Abstract
The discrepancies between polychlorinated dibenzo-p-dioxin to polychlorinated dibenzofuran (PCDD to PCDF) ratios in laboratory and field studies in the exhaust of combustion sources are not fully explained by available formation models. In this paper we present the results of experimental studies of the surface mediated formation of PCDD/F at the conditions mimicking the combustion cool zone from a mixture of 1,2-dichlorobenzene (1,2-DCBz) and 2-monochlorophenol (2-MCP) over a model surface consisting of 5% CuO/Silica. The PCDD to PCDF ratio was found to be strongly dependent on the ratio of chlorinated benzenes to chlorinated phenols and oxygen content. The higher the 1,2-DCBz to 2-MCP ratio, the lower the PCDD to PCDF ratio. PCDFs are formed predominantly from chlorinated benzenes, while chlorinated phenols are responsible for majority of PCDDs. These laboratory results are in general agreement with full-scale measurement and can be used to improve predictive models of PCDD/F formation.
Introduction
Transition metal-mediated reactions in the cool zone of the combustors account for the majority of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/F) formation in combustion systems.1−3 Both copper and iron ions, which are present in combustion generated particulate matter,5 are considered the most active metals in promoting PCDD/F formation.6−8 These transition metal-mediated reactions typically occur in the low temperature, postcombustion zone and cool zone in air pollution control devices.
Previous research on surface catalyzed reactions (precursor model of PCDD/F formation) has shown PCDD/Fs are formed primarily via two general schemes: PCDFs by a Langmuir–Hinshelwood (L–H) mechanism and PCDDs is via an Eley–Rideal (E–R) mechanism.9,10 The L–H mechanism involves the reaction of two adsorbed surface species, and the E–R mechanism is via reaction of one gas phase species with a surface-bound adsorbed species. These mechanisms are initiated by chemisorption of substituted aromatic species to metal oxide or hydroxide surface sites to form phenoxyl-type, environmentaly persistent free radicals (EPFRs), which subsequently react to form PCDD/Fs and other toxic air pollutants.12−14 Chlorinated phenols have been demonstrated to be key intermediates in essentially all pathways of PCDD/F formation.6,9,10,15−17 More recently, chlorinated benzenes have been experimentally demonstrated to be potent precursors of PCDD/Fs18,19 and are among the most abundant aromatic compounds in incinerator exhaust.22
Despite large laboratory efforts, a significant discrepancy has been observed between the field and laboratory measurements of PCDD/PCDF ratios in the surface precursor model. In general, laboratory experiments result in ratios of >1, meaning more PCDDs being formed, while incinerators measurements usually yield ratios of <1. Table 1 presents published PCDD/PCDF ratio from different commercial incineration installations. These discrepancies are strong arguments for the de novo mechanism as the main source of PCDD/F formation. This is despite the fact that PCDD/F emissions correlate very well with the concentration of chloro-benzenes and sometimes chloro-phenols in the combustors exhaust.23−27 Addressing this discrepancy has long been a major goal for PCDD/F predictive models. Our recent studies have indicated that chlorinated benzene precursors yield almost exclusively PCDFs.18 These results were in stark contrast to our earlier experiments performed at the same conditions, but in the presence of chlorophenol only (similar to most of the laboratory study focusing on precursor model). In that case PCDDs were produce with a much higher yield than PCDFs. These differences only emphasize the need of a more inclusive studies of the different precursors, especially since chlorobenzenes concentrations are usually much higher in the incinerator exhaust.22
Table 1. PCDD/PCDF Ratio Observed in Commercial Installations.
| source | PCDD:PCDF | references. |
|---|---|---|
| municipal solid waste incinerators | 0.264 | Giugliano M., et al. 2001.4 |
| 0.073 | Ryu J.,2005.11 | |
| 0.22–0.3 | Miriani, 1990.20 | |
| 0.7 | Blumenstock, 2001.21 | |
| 0.45 | Clement, 198828 | |
| 0.93 | Sakai, 200129 | |
| 0.32–1.1 | Ni, Y et al, 2009.30 | |
| hazardous waste incinerators | 0.81 | Gullet B.K., et al. 2000.31 |
| industrial boilers and furnaces | 0.22–0.58 | Everaert K., et al. 2002.23 |
| 0.2 | Duwel 1990.34 | |
| co-fired cement kilns | 0.19 | Abad E.,et al. 2004.36 |
A key question is how do chlorinated benzenes and phenols react to form PCDD/Fs when both are present as a mixture? To our knowledge, no such studies are available. This is surprising as studies of the PCDD/D formation in a complex feed streams have indicated the competition for the adsorption sites among feed constituents as one of the critical factors.32 This manuscript reports the first systematic analysis on the effect of chlorobenzene/chlorophenol ratio on PCDD/PCDF ratio. Specifically, we report the formation of PCDD/Fs from the pyrolysis and oxidation of a mixture of 2-MCP and 1,2-DCBz in the molar ratio of 1:10, 1:1 and 10:1 over 5% copper(II) oxide on silica fly ash surrogate and compare it with earlier results for pure 2-MCP and 1,2-DCBz.9,18 It has to be noted, however that during the reaction some formation of metal chlorides can occur, due to the HCl elimination reaction, and part of the activity may result from metal chloride phase.
