Abstract
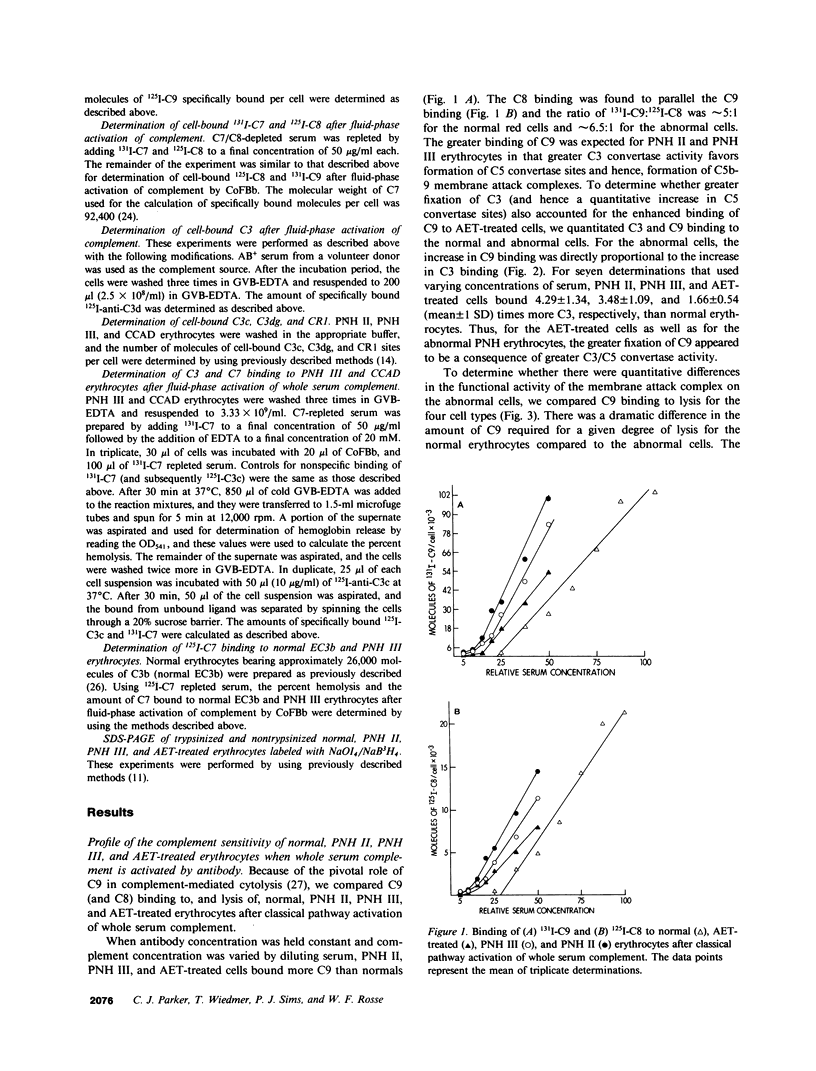
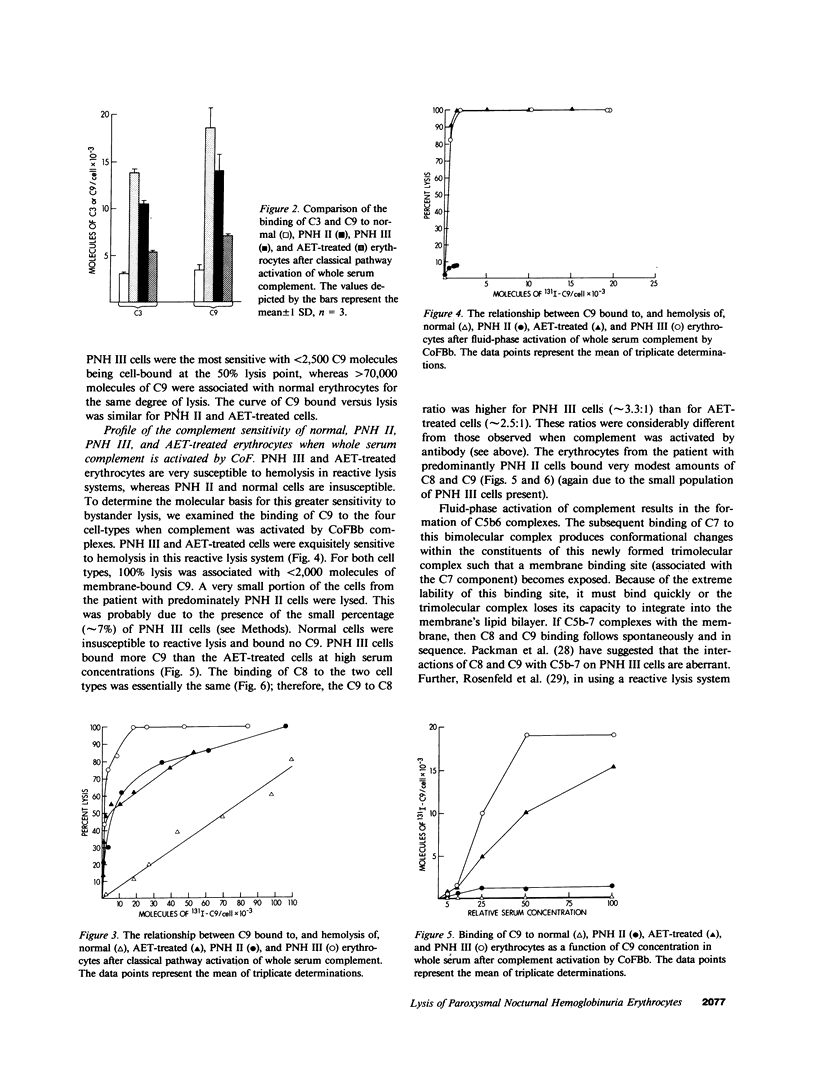
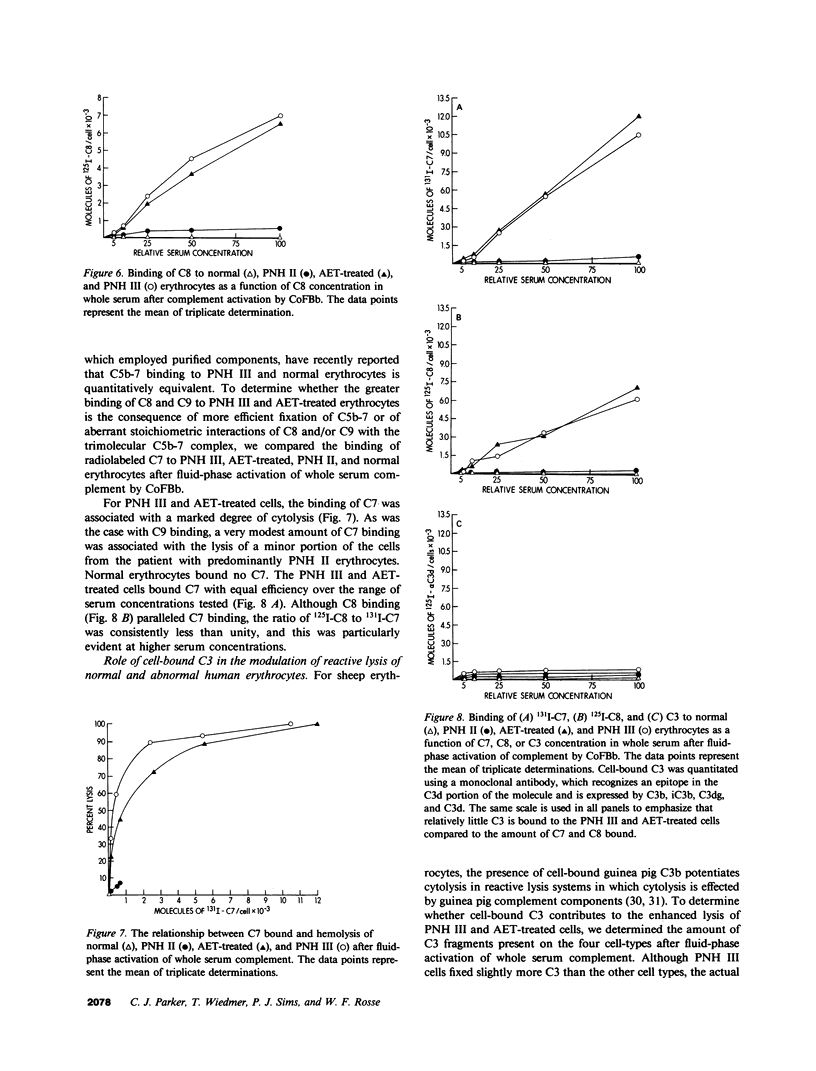
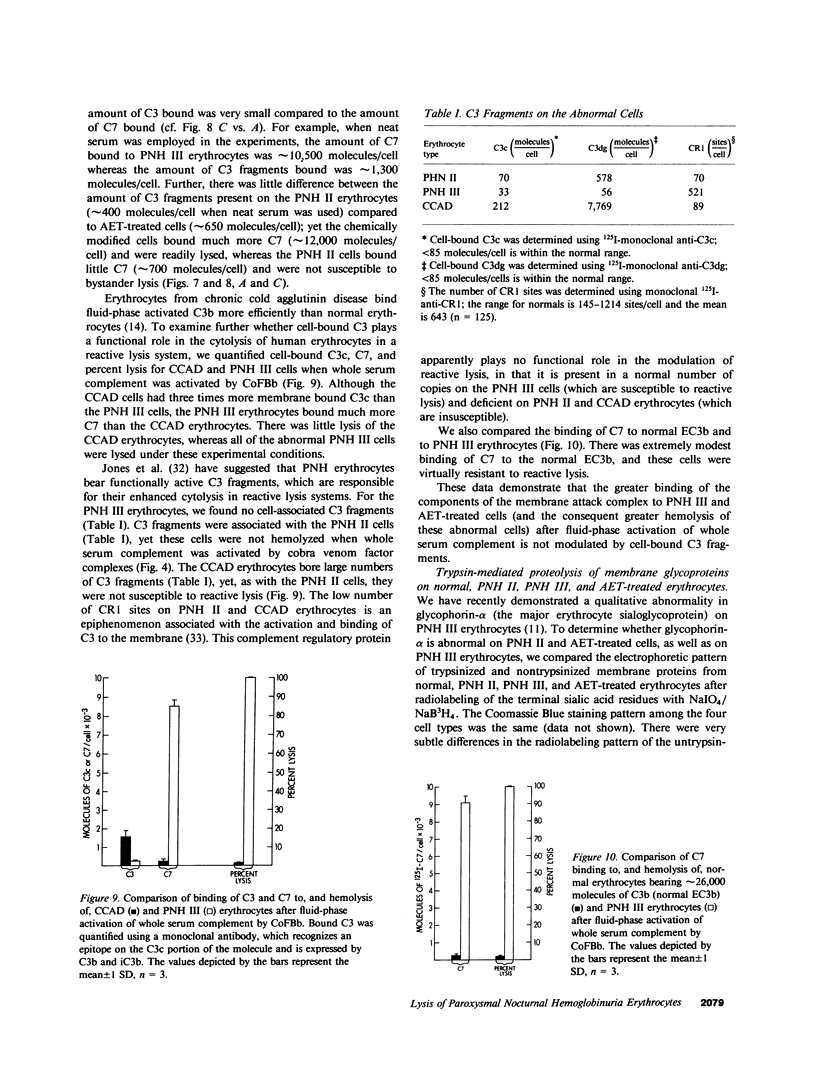
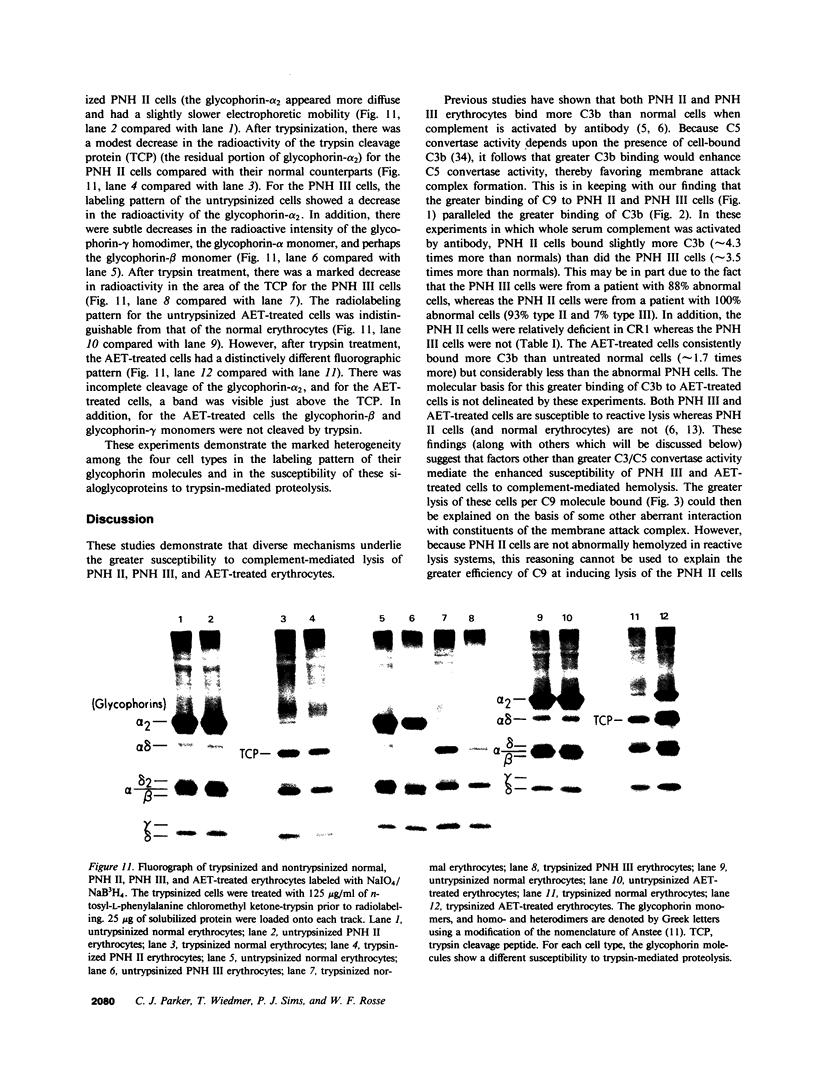
The affected erythrocytes of paroxysmal nocturnal hemoglobinuria (PNH II and PNH III cells) are abnormally sensitive to complement-mediated lysis. Normal human erythrocytes chemically modified by treatment with 2-amino-ethylisothiouronium bromide (AET) have been used as models for PNH cells inasmuch as they also exhibit an enhanced susceptibility to complement. To investigate the bases for the greater sensitivity of these abnormal cells to complement-mediated lysis, we compared binding of C3 and constituents of the membrane attack complex to normal, PNH II, PNH III, and AET-treated cells after classical pathway activation by antibody and fluid-phase activation by cobra venom factor complexes. When whole serum complement was activated by antibody, there was increased binding of C3 and C9 to PNH II, PNH III, and AET-treated cells, although the binding of these complement components to PNH II and PNH III cells was considerably greater than their binding to the AET-treated cells. In addition, all of the abnormal cell types showed a greater degree of lysis per C9 bound than did the normal erythrocytes. PNH III and AET-treated cells were readily lysed by fluid-phase activation of complement, whereas normal and PNH II erythrocytes were not susceptible to bystander lysis. The greater hemolysis of PNH III and AET-treated cells in this reactive lysis system was due to a quantitative increase in binding of constituents of the membrane attack complex. This more efficient binding of the terminal components after fluid-phase activation of whole serum complement was not mediated by cell-bound C3 fragments. These investigations demonstrate that the molecular events that characterize the enhanced susceptibility of PNH II, PNH III, and AET-treated erythrocytes to complement-mediated lysis are heterogeneous.