finally, estrogen plus progesterone, but not estrogen alone, suppressed mir-29b expression, indicating that down-regulation of mir-29b by estrogen plus progesterone is essential for uterine leiomyoma growth (Qiang et al., 2014). Given that SMAD3 can mediate transforming growth factor (TGF)-β-induced down-regulation of mir-29 via binding to the mir-29 promoter, and the TGF-β pathway plays an important role in leiomyoma growth (Lee and Nowak, 2001; Qin et al., 2011; Yin et al., 2011), it would be interesting to investigate whether progesterone/PR and TGF-β/SMAD signaling pathways interact to regulate mir-29 expression and its effect on leiomyoma growth.
Aromatase inhibitors
Based on the role of estrogen and aromatase in leiomyoma pathogenesis described above, aromatase is a logical target for treatment (Fig. 2). Non-steroidal aromatase inhibitors reversibly and competitively bind the aromatase enzyme and block its access to its natural substrates such as androstenedione or testosterone (Michaud and Buzdar, 1999; Chumsri et al., 2011). Over time, more specific aromatase inhibitors, with superior bioavailability and side-effect profiles, have been developed and the current ‘third-generation’ aromatase inhibitors (e.g. anastrozole and letrozole) have been shown to result in >98% inhibition (Chumsri et al., 2011; Lonning and Eikesdal, 2013). The role of aromatase inhibitors in the treatment of leiomyoma was first reported by Shozu et al. (2003), when they described a significant decrease in leiomyoma size and symptomatology in a perimenopausal woman treated with fadrozole. Since then, several clinical trials have demonstrated the efficacy of letrozole and anastrozole in reduction of leiomyoma volume of up to 52.5% and in providing symptomatic control (Varelas et al., 2007; Hilario et al., 2009; Parsanezhad et al., 2010; Duhan et al., 2013). Moreover, letrozole has been shown to be equivalent to GnRH agonist in reducing leiomyoma size while avoiding the side effects of the profound hypoestrogenism caused by GnRH agonists, specifically severe hot flashes (Duhan et al., 2013).
Aromatase inhibitor treatment and the subsequent decrease in circulating estrogens are not without side effects. If used in the follicular phase, aromatase inhibitors can lead to follicular stimulation and pregnancy. Therefore, women prescribed aromatase inhibitors need contraception. Additionally, there have been conflicting results regarding the level of hypoestrogenism induced by aromatase inhibitors, with some studies suggesting a more systemic effect and others a more localized effect (Duhan et al., 2013; Shozu et al., 2003). The most concerning potential consequences of long-term treatment include the possibility for increased bone loss and increased cardiovascular risk, especially in younger patients (Lonning and Eikesdal, 2013). Minor reported side effects include hot flashes and musculoskeletal pain. There is also some question as to the effectiveness of aromatase inhibitors, particularly anastrozole, in overweight and obese individuals, based on data from breast cancer trials (Lonning and Eikesdal, 2013). Moreover, developing resistance to the action of aromatase inhibitors has been reported in breast cancer (Chumsri et al., 2011; Lonning and Eikesdal, 2013). These concerns suggest that treatment with aromatase inhibitors may only be a temporary or bridging therapy for leiomyoma, as opposed to a long-term solution. Unfortunately, once aromatase inhibitors are discontinued, leiomyomas regrow, albeit to smaller volumes than the pretreatment size (Duhan et al., 2013).
Selective progesterone receptor modulators
Four SPRMs have been used in clinical trials—mifepristone (RU486), asoprisnil (J867), ulipristal acetate (CDB2914) and telapristone acetate (CDB4124)—and all of these treatments were shown to reduce leiomyoma size and improve quality of life (Chabbert-Buffet et al., 2005; Chwalisz et al., 2005; Spitz, 2009; Bouchard et al., 2011; Talaulikar and Manyonda, 2012; Islam et al., 2013; Fig. 3). In 1993, it was first suggested that mifepristone might be a novel management strategy for uterine leiomyoma (Murphy et al., 1993). Since then, a large number of clinical trials looking at various doses of mifepristone have been performed to test its effect on tumor regression and symptomatic improvement. Compared with higher doses, lower doses of mifepristone appear to have the same effectiveness for symptom control and can reduce tumor size by up to 50%, and also have better safety profiles (Eisinger et al., 2009; Islam et al., 2013).
Conversely, asoprisnil suppresses uterine bleeding in a dose-dependent manner, with decreased bleeding reported in 28, 64 and 83% of subjects at 5, 10 and 25 mg/day, respectively, and reduced fibroid volume by up to 36% at 25 mg/day (Chwalisz et al., 2007), possibly by decreasing uterine artery blood flow (Wilkens et al., 2008). Recently, follow-up studies have raised concerns about asoprisnil, in that it primarily functions as a progesterone antagonist and does not oppose estrogenic activity in endometrium (Madauss et al., 2007). Similarly, clinical trials of telapristone acetate were suspended due to concerns over liver toxicity; however, trials have recently resumed employing lower doses (Bouchard et al., 2011).
The newest antiprogestin to be studied, ulipristal acetate, has shown promising results. In a clinical trial comparing the efficacy and side-effect profile of ulipristal acetate to the GnRH agonist leuprolide acetate, ulipristal acetate provided more prolonged tumor volume reduction after termination of treatment, although leuprolide acetate caused greater reduction in fibroid volume overall (Donnez et al., 2012a, b; Talaulikar and Manyonda, 2012; Islam et al., 2013; Kim et al., 2013). Importantly, patients seem to tolerate ulipristal acetate better than leuprolide acetate with a significantly lower incidence of hot flashes, little effect on bone density and less profound suppression of E2 levels (Donnez et al., 2012a, b). Currently, ulipristal acetate 5 mg/day is approved in Europe for preoperative treatment of <3 months, but has not been approved in the USA by the Food and Drug Administration (www.fda.gov) for the treatment of leiomyoma.
Mechanisms underlying the therapeutic roles of these four antiprogestins are still being investigated. Gene expression of Kruppel-like transcription factor 11 (KLF11), a tumor suppressor, is significantly lower in leiomyoma tissues compared with adjacent myometrial tissues (Yin et al., 2010). Bisulfite sequencing revealed that the CpG island in the KLF11 gene promoter is hypermethylated in leiomyoma tissue. Therefore, dysregulation of KLF11 may be a key factor involved in uterine fibroids. Using a genome-wide approach, we found a PR-binding site located 20.5 kb upstream of the KLF11 gene promoter. Importantly, KLF11 knockdown markedly increased leiomyoma cell proliferation. Intriguingly, several investigators reported the novel role of KLF11 in cardiac and liver fibrosis through mediating extracellular matrix gene expression (Mathison et al., 2013; Zheng et al., 2014). In primary cultures of uterine leiomyoma cells, mifepristone treatment robustly regulates the protein and mRNA levels of KLF11 via enhancing the recruitment of Sp1, RNA polymerase II, PR and its coactivator SRC-2 to both the distal enhancer and basal promoter region of the KLF11 gene. It is unclear whether these activities can alter KLF11 methylation status. Furthermore, taking advantage of a robust and unbiased ChIP-sequencing assay, we recently discovered another novel PR target gene, perilipin 2 (PLIN2), in leiomyoma cells. PLIN2, an adipose differentiation-related protein, is ubiquitous in non-adipose lipid droplet-containing cells and plays important roles in lipid droplet formation and stabilization, but its loss is linked to the expression of fibrogenic genes in hepatic fibrosis. Moreover, in clear cell renal carcinoma, higher PLIN2 expression is associated with better cancer-specific survival and cancer-free survival. These findings strongly suggest that KLF11 and PLIN2 may regulate both cell proliferation and extracellular matrix formation in leiomyoma and provide novel gene therapeutic targets.