Experimental Description
The surface-mediated reactions of a mixture of 2-MCP and 1,2-DCBz over 5% CuO/silica surfaces were investigated using the System of Thermal Diagnostic Studies (STDS), which is described in detail elsewhere.33 Briefly, the system is composed of a thermal reactor located in a high-temperature furnace housed within a gas chromatographic oven that facilitates precise temperature control as well as sample introduction. A computer-interfaced control module is used to set and monitor all experimental parameters. A GC-MS system is interfaced in-line with the thermal reactor for analysis of the reactor effluents.
The method of incipient wetness was used to prepare the catalytic material that served as a surrogate for combustion-generated, copper rich fly ash. Silica gel powder (Aldrich, grade 923 100–200 mesh size) was introduced into a water solution of copper(II) nitrate (Aldrich) in the amount for incipient wetness to occur and proportion to produce 5% CuO on silica by weight. The sample was allowed to age for 24 h at room temperature and dried at 120 °C for 12 h before calcination in air for 5 h at 450 °C. The samples were then ground and sieved to a mesh size of 100–120 corresponding to a particle size of 120–150 μm.
50 mg of catalytic material was placed between quartz wool plugs in a 0.3 cm i.d. fused silica reactor in the STDS. To avoid condensation of the reaction products, all transfer lines were maintained at a constant temperature of 180 °C. Prior to each experiment, the catalytic material was oxidized in situ at 450 °C for 1 h at an air flow-rate of 5 cc/min to activate the surface of the sample. The reagent mixture samples of 2-MCP (Aldrich) and 1,2-DCBz (Aldrich) were introduced separately into the flow stream using a digital syringe pump (KD Scientific, model-100) through a vaporizer maintained at 180 °C. 20% oxygen in helium for oxidation experiments and pure helium for pyrolytic experiments were used as a carrier gas. The rate of injection was selected to maintain a constant gas phase concentration of 50 ppm of 2-MCP and 1,2-DCBz mixtures for temperatures ranging from 200 to 550 °C. The overall flow rate of the reaction gas stream was maintained at 5 cc/min and a contact time of 0.02s. The composition of the reagent mixture is described by the 1,2-DCBz/2-MCP concentration ratio in the gas phase R (cf. Table 2). Conversion of pure 2-MCP and 1,2-DCBz over exact surfaces were studied previously,9,18 and their yields are also provided with assigned R = () (due to division by 0) or 0 for comparison, respectively.
Table 2. Reaction Feed Composition.
| 1,2-DCBz (ppm) | 2-MCP (ppm) | ratio R |
|---|---|---|
| 50 | 0 | () |
| 45 | 5 | 10 |
| 25 | 25 | 1 |
| 5 | 45 | 0.1 |
| 0 | 50 | 0 |
Products from the reaction were analyzed using an in-line Agilent 6890 GC-MSD system after 60 min of the collection of reaction products at the headspace of capillary column at −60 °C. Analytical details are described elsewhere.10,35 Products were identified based on both mass spectra of the standards, comparisons to the NIST mass spectra library when available, and gas-chromatographic retention times.
The yields of the products were calculated by use of the expression: yield = ([product]/[2-MCP+1,2-DCBz]o) × 100, where [product] is the concentration of specific product formed (in moles) and [2-MCP+1,2-DCBz]o is the initial concentration of 2-MCP+1,2-DCBz mixtures (in moles) injected into the reactor. Each experimental data point presented in the manuscript is an average of three experimental runs. Quantitative standards were used to calibrate the MS response for all products. The reactor was periodically cleaned by heating in air at 800 °C, and blank runs were routinely performed to ensure no deposits or carryover of reaction products from run to run.
Results and Discussion
Our previous results have shown the surface mediated PCDD/F forming reactions of chlorinated phenols and chlorinated benzenes proceed according to the Mars-van Krevelen (MvK) mechanism.9,18 Accordingly, the oxidation process does not require gas phase oxygen for the reaction, and surface and lattice -O2– species or −OH groups are actively involved in the process. Gas phase oxygen, if present, ensures reoxidation of the surface and resupplies the consumed surface species. Current results also support this conclusion. Figure 1 presents the decomposition/oxidation profile of the 1,2-DCBz/2-MCP mixtures over CuO/Silica system under pyrolytic conditions. Despite the absence of oxygen in the gas phase, a significant fraction of the reagents were decomposed/oxidized below 350 °C, with over 80% decomposition at temperatures of 350–500 °C. Other products were also detected e.g., polychlorinated phenols, dihydroxybenzenes, polychlorinated benzenes, naphthalenes, (cf. Supporting Information for detailed product tables).
Figure 1.
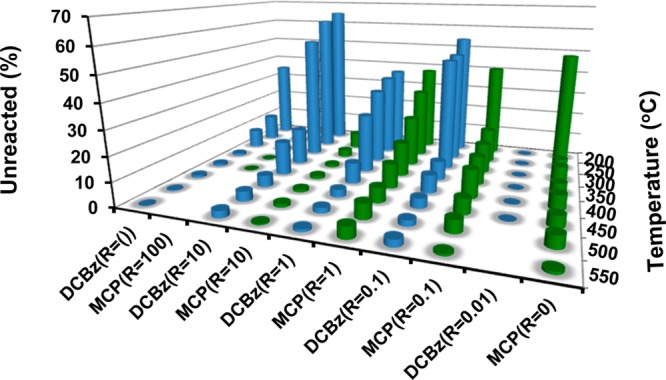
Decomposition/oxidation profiles of 1,2-DCBz/2-MCP mixtures over a CuO/Silica surface under pyrolytic conditions. The graph presents percent 1,2-DCBz and 2-MCP unreacted or formed as a reaction product.