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballow M., Cochrane C. G. Two anticomplementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol. 1969 Nov;103(5):944–952. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Membrane damage by complement. Biochim Biophys Acta. 1983 Aug 11;737(3-4):343–372. doi: 10.1016/0304-4157(83)90006-0. [DOI] [PubMed] [Google Scholar]
- Biesecker G., Müller-Eberhard H. J. The ninth component of human complement: purification and physicochemical characterization. J Immunol. 1980 Mar;124(3):1291–1296. [PubMed] [Google Scholar]
- Brauch H., Roelcke D., Rother U. Glycophorin A inhibits lysis by the complement attack phase. Immunobiology. 1983 Aug;165(2):115–120. doi: 10.1016/S0171-2985(83)80053-9. [DOI] [PubMed] [Google Scholar]
- Discipio R. G., Gagnon J. Characterization of human complement components C6 and C7. Mol Immunol. 1982 Nov;19(11):1425–1431. doi: 10.1016/0161-5890(82)90189-4. [DOI] [PubMed] [Google Scholar]
- Goldstein B. D. Production of paroxysmal nocturnal haemoglobinuria-like red cells by reducing and oxidizing agents. Br J Haematol. 1974 Jan;26(1):49–58. doi: 10.1111/j.1365-2141.1974.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. Lysis of erythrocytes by complement in the absence of antibody. J Exp Med. 1970 Nov;132(5):898–915. doi: 10.1084/jem.132.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer C. H., Abramovitz A. S., Mayer M. M. A new activity of complement component C3: cell-bound C3b potentiates lysis of erythrocytes by C5b,6 and terminal components. J Immunol. 1976 Sep;117(3):830–834. [PubMed] [Google Scholar]
