Abstract
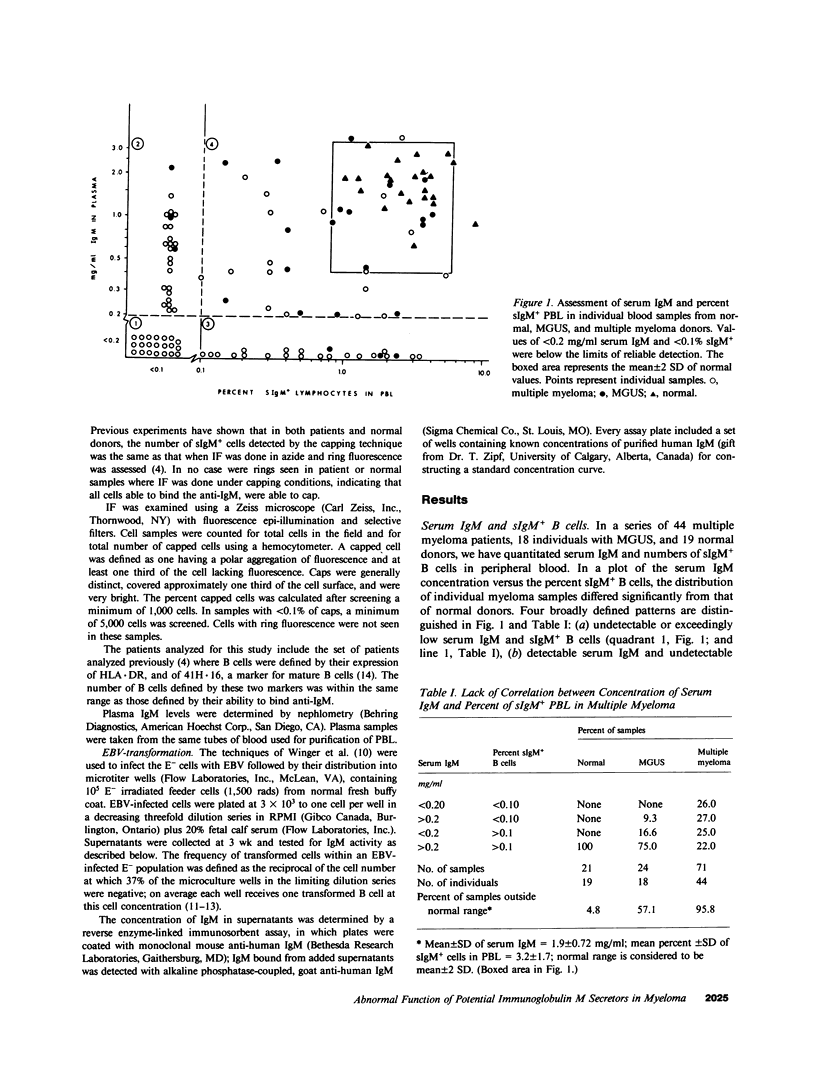
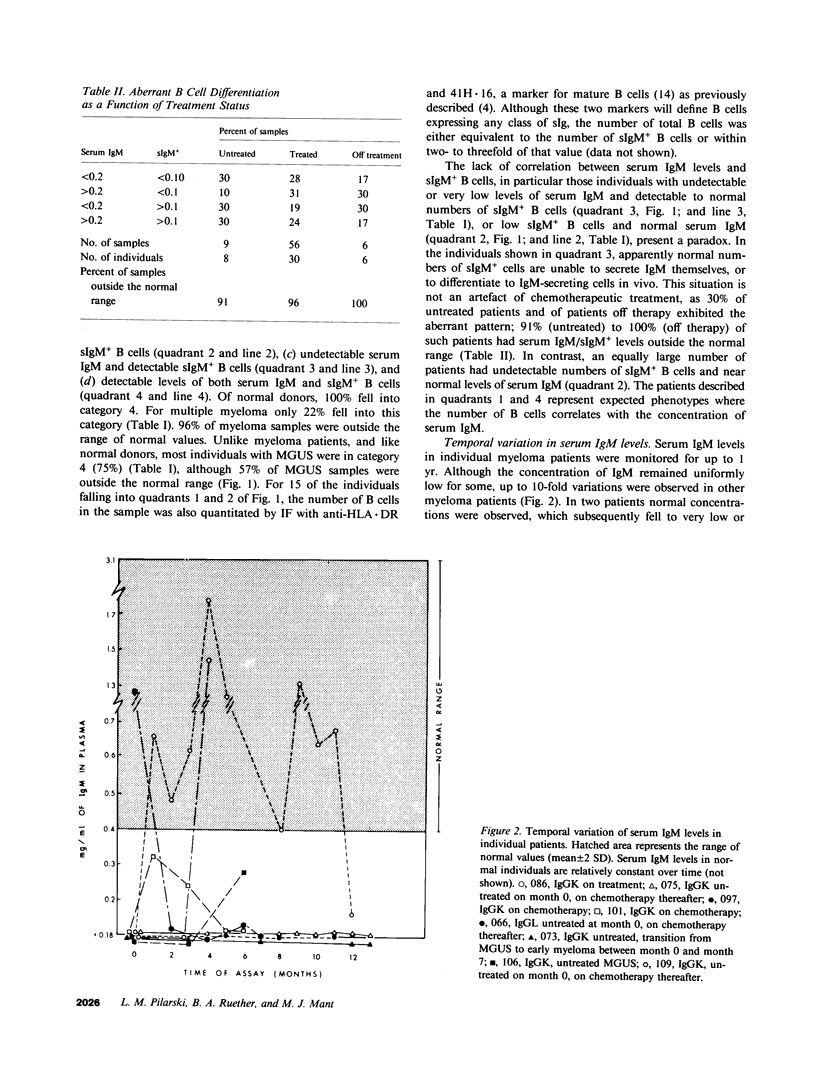
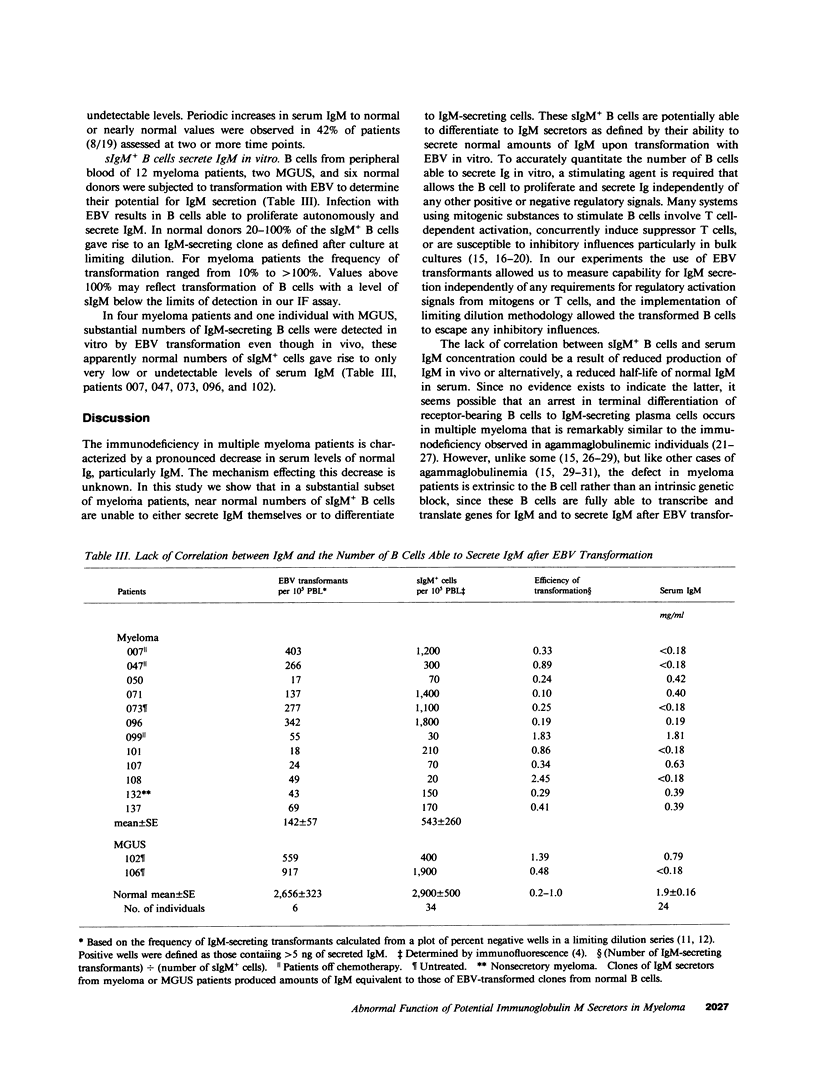
Multiple myeloma patients are deficient in normal polyclonal serum immunoglobulins. To determine the reasons for this decrease, we quantitated and compared the number of surface IgM+ B lymphocytes, and the number of B cells susceptible to transformation by Epstein-Barr virus (EBV) with the concentration of IgM in serum. Serum IgM levels varied considerably in individual patients, temporally shifting from undetectable to normal amounts and then dropping again to undetectable levels. A transient rise to normal serum IgM concentrations was seen in 42% of patients assessed at two or more time points. Of 44 patients, 52% showed a lack of correlation between the number of surface IgM+ (sIgM+) B cells and serum IgM concentration. One subset of patients (25%) had detectable to normal numbers of sIgM+ B cells in blood but undetectable levels of serum IgM. Transformation of B cells from these patients indicated a block in IgM secretion that was extrinsic to the B cells that were fully able to transcribe, translate, and secrete IgM after EBV transformation. A second subset of patients (27%) had undetectable numbers of sIgM+ B cells but near normal levels of serum IgM, suggesting abundant secretion by few clones of B cells. Of 18 patients with monoclonal gammopathy of undetermined significance (MGUS), 26% showed a lack of correlation between the numbers of sIgM+ B cells and serum IgM concentration. We suggest that in patients with multiple myeloma, and in some with MGUS, there exists a mechanism(s) extrinsic to the B cell that mediates an arrest in terminal B lymphocyte maturation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Abdou N. L. The monoclonal nature of lymphocytes in multiple myeloma. Effects of therapy. Ann Intern Med. 1975 Jul;83(1):42–45. doi: 10.7326/0003-4819-83-1-42. [DOI] [PubMed] [Google Scholar]
