Abstract
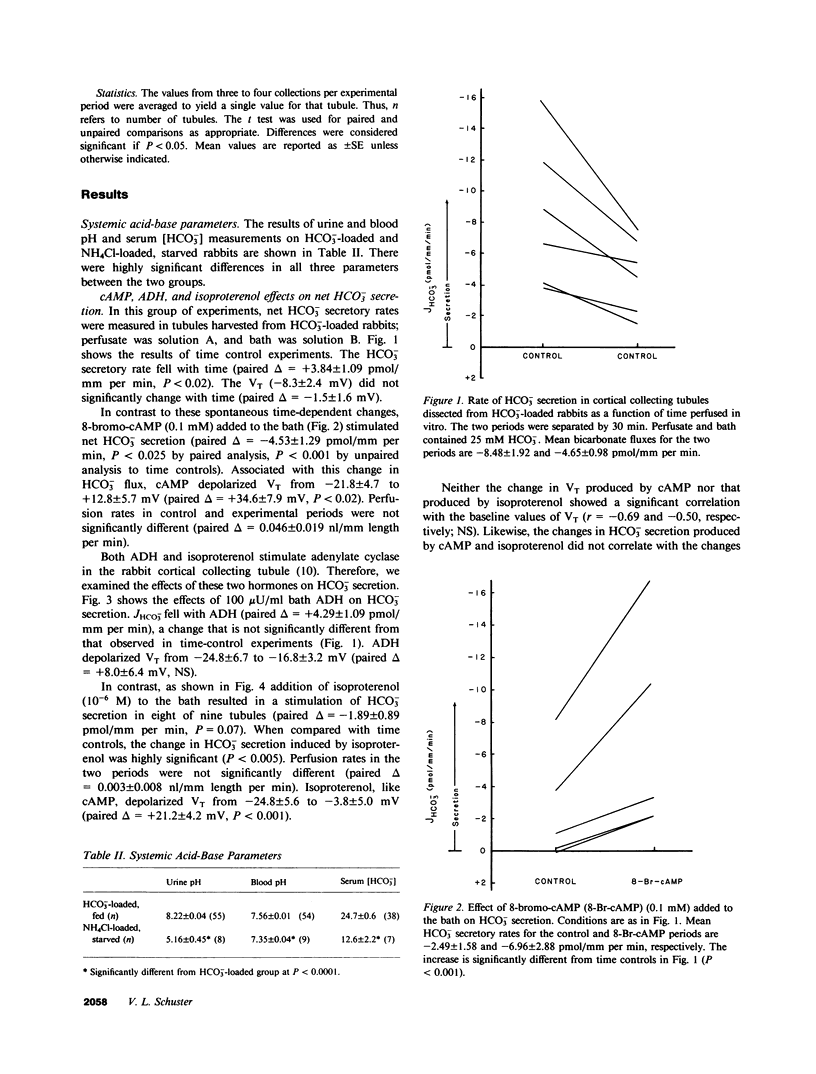
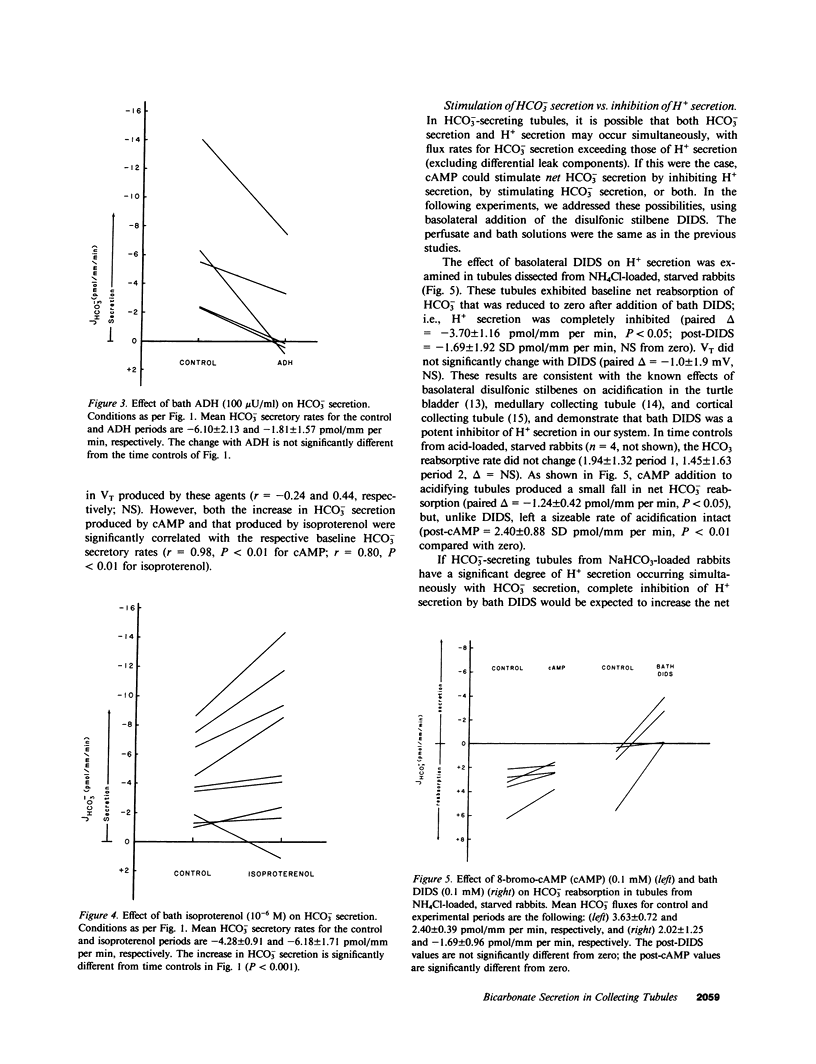
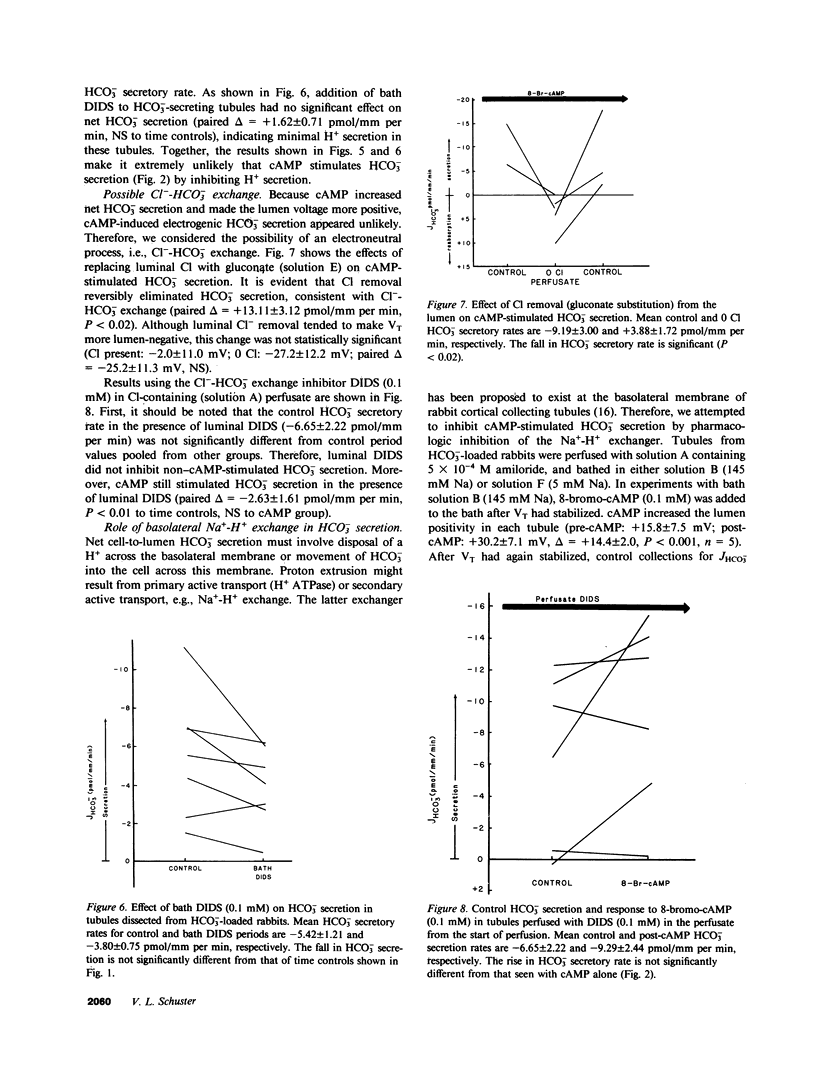
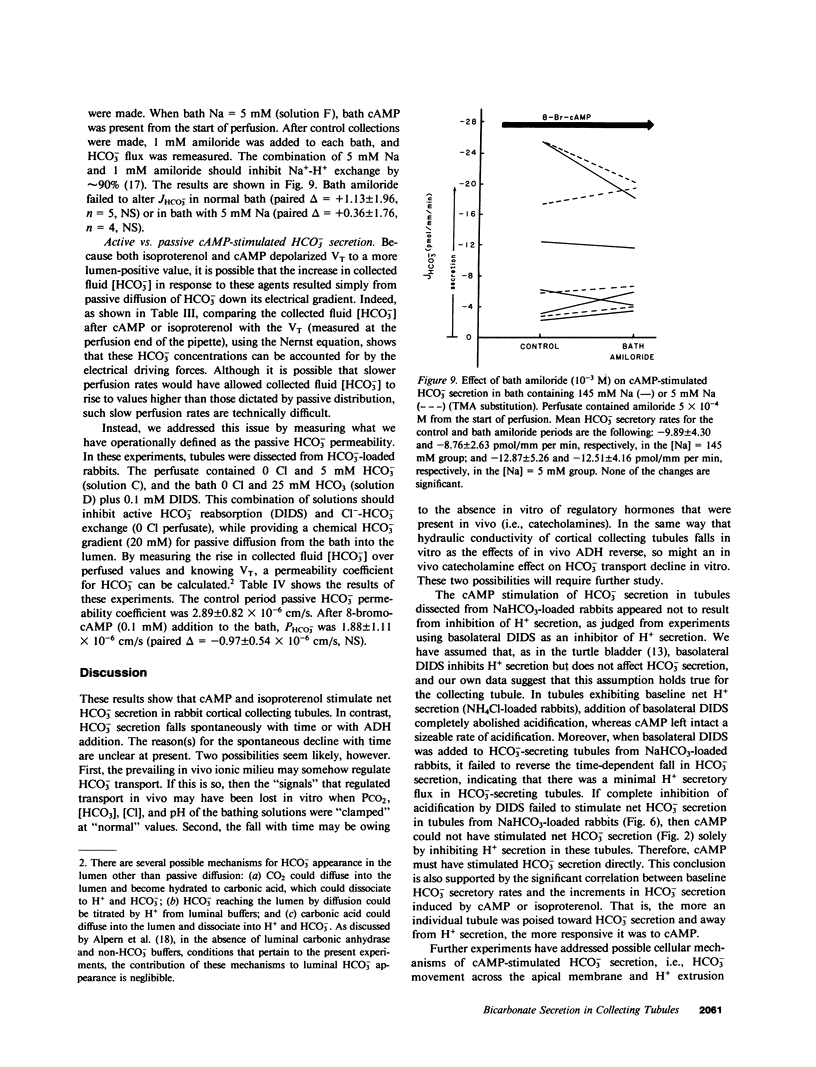
We studied the effects of cyclic AMP (cAMP) on HCO-3 transport by rabbit cortical collecting tubules perfused in vitro. Net HCO-3 secretion was observed in tubules from NaHCO3- loaded rabbits. 8-Bromo-cAMP-stimulated net HCO-3 secretion, whereas secretion fell with time in control tubules. Both isoproterenol and vasopressin (ADH) are known to stimulate adenylate cyclase in this epithelium; however, only isoproterenol stimulated net HCO-3 secretion. The mechanism of cAMP-stimulated HCO-3 secretion was examined. If both HCO-3 and H+ secretion were to occur simultaneously in tubules exhibiting net HCO-3 secretion, cAMP might increase the net HCO-3 secretory rate by inhibiting H+ secretion, by stimulating HCO-3 secretion, or both. These possibilities were examined using basolateral addition of the disulfonic stilbene (4,4'-diisothiocyanostilbene-2,2'-disulfonate (DIDS). In acidifying tubules from NH4Cl-loaded rabbits, DIDS eliminated HCO-3 reabsorption, a result consistent with known effects of DIDS as an inhibitor of H+ secretion. In contrast, cAMP left acidification (H+ secretion) intact. DIDS applied to HCO-3 secretory tubules failed to increase the HCO-3 secretory rate, indicating minimal H+ secretion in HCO-3 secreting tubules. Thus, inhibition of H+ secretion by cAMP could not account for the cAMP-induced stimulation of net HCO-3 secretion. cAMP-stimulated HCO-3 secretion was reversibly eliminated by 0 Cl perfusate, whereas luminal DIDS had no effect. Bath amiloride (1 mM) failed to eliminate cAMP-stimulated HCO-3 secretion when