Abstract
Background: animal studies suggest a neuroprotective role for leptin, but human studies have shown mixed results. We examined whether plasma leptin levels in individuals with mild cognitive impairment (MCI) were related to cognitive function at baseline and whether higher leptin levels were associated with reduced risk of dementia.
Methods: we categorised 352 MCI participants into sex-specific tertiles based on log-transformed fasting plasma leptin levels. In sex-stratified analyses, we investigated whether cognitive ability differed by leptin tertile. We also examined whether the risk of dementia over a 3-year follow-up period differed by leptin level. Analyses controlled for numerous potential confounding variables, including body mass index, hypertension and levels of blood insulin and C-reactive protein.
Results: baseline cognitive ability did not differ as a function of leptin level, nor were higher leptin levels associated with reduced hazard of developing dementia. Controlling for related co-variates did not reveal any significant associations between leptin and dementia risk.
Conclusion: in this cohort of older adults with MCI, plasma leptin level was not associated with cognitive function at baseline, nor did it predict risk of dementia. Other biological measures, such as volumetric MRI and cerebrospinal fluid protein levels, have demonstrated robust dementia prediction in this cohort. Thus, the current negative findings suggest that plasma leptin, on its own, is unlikely to become a useful clinical biomarker for Alzheimer's disease. Efforts to develop other blood-based biomarkers are needed.
Keywords: Alzheimer's disease, blood biomarker, MCI, dementia prediction, older people
Introduction
With the growing realisation that Alzheimer's disease (AD) pathology begins to accumulate years prior to the onset of dementia, there is strong interest in developing reliable tests to predict dementia risk. A blood-based test would be ideal for reasons of cost, convenience and availability. One potential serum measure that has received much recent attention is leptin, an adipocyte-secreted hormone involved in appetite regulation [1], and cognitive function (for review, see Ref. [2]). In animal models, leptin has been shown to promote synaptogenesis and neurogenesis in the hippocampus [3, 4] and to facilitate long-term potentiation—the physiologic basis of memory [5]. Leptin has been shown to be positively associated with memory performance [3] and to reverse cognitive impairment in mouse models of AD [6]. Leptin has also been shown to reduce amyloid and tau pathologies, the key pathologies of AD [7, 8]. Plasma leptin levels have thus been proposed as an early indicator of AD risk and as a potential therapeutic target [7, 9].
The association of leptin with cognitive performance in humans is not clear, however. Higher leptin levels have been associated with reduced risk of cognitive decline and dementia in some studies, but not in others [10–15]. Some studies have even reported an inverse association, with higher leptin levels associated with greater cognitive decline [15, 16]. Leptin levels have been reported to be lower in individuals with AD than in age-matched healthy controls and to correlate with disease severity in those with severe dementia [17]. However, since weight loss is common in AD [18], it is not clear whether differences in leptin levels precede or follow the onset of dementia. Few studies have examined the association of leptin with cognitive ability in individuals with mild cognitive impairment (MCI) who may be in a prodromal stage of the disorder. Here, we examined whether serum leptin levels are associated with cognitive function and dementia risk in a cohort of individuals with MCI in whom neuroimaging and CSF measures have demonstrated robust prediction of dementia risk [19–21].
Methods
Alzheimer's disease neuroimaging initiative
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI; http://adni.loni.usc.edu/). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data, but they did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf. ADNI was launched in 2003 by the National Institutes of Health, the Food and Drug Administration (FDA), private pharmaceutical companies and non-profit organisations, as a 5-year public/private partnership. ADNI's primary goal is the determination of sensitive and specific markers of very early AD progression to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials. ADNI is the result of efforts of many co-investigators from a range of academic institutions and private corporations. Participants have been recruited from over 50 sites across the United States and Canada. Participants with MCI were reassessed at ∼6-month intervals for 3 years. For further information, see www.adni-info.org.
Participants
We analysed data from 352 MCI participants who had had blood drawn for analysis of plasma proteins potentially associated with AD risk and who underwent cognitive testing and structural imaging at baseline. The criteria for a diagnosis of MCI included Mini-Mental State Exam score between 24 and 30, subjective memory complaint corroborated by an informant, objective memory loss, a Clinical Dementia Rating scale (CDR) score of 0.5, preserved activities of daily living and absence of dementia. Further details of ADNI's inclusion/exclusion criteria can be found at http://adni.loni.usc.edu/wp-content/uploads/2010/09/ADNI_GeneralProceduresManual.pdf
The research protocol was approved by each local institutional review board, and written informed consent was obtained from each participant or participant's guardian.