- Hammer C. H., Nicholson A., Mayer M. M. On the mechanism of cytolysis by complement: evidence on insertion of C5b and C7 subunits of the C5b,6,7 complex into phospholipid bilayers of erythrocyte membranes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5076–5080. doi: 10.1073/pnas.72.12.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu V. W., Esser A. F., Podack E. R., Wisnieski B. J. The membrane attack mechanism of complement: photolabeling reveals insertion of terminal proteins into target membrane. J Immunol. 1981 Jul;127(1):380–386. [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. II. C8 and C9 release C5b67 from the surface of Salmonella minnesota S218 because the terminal complex does not insert into the bacterial outer membrane. J Exp Med. 1982 Mar 1;155(3):809–819. doi: 10.1084/jem.155.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. M., Shin M. L., Mayer M. M. On the lysis of paroxysmal nocturnal hemoglobinuria erythrocytes by complement: dual role of C3b. Blut. 1982 Oct;45(4):249–259. doi: 10.1007/BF00320192. [DOI] [PubMed] [Google Scholar]
- Kabakçi T., Rosse W. F., Logue G. L. The lysis of paroxysmal nocturnal haemoglobinuria red cells by serum and cobra factor. Br J Haematol. 1972 Dec;23(6):693–705. doi: 10.1111/j.1365-2141.1972.tb03484.x. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Pangburn M. K., Oldroyd R. G. Breakdown of C3 after complement activation. Identification of a new fragment C3g, using monoclonal antibodies. J Exp Med. 1982 Jul 1;156(1):205–216. doi: 10.1084/jem.156.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Logue G. L., Rosse W. F., Adams J. P. Mechanisms of immune lysis of red blood cells in vitro. I. Paroxysmal nocturnal hemoglobinuria cells. J Clin Invest. 