bath [Na+] was 145 mM or 5 mM. cAMP depolarized the transepithelial voltage. The collected fluid [HCO-3] after cAMP could be accounted for by electrical driving forces, suggesting that cAMP stimulates passive HCO-3 secretion. However, cAMP did not alter HCO-3 permeability measured under conditions expected to inhibit transcellular HCO-3 movement (0 Cl- solutions and bath DIDS). This measured HCO-3 permeability was not high enough to account, by passive diffusion, for the HCO-3 fluxes observed in Cl-containing solutions. We conclude the following: cAMP increased net HCO3- secretion by stimulating HCO3- secretion and not by inhibiting H+ secretion; this HCO3- secretion may have occurred by Cl-HCO3- exchange; Na+-H+ exchange appeared not to play a role in basolateral H+ extrusion under these conditions; and the stimulation of HCO3- secretion by isoproterenol, but not ADH, suggests the existence of separate cell cAMP pools or cellular heterogeneity in this cAMP response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern R. J., Cogan M. G., Rector F. C., Jr Effect of luminal bicarbonate concentration on proximal acidification in the rat. Am J Physiol. 1982 Jul;243(1):F53–F59. doi: 10.1152/ajprenal.1982.243.1.F53. [DOI] [PubMed] [Google Scholar]
- Barajas L., Powers K., Wang P. Innervation of the renal cortical tubules: a quantitative study. Am J Physiol. 1984 Jul;247(1 Pt 2):F50–F60. doi: 10.1152/ajprenal.1984.247.1.F50. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Selective Cl retention in repair of metabolic alkalosis without increasing filtered load. Am J Physiol. 1970 Jan;218(1):165–170. doi: 10.1152/ajplegacy.1970.218.1.165. [DOI] [PubMed] [Google Scholar]