The presence of the co-component in the reacting mixture affects the decomposition of 1,2-DCBz, while the oxidation/degradation of 2-MCP is affected much less (cf. Figure 1). In fact, introducing even small amounts of 2-MCP into the reacting stream (R=10, 5 ppm 2-MCP) results in much higher breakthrough of 1,2-DCBz (the decomposition of 1,2-DCBz shifts toward higher temperatures). These data indicate a competitive adsorption between the 1,2-DCBz and 2-MCP, with a faster rate of 2-MCP adsorption. The same reaction under oxygen rich conditions yielded similar trends (cf. Table S3, Supporting Information). The presence of gas phase O2 under oxidative conditions enhanced the overall decomposition of each reagent by 10–20% below 350 °C. but did not affect significantly the decomposition at ≥400 °C. This further supports the MvK type reaction, with the competitive adsorption determining the relative decomposition of each component.
The inhibited adsorption of 1,2-DCBz in the copresence of 2-MCP may result from a different interaction of those molecules with the metal oxide surfaces. We have previously shown 2-MCP interacts with a surface −OH predominantly by H2O elimination, forming a monodentate surface chlorophenoxy species.9,10,37 This is not the case for 1,2-DCBz which predominantly adsorbs on to two sites forming a bidentate o-dihydroxybenzene species (i.e., chemisorbed catechol).18,37 In fact, rapid acceleration of the 1,2-DCBz decomposition above 350 °C correlated with the decomposition of the chemisorbed catechol, releasing the surface sites for more adsorbates.
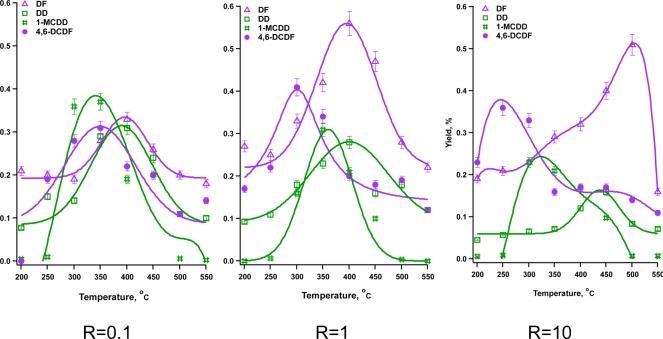
In the present study, the most interesting is the change in the yields and distribution of PCDD/F with varying dichlorobenzene to chlorophenol ratios, as presented in Figure 2. A clear shift in the maximum formation yield of 4,6-dichlorodibenzofuran (4,6-DCDF) from 350 to 250 °C can be observed with increasing 1,2-dichlorobenzene content. Concurrently, dibenzofuran yields almost double and become the most prominent PCDD/F product. It is important to note that in this particular study, we consider nonchlorinated dibenzo-p-dioxins (DD) and dibenzofuran (DF) as a representation of polychlorinated species as they represent condensation products with loss of either 1 or 2 chlorine atoms.18 This is a consequence of using a simple laboratory model with monochlorophenol or dichlorobenzene.
Figure 2.
. PCDD/F yields from mixtures of 1,2-DCBz/2-MCP over CuO/Silica at oxygen rich conditions at various reaction feed ratios.
The changing formation profile with ratio R is associated with the different formation mechanism of PCDDs and PCDFs, and different reactivity of precursors within this mechanism. Adsorbed bidentate catechol formed from 1,2-DCBz adsorption has been found to be a precursor of gas phase o-benzoquinone molecules and adsorbed phenoxyl radical and its keto- structure.11,18 Surface mediated recombination of two keto-phenoxyl radicals leads to the formation of dibenzofuran (DF). From the previous studies of surface assisted 1,2-DCBz decomposition, it is known that on CuO surface, DF is formed exclusively from decomposition of 1,2-DCBz. For the mixtures of 1,2-DCBz and 2-MCP, a correlation is observed between the DF yield (cf. Figure 2) and higher conversions of 1,2-DCBz (cf. Figure 1). In fact, DF yields, drive the total yield of PCDFs.
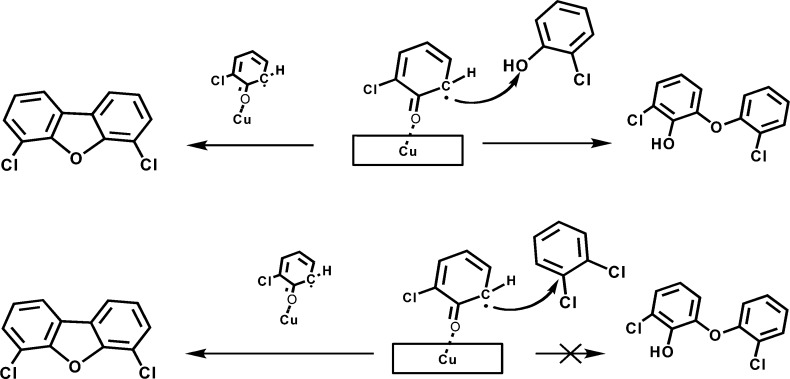
4,6-dichlorodibenzofuran is a product of the keto-structure of monochlorophenoxyl recombination, from both 1,2-DCBz and 2-MCP.9,18,37 However, in the presence of gas phase chlorophenols, two competitive reactions can occur: surface recombination of two radicals to form PCDFs (L-H mechanism) or reaction of a surface radical with a gas-phase 2-MCP to form dichlorohydroxy biphenyl ethers (E–R mechanism) (cf. Figure 3).9,18 The formation of dichlorohydroxy biphenyl ethers was not observed for 1,2-DCBz, which implies the E–R mechanism with the chlorinated benzenes is not favored (cf. Figure 3). Assuming the fast adsorption of 2-MCP, the rate expression for the L–H mechanism is zero order in the gas phase concentration of 2-MCP and first order for the E-R mechanism. As a result, with decreasing relative concentration of 2-MCP in the gas phase, the L–H mechanism becomes dominant, increasing the yield of 4,6-DCDF (cf. Figure 2). In fact, the observed 4,6-DCDF formation from 2-MCP is of −0.6 order with respect to 2-MCP.9
Figure 3.