In cultured leiomyoma cells, treatment with ulipristal acetate, telapristone acetate and asoprisnil decreases cell proliferation by down-regulating a number of growth factors and their receptors, including insulin-like growth factor-I, epidermal growth factor, TGFβ3, vascular endothelial growth factor (VEGF)-A and VEGF-B (Xu et al., 2005, 2006; Chen et al., 2006; Wang et al., 2006; Maruo et al., 2007; Luo et al., 2010; Yoshida et al., 2010). These treatments also induce apoptosis in cultured leiomyoma cells through activation of multiple differential apoptotic pathways: asoprisnil activates the tumor necrosis factor-related apoptosis-inducing ligand-mediated signaling pathway, as well as the endoplasmic reticulum stress-induced pathway (Sasaki et al., 2007; Xu et al., 2007; Yoshida et al., 2010), while ulipristal acetate induces apoptosis by reducing the anti-apoptotic protein BCL2 and stimulating expression of cleaved caspase-3 and cleaved poly ADP ribose polymerase (Xu et al., 2005; Chen et al., 2006; Yoshida et al., 2010). Furthermore, asoprisnil and ulipristal mediate extracellular matrix formation by increasing extracellular matrix metalloproteinase (MMP) inducer, MMP-1, MMP-8 and membrane type 1-MMP protein contents, and decreasing tissue inhibitor of metalloproteinase (TIMP)-1, TIMP-2, type I and type III collagen protein levels (Morikawa et al., 2008; Xu et al., 2008; Yoshida et al., 2010). Importantly, all these observations occurred in cultured leiomyoma cells, but not in cells derived from adjacent normal myometrium, suggesting the tissue-specific roles of these compounds. Both asoprisnil and ulipristal acetate demonstrated high affinity for PR (Blithe et al., 2003; DeManno et al., 2003). Further studies are needed to investigate the genome-wide binding status of PR liganded with ulipristal or asoprisnil, similar to the aforementioned studies on mifepristone.
Overall, the most commonly expressed concern during treatment of uterine leiomyoma with SPRMs is endometrial thickening from blocked progesterone action in the endometrium. A study sponsored by the US National Institutes of Health specifically evaluated the endometrial histological changes following treatment with mifepristone, asoprisnil or ulipristal acetate and reported little evidence of mitosis and atypical hyperplasia; however, there was asymmetry of stromal and epithelial growth and prominent cystic, dilated glands. These novel endometrial changes represent a new morphological category designated as progesterone receptor modulator-associated endometrial change (Horne and Blithe, 2007; Mutter et al., 2008; Spitz, 2009; Williams et al., 2012). Of note, most of the existing studies have described endometrial changes over a short period (months) of follow-up, with one group reporting return to normal endometrial histology 6 months after cessation of ulipristal acetate (Williams et al., 2012). Although it has not yet been tested in humans, a promising new SPRM, CP8947, was shown to inhibit leiomyoma cell proliferation and extracellular matrix gene expression in vitro without blocking progesterone action in the endometrium in an animal model (Catherino et al., 2010). More long-term studies are necessary to understand the full side-effect profile of SPRMs.
Stem-progenitor cells
Somatic stem cells (also called adult stem cells or tissue-specific stem cells) are a small group of cells present throughout the body that undergo asymmetric division, allowing self-renewal and the production of daughter cells that can go on and differentiate into tissue-specific cell types. These cells are necessary for tissue regeneration and repair, which is critical for maintaining organ function (Weissman, 2000; Li and Clevers, 2010). Similarly, tumor-initiating cells (also called cancer stem cells or tumor progenitor cells) are a small group of cells within a tumor also capable of asymmetric division, and thereby have the ability for self-renewal and tumor maintenance and growth (Schofield, 1978; Jordan et al., 2006). Recently, human and mouse myometrial tissues have been found to contain a subset of cells characteristic of somatic stem cells, which self-renew and produce daughter cells in a hormone-dependent manner (Arango et al., 2005; Ono et al., 2007; Szotek et al., 2007). Subsequently, we and others have been able to isolate a small population of leiomyoma cells consistent with undifferentiated somatic stem cells or tumor progenitor cells (Mas et al., 2012; Ono et al., 2012).
Stem cells derived from leiomyoma tissue, but not myometrium, carry mediator complex subunit 12 (MED12) mutations, leading some to hypothesize that at least one genetic hit may transform a myometrial stem cell into a leiomyoma tumor progenitor cell, which then interacts with the surrounding myometrial tissue to give rise to a leiomyoma tumor (Ono et al., 2012). Interestingly, mutations in the MED12 gene have been reported in ∼70% of uterine leiomyomas (Makinen et al., 2011; McGuire et al., 2012). Mutations affecting the expression of the high mobility AT-hook 2 (HMGA2) gene have also been reported, and appear to be mutually exclusive with MED12 mutations, suggesting the possibility of different pathophysiologies behind leiomyomas harboring different mutations (Bertsch et al., 2014). Alterations in HMGA2 and MED12 expression and function have also been hypothesized to support leiomyoma stem-progenitor cell self-renewal and cell proliferation (Bulun, 2013), making it unclear whether these genetic alterations cause the transformation of a myometrial stem cell or simply support already existing leiomyoma stem-progenitor cells. Importantly, it has alternatively been hypothesized that uterine hypoxia, aberrant methylation or abnormal estrogen signaling could play a critical role in the transformation of a myometrial stem cell into a leiomyoma (Zhou et al., 2011; Maruyama et al., 2013). Further research into the cellular insult leading to this transformation event in myometrial stem cells could reveal important therapeutic targets.
Evidence suggests that leiomyomas possess much smaller populations of stem cells compared with the myometrium (Chang et al., 2010); however, the leiomyoma stem cell population is likely essential for steroid-dependent leiomyoma growth (Mas et al., 2012; Ono et al., 2012). When cell suspensions containing leiomyoma stem-progenitor cells and mixed myometrial cells are injected under the kidney capsules of immunodeficient mice treated with estrogen and progesterone, they grow into significantly larger tumors than those containing differentiated leiomyoma cells and mixed myometrial cells. The tumors derived from leiomyoma stem-progenitor cells also have a much higher proliferation index than the tumors that do not contain these cells (Ono et al., 2012). Interestingly, leiomyoma stem-progenitor cells appear to be deficient in ERs and PRs, but have tumorigenic capabilities when stimulated by estrogen and progesterone. Moreover, leiomyoma stem-progenitor cells seem to require the presence of either mature leiomyoma or myometrial cells for proliferation and growth. We hypothesize that these cells rely on strikingly higher levels of steroid hormone receptors in surrounding differentiated myometrial and leiomyoma cells to mediate estrogen and progesterone action via paracrine signaling (Ono et al., 2012; Fig. 4).
