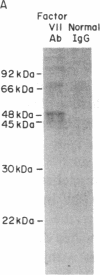
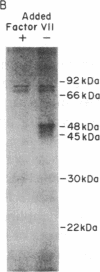
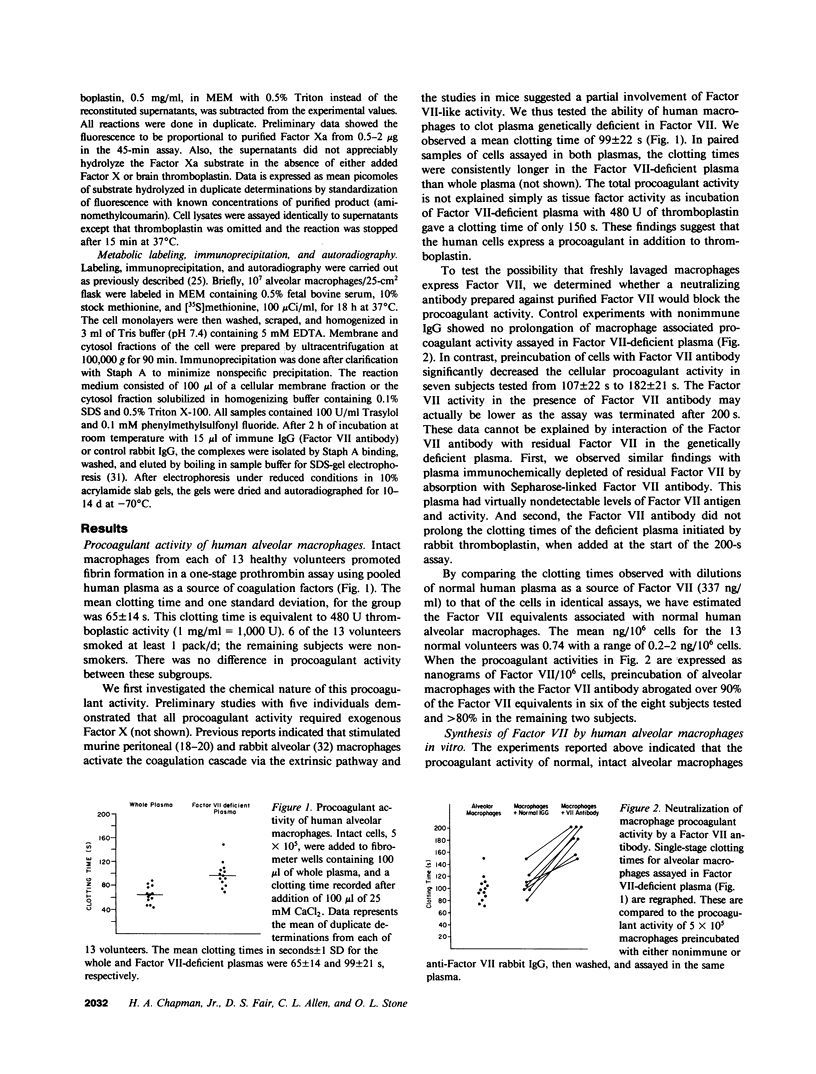
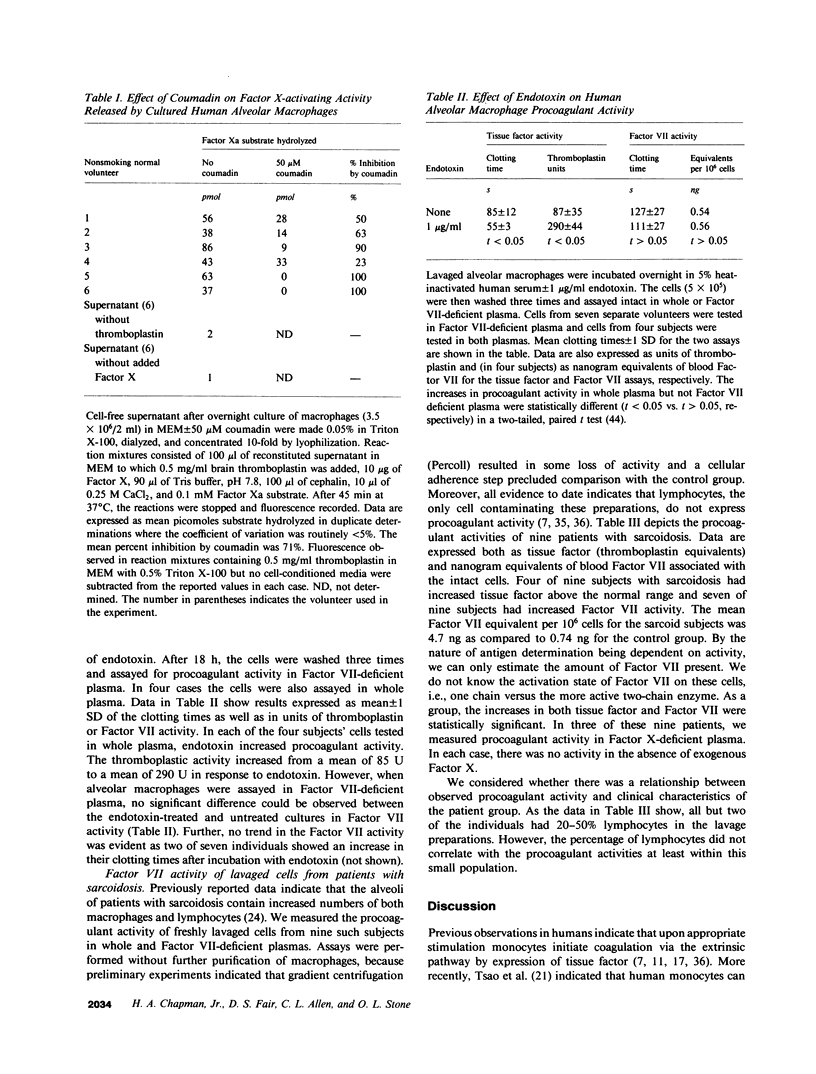
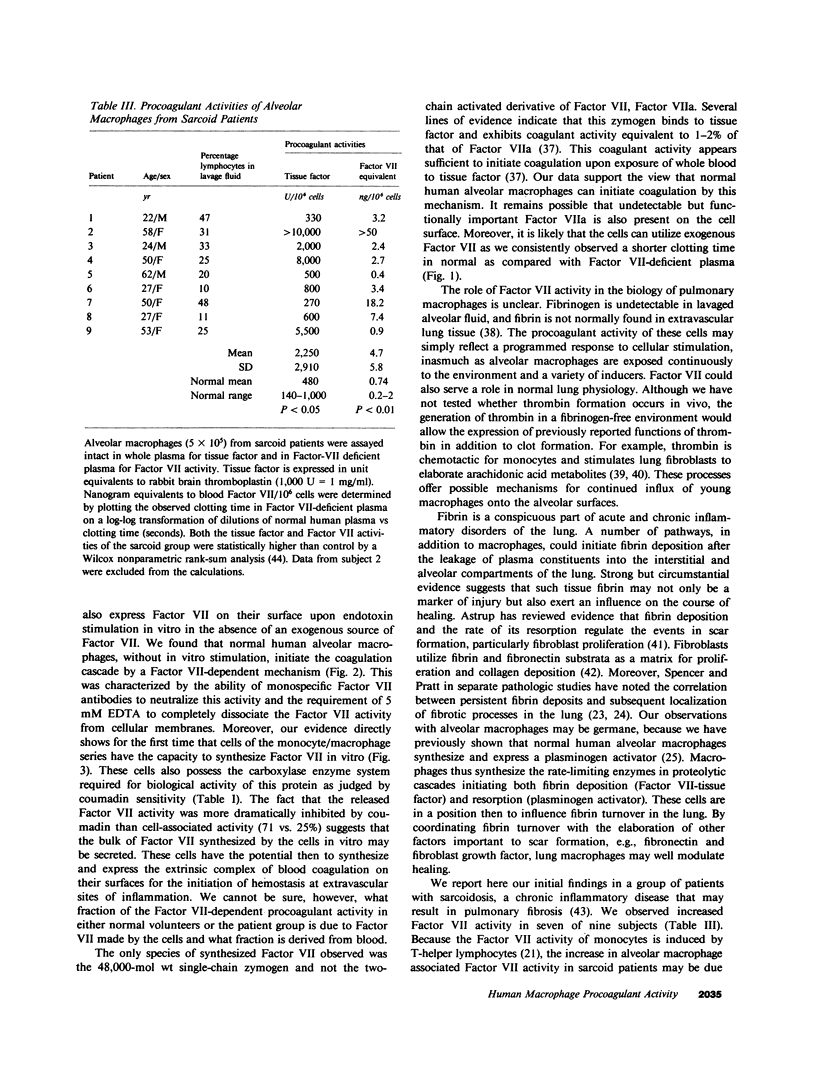
Abstract
Both fibrin and tissue macrophages are prominent in the histopathology of chronic inflammatory pulmonary disease. We therefore examined the procoagulant activity of freshly lavaged human alveolar macrophages in vitro. Intact macrophages (5 X 10(5) cells) from 13 healthy volunteers promoted clotting of whole plasma in a mean of 65 s. Macrophage procoagulant activity was at least partially independent of exogenous Factor VII as judged by a mean clotting time of 99 s in Factor VII-deficient plasma and by neutralization of procoagulant activity by an antibody to Factor VII. Immunoprecipitation of extracts of macrophages metabolically labeled with [35S]methionine by Factor VII antibody and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed a labeled protein consistent in size with the known molecular weight of blood Factor VII, 48,000. The addition of 50 micrograms of unlabeled, purified Factor VII blocked recovery of the 48,000-mol wt protein. In addition, supernatants of cultured macrophages from six normal volunteers had Factor X-activating activity that was suppressed an average of 71% after culture in the presence of 50 microM coumadin or entirely by the Factor VII antibody indicating that Factor VII synthesized by the cell was biologically active. Endotoxin in vitro induced increases in cellular tissue factor but had no consistent effect on macrophage Factor VII activity. We also examined the tissue factor and Factor VII activities of freshly lavaged alveolar cells from nine subjects with clinical and/or histologic evidence of sarcoidosis. Four of the nine subjects expressed increased tissue factor and seven of nine had increased Factor VII activity over the normal range (P less than 0.01). We estimate the mean Factor VII associated with the cells of sarcoid patients to be 4.7 ng/10(6) cells (range 0.4-20) as compared to a mean of 0.74 