Schematic representation of the two competing mechanisms of pre-DCDF species in the excess of gas phase 2-MCP (top) and 1,2-DCBz (bottom). Reaction to the left is a L–H surface condensation. Reaction to the right is an E–R reaction with gas phase precursor.
Decreasing the 1,2-DCBz/2-MCP ratio, R, had also an impact on the yields of PCDDs. PCDD formation is exclusively a result of 2-MCP reaction with an experimental reaction order of 0.6–0.7, with respect to 2-MCP. Observed reduction in both 1-MCDD and DD yield with increasing R is a direct result of the decreasing relative concentration of 2-MCP.
A closer look at the total PCDD and PCDF yields as a function of chlorinated benzenes to phenols ratio R indicates interesting differences in reactivity. Figure 4 presents average total yields of the particular group of products over entire temperature range (where average is defined as a sum of all product in a category divided by the number of measurement points). For PCDD sum include DD, 1-MCDD, for PCDF sum include DF and 4,6-DCDF.
Figure 4.
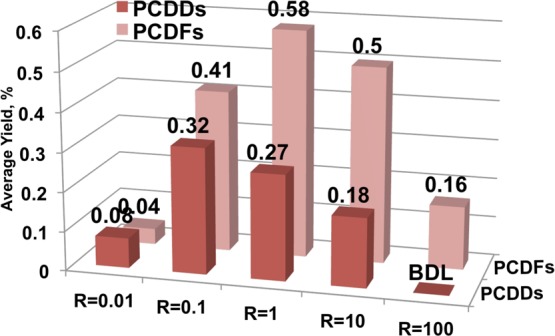
Average yield of PCDDs and PCDFs depending on the feed composition at oxygen rich conditions.
A maximum of the PCDF formation can be observed for the reaction feed composed of equal amounts of both 1,2-dichlorobenzene and 2-monochlorophenol. In the case of PCDD, a decreasing trend of PCDD yields with increasing R is prominent, with the exception of pure 2-MCP feed. The sharp drop in the average PCDD yield for pure chlorophenol feed is however an averaging artifact, as in this case a very narrow PCDD formation window was observed, however, with significant yield (at 300–320 °C, ∼0.3% yield),9
Study Implications
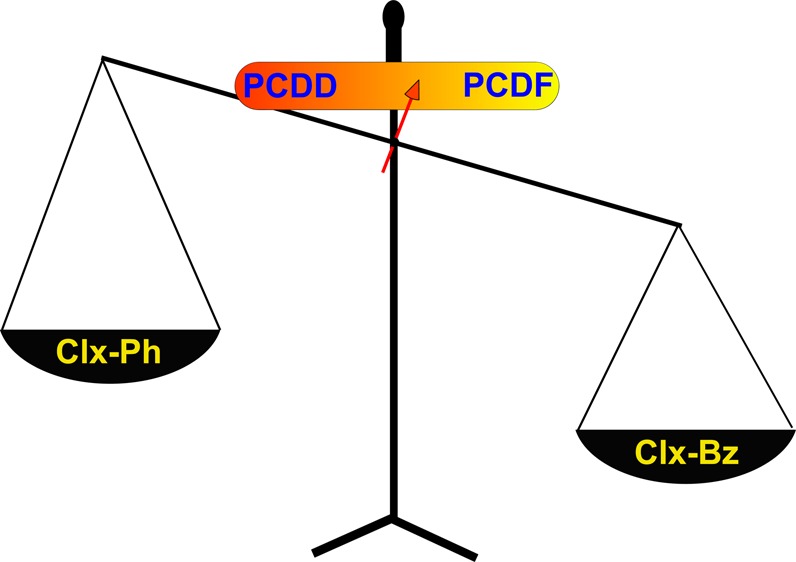
The PCDD/PCDF ratio is one of the most important indicators of the performance of predictive models. Until now, the laboratory studies using a precursor model of PCDD/F formation were not able to mimic the test fields in the incinerators, where PCDFs are dominant product. As a result it is a common interpretation that in the incinerator systems where PCDF are a major dioxin product, carbonaceous deposits are responsible through a de novo process. Present results demonstrate the overreaching conclusions of such assumptions. The presented in here results indicate the ratio of chlorinated benzenes to chlorinated phenols in the reaction feed of precursor model as a key factor determining the PCDD to PCDF ratio. Since PCDF formation is observed over a broad temperature range and the maximum formation windows for PCDFs and PCDDs do not always overlap, it is necessary to compare the yields integrated for the entire temperature range of surface mediated reactions. The advantage of such an approach is accommodation of the yields from the entire postcombustion, cool-zone. Again, in our calculations, we included the nonchlorinated DDs and DFs in their chlorinated group. In full-scale combustor exhausts, where higher chlorinated precursors will be present, the respective products will be (x-1)-chlorodibenzo-p-dioxins for x-chlorophenol precursors and (y-2)-chlorodibenzofuran for y-chlorobenzene precursors, where x and y represent number of chlorine atoms per precursor molecule. Table 3 presents the integrated PCDD to PCDF ratios for different feed composition ratios, R, for both oxygen rich and pyrolytic conditions. The PCDD to PCDF ratio is decreasing “diagonally” from top-left to bottom-right, i.e. with decreasing precursor ratio R and oxygen content. One can claim that the oxygen effect on PCDD/PCDF ratio is anticipated and known due to more oxidized form of the product (2 oxygen atoms included in the structure of PCDD vs 1 for PCDF), however studies indicate that gas phase oxygen is not directly involved in the dioxin formation. The observed PCDD/PCDF ratio depends on the relative concentrations of chlorinated phenols to chlorinated benzenes. The provided matrix can be used as a guide in developing predictive models PCDD/F emissions for copper rich fly ashes. Much of the research has been focused on the correlation between the particular component of the exhaust stream, however this studies indicate that rather a benzenes to phenols (BtP) ratio should be evaluated in field studies. Most importantly, this study shows that the precursor model is absolutely valid in cases where a large excess of PCDFs is formed over PCDDs. It has to be mentioned that other factors such as the presence of different than copper metals can also contribute to the varying PCDD/F ratio. In particular we have recently reported the Iron Oxides are more prone to the formation of PCDF than PCDDs. In fact the effects of chlorobenzene/chlorophenol ratio on PCDD to PCDF ratio over iron oxide surfaces is currently under investigation.