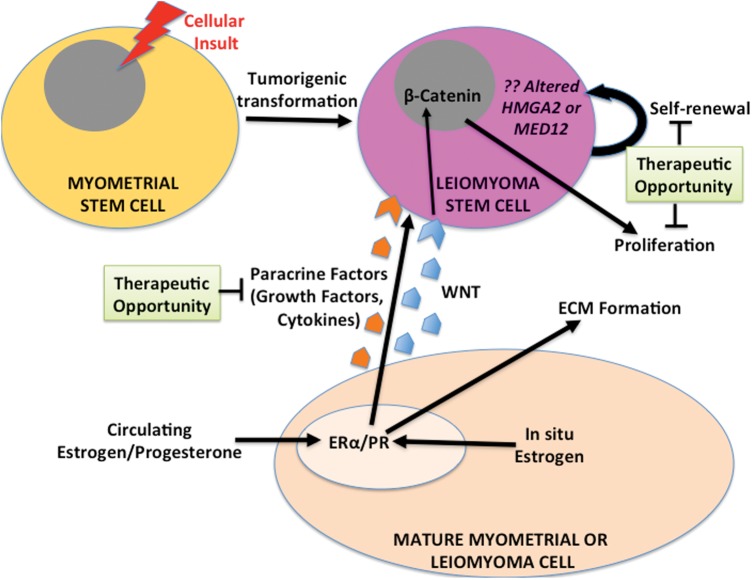
Figure 4.
Proposed role of stem-progenitor cells in leiomyoma pathogenesis. It has been proposed that a single myometrial stem-progenitor cell goes through tumorigenic transformation following a cellular insult and gives rise to daughter leiomyoma stem-progenitor cells, which proliferate, undergo self-renewal and clonally expand in response to steroid hormones via paracrine signaling from surrounding differentiated myometrial and leiomyoma cells. Pathways involved in the paracrine signaling have not been fully elucidated, but there is recent evidence for a critical role of the WNT/β-catenin pathway. Treatments targeting these paracrine interactions, or the stem-progenitor cells themselves, could not only treat existing leiomyoma, but also possibly prevent the development and growth of new tumors.
The mechanism of steroid hormone paracrine action on leiomyoma stem-progenitor cells has not been fully elucidated. Recently, Ono et al. (2013) reported a critical role for the wingless-type (WNT)/β-catenin pathway in the communication between leiomyoma stem-progenitor cells and the surrounding differentiated cells. Treatment of mature myometrial cells with estrogen and progesterone resulted in secretion of WNT ligands, which induced nuclear translocation of β-catenin in neighboring leiomyoma stem-progenitor cells and ultimately activated the expression of genes critical for growth and proliferation. Moreover, selective inhibition of WNT binding or β-catenin in leiomyoma stem-progenitor cells, but not in fully differentiated leiomyoma cells, significantly decreased tumor growth (Ono et al., 2013). The importance of β-catenin in myometrial stem cell function has previously been shown in an animal model, where myometrial stem cells differentiate into adipocytes in β-catenin-deficient uteri (Arango et al., 2005; Szotek et al., 2007). These findings are consistent with previous reports implicating the WNT/β-catenin pathway in leiomyoma formation and fibrogenesis (Tanwar et al., 2009; Lam and Gottardi, 2011; Gottardi and Konigshoff, 2013). Additionally, MED12 has previously been shown to regulate β-catenin/WNT signaling, further supporting its role in leiomyoma pathogenesis (Kim et al., 2006). On the other hand, treatment with progesterone has previously been shown to decrease WNT expression in the ovine uterus (Satterfield et al., 2008), so the role of steroid hormones in the WNT signaling pathway needs to be further clarified.
Much remains to be explored in leiomyoma stem-progenitor cells. Thus far, the presence of leiomyoma stem-progenitor cells has been identified as the leiomyoma-derived side population (SP) cells, based on the universal ability of somatic stem cells to extrude Hoechst dye (Challen and Little, 2006; Jordan et al., 2006). This technique has been successfully employed in multiple tissues throughout the body (Goodell et al., 1996; Gargett and Masuda, 2010), however, the pitfalls of the SP technique for stem cell isolation are its expense and high sensitivity to slight variations in staining conditions, as well as the profound toxic effects of Hoechst staining on cell survival (Golebiewska et al., 2011). To avoid these consequences and to further study the function and regulation of leiomyoma stem-progenitor cells, cell surface markers should be identified and used for isolation. Additionally, the molecular characteristics of leiomyoma stem-progenitor cells remain unknown. To further examine the function and regulation of this population of cells, an unbiased genome-wide investigation of their gene expression should be undertaken and will likely lead to new therapeutic targets (Fig. 4). Finally, the pathways that mediate paracrine signaling between leiomyoma cells and surrounding differentiated cells need to be further explored. Scientific direction may be taken from the breast cancer literature, which is a better-studied model of stem-progenitor cells that are deficient in ERs and PRs, but are hormone responsive via paracrine interaction with surrounding differentiated cells (Asselin-Labat et al., 2010; Fillmore et al., 2010; Roarty and Rosen, 2010; Montales et al., 2012; Cittelly et al., 2013; Vares et al., 2013). For example, Cittelly et al. (2013) discovered that progesterone leads to expansion of stem-progenitor cells in breast cancer via down-regulation of miR-29 and subsequent up-regulation of KLF4. These results are particularly interesting for leiomyoma researchers in light of the aforementioned findings from Qiang et al. (2014) that steroid hormone suppression of miR-29 is necessary for leiomyoma growth, and may indicate that a similar mechanism is at play in leiomyoma stem-progenitor cells. Moreover, research performed on breast cancer stem-progenitor cells may shed light on potential therapeutics targeting leiomyoma progenitor-stem cells. For example, Montales et al. (2012) showed that dietary factors targeting the phosphatidylinositol 3-kinase/Akt signaling pathway can decrease expansion of stem-progenitor cells, and therefore decrease progression and recurrence in breast cancer.
Conclusions
Until very recently, the knowledge that leiomyoma cells contained ERs and PRs led to assumptions about their pathophysiology involving primarily sex steroid stimulation. Over the past several years, the concept of the importance of stem-progenitor cells in the tumorigenesis of many cancers has been evolving, and this has recently been applied to leiomyomas, indicating that the story behind their growth is much more complex than previously thought. Much research has been dedicated to understanding the role of ovarian steroids in the pathogenesis of leiomyoma, and has led to the development of medical treatment options, such as aromatase inhibitors and antiprogestins. Unfortunately, none of these treatments result in complete regression of leiomyoma, and tumors recur after treatment is stopped. Finding an effective, long-term treatment for leiomyoma could have great public health implications, given their high prevalence and associated medical costs. The recent discovery of stem cells and their paracrine interactions with more differentiated cell populations within leiomyoma may provide the missing link between developing therapeutics that temper leiomyoma growth and those that eradicate them.
Authors' roles
M.B.M., P.Y. and S.E.B. drafted the original manuscript. M.B.M., P.Y., M.O., J.S.C., M.T.D., A.N., E.E.M., D.C., J.J.K., J.J.W. and S.E.B. contributed to the research and interpretations of data discussed in the manuscript, revised the manuscript and approved the final version.
