Introduction
Psoriasis is a common, chronic inflammatory skin disorder that occurs worldwide. However, epidemiological, clinical, and therapeutic data pertaining to psoriasis in non-Caucasian racial/ethnic groups are currently limited. Psoriasis in darker skin types can present diagnostic challenges due to overlapping features with other papulosquamous disorders and less conspicuous erythema. Variations in clinical presentation and quality-of-life impact of psoriasis may contribute to nuances in the approach to treatment in patients with skin of color.
Epidemiology
Estimates of the prevalence of psoriasis in adults vary globally, with higher rates reported at higher latitudes and Western countries.1 Epidemiological data on psoriasis in non-Caucasian populations are limited, but suggest a lower prevalence compared to Caucasians (Table 1). A 2013 systematic review of published population-based studies found the prevalence of psoriasis in all ages to be lower overall in non-Caucasian populations, with zero cases in the Indian population of Latin America, 0.19 percent in Egypt, 0.44 percent in Sri-Lanka, 0.23 percent in Taiwan, and 0.123 to 0.35 percent in China.1 Studies in Australia found a prevalence of 2.6 percent in Caucasians and no cases in the Aboriginal population.2
TABLE 1.
Prevalence of psoriasis in non-Caucasian populations
| ESTIMATED PREVALENCE | STUDY DESIGN | REFERENCE |
|---|---|---|
| 1.9% in African Americans 3.6% in Caucasians | Cross-sectional study of 6,216 US Adults | Rachakonda et al, 20143 |
| 1.3% in African Americans 2.5% in Caucasians | Population-based study of 27,220 adults in the US | Gelfand et al, 20054 |
| 0.06% in African Americans 0.225% in Caucasians | Multiethnic population-based cohort of 710,949 children in southern California | Wu et al, 20115 |
| 0.22% in African Americans 1.28% in Caucasians | In-home interviews of 264,259 individuals in the US (all ages) | Adams et al, 19996 |
| 0% in Indians of Latin America | 25,915 individuals | Convit, 196234 |
| 0.19% in Egypt | Cross-sectional community-based survey of 8,008 individuals | Abdel-Hafez et al, 200335 |
| 2.6% in Kenya | Clinic-based investigation of 1,230 individuals | Verhagen and Koten, 196736 |
| 2.8% in Uganda | Clinic-based study of 3,371 individuals | Lomholt, 197137 |
| 3.3% in Tanzania | Clinic-based study of 990 individuals | Masawe, 197338 |
| 0.09% in Nigeria | Clinic record analysis of 5,459 individuals | Emokpare, 197639 |
| 0.05% in Mali | Clinic-based study of 20,000 individuals | Saint-Andre and Louvet, 197640 |
| 0.3% in Angola | Clinic-based study of 1,459 individuals | Oliveira, 195741 |
| 0.44% in Sri Lanka | Survey of 1,806 individuals in 418 households | Perera et al, 200042 |
| 0.23% in Taiwan | Database search of Taiwan National Health Insurance claims of 23 million individuals | Tsai et al, 201143 |
| 0.123% in China | Clinic-based study of 320,906 individuals and Population surveys of 674,245 individuals | Yip, 198444 |
Previously published reports found psoriasis to be uncommon (<1%) among African Americans, including those residing in the United States and in continental Africa. More recent population-based studies in the United States—despite showing a lower prevalence of psoriasis in blacks compared to Caucasians—have observed a higher prevalence of psoriasis among blacks than previously reported.3-5 In the 2009-2010 National Health and Nutrition Examination Survey (NHANES), which estimated the prevalence of psoriasis in US adults age 20 and older to be 3.2 percent, psoriasis was most common in Caucasians (3.6%), followed by African Americans (1.9%), and Hispanics (1.6%).3 The above prevalence among African Americans was slightly greater than that reported in an earlier study by Gelfand et al4 (1.3% in African Americans vs. 2.5% in Caucasians) and considerably higher than a 1996 National Health Interview Survey (0.22% in African Americans vs. 1.28% in Caucasians).6 However, different methodologies as well as variable inclusion of children and adolescents limit comparison of the above prevalence rates.