1973 May;52(5):1129–1137. doi: 10.1172/JCI107279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod B., Baker P., Gewurz H. Studies on the inhibition of C56-initiated lysis (reactive lysis). III. Characterization of the inhibitory activity C567-INH and its mode of action. Immunology. 1975 Jan;28(1):133–149. [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Prince G. M., Mold C. Release of soluble immune complexes from immune adherence receptors on human erythrocytes is mediated by C3b inactivator independently of Beta 1H and is accompanied by generation of C3c. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5047–5051. doi: 10.1073/pnas.79.16.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Schreiber R. D. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosenfeld S. I., Austen K. F. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5066–5070. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H., Tanaka H. Species-specific inhibition by glycophorins of complement activation via the alternative pathway. Mol Immunol. 1983 Nov;20(11):1233–1236. doi: 10.1016/0161-5890(83)90148-7. [DOI] [PubMed] [Google Scholar]
- Packman C. H., Rosenfeld S. I., Jenkins D. E., Jr, Leddy J. P. Complement lysis of human erythrocytes. II. A unique interaction of human C8 and C9 with paroxysmal nocturnal hemoglobinuria erythrocytes. J Immunol. 1980 Jun;124(6):2818–2823. [PubMed] [Google Scholar]
- Packman C. H., Rosenfeld S. I., Jenkins D. E., Jr, Thiem P. A., Leddy J. P. Complement lysis of human erythrocytes. Differeing susceptibility of two types of paroxysmal nocturnal hemoglobinuria cells to C5b-9. J Clin Invest. 1979 Aug;64(2):428–433. doi: 10.1172/JCI109479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J Exp Med. 1980 Oct 1;152(4):1102–1114. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Trombold J. S., Müller-Eberhard H. J. Paroxysmal nocturnal hemoglobinuria: deficiency in factor H-like functions of the abnormal erythrocytes. J Exp Med. 1983 Jun 1;157(6):1971–1980. doi: 10.1084/jem.157.6.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. J., Baker P. J., Rosse W. F. Comparison of binding characteristics of factors B and H to C3b on normal and paroxysmal nocturnal hemoglobinuria erythrocytes. J Immunol. 1983 Nov;131(5):2484–2489. [PubMed] [Google Scholar]
- Parker C. J., Baker P. J., Rosse W. F. Increased enzymatic activity of the alternative pathway convertase when bound to the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1982 Feb;69(2):337–346. doi: 10.1172/JCI110457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. J., Soldato C. M., Rosse W. F. Abnormality of glycophorin-alpha on paroxysmal nocturnal hemoglobinuria erythrocytes. J Clin Invest. 1984 Apr;73(4):1130–1143. doi: 10.1172/JCI111299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. J., Soldato C. M., Telen M. J. Increased efficiency of binding of nascent C3b to the erythrocytes of chronic cold agglutinin disease. J Clin Invest. 1984 Sep;74(3):1050–1062. doi: 10.1172/JCI111472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Biesecker G., Kolb W. P., Müller-Eberhard H. J. The C5b-6 complex: reaction with C7, C8, C9. J Immunol. 1978 Aug;121(2):484–490. [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Esser A. F., Müller-Eberhard H. J. Structural similarities between C6 and C7 of human complement. J Immunol. 1979 Sep;123(3):1071–1077. [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. The C5b-6 complex: formation, isolation, and inhibition of its activity by lipoprotein and the S-protein of human serum. J Immunol. 1978 Jun;120(6):1841–1848. [PubMed] [Google Scholar]