Table 3. PCDD to PCDF Ratio from Precursor Model in Laboratory Experiments Based on the Integrated PCDD/PCDF Yields (± 0.01).
| PCDD:PCDF
Ratio |
||
|---|---|---|
| precursors ratio R | oxidation | pyrolysis |
| 0 | 1.88 | 1.35 |
| 0.1 | 0.78 | 0.64 |
| 1 | 0.46 | 0.27 |
| 10 | 0.35 | 0.25 |
| () | <0.01 | <0.01 |
To verify the chlorobenzenes effect theory, it is necessary to compare the emissions of PCDD/F, chlorinated benzenes and chlorinated phenols from actual field studies. Unfortunately, studies reporting all those emissions are limited. In here we would like to focus on a manuscript reporting the emissions from municipal solid waste incinerators (MSWI).38 General trends in the PCDD/F emissions from different installations indicate that OCDD is the most dominant congener of polychlorinated dibenzo-p-dioxins.39 As for the polychlorinated dibenzofurans, the dominant congener group is sensitive to the type of facility and combusted material.39,40 This is in agreement with the proposed surface mechanism that indicates surface retention of PCDD molecules during ring closure reaction and their chlorination, while PCDFs are liberated to the gas phase while formed.41 Takaoka et all38 reported gas phase concentrations of chlorinated benzenes and chlorinated phenols for 2 different MSWI and in both cases BtP ratio was close to 1 before scrubbers and ∼2 after scrubbers. The observed PCDD/F concentrations corresponded to the PCDD to PCDF ratio of 0.2–0.25 for before scrubbers and 0.37–0.5 behind scrubbers. Comparing it with the data in Table 3 a good prediction can be concluded (for R between 1 and 10). It is interesting to notice that before scrubber conditions fit better to pyrolytic, while after scrubber conditions fit better oxidation condition predictions.
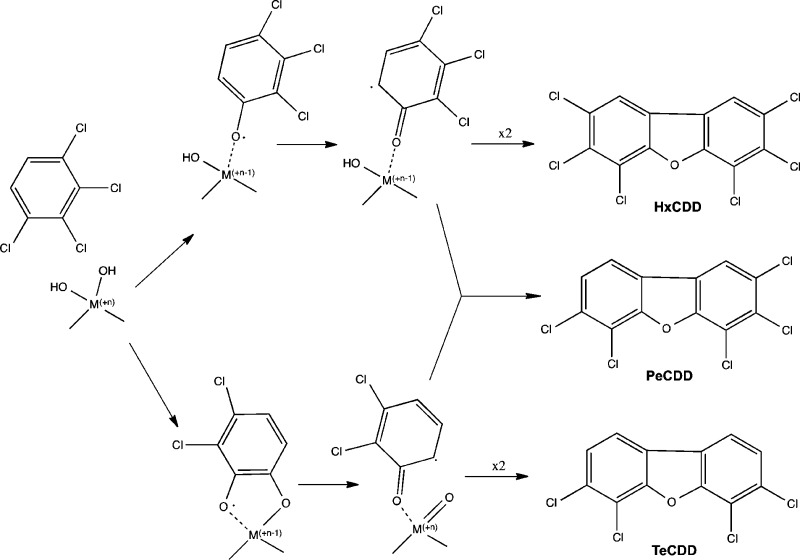
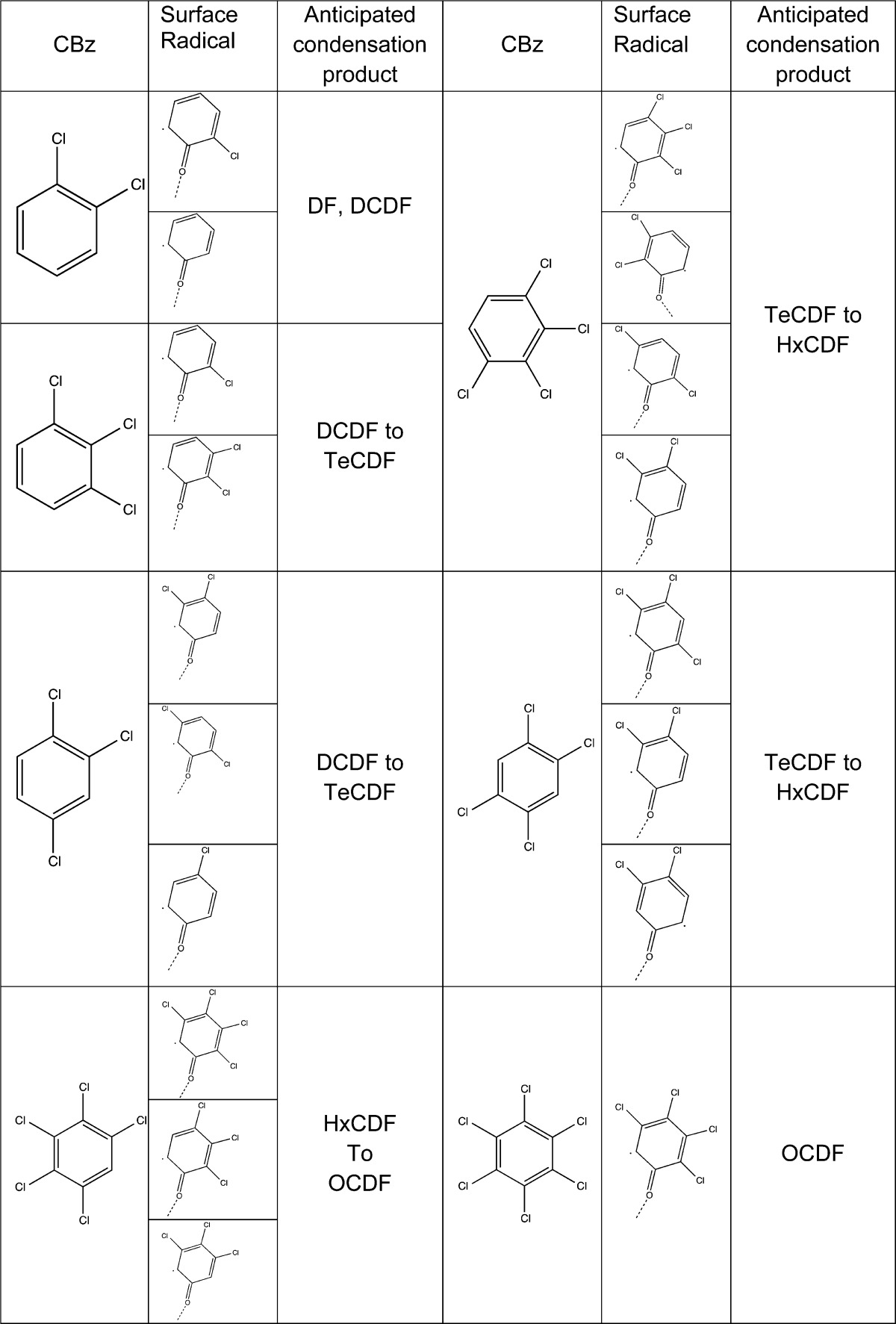