Though population-based studies on the prevalence of psoriasis have not been conducted in Africa, clinic-based studies have found differing prevalence rates dependent upon location, with higher prevalences (2.6-3.3%) in eastern African countries (Kenya, Uganda, and Tanzania), compared to western African countries (0.05-0.3% in Nigeria, Mali, Angola).2
Under-reporting and selection bias may contribute to observed differences in prevalence. Racial/ethnic disparities in access to a dermatologist have been reported in the United States and the likelihood of having undiagnosed psoriasis was higher among African Americans in a national study analyzing NHANES data from 2003 to 2004.7 Using data from 2,984 patients with an accompanying psoriasis questionnaire and standardized clinical photographs, the authors found that undiagnosed patients were more likely to be male, non-Caucasian, less educated, and unmarried.
The wide variation seen in prevalence worldwide is likely influenced, at least in part, by the environment, with higher frequencies seen in areas further from the equator.1,2 Reasons for this are likely multifactorial and include differences in sun exposure and other climate differences, but the role of genetics cannot be excluded. Diet may also play a role, with diets high in lineolic acid, as is seen in parts of Africa, possibly being protective according to one study.8
Genetics
While research over the last few decades has begun to elucidate psoriasis susceptibility genes, these studies have been composed primarily of individuals of European or Asian descent. A recent review of psoriasis research identified five genome-wide association studies (GWAS) covering more than 30 psoriasis susceptibility regions currently implicated in the disease, four of which were comprised solely of patients of European descent, while one studied European and Asian populations (Table 2).9
TABLE 2.
Genetics
| GENES | STUDY POPULATION | REFERENCE |
|---|---|---|
| HLA-C, IL23A, IL23R, IL23B, TNIP1, TNAIP3, IL4, IL13 | Initial set: 1,409 psoriasis cases, 1,436 controls Follow up: 5,048 psoriasis cases, 5,041 controls All individuals were of Caucasian European ancestry | Nair et al, 200945 |
| LCE3C, LCE3B | 9,389 psoriasis cases, 9,477 controls Individuals were of either European or Asian ancestry | Riveira-Munoz et al, 201146 |
| NOS2, FBXL19, NFKBIA | Initial set: 1,831 psoriasis cases, 2,546 controls Follow up: 4,064 psoriasis cases, 4,685 controls Meta-analysis of two previous studies45,47—all patients of European ancestry | Stuart et al, 201048 |
| Same set as Nair et al | Elder et al, 201049 | |
| IL28RA, REL, IFIH1, ERAP1, TRAF3IP2, NFK-BIA, TYK2 | 2,278 psoriasis cases, 5,175 controls Individuals were of self-reported European ancestry | Strange et al, 201050 |
Among the most strongly associated susceptibilities to psoriasis is HLA-Cw6, a gene encoding the major histocompatibility complex, important in the adaptive immune response.10 Despite this association, the prevalence of this allele is actually higher in Africans (15.09%) compared to Caucasians (9.62%).11,12 HLA-Cw6 distribution is also similar among Eastern and Western Africans, further complicating the links between observed differences in prevalence of psoriasis among these groups and genetic links.11
Interestingly, African Americans are more closely linked to Western Africans genetically, a group that has a lower incidence of psoriasis than those in Eastern African, despite similar climates, suggesting potential differences in susceptibility genes between East and West African populations.2,11,13
Studies conducted in other populations suggest that genetic markers may be dependent upon ethnicity. A small study (N=54) done with Arab Omani patients with plaque psoriasis found a significant association with HLA-B52 but not Cw6.14 A larger study involving a meta-analysis of eight studies including more than 1,000 psoriasis patients with controls also found ethnic differences, implicating IL-10 polymorphisms as significantly associated with psoriasis among Asians but not Europeans.15 Taken together, genetic and environmental factors likely contribute to observed epidemiological differences in psoriasis, and may potentially have implications on clinical presentation and responses to treatment.
Clinical Presentation
Psoriasis presentation among Caucasians and African blacks has been previously described as almost identical,16 but multiple cases of complicated presentations of psoriasis in Africans and African Americans have been reported.17-19 Differences can be seen not only in the presentation itself (erythema and pigment changes), but also in disease distribution and severity (Table 3).