Plasma proteins
Plasma samples were obtained in the morning following an overnight fast. Samples were frozen, most within 120 min of collection. Plasma proteins were assessed with the Human Discovery Multi-Analyte Profile panel developed by Rules-Based Medicine (RBM, Austin, TX, USA) on the Luminex xMAP platform. Details of the quantification of plasma proteins are available at http://adni.loni.usc.edu/wp-content/uploads/2010/11/BC_Plasma_Proteomics_Data_Primer.pdf. We downloaded the quality-controlled plasma measures from the public ADNI website. As part of quality control, measures that were not normally distributed, such as leptin, were log-transformed. Log-transformed insulin and C-reactive protein (CRP) levels were included as co-variates in adjusted analyses.
Assessment of cognitive function
A battery of cognitive function tests were administered to ADNI participants. We focus here on measures that are particularly vulnerable to early AD, including global measures of function (the Clinical Dementia Rating Scale, Sum of Boxes Score, CDR-SB) and cognition (the Alzheimer's Disease Assessment Scale, Cognitive Subscale, ADAS-Cog). We also included measures of learning and memory assessed with the Rey Auditory Learning Verbal Test (AVLT). Learning was assessed as the sum of the correct responses across the five AVLT learning trails; recall was assessed as the number of words correctly recalled after a 20-min delay.
Statistical analysis
Since prior evidence suggests that leptin may not be linearly related to cognitive function [11], we categorised participants into sex-specific leptin tertiles. We used analyses of co-variance (ANCOVAs) to examine differences in demographics, clinical measures and neuropsychological task performance across leptin tertile groups. Since leptin levels differ by sex, we analysed data from men and women separately. Since cognitive performance is affected by age and education, we controlled for these effects by including age and education as co-variates. In secondary analyses, we examined whether control for additional factors plausibly related to leptin or cognitive function affected the results by including, in separate ANCOVAs, body mass index (BMI), hypertension as assessed by the use of any antihypertensive medication, CRP and insulin levels as co-variates. To explore whether the association of leptin with cognitive performance differed by obesity [13, 14], we classified participants into normal weight (BMI < 25) and overweight groups (BMI ≥ 25) and included BMI group as an interaction term in the models.
We examined risk of developing dementia over 3 years across leptin tertiles using Cox regression models, with age as a co-variate. Hazard ratios (HRs) of developing dementia were calculated for the two lowest leptin tertiles relative to the highest tertile. In secondary analyses, we examined whether inclusion of any of the co-variates described above (BMI, hypertension, CRP and insulin level) affected the results of the Cox regression analyses.
To determine whether results differed when leptin was treated as a continuous rather than categorical variable, analyses were repeated treating leptin as a continuous measure. These analyses used linear regression models and included the same co-variates as described above.
Results
Participant characteristics
In this sample of 222 men and 128 women, leptin levels were higher in women than in men (F(1,347) = 209; P < 0.001; controlling for age and BMI) and strongly correlated with BMI (Pearson's partial r = 0.63; P < 0.001; controlling for sex and age) and insulin levels (Pearson's partial r = 0.43; P < 0.001 controlling for sex and age). More men than women in this study were overweight (χ2 = 9.15; P = 0.002; 63.8% of men and 47.2% of women had a BMI ≥ 25), and more men than women were taking antihypertensive medications (χ2 = 16.66; P = 0.001; 54.9% men and 48.4% women).
Table 1 shows participant demographics as a function of sex-specific leptin tertiles. Age and education levels did not differ across leptin levels for men or women. As expected, BMI increased with increasing leptin level, as did insulin levels. In men, the proportion of participants being treated for hypertension increased with increasing leptin level. For women, this effect approached significance. In women, CRP level increased with increasing leptin tertile; in men, CRP level did not differ across leptin tertiles.
Table 1.