The proposed surface model can also predict the dominant PCDD/F congener group, based on the prevalent CBz concentrations. According to the surface model, PCDFs result from the surface condensation of predioxin radicals.41 Such radicals are formed due to the chemisorption of precursors either as monodentate or bidentate species.18 In the case of 1,2-dichlorobenzene (model compound used in here) this leads to the formation of DCDF and DF.18 The same model can be adapted to higher chlorinated benzenes as depicted in Figure 5 for 1,2,3,4-tetrachlorobenzene. The presence of tetrachlorobenzene will yield the formation of tetra to hexa CDF. Unlike chlorophenols, chlorobenzenes, have a propensity to undergo a bidentate adsorption,37 which results in bottom pathway in Figure 5 (Through bidentate species). As a result PeCDF and TeCDF would dominate the emissions from TeCBz. Table 4 presents selected CBz and their surface radicals as well as anticipated PCDF congeners as their condensation products. This table can serve as a guide for the estimation of PCDF congener group distribution based on the PCBz concentrations. In the studies reported by Takaoka et all38 chlorobenzenes concentration in the gas phase in one of tested MSWI was heavily skewed toward the lower chlorinated benzenes (up to Tetrachlorobenzene)—the ratio of Tri+Tetra CBz to PeCBz is ∼9. According to our prediction table this would correspond to higher concentrations of TeCDF and PeCDF, as authors reported only the TeCDF-OCDF. In fact their measurement indicate TCDF to be the highest concentration, with PeCDF following and the remaining congener groups to be at much lower concentrations. The other studied MSWI in38 have shown similar trends in the inlet to the scrubber. However, with the changing Tri+Tetra/Penta CBz to 5.4, as in the case of the outlet from the scrubber, the contribution of higher chlorinated PCDF is increasing with hexa and hepta congeners becoming doiminant. Such behavior can be predicted based on Table 4.
Figure 5.
Surface condensation model of 1,2,3,4-tetrachlorobenzne to yield PCDF.
Table 4. Selected Chlorobenznes and Their Surface Radicals and Anticipated PCDFs Formed Due to the Surface Condensation Mechanism.