TABLE 3.
Key nuances in clinical presentation of psoriasis in skin of color
|
Recent published survey results from national psoriasis experts who worked frequently with African American patients found that over half notice differences in presentations among their African American patients compared to Caucasians, with their African American patients experiencing a higher degree of dyspigmentation (41% of responders), but a lower amount of erythema (24% of responders).20
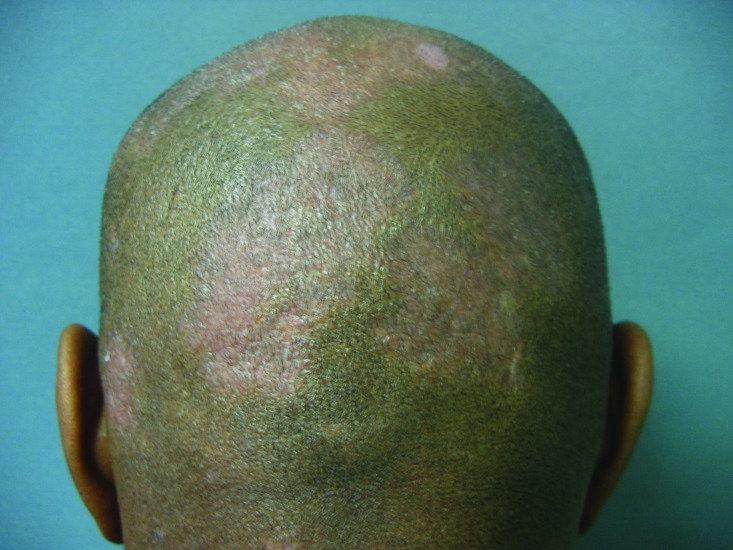
African American patients have also been reported to have more extensive disease involvement, with patients reporting 3 to 10 palms of involvement (3-10% of body surface area [BSA]) as compared to only 1 or 2 palms (1-2% of BSA) for Caucasian patients.4 When involvement includes the scalp, anecdotal clinical experience suggests that the severity tends to be increased in women of African ancestry, likely due to less frequent hair washing in women with naturally Afro-textured hair (Figure 1).
Figure 1.
Severe scalp psoriasis in an African American woman
In a post-hoc analysis of a community-based trial of etanercept, Shah et al25 reported significant differences in baseline BSA affected between racial/ethnic groups, with Caucasians having the lowest BSA of involvement at baseline. The mean baseline BSA in each of the racial/ethnic groups studied was 28.07, 31.71, 32.69, and 41.35 percent in Caucasians, African Americans, Hispanic/Latinos and Asians, respectively. Disparities in access to care and ethnic differences in utilization of dermatology services could contribute to the observed differences in baseline severity.
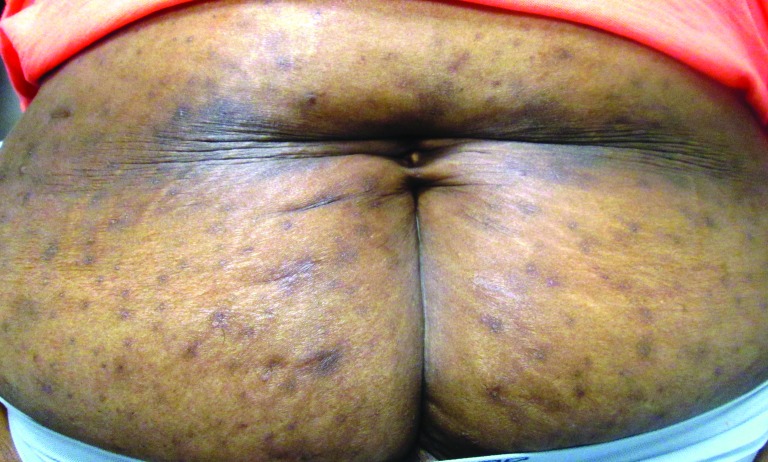
Dyspigmentation is a common concern among patients with skin of color21,22 and is due to effects of inflammatory mediators on melanogenesis.21 The mechanisms underlying psoriasis-associated dyspigmentation, which can be either postinflammatory hypo- or hyperpigmentation, have not been fully elucidated. However, new data suggest that IL-17 and tumor necrosis factor (TNF) work synergistically to inhibit melanogenesis, while also inducing numerous melanocyte mitogens.23 These findings suggest that in psoriasis, dyspigmentation may be related to alterations in melanin production, but also an increased number of melanocytes.