- Bona C., Broder S., Dimitriu A., Waldmann T. A. Polyclonal activation of human B lymphocytes by Nocardia water soluble mitogen (NWSM). Immunol Rev. 1979;45:69–92. doi: 10.1111/j.1600-065x.1979.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Lawton A. R., Bockman D. E. Agammaglobulinaemia with B lymphocytes. Specific defect of plasma-cell differentiation. Lancet. 1971 Oct 9;2(7728):791–794. doi: 10.1016/s0140-6736(71)92742-5. [DOI] [PubMed] [Google Scholar]
- Denis K. A., Wall R., Saxon A. Human-human B cell hybridomas from in vitro stimulated lymphocytes of patients with common variable immunodeficiency. J Immunol. 1983 Nov;131(5):2273–2278. [PubMed] [Google Scholar]
- Dosch H. M., Percy M. E., Gelfand E. W. Functional differentiation of B lymphocytes in congenital agammaglobulinemia. I. Generation of hemolytic plaque-forming cells. J Immunol. 1977 Dec;119(6):1959–1964. [PubMed] [Google Scholar]
- Dwyer J. M. Identifying and enumerating human T and B lymphocytes. A review of techniques, problems, and progress in clinical studies. Prog Allergy. 1976;21:178–260. [PubMed] [Google Scholar]
- Falkoff R. J., Zhu L. P., Fauci A. S. Separate signals for human B cell proliferation and differentiation in response to Staphylococcus aureus: evidence for a two-signal model of B cell activation. J Immunol. 1982 Jul;129(1):97–102. [PubMed] [Google Scholar]
- Fleisher T. A., Greene W. C., Uchiyama T., Goldman C. K., Nelson D. L., Blaese R. M., Waldmann T. A. Characterization of a soluble suppressor of human B cell immunoglobulin biosynthesis produced by a continuous human suppressor T cell line. J Exp Med. 1981 Jul 1;154(1):156–167. doi: 10.1084/jem.154.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajl-Peczalska K. J., Park B. H., Biggar W. D., Good R. A. B and T lymphocytes in primary immunodeficiency disease in man. J Clin Invest. 1973 Apr;52(4):919–928. doi: 10.1172/JCI107257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblum R. M., Lord R. A., Cooper M. D., Gathings W. E., Goldman A. S. X-linked B lymphocyte deficiency. I. Panhypo-gamma-globulinemia and dys-gamma-globulinemia in siblings. J Pediatr. 1974 Aug;85(2):188–191. doi: 10.1016/s0022-3476(74)80390-2. [DOI] [PubMed] [Google Scholar]