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences, Superfund Research Program, Grant # 2P42ES013648-03
Supporting Information Available
Additional information as noted in the text. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
§ Currently at the South Louisiana Community College.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Olie K.; Addink R.; Schoonenboom M. Metals as catalysts during the formation and decomposition of chlorinated dioxins and furans in incineration processes. J. Air Waste Manage 1998, 482101–105. [DOI] [PubMed] [Google Scholar]
- Altwicker E. R.; Schonberg J. S.; Konduri R.; Milligan M. S. POLYCHLORINATED DIOXIN FURAN FORMATION IN INCINERATORS. Hazard. Waste Hazard. Mater. 1990, 7173–87. [Google Scholar]
- Ghorishi S. B.; Altwicker E. R. Formation of polychlorinated dioxins, furans, benzenes and phenols in the postcombustion region of a heterogeneous combustor—Effect of bed material and postcombustion temperature. Environ. Sci. Technol. 1995, 2951156–1162. [DOI] [PubMed] [Google Scholar]
- Giugliano M.; Cernuschi S.; Grosso M.; Aloigi E.; Miglio R. The flux and mass balance of PCDD/F in a MSW incineration full scale plant. Chemosphere 2001, 434–7743–750. [DOI] [PubMed] [Google Scholar]
- Cains P. W.; McCausland L. J.; Fernandes A. R.; Dyke P. Polychlorinated dibenzo-p-dioxins and dibenzofurans formation in incineration: Effects of fly ash and carbon source. Environ. Sci. Technol. 1997, 313776–785. [Google Scholar]
- Nganai S.; Lomnicki S.; Dellinger B. Ferric oxide mediated formation of PCDD/Fs from 2-monochlorophenol. Environ. Sci. Technol. 2009, 432368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz L.; V H.; Zwick G.; Beck J.; Bautz H. On formation conditions of organohalogen compounds from particulate carbon of fly ash. Chemosphere 1991, 238–101255–64. [Google Scholar]
- Ismo H.; Kari T.; Juhani R. Formation of aromatic chlorinated compounds catalyzed by copper and iron. Chemosphere 1997, 34122649–2662. [Google Scholar]
- Lomnicki S.; Dellinger B. A detailed mechanism of the surface-mediated formation of PCDD/F from the oxidation of 2-chlorophenol on a CuO/silica surface. J. Phys. Chem. A 2003, 107224387–4395. [Google Scholar]
- Lomnicki S.; Dellinger B. Formation of PCDD/F from the pyrolysis of 2-chlorophenol on the surface of dispersed copper oxide particles. Proc. Combust. Inst. 2003, 29, 2463–2468. [Google Scholar]
- Ryu J. Y.; Mulholland J. A.; Kim D. H.; Takeuchi M. Homoloque and isomer patterns of polychlorinated dibenzo-p-dioxins and dihenzofurans from phenol precursors: Comparison with municipal waste incinerator data. Environ. Sci. Technol. 2005, 39124398–4406. [DOI] [PubMed] [Google Scholar]
- Vejerano E.; Lomnicki S.; Dellinger B. Formation and stabilization of combustion-generated environmentally persistent free radicals on an Fe(III)2O3/silica surface. Environ. Sci. Technol. 2011, 452589–594. [DOI] [PubMed] [Google Scholar]
- Lomnicki S.; Truong H.; Vejerano E.; Dellinger B. Copper oxide-based model of persistent free radical formation on combustion-derived particulate matter. Environ. Sci. Technol. 2008, 42134982–4988. [DOI] [PubMed] [Google Scholar]
- Dellinger B.; Loninicki S.; Khachatryan L.; Maskos Z.; Hall R. W.; Adounkpe J.; McFerrin C.; Truong H. Formation and stabilization of persistent free radicals. Proc. Combust. Inst. 2007, 31, 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson L. C.; Lenoir D.; Hutzinger O. Quantitative comparison of denovo and precursor formation of polychlorinated dibenzo-p-dioxins under simulated municipal solid-waste incinerator postcombustion conditions. Environ. Sci. Technol. 1992, 2691822–1828. [Google Scholar]
- Altwicker E. R.; Milligan M. S. Formation of dioxins—Competing rates between chemically similar precursors and de-novo reactions. Chemosphere 1993, 271–3301–307. [Google Scholar]
- Karasek F. W.; Dickson L. C. Model studies of polychlorinated dibenzo-para-dioxin formation during municipal refuse incineration. Science 1987, 2374816754–756. [DOI] [PubMed] [Google Scholar]
- Nganai S.; Lomnicki S. M.; Dellinger B. Formation of PCDD/Fs from the copper oxide-mediated pyrolysis and oxidation of 1,2-dichlorobenzene. Environ. Sci. Technol. 2011, 4531034–1040. [DOI] [PubMed] [Google Scholar]
- Stanmore B. R. The formation of dioxins in combustion systems. Combust. Flame 2004, 136, 368–427. [Google Scholar]
- Mariani G.; Benfenati E.; Fanelli R. Concentrations of PCDD and PCDF in different points of a modern refuse incinerator. Chemosphere 1990, 214–5507–517. [Google Scholar]