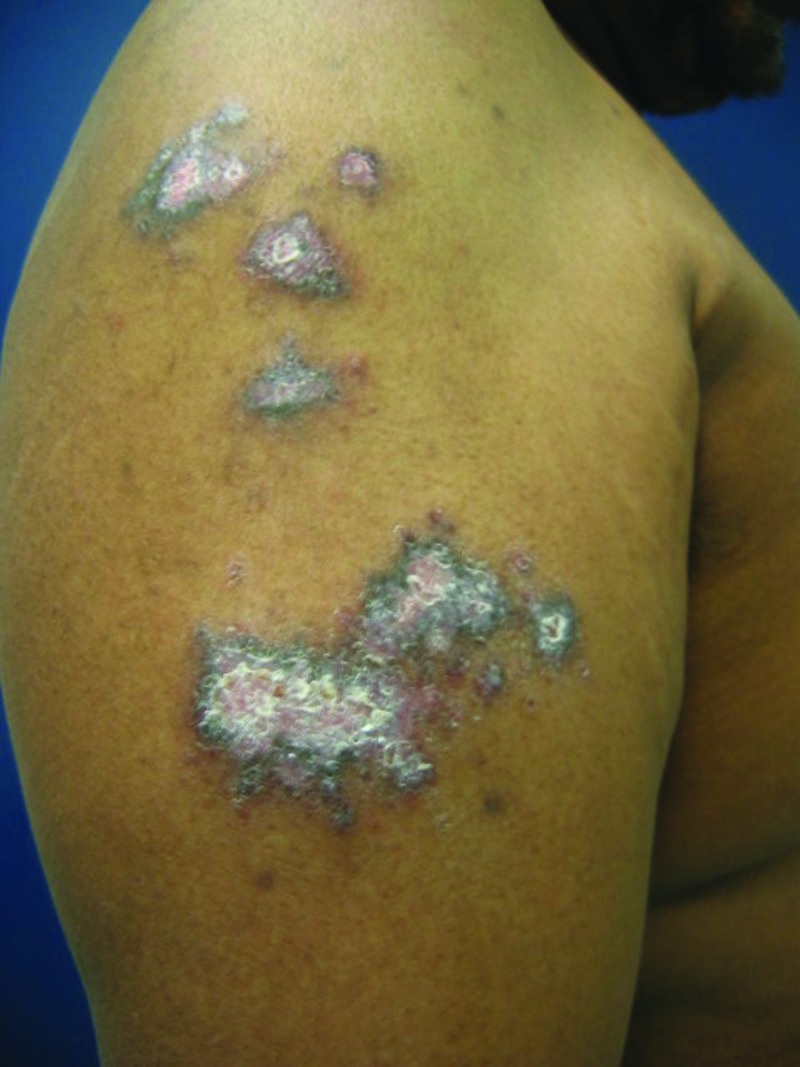
The frequent development of pigmentary abnormalities is a point to consider when assessing disease burden in darker skinned patients. Plaques of psoriasis in patients with skin of color tend to resolve with hyper- or hypopigmented patches that may take 3 to 12 months to resolve depending on the severity and anatomic location (Figure 2). This can be of equal or greater concern to the patient as the psoriasis itself.
Figure 2.
Postinflammatory hyperpigmentation secondary to psoriasis (persistent after complete clearance of psoriatic plaques).
In clinical research, the severity of psoriasis is commonly assessed using the PASI score (psoriasis area and severity index), which uses erythema as one of its indices. However, erythema can be more challenging to detect in darker skinned individuals, as involved areas may have a dark brown or violaceous hue instead of the pink or red color typically observed in patients with lighter complexions (Figure 3). This can be mistaken for postinflammatory hyperpigmentation, when in fact it may be a marker of active inflammation.24
Figure 3.