- Cohen L. H., Mueller A., Steinmetz P. R. Inhibition of the bicarbonate exit step in urinary acidification by a disulfonic stilbene. J Clin Invest. 1978 Apr;61(4):981–986. doi: 10.1172/JCI109023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobyan D. C., Bulger R. E. Renal carbonic anhydrase. Am J Physiol. 1982 Oct;243(4):F311–F324. doi: 10.1152/ajprenal.1982.243.4.F311. [DOI] [PubMed] [Google Scholar]
- Galla J. H., Bonduris D. N., Luke R. G. Correction of acute chloride-depletion alkalosis in the rat without volume expansion. Am J Physiol. 1983 Feb;244(2):F217–F221. doi: 10.1152/ajprenal.1983.244.2.F217. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Orloff J. Effect of prostaglandin E1 on the permeability response of the isolated collecting tubule to vasopressin, adenosine 3',5'-monophosphate, and theophylline. J Clin Invest. 1968 May;47(5):1154–1161. doi: 10.1172/JCI105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter-Smith P. J., White J. F. Response of Amphiuma small intestine to theophylline: effect on bicarbonate transport. Am J Physiol. 1979 Jun;236(6):E775–E783. doi: 10.1152/ajpendo.1979.236.6.E775. [DOI] [PubMed] [Google Scholar]
- Husted R. F., Cohen L. H., Steinmetz P. R. Pathways for bicarbonate transfer across the serosal membrane of turtle urinary bladder: studies with a disulfonic stilbene. J Membr Biol. 1979 May 7;47(1):27–37. doi: 10.1007/BF01869045. [DOI] [PubMed] [Google Scholar]
- Iino Y., Troy J. L., Brenner B. M. Effects of catecholamines on electrolyte transport in cortical collecting tubule. J Membr Biol. 1981;61(2):67–73. doi: 10.1007/BF02007632. [DOI] [PubMed] [Google Scholar]