ng/10(6) cells (range 0.2-2) for that of normal subjects. Along with previous data showing synthesis of plasminogen activator, these findings indicate that human alveolar macrophages normally synthesize and express measurable amounts of the initial enzymes of proteolytic reactions regulating both fibrin deposition and fibrin resorption. Abnormalities in Factor VII activity in a small group of patients with sarcoidosis raise the possibility that modulation of fibrin turnover by macrophages may contribute to the pathology of this and perhaps other interstitial lung diseases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrup T. Blood coagulation and fibrinolysis in tissue culture and tissue repair. Biochem Pharmacol. 1968 Mar;(Suppl):241–257. doi: 10.1016/0006-2952(68)90310-9. [DOI] [PubMed] [Google Scholar]
- Bach R., Nemerson Y., Konigsberg W. Purification and characterization of bovine tissue factor. J Biol Chem. 1981 Aug 25;256(16):8324–8331. [PubMed] [Google Scholar]
- Bajaj S. P., Rapaport S. I., Brown S. F. Isolation and characterization of human factor VII. Activation of factor VII by factor Xa. J Biol Chem. 1981 Jan 10;256(1):253–259. [PubMed] [Google Scholar]
- Bar-Shavit R., Kahn A., Wilner G. D., Fenton J. W., 2nd Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science. 1983 May 13;220(4598):728–731. doi: 10.1126/science.6836310. [DOI] [PubMed] [Google Scholar]
- Bell D. Y., Haseman J. A., Spock A., McLennan G., Hook G. E. Plasma proteins of the bronchoalveolar surface of the lungs of smokers and nonsmokers. Am Rev Respir Dis. 1981 Jul;124(1):72–79. doi: 10.1164/arrd.1981.124.1.72. [DOI] [PubMed] [Google Scholar]
- Broze G. J., Jr Binding of human factor VII and VIIa to monocytes. J Clin Invest. 1982 Sep;70(3):526–535. doi: 10.1172/JCI110644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broze G. J., Jr, Majerus P. W. Purification and properties of human coagulation factor VII. J Biol Chem. 1980 Feb 25;255(4):1242–1247. [PubMed] [Google Scholar]