Demographic and clinical characteristics of participants by sex-specific leptin tertiles
| Leptin tertile | T1 | T2 | T3 | Significance, P |
|---|---|---|---|---|
| Men (N) | 75 | 75 | 74 | |
| Age | 75.45 (7.48) | 75.17 (7.36) | 75.36 (7.22) | 0.972 |
| Education | 16.39 (3.22) | 15.85 (2.79) | 15.35 (3.09) | 0.117 |
| BMI | 24.12 (2.59) | 26.16 (2.75) | 29.08 (3.46) | <0.001 |
| Hypertension (%) | 40.00 | 52.00 | 72.97 | 0.001 |
| Log CRP (µg/ml) | −0.01 (0.55) | 0.04 (0.48) | 0.14 (0.50) | 0.267 |
| Log insulin (µIU/ml) | 0.16 (0.36) | 0.33 (0.25) | 0.47 (0.27) | <0.001 |
| Log leptin (ng/ml) | 0.39 (0.23) | 0.79 (0.08) | 1.15 (0.15) | – |
| Women (N) | 42 | 45 | 41 | |
| Age | 73.26 (6.76) | 74.09 (8.13) | 73.08 (7.73) | 0.803 |
| Education | 16.19 (2.44) | 14.71 (3.38) | 15.17 (3.04) | 0.067 |
| BMI | 22.46 (3.35) | 24.29 (2.36) | 28.90 (3.61) | <0.001 |
| Hypertension (%) | 38.09 | 46.66 | 60.97 | 0.109 |
| Log CRP (µg/ml) | −0.10 (0.56) | 0.15 (0.47) | 0.40 (0.40) | <0.001 |
| Log insulin (µIU/ml) | 0.17 (0.27) | 0.33 (0.28) | 0.47 (0.27) | <0.001 |
| Log leptin (ng/ml) | 0.74 (0.21) | 1.17 (0.11) | 1.56 (0.16) | – |
Unless otherwise indicated, values are mean (standard deviation).
T, leptin tertile; tertiles are not equal due to tied values.
Association of leptin with cognitive function
The relation of leptin tertiles to measures of global cognitive (ADAS-Cog) and functional (CDR-SB) impairment, learning and recall is shown in Table 2. In primary analyses, with age and education as co-variates in the ANCOVA, the only significant association to emerge was for ADAS-Cog score in men (P = 0.047). Men with leptin levels in the highest tertile showed less impairment than men with leptin levels in the two lower tertiles. However, with BMI included as a co-variate, ADAS-Cog score did not differ across leptin levels (P = 0.271). For women, none of the test scores showed significant differences across leptin tertiles, with or without control for additional co-variates. Inclusion of body weight (normal versus overweight) as an interaction term revealed no significant interactions of leptin with body weight for any outcome measure, in men or women. Treating leptin as a continuous rather than a categorical variable did not reveal any significant associations between leptin level and any outcome measure for men or women.
Table 2.
Mean (standard deviation) scores on clinical and cognitive tests across sex-specific leptin tertiles (T)
| T1 | T2 | T3 | Significance, P | |
|---|---|---|---|---|
| Men | ||||
| CDR_SB | 1.62 (0.93) | 1.45 (0.93) | 1.65 (0.93) | 0.349 |
| ADAS-cog | 12.15 (4.07) | 11.99 (4.05) | 10.63 (4.07) | 0.047 |
| Learning | 28.82 (7.99) | 29.55 (7.93) | 29.69 (7.96) | 0.777 |
| Delayed recall | 2.26 (2.99) | 2.49 (2.97) | 3.091 (2.99) | 0.224 |
| Women | ||||
| CDR_SB | 1.54 (0.83) | 1.57 (0.83) | 1.72 (0.81) | 0.554 |
| ADAS-cog | 11.24 (4.77) | 12.21 (4.75) | 11.08 (4.72) | 0.492 |
| Learning | 34.57 (9.94) | 32.11 (9.89) | 32.01 (9.82) | 0.415 |
| Delayed recall | 3.29 (3.83) | 3.22 (3.82) | 3.20 (3.79) | 0.995 |
Analyses included age and education level as co-variates.
CDR-SB, Clinical Dementia Rating Scale, Sum of Boxes; ADAS-Cog, Cognitive Subscale of the Alzheimer's Disease Assessment Scale; T, leptin tertile.
Association of leptin with 3-year risk of dementia
Over the 3-year follow-up period, 38% of men and 45% of women with MCI developed dementia. In men, 35% of those in the lowest leptin tertile, 30% of those in the middle tertile and 38% of those in the highest tertile developed dementia. In women, the corresponding percentages were 60, 40 and 37%. HRs of developing dementia with lower leptin levels were not significant in men or women (Figure 1). The HR of developing dementia was 0.90 (95% CI = 0.48–1.70) for men with leptin levels in the lowest tertile, and HR = 0.95 (0.54–1.65) for men with leptin levels in the second tertile, relative to the highest tertile. For women, the corresponding HRs were 1.71 (0.74–3.95) and 1.25, (0.56–2.77). Including additional co-variates in the Cox regression analyses did not yield significant findings, nor did treating leptin as a continuous rather than a categorical variable.