- KASSIRER J. P., BERKMAN P. M., LAWRENZ D. R., SCHWARTZ W. B. THE CRITICAL ROLE OF CHLORIDE IN THE CORRECTION OF HYPOKALEMIC ALKALOSIS IN MAN. Am J Med. 1965 Feb;38:172–189. doi: 10.1016/0002-9343(65)90172-5. [DOI] [PubMed] [Google Scholar]
- Kimmel P. L., Goldfarb S. Effects of isoproterenol on potassium secretion by the cortical collecting tubule. Am J Physiol. 1984 Jun;246(6 Pt 2):F804–F810. doi: 10.1152/ajprenal.1984.246.6.F804. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1981 Oct;241(4):F374–F379. doi: 10.1152/ajprenal.1981.241.4.F374. [DOI] [PubMed] [Google Scholar]
- Koeppen B. M., Biagi B. A., Giebisch G. H. Intracellular microelectrode characterization of the rabbit cortical collecting duct. Am J Physiol. 1983 Jan;244(1):F35–F47. doi: 10.1152/ajprenal.1983.244.1.F35. [DOI] [PubMed] [Google Scholar]
- Koeppen B. M., Helman S. I. Acidification of luminal fluid by the rabbit cortical collecting tubule perfused in vitro. Am J Physiol. 1982 May;242(5):F521–F531. doi: 10.1152/ajprenal.1982.242.5.F521. [DOI] [PubMed] [Google Scholar]
- Laski M. E., Warnock D. G., Rector F. C., Jr Effects of chloride gradients on total CO2 flux in the rabbit cortical collecting tubule. Am J Physiol. 1983 Feb;244(2):F112–F121. doi: 10.1152/ajprenal.1983.244.2.F112. [DOI] [PubMed] [Google Scholar]
- LeFurgey A., Tisher C. C. Morphology of rabbit collecting duct. Am J Anat. 1979 May;155(1):111–124. doi: 10.1002/aja.1001550108. [DOI] [PubMed] [Google Scholar]
- Lief P. D., Mutz B. F., Bank N. Effect of cyclic AMP on hydrogen ion secretion by turtle urinary bladder. Kidney Int. 1979 Aug;16(2):103–112. doi: 10.1038/ki.1979.111. [DOI] [PubMed] [Google Scholar]
- Lombard W. E., Kokko J. P., Jacobson H. R. Bicarbonate transport in cortical and outer medullary collecting tubules. Am J Physiol. 1983 Mar;244(3):F289–F296. doi: 10.1152/ajprenal.1983.244.3.F289. [DOI] [PubMed] [Google Scholar]