- Chapman H. A., Jr, Stone O. L., Vavrin Z. Degradation of fibrin and elastin by intact human alveolar macrophages in vitro. Characterization of a plasminogen activator and its role in matrix degradation. J Clin Invest. 1984 Mar;73(3):806–815. doi: 10.1172/JCI111275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Vavrin Z., Hibbs J. B., Jr Coordinate expression of macrophage procoagulant and fibrinolytic activity in vitro and in vivo. J Immunol. 1983 Jan;130(1):261–266. [PubMed] [Google Scholar]
- Colvin R. B., Johnson R. A., Mihm M. C., Jr, Dvorak H. F. Role of the clotting system in cell-mediated hypersensitivity. I. Fibrin deposition in delayed skin reactions in man. J Exp Med. 1973 Sep 1;138(3):686–698. doi: 10.1084/jem.138.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin R. B., Mosesson M. W., Dvorak H. F. Delayed-type hypersensitivity skin reactions in congenital afibrinogenemia lack fibrin deposition and induration. J Clin Invest. 1979 Jun;63(6):1302–1306. doi: 10.1172/JCI109425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. L., Rickles F. R., Bobrove A. M. Mononuclear cell tissue factor: cell of origin and requirements for activation. Blood. 1979 Aug;54(2):359–370. [PubMed] [Google Scholar]
- Edwards R. L., Rickles F. R. The role of human T cells (and T cell products) for monocyte tissue factor generation. J Immunol. 1980 Aug;125(2):606–609. [PubMed] [Google Scholar]
- Fair D. S., Plow E. F., Edgington T. S. Combined functional and immunochemical analysis of normal and abnormal human factor X. J Clin Invest. 1979 Oct;64(4):884–894. doi: 10.1172/JCI109554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D. S. Quantitation of factor VII in the plasma of normal and warfarin-treated individuals by radioimmunoassay. Blood. 1983 Oct;62(4):784–791. [PubMed] [Google Scholar]
- Feinmark S. J., Bailey J. M. Lipid metabolism in cultured cells. Activators of endogenous thromboxane A2 synthesis in cultured lung fibroblasts. J Biol Chem. 1982 Mar 25;257(6):2816–2821. [PubMed] [Google Scholar]
- Geczy C. L., Hopper K. E. A mechanism of migration inhibition in delayed-type hypersensitivity reactions. II. Lymphokines promote procoagulant activity of macrophages in vitro. J Immunol. 1981 Mar;126(3):1059–1065. [PubMed] [Google Scholar]