Funding
National Institutes of Health Grant NIH/NICHD P01-HD057877.
Conflict of interest
None declared.
References
- Al Hilli MM, Stewart EA. Magnetic resonance-guided focused ultrasound surgery. Sem Reprod Med. 2010;28:242–249. doi: 10.1055/s-0030-1251481. [DOI] [PubMed] [Google Scholar]
- Al-Hendy A, Salama SA. Ethnic distribution of estrogen receptor-alpha polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans. Fertil Steril. 2006;86:686–693. doi: 10.1016/j.fertnstert.2006.01.052. [DOI] [PubMed] [Google Scholar]
- Andersen J, Barbieri RL. Abnormal gene expression in uterine leiomyomas. J Soc Gynecol Invest. 1995;2:663–672. doi: 10.1016/1071-5576(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Andersen J, DyReyes VM, Barbieri RL, Coachman DM, Miksicek RJ. Leiomyoma primary cultures have elevated transcriptional response to estrogen compared with autologous myometrial cultures. J Soc Gynecol Invest. 1995;2:542–551. doi: 10.1016/1071-5576(94)00053-4. [DOI] [PubMed] [Google Scholar]
- Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Bio. 2005;288:276–283. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- Benassayag C, Leroy MJ, Rigourd V, Robert B, Honore JC, Mignot TM, Vacher-Lavenu MC, Chapron C, Ferre F. Estrogen receptors (ERalpha/ERbeta) in normal and pathological growth of the human myometrium: pregnancy and leiomyoma. Am J Physiol. 1999;276:E1112–E1118. doi: 10.1152/ajpendo.1999.276.6.E1112. [DOI] [PubMed] [Google Scholar]
- Bertsch E, Qiang W, Zhang Q, Espona-Fiedler M, Druschitz S, Liu Y, Mittal K, Kong B, Kurita T, Wei JJ. MED12 and HMGA2 mutations: two independent genetic events in uterine leiomyoma and leiomyosarcoma. Mod Pathol. 2014;27:1144–1153. doi: 10.1038/modpathol.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blithe DL, Nieman LK, Blye RP, Stratton P, Passaro M. Development of the selective progesterone receptor modulator CDB-2914 for clinical indications. Steroids. 2003;68:1013–1017. doi: 10.1016/s0039-128x(03)00118-1. [DOI] [PubMed] [Google Scholar]
- Bouchard P, Chabbert-Buffet N, Fauser BC. Selective progesterone receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety. Fertil Steril. 2011;96:1175–1189. doi: 10.1016/j.fertnstert.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Brandon DD, Bethea CL, Strawn EY, Novy MJ, Burry KA, Harrington MS, Erickson TE, Warner C, Keenan EJ, Clinton GM. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol. 1993;169:78–85. doi: 10.1016/0002-9378(93)90135-6. [DOI] [PubMed] [Google Scholar]
- Bulun SE. Uterine fibroids. New Engl J Med. 2013;369:1344–1355. doi: 10.1056/NEJMra1209993. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Simpson ER, Word RA. Expression of the CYP19 gene and its product aromatase cytochrome P450 in human uterine leiomyoma tissues and cells in culture. J Clin Endocrinol Metabol. 1994;78:736–743. doi: 10.1210/jcem.78.3.8126151. [DOI] [PubMed] [Google Scholar]
- Cakmak H, Taylor HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update. 2011;17:242–253. doi: 10.1093/humupd/dmq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206:211 e211–219. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catherino WH, Malik M, Driggers P, Chappel S, Segars J, Davis J. Novel, orally active selective progesterone receptor modulator CP8947 inhibits leiomyoma cell proliferation without adversely affecting endometrium or myometrium. J Steroid Biochem Mol Biol. 2010;122:279–286. doi: 10.1016/j.jsbmb.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermik D, Arici A, Taylor HS. Coordinated regulation of HOX gene expression in myometrium and uterine leiomyoma. Fertil Steril. 2002;78:979–984. doi: 10.1016/s0015-0282(02)03366-6. [DOI] [PubMed] [Google Scholar]
- Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM. Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications. Hum Reprod Update. 2005;11:293–307. doi: 10.1093/humupd/dmi002. [DOI] [PubMed] [Google Scholar]
- Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- Chang HL, Senaratne TN, Zhang L, Szotek PP, Stewart E, Dombkowski D, Preffer F, Donahoe PK, Teixeira J. Uterine leiomyomas exhibit fewer stem/progenitor cell characteristics when compared with corresponding normal myometrium. Reprod Sci. 2010;17:158–167. doi: 10.1177/1933719109348924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Ohara N, Wang J, Xu Q, Liu J, Morikawa A, Sasaki H, Yoshida S, Demanno DA, Chwalisz K, et al. A novel selective progesterone receptor modulator asoprisnil (J867) inhibits proliferation and induces apoptosis in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metabol. 2006;91:1296–1304. doi: 10.1210/jc.2005-2379. [DOI] [PubMed] [Google Scholar]
- Chumsri S, Howes T, Bao T, Sabnis G, Brodie A. Aromatase, aromatase inhibitors, and breast cancer. J Steroid Biochem Mol Biol. 2011;125:13–22. doi: 10.1016/j.jsbmb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26:423–438. doi: 10.1210/er.2005-0001. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Larsen L, Mattia-Goldberg C, Edmonds A, Elger W, Winkel CA. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertil Steril. 2007;87:1399–1412. doi: 10.1016/j.fertnstert.2006.11.094. [DOI] [PubMed] [Google Scholar]
- Cittelly DM, Finlay-Schultz J, Howe EN, Spoelstra NS, Axlund SD, Hendricks P, Jacobsen BM, Sartorius CA, Richer JK. Progestin suppression of miR-29 potentiates dedifferentiation of breast cancer cells via KLF4. Oncogene. 2013;32:2555–2564. doi: 10.1038/onc.2012.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94:435–438. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lu J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- DeManno D, Elger W, Garg R, Lee R, Schneider B, Hess-Stumpp H, Schubert G, Chwalisz K. Asoprisnil (J867): a selective progesterone receptor modulator for gynecological therapy. Steroids. 2003;68:1019–1032. doi: 10.1016/j.steroids.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, Ugocsai G, Mara M, Jilla MP, Bestel E, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. New Engl J Med. 2012a;366:409–420. doi: 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tomaszewski J, Vazquez F, Bouchard P, Lemieszczuk B, Baro F, Nouri K, Selvaggi L, Sodowski K, Bestel E, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. New Engl J Med. 2012b;366:421–432. doi: 10.1056/NEJMoa1103180. [DOI] [PubMed] [Google Scholar]
- Duhan N, Madaan S, Sen J. Role of the aromatase inhibitor letrozole in the management of uterine leiomyomas in premenopausal women. Eur J Obstet, Gynecol Reprod Biol. 2013;171:329–332. doi: 10.1016/j.ejogrb.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Edwards DP. The role of coactivators and corepressors in the biology and mechanism of action of steroid hormone receptors. J Mammary Gland Biol Neoplasia. 2000;5:307–324. doi: 10.1023/a:1009503029176. [DOI] [PubMed] [Google Scholar]