Figure 1.
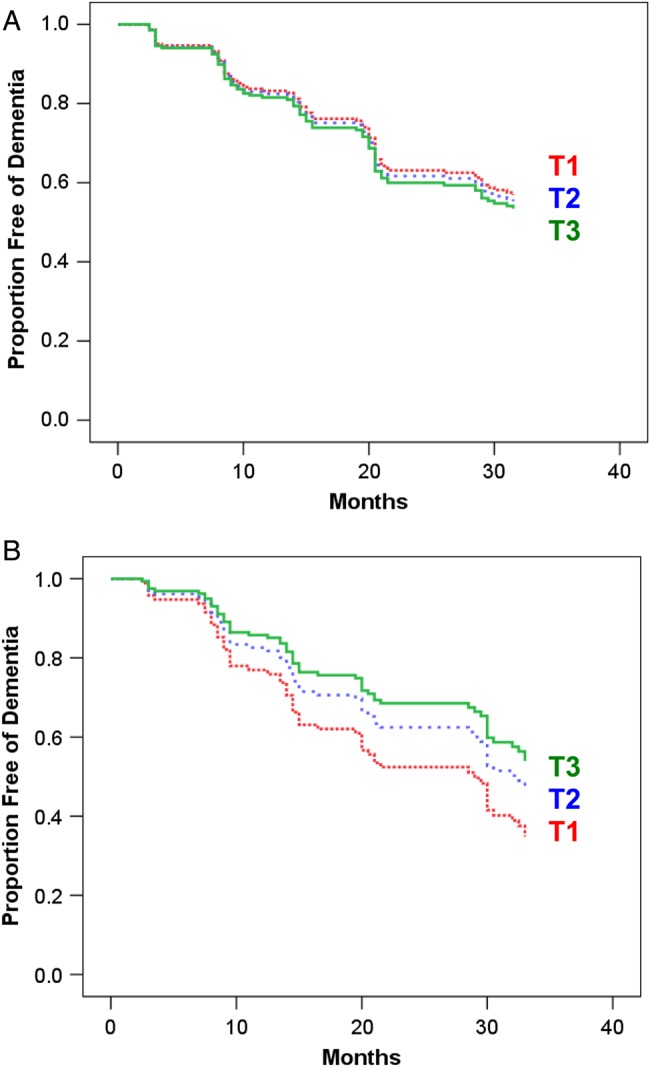
Survival curves from the Cox regression analysis showing risk of developing dementia over the 3-year follow-up period by sex-specific leptin tertile (T) for men (A) and women (B). Analyses controlled for age and BMI. There were no significant differences in hazard of developing dementia across leptin tertiles.
Discussion
We examined the relation of baseline serum leptin levels to measures of cognitive impairment at baseline and with risk of decline to dementia among individuals with MCI. Leptin levels were weakly associated with general cognitive function at baseline in men, as evidenced by better performance on the ADAS-Cog in men with the highest level of leptin relative to those with lower levels. However, this effect was not independent of BMI. Leptin levels did not predict risk of dementia over the 3-year follow-up period in either men or women. Thus, unlike several other biological measures that have been found to significantly predict dementia in this cohort, such as structural brain atrophy, regional cerebral hypometabolism, amyloid and tau levels in cerebrospinal fluid [19, 20, 22, 23], serum leptin levels do not appear to be a robust measure, on their own, for identifying those individuals with MCI who are at a highest risk of developing dementia.
Despite evidence from animal studies that higher leptin levels are associated with better memory task performance [3, 6], evidence that leptin protects against cognitive decline in humans has been less consistent. Higher plasma leptin levels were associated with reduced risk of cognitive decline over a 4-year period in one study of older adults [11] and with reduced risk of AD over an 8-year period in another study [10]. In two other studies, an association of high leptin level with reduced risk of dementia or cognitive decline was found only among normal weight but not among overweight individuals [13, 14]. A study of middle-aged women found that mid-life leptin levels were not associated with risk of dementia over a 32-year follow-up [12]. A study of middle-aged men and women reported that leptin levels were not associated with cognitive performance in women, but showed an inconsistent relation with cognitive performance in men, with high leptin levels associated with better cognitive performance 8 years later in white men but with worse cognitive performance 8 years later in black men [15]. A large study of older adults with Type II diabetes found that higher leptin levels were associated with worse cognitive function in men, but they were not associated with cognitive function in women [16]. Thus, across studies, the association of leptin with cognitive function or risk of dementia varied by age, sex, race and health status.