- Reid K. B., Lowe D. M., Porter R. R. Isolation and characterization of C1q, a subcomponent of the first component of complement, from human and rabbit sera. Biochem J. 1972 Dec;130(3):749–763. doi: 10.1042/bj1300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld S. I., Jenkins D. E., Jr, Leddy J. P. Enhanced reactive lysis of paroxysmal nocturnal hemoglobinuria erythrocytes by C5b-9 does not involve increased C7 binding or cell-bound C3b. J Immunol. 1985 Jan;134(1):506–511. [PubMed] [Google Scholar]
- Ross G. D., Lambris J. D., Cain J. A., Newman S. L. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs CR1 cofactor activity. J Immunol. 1982 Nov;129(5):2051–2060. [PubMed] [Google Scholar]
- Ross G. D., Newman S. L., Lambris J. D., Devery-Pocius J. E., Cain J. A., Lachmann P. J. Generation of three different fragments of bound C3 with purified factor I or serum. II. Location of binding sites in the C3 fragments for factors B and H, complement receptors, and bovine conglutinin. J Exp Med. 1983 Aug 1;158(2):334–352. doi: 10.1084/jem.158.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F., Adams J. P., Thorpe A. M. The population of cells in paroxysmal nocturnal haemoglobinuria of intermediate sensitivity to complement lysis: significance and mechanism of increased immune lysis. Br J Haematol. 1974 Oct;28(2):181–190. doi: 10.1111/j.1365-2141.1974.tb06652.x. [DOI] [PubMed] [Google Scholar]
- Rosse W. F., Dacie J. V. Immune lysis of normal human and paroxysmal nocturnal hemoglobinuria (PNH) red blood cells. I. The sensitivity of PNH red cells to lysis by complement and specific antibody. J Clin Invest. 