- Gordon J., Holden H. T., Segal S., Feldman M. Anti-tumor immunity in B-lymphocyte-deprived mice. III. Immunity to primary Moloney sarcoma virus-induced tumors. Int J Cancer. 1982 Mar 15;29(3):351–357. doi: 10.1002/ijc.2910290320. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Rabellino E., Pirofsky B. Immunoglobulins on the surface of lymphocytes. IV. Distribution in hypogammaglobulinemia, cellular immune deficiency, and chronic lymphatic leukemia. J Clin Invest. 1971 Nov;50(11):2368–2375. doi: 10.1172/JCI106735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshgegian A. A. Suppression of one monoclonal immunoglobulin in the presence of another in multiple myeloma. Evidence for benign B-cell neoplasia. Cancer. 1983 Mar 15;51(6):1097–1100. doi: 10.1002/1097-0142(19830315)51:6<1097::aid-cncr2820510621>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Self K. S., Cooper M. D. Survival and function of bursa-derived cells in bursectomized chickens. Cell Immunol. 1973 Jul;8(1):93–102. doi: 10.1016/0008-8749(73)90096-8. [DOI] [PubMed] [Google Scholar]
- Kubagawa H., Vogler L. B., Capra J. D., Conrad M. E., Lawton A. R., Cooper M. D. Studies on the clonal origin of multiple myeloma. Use of individually specific (idiotype) antibodies to trace the oncogenic event to its earliest point of expression in B-cell differentiation. J Exp Med. 1979 Oct 1;150(4):792–807. doi: 10.1084/jem.150.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström F. D., Hardy W. R., Eberle B. J., Williams R. C., Jr Multiple myeloma and benign monoclonal gammopathy: differentiation by immunofluorescence of lymphocytes. Ann Intern Med. 1973 Jun;78(6):837–844. doi: 10.7326/0003-4819-78-6-837. [DOI] [PubMed] [Google Scholar]
- Louie S., Daoust P. R., Schwartz R. S. Immunodeficiency and the pathogenesis of non-Hodgkin's lymphoma. Semin Oncol. 1980 Sep;7(3):267–284. [PubMed] [Google Scholar]
- Martínez-Maza O., Britton S. Frequencies of the separate human B cell subsets activatable to Ig secretion by Epstein-Barr virus and pokeweed mitogen. J Exp Med. 1983 Jun 1;157(6):1808–1814. doi: 10.1084/jem.157.6.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayumi M., Kuritani T., Kubagawa H., Cooper M. D. IgG subclass expression by human B lymphocytes and plasma cells: B lymphocytes precommitted to IgG subclass can be preferentially induced by polyclonal mitogens with T cell help. J Immunol. 1983 Feb;130(2):671–677. [PubMed] [Google Scholar]