- Eisinger SH, Fiscella J, Bonfiglio T, Meldrum S, Fiscella K. Open-label study of ultra low-dose mifepristone for the treatment of uterine leiomyomata. Eur J Obstet Gynecol Reprod Biol. 2009;146:215–218. doi: 10.1016/j.ejogrb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Englund K, Blanck A, Gustavsson I, Lundkvist U, Sjoblom P, Norgren A, Lindblom B. Sex steroid receptors in human myometrium and fibroids: changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J Clin Endocrinol Metabol. 1998;83:4092–4096. doi: 10.1210/jcem.83.11.5287. [DOI] [PubMed] [Google Scholar]
- Feng Y, Lin X, Zhou S, Xu N, Yi T, Zhao X. The associations between the polymorphisms of the ER-alpha gene and the risk of uterine leiomyoma (ULM) Tumour Biol. 2013;34:3077–3082. doi: 10.1007/s13277-013-0874-0. [DOI] [PubMed] [Google Scholar]
- Fillmore CM, Gupta PB, Rudnick JA, Caballero S, Keller PJ, Lander ES, Kuperwasser C. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc Natl Acad Sci USA. 2010;107:21737–21742. doi: 10.1073/pnas.1007863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flake GP, Moore AB, Sutton D, Kissling GE, Horton J, Wicker B, Walmer D, Robboy SJ, Dixon D. The natural history of uterine leiomyomas: light and electron microscopic studies of fibroid phases, interstitial ischemia, inanosis, and reclamation. Obstet Gynecol Int. 2013;2013:528376. doi: 10.1155/2013/528376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkerd EJ, Newton CJ, Davidson K, Anderson MC, James VH. Aromatase activity in uterine leiomyomata. J Steroid Biochem. 1984;20:1195–1200. doi: 10.1016/0022-4731(84)90366-2. [DOI] [PubMed] [Google Scholar]
- Freed MM, Spies JB. Uterine artery embolization for fibroids: a review of current outcomes. Sem Reprod Med. 2010;28:235–241. doi: 10.1055/s-0030-1251480. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod. 2010;16:818–834. doi: 10.1093/molehr/gaq061. [DOI] [PubMed] [Google Scholar]
- Golebiewska A, Brons NH, Bjerkvig R, Niclou SP. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell. 2011;8:136–147. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Konigshoff M. Considerations for targeting beta-catenin signaling in fibrosis. Am J Respir Crit Care Med. 2013;187:566–568. doi: 10.1164/rccm.201301-0144ED. [DOI] [PubMed] [Google Scholar]
- Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18:502–519. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H, Meyer ME, Bocquel MT, Kastner P, Turcotte B, Chambon P. Progestin receptors: isoforms and antihormone action. J Steroid Biochem Mol Biol. 1991;40:271–278. doi: 10.1016/0960-0760(91)90192-8. [DOI] [PubMed] [Google Scholar]
- Halder SK, Sharan C, Al-Hendy A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol Reprod. 2012;86:116. doi: 10.1095/biolreprod.111.098145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder SK, Osteen KG, Al-Hendy A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum Reprod. 2013;28:2407–2416. doi: 10.1093/humrep/det265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MH, Salama SA, Arafa HM, Hamada FM, Al-Hendy A. Adenovirus-mediated delivery of a dominant-negative estrogen receptor gene in uterine leiomyoma cells abrogates estrogen- and progesterone-regulated gene expression. J Clin Endocrinol Metabol. 2007;92:3949–3957. doi: 10.1210/jc.2007-0823. [DOI] [PubMed] [Google Scholar]
- Hermon TL, Moore AB, Yu L, Kissling GE, Castora FJ, Dixon D. Estrogen receptor alpha (ERalpha) phospho-serine-118 is highly expressed in human uterine leiomyomas compared to matched myometrium. Virchows Arch. 2008;453:557–569. doi: 10.1007/s00428-008-0679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario SG, Bozzini N, Borsari R, Baracat EC. Action of aromatase inhibitor for treatment of uterine leiomyoma in perimenopausal patients. Fertil Steril. 2009;91:240–243. doi: 10.1016/j.fertnstert.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Horne FM, Blithe DL. Progesterone receptor modulators and the endometrium: changes and consequences. Hum Reprod Update. 2007;13:567–580. doi: 10.1093/humupd/dmm023. [DOI] [PubMed] [Google Scholar]
- Imir AG, Lin Z, Yin P, Deb S, Yilmaz B, Cetin M, Cetin A, Bulun SE. Aromatase expression in uterine leiomyomata is regulated primarily by proximal promoters I.3/II. J Clin Endocrinol Metabol. 2007;92:1979–1982. doi: 10.1210/jc.2006-2482. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Fenkci V, Marsh EE, Yin P, Chen D, Cheng YH, Reisterd S, Lin Z, Bulun SE. CCAAT/enhancer binding protein beta regulates aromatase expression via multiple and novel cis-regulatory sequences in uterine leiomyoma. J Clin Endocrinol Metabol. 2008;93:981–991. doi: 10.1210/jc.2007-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Reierstad S, Demura M, Rademaker AW, Kasai T, Inoue M, Usui H, Shozu M, Bulun SE. High aromatase expression in uterine leiomyoma tissues of African-American women. J Clin Endocrinol Metabol. 2009;94:1752–1756. doi: 10.1210/jc.2008-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151:2433–2442. doi: 10.1210/en.2009-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Protic O, Giannubilo SR, Toti P, Tranquilli AL, Petraglia F, Castellucci M, Ciarmela P. Uterine leiomyoma: available medical treatments and new possible therapeutic options. J Clin Endocrinol Metabol. 2013;98:921–934. doi: 10.1210/jc.2012-3237. [DOI] [PubMed] [Google Scholar]
- Jakimiuk AJ, Bogusiewicz M, Tarkowski R, Dziduch P, Adamiak A, Wrobel A, Haczynski J, Magoffin DA, Jakowicki JA. Estrogen receptor alpha and beta expression in uterine leiomyomas from premenopausal women. Fertil Steril. 2004;82(Suppl 3):1244–1249. doi: 10.1016/j.fertnstert.2004.02.130. [DOI] [PubMed] [Google Scholar]
- Jordan CT, Guzman ML, Noble M. Cancer stem cells. New Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi K, Fujii S, Konishi I, Nanbu Y, Nonogaki H, Mori T. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol. 1989;160:637–641. doi: 10.1016/s0002-9378(89)80046-8. [DOI] [PubMed] [Google Scholar]
- Kim S, Xu X, Hecht A, Boyer TG. Mediator is a transducer of Wnt/beta-catenin signaling. J Biol Chem. 2006;281:14066–14075. doi: 10.1074/jbc.M602696200. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34:130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AP, Gottardi CJ. beta-catenin signaling: a novel mediator of fibrosis and potential therapeutic target. Curr Opin Rheumatol. 2011;23:562–567. doi: 10.1097/BOR.0b013e32834b3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamminen S, Rantala I, Helin H, Rorarius M, Tuimala R. Proliferative activity of human uterine leiomyoma cells as measured by automatic image analysis. Gynecol Obstet Invest. 1992;34:111–114. doi: 10.1159/000292738. [DOI] [PubMed] [Google Scholar]
- Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J Clin Endocrinol Metabol. 2001;86:913–920. doi: 10.1210/jcem.86.2.7237. [DOI] [PubMed] [Google Scholar]
- Lee K, Jeong J, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ. Molecular mechanisms involved in progesterone receptor regulation of uterine function. J Steroid Biochem Mol Biol. 2006;102:41–50. doi: 10.1016/j.jsbmb.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA, Alexander PS, Horwitz KB. The subunit structure of human breast cancer progesterone receptors: characterization by chromatography and photoaffinity labeling. Endocrinology. 1983;112:1267–1274. doi: 10.1210/endo-112-4-1267. [DOI] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, McLachlan JA. Estrogen-associated genes in uterine leiomyoma. Ann N Y Acad Sci. 2001;948:112–120. doi: 10.1111/j.1749-6632.2001.tb03992.x. [DOI] [PubMed] [Google Scholar]
- Linder D, Gartler SM. Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science. 1965;150:67–69. doi: 10.1126/science.150.3692.67. [DOI] [PubMed] [Google Scholar]
- Lonning PE, Eikesdal HP. Aromatase inhibition 2013: clinical state of the art and questions that remain to be solved. Endocr Relat Cancer. 2013;20:R183–R201. doi: 10.1530/ERC-13-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Yin P, Coon VJ, Cheng YH, Wiehle RD, Bulun SE. The selective progesterone receptor modulator CDB4124 inhibits proliferation and induces apoptosis in uterine leiomyoma cells. Fertil Steril. 2010;93:2668–2673. doi: 10.1016/j.fertnstert.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Madauss KP, Grygielko ET, Deng SJ, Sulpizio AC, Stanley TB, Wu C, Short SA, Thompson SK, Stewart EL, Laping NJ, et al. A structural and in vitro characterization of asoprisnil: a selective progesterone receptor modulator. Mol Endocrinol. 2007;21:1066–1081. doi: 10.1210/me.2006-0524. [DOI] [PubMed] [Google Scholar]
- Makinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, Gentile M, Yan J, Enge M, Taipale M, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334:252–255. doi: 10.1126/science.1208930. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh EE, Lin Z, Yin P, Milad M, Chakravarti D, Bulun SE. Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertil Steril. 2008;89:1771–1776. doi: 10.1016/j.fertnstert.2007.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, Willett WC, Hunter DJ. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- Maruo T, Ohara N, Wang J, Matsuo H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum Reprod Update. 2004;10:207–220. doi: 10.1093/humupd/dmh019. [DOI] [PubMed] [Google Scholar]
- Maruo T, Ohara N, Matsuo H, Xu Q, Chen W, Sitruk-Ware R, Johansson ED. Effects of levonorgestrel-releasing IUS and progesterone receptor modulator PRM CDB-2914 on uterine leiomyomas. Contraception. 2007;75:S99–103. doi: 10.1016/j.contraception.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Miyazaki K, Masuda H, Ono M, Uchida H, Yoshimura Y. Review: Human uterine stem/progenitor cells: Implications for uterine physiology and pathology. Placenta. 2013;34(Suppl):S68–S72. doi: 10.1016/j.placenta.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Mas A, Cervello I, Gil-Sanchis C, Faus A, Ferro J, Pellicer A, Simon C. Identification and characterization of the human leiomyoma side population as putative tumor-initiating cells. Fertil Steril. 2012;98:741––751 e746. doi: 10.1016/j.fertnstert.2012.04.044. [DOI] [PubMed] [Google Scholar]
- Mathison A, Grzenda A, Lomberk G, Velez G, Buttar N, Tietz P, Hendrickson H, Liebl A, Xiong YY, Gores G, et al. Role for Kruppel-like transcription factor 11 in mesenchymal cell function and fibrosis. PloS one. 2013;8:e75311. doi: 10.1371/journal.pone.0075311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell DP, Shahbaz MM, Vegeto E, Goldman ME. The human progesterone receptor A-form functions as a transcriptional modulator of mineralocorticoid receptor transcriptional activity. J Steroid Biochem Mol Biol. 1994;48:425–432. doi: 10.1016/0960-0760(94)90190-2. [DOI] [PubMed] [Google Scholar]
- McGuire MM, Yatsenko A, Hoffner L, Jones M, Surti U, Rajkovic A. Whole exome sequencing in a random sample of North American women with leiomyomas identifies MED12 mutations in majority of uterine leiomyomas. PLoS One. 2012;7:e33251. doi: 10.1371/journal.pone.0033251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehine M, Kaasinen E, Makinen N, Katainen R, Kampjarvi K, Pitkanen E, Heinonen HR, Butzow R, Kilpivaara O, Kuosmanen A, et al. Characterization of uterine leiomyomas by whole-genome sequencing. New Engl J Med. 2013;369:43–53. doi: 10.1056/NEJMoa1302736. [DOI] [PubMed] [Google Scholar]
- Michaud LB, Buzdar AU. Risks and benefits of aromatase inhibitors in postmenopausal breast cancer. Drug Saf: Int J Med Toxicol Drug Exp. 1999;21:297–309. doi: 10.2165/00002018-199921040-00005. [DOI] [PubMed] [Google Scholar]
- Montales MT, Rahal OM, Kang J, Rogers TJ, Prior RL, Wu X, Simmen RC. Repression of mammosphere formation of human breast cancer cells by soy isoflavone genistein and blueberry polyphenolic acids suggests diet-mediated targeting of cancer stem-like/progenitor cells. Carcinogenesis. 2012;33:652–660. doi: 10.1093/carcin/bgr317. [DOI] [PubMed] [Google Scholar]
- Morikawa A, Ohara N, Xu Q, Nakabayashi K, DeManno DA, Chwalisz K, Yoshida S, Maruo T. Selective progesterone receptor modulator asoprisnil down-regulates collagen synthesis in cultured human uterine leiomyoma cells through up-regulating extracellular matrix metalloproteinase inducer. Hum Reprod. 2008;23:944–951. doi: 10.1093/humrep/den025. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AA, Kettel LM, Morales AJ, Roberts VJ, Yen SS. Regression of uterine leiomyomata in response to the antiprogesterone RU 486. J Clin Endocrinol Metabol. 1993;76:513–517. doi: 10.1210/jcem.76.2.8432797. [DOI] [PubMed] [Google Scholar]
- Mutter GL, Bergeron C, Deligdisch L, Ferenczy A, Glant M, Merino M, Williams AR, Blithe DL. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol. 2008;21:591–598. doi: 10.1038/modpathol.2008.19. [DOI] [PubMed] [Google Scholar]
- Myers ER, Barber MD, Gustilo-Ashby T, Couchman G, Matchar DB, McCrory DC. Management of uterine leiomyomata: what do we really know? Obstet Gynecol. 2002;100:8–17. doi: 10.1016/s0029-7844(02)02019-7. [DOI] [PubMed] [Google Scholar]