- McKinney T. D., Burg M. B. Bicarbonate secretion by rabbit cortical collecting tubules in vitro. J Clin Invest. 1978 Jun;61(6):1421–1427. doi: 10.1172/JCI109061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel F., Imbert-Teboul M., Chabardès D. Distribution of hormone-dependent adenylate cyclase in the nephron and its physiological significance. Annu Rev Physiol. 1981;43:569–581. doi: 10.1146/annurev.ph.43.030181.003033. [DOI] [PubMed] [Google Scholar]
- Münzel P. A., Healy D. P., Insel P. A. Autoradiographic localization of beta-adrenergic receptors in rat kidney slices using [125I]iodocyanopindolol. Am J Physiol. 1984 Feb;246(2 Pt 2):F240–F245. doi: 10.1152/ajprenal.1984.246.2.F240. [DOI] [PubMed] [Google Scholar]
- Nagel W., Reinach P. Mechanism of stimulation by epinephrine of active transepithelial Cl transport in isolated frog cornea. J Membr Biol. 1980 Aug 21;56(1):73–79. doi: 10.1007/BF01869354. [DOI] [PubMed] [Google Scholar]
- Petersen K. U., Reuss L. Cyclic AMP-induced chloride permeability in the apical membrane of Necturus gallbladder epithelium. J Gen Physiol. 1983 May;81(5):705–729. doi: 10.1085/jgp.81.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S. Effect of cyclic AMP on acidification in the isolated turtle bladder. Kidney Int. 1985 Jan;27(1):25–30. doi: 10.1038/ki.1985.5. [DOI] [PubMed] [Google Scholar]
- Saito Y., Wright E. M. Bicarbonate transport across the frog choroid plexus and its control by cyclic nucleotides. J Physiol. 1983 Mar;336:635–648. doi: 10.1113/jphysiol.1983.sp014602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom S. C., Weinman E. J., O'Neil R. G. Microelectrode assessment of chloride-conductive properties of cortical collecting duct. Am J Physiol. 1984 Aug;247(2 Pt 2):F291–F302. doi: 10.1152/ajprenal.1984.247.2.F291. [DOI] [PubMed] [Google Scholar]