1966 May;45(5):736–748. doi: 10.1172/JCI105388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F., Logue G. L., Adams J., Crookston J. H. Mechanisms of immune lysis of the red cells in hereditary erythroblastic multinuclearity with a positive acidified serum test and paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1974 Jan;53(1):31–43. doi: 10.1172/JCI107551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F. Variations in the red cells in paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1973 Mar;24(3):327–342. doi: 10.1111/j.1365-2141.1973.tb01657.x. [DOI] [PubMed] [Google Scholar]
- SIRCHIA G., FERRONE S., MERCURIALI F. THE ACTION OF TWO SULFHYDRYL COMPOUNDS ON NORMAL HUMAN RED CELLS. RELATIONSHIP TO RED CELLS OF PAROXYSMAL NOCTURNAL HEMOGLOBINURIA. Blood. 1965 Apr;25:502–510. [PubMed] [Google Scholar]
- Sims P. J. Complement pores in erythrocyte membranes. Analysis of C8/C9 binding required for functional membrane damage. Biochim Biophys Acta. 1983 Aug 10;732(3):541–552. doi: 10.1016/0005-2736(83)90230-4. [DOI] [PubMed] [Google Scholar]
- Sims P. J. Complement protein C9 labeled with fluorescein isothiocyanate can be used to monitor C9 polymerization and formation of the cytolytic membrane lesion. Biochemistry. 1984 Jul 3;23(14):3248–3260. doi: 10.1021/bi00309a020. [DOI] [PubMed] [Google Scholar]
- Steckel E. W., Welbaum B. E., Sodetz J. M. Evidence of direct insertion of terminal complement proteins into cell membrane bilayers during cytolysis. Labeling by a photosensitive membrane probe reveals a major role for the eighth and ninth components. J Biol Chem. 1983 Apr 10;258(7):4318–4324. [PubMed] [Google Scholar]
- Steckel E. W., York R. G., Monahan J. B., Sodetz J. M. The eighth component of human complement. Purification and physicochemical characterization of its unusual subunit structure. J Biol Chem. 1980 Dec 25;255(24):11997–12005. [PubMed] [Google Scholar]
- Tenner A. J., Lesavre P. H., Cooper N. R. Purification and radiolabeling of human C1q. J Immunol. 1981 Aug;127(2):648–653. [PubMed] [Google Scholar]