- Mitsuya H., Osaki K., Tomino S., Katsuki T., Kishimoto S. Pathophysiologic analysis of peripheral blood lymphocytes from patients with primary immunodeficiency. I. Ig synthesis by peripheral blood lymphocytes stimulated with either pokeweed mitogen or Epstein-Barr virus in vitro. J Immunol. 1981 Jul;127(1):311–315. [PubMed] [Google Scholar]
- Peest D., Brunkhorst U., Schedel I., Deicher H. In vitro immunoglobulin production by peripheral blood mononuclear cells from multiple myeloma patients and patients with benign monoclonal gammopathy. Regulation by cell subsets. Scand J Immunol. 1984 Feb;19(2):149–157. doi: 10.1111/j.1365-3083.1984.tb00911.x. [DOI] [PubMed] [Google Scholar]
- Perri R. T., Oken M. M., Kay N. E. Enhanced T cell suppression is directed toward sensitive circulating B cells in multiple myeloma. J Lab Clin Med. 1982 Apr;99(4):512–519. [PubMed] [Google Scholar]
- Pilarski L. M., Mant M. J., Ruether B. A., Belch A. Severe deficiency of B lymphocytes in peripheral blood from multiple myeloma patients. J Clin Invest. 1984 Oct;74(4):1301–1306. doi: 10.1172/JCI111540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preud'homme J. L., Seligmann M. Primary immunodeficiency with increased numbers of circulating B lymphocytes contrasting with hypogammaglobulinaemia. Lancet. 1972 Feb 19;1(7747):442–442. doi: 10.1016/s0140-6736(72)90898-7. [DOI] [PubMed] [Google Scholar]
- Pruzanski W., Gidon M. S., Roy A. Suppression of polyclonal immunoglobulins in multiple myeloma: relationship to the staging and other manifestations at diagnosis. Clin Immunol Immunopathol. 1980 Oct;17(2):280–286. doi: 10.1016/0090-1229(80)90097-5. [DOI] [PubMed] [Google Scholar]
- Schwaber J. F., Klein G., Ernberg I., Rosen A., Lazarus H., Rosen F. S. Deficiency of Epstein-Barr virus (EBV) receptors on B lymphocytes from certain patients with common varied agammaglobulinemia. J Immunol. 1980 May;124(5):2191–2196. [PubMed] [Google Scholar]