- Satake N., Durham J. H., Ehrenspeck G., Brodsky W. A. Active electrogenic mechanisms for alkali and acid transport in turtle bladders. Am J Physiol. 1983 Mar;244(3):C259–C269. doi: 10.1152/ajpcell.1983.244.3.C259. [DOI] [PubMed] [Google Scholar]
- Schuster V. L., Kokko J. P., Jacobson H. R. Interactions of lysyl-bradykinin and antidiuretic hormone in the rabbit cortical collecting tubule. J Clin Invest. 1984 Jun;73(6):1659–1667. doi: 10.1172/JCI111372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D. K., Seldin D. W., Kokko J. P., Jacobson H. R. Anion dependence of rabbit medullary collecting duct acidification. J Clin Invest. 1983 May;71(5):1505–1508. doi: 10.1172/JCI110905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam S. C., Goldstein M. B., Stinebaugh B. J., Chen C. B., Gougoux A., Halperin M. L. Studies on the regulation of hydrogen ion secretion in the collecting duct in vivo: evaluation of factors that influence the urine minus blood PCO2 difference. Kidney Int. 1981 Nov;20(5):636–642. doi: 10.1038/ki.1981.187. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Smith P. L., Frizzell R. A. Chloride secretion by canine tracheal epithelium: II. The cellular electrical potential profile. J Membr Biol. 1982;70(3):227–238. doi: 10.1007/BF01870565. [DOI] [PubMed] [Google Scholar]
- Woodruff C. R., Carpenter F. G. Actions of catecholamines and physostigmine on rat parotid salivary secretion. Am J Physiol. 1973 Dec;225(6):1449–1453. doi: 10.1152/ajplegacy.1973.225.6.1449. [DOI] [PubMed] [Google Scholar]