Psoriasis in an African American woman with Fitzpatrick skin phototype VI; note the violaceous hue
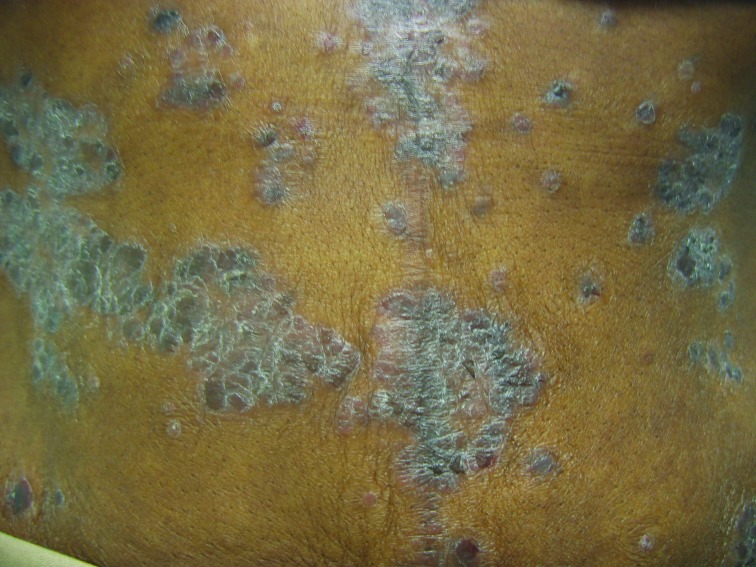
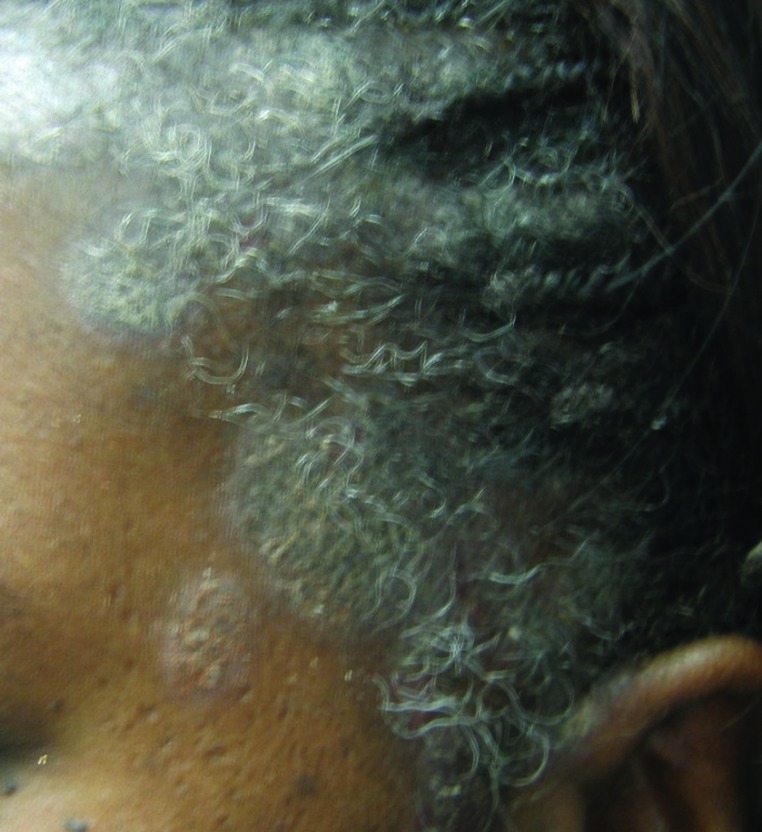
Further challenges can arise in those with Fitzpatrick skin type VI or heavily-pigmented skin, where distinguishing psoriasis from lichen planus (especially the hypertrophic type), sarcoidosis, and cutaneous lupus can be difficult and may require a biopsy (Figures 4A and 4B). A thorough skin examination is paramount such that diagnostic clues for psoriasis or its clinical mimickers can be identified. For example, identifying characteristic nail changes, the presence of sharply demarcated scaly plaques, or involvement of anatomic areas typical of psoriasis (such as the scalp, extensor surfaces, palms, soles, and intergluteal cleft) can help to confirm the diagnosis of psoriasis.