Since leptin is secreted in proportion to adipose mass, leptin levels are positively correlated with obesity and with obesity-related health conditions, such as cardiovascular and metabolic diseases. The prevalence of cardiovascular and metabolic diseases varies by age, race and sex. Many cardiovascular and metabolic risk factors have themselves been found to relate to cognitive impairment and dementia risk [24–26]. There is thus strong potential for these risk factors to mask any beneficial effect of leptin on cognition. Although most studies control some of these risk factors, it is not possible to control all potentially related factors. The variability in the co-variates included in the analyses and in the prevalence of these diseases across study samples likely contributes to the inconsistent results across studies and within study subsamples.
Another factor that makes it difficult to assess the relation of serum leptin to cognitive function in humans, and that is likely to contribute to discrepancies across studies, is leptin resistance. Leptin is transported into the brain through a saturable mechanism involving triglycerides [27, 28]. In conditions associated with high-circulating leptin levels, such as obesity, leptin transport across the blood–brain barrier is reduced [28]. Within the central nervous system, leptin resistance arises from reduction of leptin receptors and impaired leptin signalling [2]. Obesity, age and oestrogen insufficiency have all been associated with central leptin resistance [29]. Thus, the relation between peripheral leptin levels and central leptin activity likely differs by age, sex and disease states. We found that leptin levels were positively correlated with CRP in women but not in men, a finding that has been reported previously [30]. CRP binds to leptin, potentially contributing to leptin resistance [30], and this may contribute to the sex difference observed here in the relation of leptin to baseline cognitive function. The phenomenon of leptin resistance poses challenges for the viability of therapies aimed at increasing leptin levels to prevent or treat AD.
A strength of this study is the use of a cohort that was highly selected to increase the probability that participants are in a prodromal stage of AD, allowing us to examine the predictive ability of leptin in a population at high risk for dementia, and for whom the predictive ability of other biological measures has been assessed. However, the sample size is relatively small compared with larger population-based studies, and the length of follow-up is limited. Given the findings of a weak association of leptin level with baseline cognitive function in men, and an orderly, albeit insignificant, increase in HR of developing dementia with decreasing leptin level in women, it is possible that a study with a larger sample size and longer follow-up would have observed significant effects. It is also possible that the combination of leptin with other blood biochemical measures would show greater association with baseline cognitive impairment and greater ability to predict dementia risk.
Nevertheless, the results of this study, along with the inconsistencies of results across prior studies, suggest that plasma leptin levels are not useful, on their own, for predicting dementia risk in older adults. Further efforts to develop other blood-based tests of AD, potentially involving combinations of measures, are needed.
Key points.
Neuroimaging and cerebrospinal fluid biomarkers exist for predicting dementia risk in older adults with MCI.
A blood-based test of dementia risk would be more economical and have greater availability.
Evidence suggests that leptin may facilitate cognitive function and may play a neuroprotective role in AD.
Serum leptin levels in older adults with MCI were weakly associated with cognitive ability at baseline in men but not in women.
High serum leptin levels were not associated with reduced risk of dementia in patients with MCI.
Conflicts of interest
None declared.
Funding
L.K.M. was supported by National Institute on Aging (NIA) NIA K01AG029218 and National Institute on Alcohol Abuse and Alcoholism (NIAAA) NIAAA R01AA021187. R.O. was supported by NIA T35 grant AG26757. Data collection and sharing for this project were funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering and through generous contributions from the following: Alzheimer's Association; Alzheimer's Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc. and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organisation is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. This research was also supported by NIH grants P30 AG010129 and K01 AG030514.
Authors’ Contributions
R.O. and L.K.M. jointly conceived the study. R.O. analysed the data and drafted the manuscript. L.K.M. oversaw data analysis and edited the manuscript for scientific content.