- Geczy C. L., Meyer P. A. Leukocyte procoagulant activity in man: an in vitro correlate of delayed-type hypersensitivity. J Immunol. 1982 Jan;128(1):331–336. [PubMed] [Google Scholar]
- Grinnell F., Feld M., Minter D. Fibroblast adhesion to fibrinogen and fibrin substrata: requirement for cold-insoluble globulin (plasma fibronectin). Cell. 1980 Feb;19(2):517–525. doi: 10.1016/0092-8674(80)90526-7. [DOI] [PubMed] [Google Scholar]
- Helin H., Edgington T. S. A distinct "slow" cellular pathway involving soluble mediators for the T cell-instructed induction of monocyte tissue factor activity in an allogeneic immune response. J Immunol. 1984 May;132(5):2457–2463. [PubMed] [Google Scholar]
- Holdsworth S. R., Neale T. J., Wilson C. B. Abrogation of macrophage-dependent injury in experimental glomerulonephritis in the rabbit. Use of an antimacrophage serum. J Clin Invest. 1981 Sep;68(3):686–698. doi: 10.1172/JCI110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth S. R., Thomson N. M., Glasgow E. F., Atkins R. C. The effect of defibrination on macrophage participation in rabbit nephrotoxic nephritis: studies using glomerular culture and electronmicroscopy. Clin Exp Immunol. 1979 Jul;37(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981 Aug 20;305(8):429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin J., Poore T. E., Young N. S., Margolis S., Zauber N. P., Townes A. S., Bell W. R. Gram-negative sepsis: detection of endotoxemia with the limulus test. With studies of associated changes in blood coagulation, serum lipids, and complement. Ann Intern Med. 1972 Jan;76(1):1–7. doi: 10.7326/0003-4819-76-1-1. [DOI] [PubMed] [Google Scholar]