- Nisolle M, Gillerot S, Casanas-Roux F, Squifflet J, Berliere M, Donnez J. Immunohistochemical study of the proliferation index, oestrogen receptors and progesterone receptors A and B in leiomyomata and normal myometrium during the menstrual cycle and under gonadotrophin-releasing hormone agonist therapy. Hum Reprod. 1999;14:2844–2850. doi: 10.1093/humrep/14.11.2844. [DOI] [PubMed] [Google Scholar]
- Ono M, Maruyama T, Masuda H, Kajitani T, Nagashima T, Arase T, Ito M, Ohta K, Uchida H, Asada H, et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc Natl Acad Sci USA. 2007;104:18700–18705. doi: 10.1073/pnas.0704472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Qiang W, Serna VA, Yin P, Coon JSt, Navarro A, Monsivais D, Kakinuma T, Dyson M, Druschitz S, et al. Role of stem cells in human uterine leiomyoma growth. PLoS One. 2012;7:e36935. doi: 10.1371/journal.pone.0036935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Yin P, Navarro A, Moravek MB, Coon JSt, Druschitz SA, Serna VA, Qiang W, Brooks DC, Malpani SS, et al. Paracrine activation of WNT/beta-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proc Nat Acad Sci USA. 2013;110:17053–17058. doi: 10.1073/pnas.1313650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725–736. doi: 10.1016/j.fertnstert.2007.01.093. [DOI] [PubMed] [Google Scholar]
- Parsanezhad ME, Azmoon M, Alborzi S, Rajaeefard A, Zarei A, Kazerooni T, Frank V, Schmidt EH. A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (letrozole) and gonadotropin-releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertil Steril. 2010;93:192–198. doi: 10.1016/j.fertnstert.2008.09.064. [DOI] [PubMed] [Google Scholar]
- Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, Semelka RC, Kowalik A, Armao D, Davis B, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:19887–19892. doi: 10.1073/pnas.0808188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedeutour F, Quade BJ, Weremowicz S, Dal Cin P, Ali S, Morton CC. Localization and expression of the human estrogen receptor beta gene in uterine leiomyomata. Genes Chromosomes Cancer. 1998;23:361–366. doi: 10.1002/(sici)1098-2264(199812)23:4<361::aid-gcc12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Qiang W, Liu Z, Serna VA, Ann Druschitz S, Liu Y, Espona-Fiedler M, Wei JJ, Kurita T. Downregulation of miR-29b is essential for pathogenesis of uterine leiomyoma. Endocrinology. 2014;155:663–669. doi: 10.1210/en.2013-1763. en20131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM, Sung JJ, Lan HY. TGF-beta/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol. 2011;22:1462–1474. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roarty K, Rosen JM. Wnt and mammary stem cells: hormones cannot fly wingless. Curr Opin Pharmacol. 2010;10:643–649. doi: 10.1016/j.coph.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Rechavi M, Escriva Garcia H, Laudet V. The nuclear receptor superfamily. J Cell Sci. 2003;116:585–586. doi: 10.1242/jcs.00247. [DOI] [PubMed] [Google Scholar]
- Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- Roshdy E, Rajaratnam V, Maitra S, Sabry M, Allah AS, Al-Hendy A. Treatment of symptomatic uterine fibroids with green tea extract: a pilot randomized controlled clinical study. Int J Women's Health. 2013;5:477–486. doi: 10.2147/IJWH.S41021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabry M, Al-Hendy A. Innovative oral treatments of uterine leiomyoma. Obstet Gynecol Int. 2012a;2012:943635. doi: 10.1155/2012/943635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabry M, Al-Hendy A. Medical treatment of uterine leiomyoma. Reprod Sci. 2012b;19:339–353. doi: 10.1177/1933719111432867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Ohara N, Xu Q, Wang J, DeManno DA, Chwalisz K, Yoshida S, Maruo T. A novel selective progesterone receptor modulator asoprisnil activates tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated signaling pathway in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metabol. 2007;92:616–623. doi: 10.1210/jc.2006-0898. [DOI] [PubMed] [Google Scholar]
- Satterfield MC, Song G, Hayashi K, Bazer FW, Spencer TE. Progesterone regulation of the endometrial WNT system in the ovine uterus. Reprod Fertil Dev. 2008;20:935–946. doi: 10.1071/rd08069. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Shozu M, Sumitani H, Segawa T, Yang HJ, Murakami K, Kasai T, Inoue M. Overexpression of aromatase P450 in leiomyoma tissue is driven primarily through promoter I.4 of the aromatase P450 gene (CYP19) J Clin Endocrinol Metabol. 2002;87:2540–2548. doi: 10.1210/jcem.87.6.8533. [DOI] [PubMed] [Google Scholar]
- Shozu M, Murakami K, Segawa T, Kasai T, Inoue M. Successful treatment of a symptomatic uterine leiomyoma in a perimenopausal woman with a nonsteroidal aromatase inhibitor. Fertil Steril. 2003;79:628–631. doi: 10.1016/s0015-0282(02)04761-1. [DOI] [PubMed] [Google Scholar]
- Shozu M, Murakami K, Inoue M. Aromatase and leiomyoma of the uterus. Sem Reprod Med. 2004;22:51–60. doi: 10.1055/s-2004-823027. [DOI] [PubMed] [Google Scholar]
- Sinclair DC, Mastroyannis A, Taylor HS. Leiomyoma simultaneously impair endometrial BMP-2-mediated decidualization and anticoagulant expression through secretion of TGF-beta3. J Clin Endocrinol Metabol. 2011;96:412–421. doi: 10.1210/jc.2010-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz IM. Clinical utility of progesterone receptor modulators and their effect on the endometrium. Curr Opin Obstet Gynecol. 2009;21:318–324. doi: 10.1097/GCO.0b013e32832e07e8. [DOI] [PubMed] [Google Scholar]
- Sumitani H, Shozu M, Segawa T, Murakami K, Yang HJ, Shimada K, Inoue M. In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology. 2000;141:3852–3861. doi: 10.1210/endo.141.10.7719. [DOI] [PubMed] [Google Scholar]
- Szotek PP, Chang HL, Zhang L, Preffer F, Dombkowski D, Donahoe PK, Teixeira J. Adult mouse myometrial label-retaining cells divide in response to gonadotropin stimulation. Stem Cells. 2007;25:1317–1325. doi: 10.1634/stemcells.2006-0204. [DOI] [PubMed] [Google Scholar]
- Tal R, Segars JH. The role of angiogenic factors in fibroid pathogenesis: potential implications for future therapy. Hum Reprod Update. 2014;20:194–216. doi: 10.1093/humupd/dmt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaulikar VS, Manyonda IT. Ulipristal acetate: a novel option for the medical management of symptomatic uterine fibroids. Adv Therapy. 2012;29:655–663. doi: 10.1007/s12325-012-0042-8. [DOI] [PubMed] [Google Scholar]