References
- 1.Kalra SP, Kalra PS. Neuroendocrine control of energy homeostasis: update on new insights. Prog Brain Res. 2010;181:17–33. doi: 10.1016/S0079-6123(08)81002-3. [DOI] [PubMed] [Google Scholar]
- 2.Fadel JR, Jolivalt CG, Reagan LP. Food for thought: the role of appetitive peptides in age-related cognitive decline. Ageing Res Rev. 2013;12:764–76. doi: 10.1016/j.arr.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–5. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Oomura Y, Hori N, Shiraishi T, et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–49. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greco SJ, Bryan KJ, Sarkar S, et al. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis. 2010;19:1155–67. doi: 10.3233/JAD-2010-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tezapsidis N, Johnston JM, Smith MA, et al. Leptin: a novel therapeutic strategy for Alzheimer's disease. J Alzheimers Dis. 2009;16:731–40. doi: 10.3233/JAD-2009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Gonzalez R, Antequera D, Vargas T, Spuch C, Bolos M, Carro E. Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2011;24(Suppl. 2):17–25. doi: 10.3233/JAD-2011-102070. [DOI] [PubMed] [Google Scholar]
- 9.Johnston JM, Hu WT, Fardo DW, et al. Low plasma leptin in cognitively impaired ADNI subjects—Gender differences and diagnostic and therapeutic potential. Curr Alzheimer Res. 2014;11:165–74. doi: 10.2174/1567205010666131212114156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieb W, Beiser AS, Vasan RS, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2565–72. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly: findings from the Health ABC Study. Neurobiol Aging. 2009;30:1483–9. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gustafson DR, Backman K, Lissner L, et al. Leptin and dementia over 32 years-The Prospective Population Study of Women. Alzheimers Dement. 2012;8:272–7. doi: 10.1016/j.jalz.2011.05.2411. [DOI] [PubMed] [Google Scholar]
- 13.Zeki Al Hazzouri A, Haan MN, Whitmer RA, Yaffe K, Neuhaus J. Central obesity, leptin and cognitive decline: the Sacramento Area Latino Study on Aging. Dement Geriatr Cogn Disord. 2012;33:400–9. doi: 10.1159/000339957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeki Al Hazzouri A, Stone KL, Haan MN, Yaffe K. Leptin, mild cognitive impairment, and dementia among elderly women. J Gerontol A Biol Sci Med Sci. 2013;68:175–80. doi: 10.1093/gerona/gls155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren MW, Hynan LS, Weiner MF. Leptin and cognition. Dement Geriatr Cogn Disord. 2012;33:410–5. doi: 10.1159/000339956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labad J, Price JF, Strachan MW, et al. Serum leptin and cognitive function in people with type 2 diabetes. Neurobiol Aging. 2012;33:2938–41. doi: 10.1016/j.neurobiolaging.2012.02.026. e2. [DOI] [PubMed] [Google Scholar]
- 17.Khemka VK, Bagchi D, Bandyopadhyay K, et al. Altered serum levels of adipokines and insulin in probable Alzheimer's disease. J Alzheimers Dis. 2014;41:525–33. doi: 10.3233/JAD-140006. [DOI] [PubMed] [Google Scholar]
- 18.Gillette-Guyonnet S, Nourhashemi F, Andrieu S, et al. Weight loss in Alzheimer disease. Am J Clin Nutr. 2000;71:637S–42S. doi: 10.1093/ajcn/71.2.637s. [DOI] [PubMed] [Google Scholar]
- 19.Landau SM, Harvey D, Madiso CM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–8. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heister D, Brewer JB, Magda S, Blennow K, McEvoy LK. Predicting MCI outcome with clinically available MRI and CSF biomarkers. Neurology. 2011;77:1619–28. doi: 10.1212/WNL.0b013e3182343314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology. 2009;73:294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol. 2011;121:597–609. doi: 10.1007/s00401-011-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerola T, Kettunen R, Nieminen T. The complex interplay of cardiovascular system and cognition: how to predict dementia in the elderly? Int J Cardiol. 2011;150:123–9. doi: 10.1016/j.ijcard.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Laughlin GA, McEvoy LK, von Muhlen D, et al. Sex differences in the association of Framingham Cardiac Risk Score with cognitive decline in community-dwelling elders without clinical heart disease. Psychosom Med. 2011;73:683–9. doi: 10.1097/PSY.0b013e31822f9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McEvoy LK, Laughlin GA, Barrett-Connor E, et al. Metabolic syndrome and 16-year cognitive decline in community-dwelling older adults. Ann Epidemiol. 2012;22:310–7. doi: 10.1016/j.annepidem.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2:589–93. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 28.Banks WA, Coon AB, Robinson SM, et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–60. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 29.Scarpace PJ, Matheny M, Shek EW. Impaired leptin signal transduction with age-related obesity. Neuropharmacology. 2000;39:1872–9. doi: 10.1016/s0028-3908(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 30.Abdullah SM, Khera A, Leonard D, et al. Sex differences in the association between leptin and CRP: results from the Dallas Heart Study. Atherosclerosis. 2007;195:404–10. doi: 10.1016/j.atherosclerosis.2006.10.022. [DOI] [PubMed] [Google Scholar]