- Levy G. A., Edgington T. S. Lymphocyte cooperation is required for amplification of macrophage procoagulant activity. J Exp Med. 1980 May 1;151(5):1232–1244. doi: 10.1084/jem.151.5.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemetz J. Coagulant activity of leukocytes. Tissue factor activity. J Clin Invest. 1972 Feb;51(2):307–313. doi: 10.1172/JCI106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterud B., Lindahl U., Seljelid R. Macrophages produce blood coagulation factors. FEBS Lett. 1980 Oct 20;120(1):41–43. doi: 10.1016/0014-5793(80)81041-6. [DOI] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe R., Nemerson Y. Mechanism of activation of bovine factor VII. Products of cleavage by factor Xa. J Biol Chem. 1976 Aug 25;251(16):4749–4802. [PubMed] [Google Scholar]
- Rickles F. R., Levin J., Hardin J. A., Barr C. F., Conrad M. E., Jr Tissue factor generation by human mononuclear cells: effects of endotoxin and dissociation of tissue factor generation from mitogenic response. J Lab Clin Med. 1977 Apr;89(4):792–803. [PubMed] [Google Scholar]
- Schwartz B. S., Edgington T. S. Lymphocyte collaboration is required for induction of murine monocyte procoagulant activity by immune complexes. J Immunol. 1981 Aug;127(2):438–443. [PubMed] [Google Scholar]
- Shands J. W., Jr The endotoxin-induced procoagulant of mouse exudate macrophages: a factor-X activator. Blood. 1983 Aug;62(2):333–340. [PubMed] [Google Scholar]
- Sitrin R. G., Kaltreider H. B., Ansfield M. J., Webster R. O. Procoagulant activity of rabbit alveolar macrophages. Am Rev Respir Dis. 1983 Aug;128(2):282–287. doi: 10.1164/arrd.1983.128.2.282. [DOI] [PubMed] [Google Scholar]
- Tsao B. P., Fair D. S., Curtiss L. K., Edgington T. S. Monocytes can be induced by lipopolysaccharide-triggered T lymphocytes to express functional factor VII/VIIa protease activity. J Exp Med. 1984 Apr 1;159(4):1042–1057. doi: 10.1084/jem.159.4.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbauer R. H., Belic N., Moores K. D. Clinical interpretation of bilateral hilar adenopathy. Ann Intern Med. 1973 Jan;78(1):65–71. doi: 10.7326/0003-4819-78-1-65. [DOI] [PubMed] [Google Scholar]
- Zur M., Radcliffe R. D., Oberdick J., Nemerson Y. The dual role of factor VII in blood coagulation. Initiation and inhibition of a proteolytic system by a zymogen. J Biol Chem. 1982 May 25;257(10):5623–5631. [PubMed] [Google Scholar]