- Blumenstock M.; Zimmermann R.; Schramm K. W.; Kettrup A. Identification of surrogate compounds for the emission of PCDD/F (I-TEQ value) and evaluation of their on-line realtime detectability in flue gases of waste incineration plants by REMPI-TOFMS mass spectrometry. Chemosphere 2001, 425–7507–518. [DOI] [PubMed] [Google Scholar]
- Zimmermann R.; Blumenstock M.; Heger H. J.; Schramm K. W.; Kettrup A. Emission of nonchlorinated and chlorinated aromatics in the flue gas of incineration plants during and after transient disturbances of combustion conditions: Delayed emission effects. Environ. Sci. Technol. 2001, 3561019–1030. [DOI] [PubMed] [Google Scholar]
- Everaert K.; Baeyens J. The formation and emission of dioxins in large scale thermal processes. Chemosphere 2002, 463439–448. [DOI] [PubMed] [Google Scholar]
- Neuer-Etscheidt K.; Orasche J.; Nordsieck H.; Streibel T.; Zimmermann R.; Kettrup A. Changes in PCDD/PCDF formation processes during instationary phases of combustor operation - Exemplified by the use of C14DD isomer patterns. Chemosphere 2007, 679S205–S216. [DOI] [PubMed] [Google Scholar]
- Tupporainen K. A.; Ruokojarvi P. H.; Asikainen A. H.; Aatamila M.; Ruuskinen J. Chlorophenols as Precursors of PCDD/Fs in incineration processes: Correlations, PLS modeling, and reaction mechanisms. Environ. Sci. Technol. 2000, 34, 4958–4962. [Google Scholar]
- Pandelova M.; Stanev I.; Henkelmann B.; Lenoir D.; Schramm K.-W. Correlation of PCDD/F and PCB at combustion experiments using wood and hospital waste. Influence of (NH4)2SO4 as additive on PCDD/F and PCB emissions. Chemosphere 2009, 755685–691. [DOI] [PubMed] [Google Scholar]
- Weber R.; Hagenmaier H. PCDD/PCDF formation in fluidized bed incineration. Chemosphere 1999, 38112643–2654. [Google Scholar]
- Clement R. E.; Tosine H. M.; Osborne J.; Ozvacic V.; Wong G. Gas-Chromatographic mass-spectrometric determination of chlorinated dibenzo-para-dioxins and dibenzofurans in incinerator stack emissions and Fly-Ash—A 13-test study. Biomed Environ. Mass 1988, 17281–96. [Google Scholar]
- Sakai S. I.; Hayakawa K.; Takatsuki H.; Kawakami I. Dioxin-like PCBs released from waste incineration and their deposition flux. Environ. Sci. Technol. 2001, 35183601–3607. [DOI] [PubMed] [Google Scholar]
- Ni Y.; Zhang H.; Fan S.; Zhang X.; Zhang Q.; Chen J. Emissions of PCDD/Fs from municipal solid waste incinerators in China. Chemosphere 2009, 7591153–1158. [DOI] [PubMed] [Google Scholar]
- Gullett B. K.; Touati A.; Lee C. W. Formation of chlorinated dioxins and furans in a hazardous-waste-firing industrial boiler. Environ. Sci. Technol. 2000, 34112069–2074. [Google Scholar]
- Cieplik M. K.; De Jong V.; Bozovic J.; Liljelind P.; Marklund S.; Louw R. Formation of dioxins from combustion micropollutants over MSWI fly ash. Environ. Sci. Technol. 2006, 4041263–1269. [DOI] [PubMed] [Google Scholar]
- Rubey W. A.; Grant R. A. Design aspects of a modular instrumentation system for thermal diagnostic studies. Rev. Sci. Instrum. 1988, 592265–269. [Google Scholar]
- Duwel U.; Nottrodt A.; Ballschmiter K. Simultaneous sampling of PCDD PCDF inside the combustion-chamber and on 4 boiler levels of a waste incineration plant. Chemosphere 1990, 2010–121839–1846. [Google Scholar]
- Nganai S.; Lomnicki S.; Dellinger B. Formation of PCDD/Fs from oxidation of 2-monochlorophenol over an Fe2O3/silica surface. Chemosphere 2012, 883371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abad E.; Martinez K.; Caixach J.; Rivera J. Polychlorinated dibenzo-p-dioxin/polychlorinated dibenzofuran releases into the atmosphere from the use of secondary fuels in cement kilns during clinker formation. Environ. Sci. Technol. 2004, 38184734–4738. [DOI] [PubMed] [Google Scholar]
- Lomnicki S.; Truong H.; Vajereno E.; Dellinger B. A copper oxide-based model of persistent free radical formation on combustion derived particulate matter. Environ. Sci. Technol. 2008, 42134982–4988. [DOI] [PubMed] [Google Scholar]
- Takaoka M.; Liao P.; Takeda N.; Fujiwara T.; Oshita K. The behavior of PCDD/Fs, PCBs, chlorobenzenes and chlorophenols in wet scrubbing system of municipal solid waste incinerator. Chemosphere 2003, 532153–161. [DOI] [PubMed] [Google Scholar]
- Jen-Ho K.; Kang-Shin Chen; Guo-Ping C.-C.; Chou I.-C. Emissions of polychlorinated dibenzo-p-dioxins and dibenzofurans from various stationary sources. Aerosol Air Qual. Res. 2006, 62170–179. [Google Scholar]
- Ni Y. W.; Zhang H. J.; Fan S.; Zhang X. P.; Zhang Q.; Chen J. P. Emissions of PCDD/Fs from municipal solid waste incinerators in China. Chemosphere 2009, 7591153–1158. [DOI] [PubMed] [Google Scholar]
- Lomnicki S.; Dellinger B. A detailed mechanism of the surface-mediated formation of PCDD/F from the oxidation of 2-chlorophenol on CuO/ silica surface. J. Phys. Chem. A 2003, 107224387–4395. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.