- Tamaya T, Fujimoto J, Okada H. Comparison of cellular levels of steroid receptors in uterine leiomyoma and myometrium. Acta Obstet Gynecolo Scandin. 1985;64:307–309. doi: 10.3109/00016348509155136. [DOI] [PubMed] [Google Scholar]
- Tanwar PS, Lee HJ, Zhang L, Zukerberg LR, Taketo MM, Rueda BR, Teixeira JM. Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod. 2009;81:545–552. doi: 10.1095/biolreprod.108.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetel MJ, Giangrande PH, Leonhardt SA, McDonnell DP, Edwards DP. Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo. Mol Endocrinol. 1999;13:910–924. doi: 10.1210/mend.13.6.0300. [DOI] [PubMed] [Google Scholar]
- Townsend DE, Sparkes RS, Baluda MC, McClelland G. Unicellular histogenesis of uterine leiomyomas as determined by electrophoresis by glucose-6-phosphate dehydrogenase. Am J Obstet Gynecol. 1970;107:1168–1173. doi: 10.1016/s0002-9378(15)30365-3. [DOI] [PubMed] [Google Scholar]
- Tung L, Mohamed MK, Hoeffler JP, Takimoto GS, Horwitz KB. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol. 1993;7:1256–1265. doi: 10.1210/mend.7.10.8123133. [DOI] [PubMed] [Google Scholar]
- Tung L, Abdel-Hafiz H, Shen T, Harvell DM, Nitao LK, Richer JK, Sartorius CA, Takimoto GS, Horwitz KB. Progesterone receptors (PR)-B and -A regulate transcription by different mechanisms: AF-3 exerts regulatory control over coactivator binding to PR-B. Mol Endocrinol. 2006;20:2656–2670. doi: 10.1210/me.2006-0105. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas FK, Papanicolaou AN, Vavatsi-Christaki N, Makedos GA, Vlassis GD. The effect of anastrozole on symptomatic uterine leiomyomata. Obstet Gynecol. 2007;110:643–649. doi: 10.1097/01.AOG.0000279151.20878.60. [DOI] [PubMed] [Google Scholar]
- Vares G, Cui X, Wang B, Nakajima T, Nenoi M. Generation of breast cancer stem cells by steroid hormones in irradiated human mammary cell lines. PLoS One. 2013;8:e77124. doi: 10.1371/journal.pone.0077124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- Wang J, Ohara N, Wang Z, Chen W, Morikawa A, Sasaki H, DeManno DA, Chwalisz K, Maruo T. A novel selective progesterone receptor modulator asoprisnil (J867) down-regulates the expression of EGF, IGF-I, TGFbeta3 and their receptors in cultured uterine leiomyoma cells. Hum Reprod. 2006;21:1869–1877. doi: 10.1093/humrep/del035. [DOI] [PubMed] [Google Scholar]
- Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, Mittal K, Soteropoulos P, Wei JJ. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer. 2007;46:336–347. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- West CP, Lumsden MA, Lawson S, Williamson J, Baird DT. Shrinkage of uterine fibroids during therapy with goserelin (Zoladex): a luteinizing hormone-releasing hormone agonist administered as a monthly subcutaneous depot. Fertil Steril. 1987;48:45–51. doi: 10.1016/s0015-0282(16)59288-7. [DOI] [PubMed] [Google Scholar]
- Wilkens J, Chwalisz K, Han C, Walker J, Cameron IT, Ingamells S, Lawrence AC, Lumsden MA, Hapangama D, Williams AR, et al. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. J Clin Endocrinol Metabol. 2008;93:4664–4671. doi: 10.1210/jc.2008-1104. [DOI] [PubMed] [Google Scholar]
- Williams AR, Bergeron C, Barlow DH, Ferenczy A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Int J Gynecol Pathol. 2012;31:556–569. doi: 10.1097/PGP.0b013e318251035b. [DOI] [PubMed] [Google Scholar]
- Wilson EA, Yang F, Rees ED. Estradiol and progesterone binding in uterine leiomyomata and in normal uterine tissues. Obstet Gynecol. 1980;55:20–24. [PubMed] [Google Scholar]
- Xu Q, Takekida S, Ohara N, Chen W, Sitruk-Ware R, Johansson ED, Maruo T. Progesterone receptor modulator CDB-2914 down-regulates proliferative cell nuclear antigen and Bcl-2 protein expression and up-regulates caspase-3 and poly(adenosine 5′-diphosphate-ribose) polymerase expression in cultured human uterine leiomyoma cells. J Clin Endocrinol Metabol. 2005;90:953–961. doi: 10.1210/jc.2004-1569. [DOI] [PubMed] [Google Scholar]
- Xu Q, Ohara N, Chen W, Liu J, Sasaki H, Morikawa A, Sitruk-Ware R, Johansson ED, Maruo T. Progesterone receptor modulator CDB-2914 down-regulates vascular endothelial growth factor, adrenomedullin and their receptors and modulates progesterone receptor content in cultured human uterine leiomyoma cells. Hum Reprod. 2006;21:2408–2416. doi: 10.1093/humrep/del159. [DOI] [PubMed] [Google Scholar]
- Xu Q, Ohara N, Liu J, Nakabayashi K, DeManno D, Chwalisz K, Yoshida S, Maruo T. Selective progesterone receptor modulator asoprisnil induces endoplasmic reticulum stress in cultured human uterine leiomyoma cells. Am J Physiol Endocrinol Metabol. 2007;293:E1002–E1011. doi: 10.1152/ajpendo.00210.2007. [DOI] [PubMed] [Google Scholar]
- Xu Q, Ohara N, Liu J, Amano M, Sitruk-Ware R, Yoshida S, Maruo T. Progesterone receptor modulator CDB-2914 induces extracellular matrix metalloproteinase inducer in cultured human uterine leiomyoma cells. Mol Hum Reprod. 2008;14:181–191. doi: 10.1093/molehr/gan004. [DOI] [PubMed] [Google Scholar]
- Yin P, Lin Z, Reierstad S, Wu J, Ishikawa H, Marsh EE, Innes J, Cheng Y, Pearson K, Coon JSt, et al. Transcription factor KLF11 integrates progesterone receptor signaling and proliferation in uterine leiomyoma cells. Cancer Res. 2010;70:1722–1730. doi: 10.1158/0008-5472.CAN-09-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P, Navarro A, Fang F, Xie A, Coon JS, Richardson C, Bulun SE. Early growth response-2 expression in uterine leiomyoma cells: regulation and function. Fertil Steril. 2011;96:439–444. doi: 10.1016/j.fertnstert.2011.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Ohara N, Xu Q, Chen W, Wang J, Nakabayashi K, Sasaki H, Morikawa A, Maruo T. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Sem Reprod Med. 2010;28:260–273. doi: 10.1055/s-0030-1251483. [DOI] [PubMed] [Google Scholar]
- Zaitseva M, Vollenhoven BJ, Rogers PA. In vitro culture significantly alters gene expression profiles and reduces differences between myometrial and fibroid smooth muscle cells. Mol Hum Reprod. 2006;12:187–207. doi: 10.1093/molehr/gal018. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Kong Y, Li F. Kruppel-like transcription factor 11 (KLF11) overexpression inhibits cardiac hypertrophy and fibrosis in mice. Biochem Biophys Res Commun. 2014;443:683–688. doi: 10.1016/j.bbrc.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Zhou S, Yi T, Shen K, Zhang B, Huang F, Zhao X. Hypoxia: the driving force of uterine myometrial stem cells differentiation into leiomyoma cells. Med Hypotheses. 2011;77:985–986. doi: 10.1016/j.mehy.2011.08.026. [DOI] [PubMed] [Google Scholar]