- Spengler G. A., Steinberg A. G., Skvaril F. Development of a second monoclonal immunoglobulin G in a patient with late manifestation of myeloma. Acta Med Scand. 1972 Oct;192(4):309–314. doi: 10.1111/j.0954-6820.1972.tb04820.x. [DOI] [PubMed] [Google Scholar]
- Stein L. D., Ledgley C. J., Sigal N. H. Patterns of isotype commitment in human B cells: limiting dilution analysis of Epstein Barr virus-infected cells. J Immunol. 1983 Apr;130(4):1640–1645. [PubMed] [Google Scholar]
- Turesson I. Distribution of immunoglobulin-containing cells in bone marrow and lymphoid tissues in patients with monoclonal gammapathy. Acta Med Scand. 1978;203(4):247–255. doi: 10.1111/j.0954-6820.1978.tb14868.x. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Winger L., Winger C., Shastry P., Russell A., Longenecker M. Efficient generation in vitro, from human peripheral blood cells, of monoclonal Epstein-Barr virus transformants producing specific antibody to a variety of antigens without prior deliberate immunization. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4484–4488. doi: 10.1073/pnas.80.14.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachie A., Miyawaki T., Yokoi T., Nagaoki T., Taniguchi N. Ia-positive cells generated by PWM-stimulation within OKT4+ subset interact with OKT8+ cells for inducing active suppression on B cell differentiation in vitro. J Immunol. 1982 Jul;129(1):103–106. [PubMed] [Google Scholar]
- Yarchoan R., Tosato G., Blaese R. M., Simon R. M., Nelson D. L. Limiting dilution analysis of Epstein-Barr virus-induced immunoglobulin production by human B cells. J Exp Med. 1983 Jan 1;157(1):1–14. doi: 10.1084/jem.157.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipf T. F., Lauzon G. J., Longenecker B. M. A monoclonal antibody detecting a 39,000 m.w. molecule that is present on B lymphocytes and chronic lymphocytic leukemia cells but is rare on acute lymphocytic leukemia blasts. J Immunol. 1983 Dec;131(6):3064–3072. [PubMed] [Google Scholar]
- de la Concha E. G., Oldham G., Webster A. D., Asherson G. L., Platts-Mills T. A. Quantitative measurements of T- and B-cell function in "variable" primary hypogammaglobulinaemia: evidence for a consistent B-cell defect. Clin Exp Immunol. 1977 Feb;27(2):208–215. [PMC free article] [PubMed] [Google Scholar]