Figure 4.
Psoriasis mimickers: A) Sarcoidosis of the scalp; B) hypertrophic discoid lupus erythematosus
Quality-of-Life Impact
Shah et al25 identified differences in the quality-of-life impact of psoriasis patients enrolled in a community-based etanercept trial. In this study, African American and Hispanic/Latino psoriasis patients experienced a greater impairment in health-related quality-of-life compared to Caucasians, even when controlling for percent BSA affected and physicians global assessment (PGA).25 Impact was assessed using the Dermatology Life Quality Index (DLQI), a short questionnaire completed by patients that addresses the impact of disease and treatment on the patient’s life and includes both physical symptoms of itchiness and pain, as well as psychosocial impacts of embarrassment and interference with work and studying.
Observed racial/ethnic differences in health-related quality-of-life among psoriasis patients may be related to cultural variations in perceptions of skin disorders, such as psoriasis, as well as the potential impact of associated dyspigmentation commonly seen in patient populations with skin of color.
Further support for differences in quality of life between ethnicities is seen from a study conducted by the National Psoriasis Foundation that investigated the psychosocial impacts of psoriasis on 4,725 individuals.26 In that study, 72 percent of minorities reported an impact in their quality of life due to psoriasis, compared to 54 percent of Caucasian respondents. Among the quality-of-life complaints were feelings of self-consciousness, embarrassment, anger, frustration, and helplessness. A higher percentage of African American patients (23%) also reported having very severe psoriasis compared to Caucasian patients (8%).
Nuances to Treatment
Skin phototype, as well as ethnic and cultural factors, are potential considerations when making treatment recommendations for patients. For example, consideration of phototherapy in darker skin types should include a brief discussion about increased pigmentation (i.e., tanning) in exposed areas, which may be a concern for some patients depending on their own perceptions of ideal complexion. Cultural ideals regarding desirable skin complexion can vary in different populations.27,28
Also important, is to discuss the risk of postinflammatory hyperpigmentation, which can result if phototherapy-associated burns occur during the course of treatment. Furthermore, while the effects of phototherapy on preexisting postinflammatory hyperpigmentation have not been studied, it is possible that exposure to ultraviolet radiation may exacerbate hyperpigmentation. Notwithstanding these potential limitations, phototherapy remains a safe and effective treatment option for patients with skin of color.29 Including a discussion of the above considerations in patients with skin of color can help to improve treatment adherence and satisfaction.
When managing scalp psoriasis in patients of color—especially those of African ancestry—effort should be made to select a topical treatment regimen that is compatible with hair care practices and cultural preferences. This involves taking into consideration hair texture (e.g., Afro-textured vs. straight), styling (e.g., chemical relaxers, braids, weaves, locks, etc.), and washing frequency. Treatment that includes daily hair washing with a prescription shampoo is often not a suitable option for African American female patients, as such frequent hair washing may cause hair dryness, breakage, and interference with usual hair styling, ultimately leading to noncompliance and patient dissatisfaction. Instead, a more optimal treatment regimen for African American women may be weekly washing with a medicated shampoo in conjunction with once-to twice-daily application of a topical corticosteroid in a vehicle that does not adversely affect hair texture or preferred hair styling practices. In the current author’s (AFA) clinical experience, oils or oil-based suspensions, lotions, and emollient foams are generally the best suited vehicles for the majority of hair textures and styles observed in the African American patient population (although individual patient preference will vary and can include other vehicles). Treatment adherence and patient satisfaction can be improved by taking the above nuances into consideration. With this in mind, having a brief discussion about vehicle options and eliciting patient preferences is a useful approach to managing scalp psoriasis, especially in African American patients and other populations of African ancestry.
Clinicians should also inquire about the patient’s use of traditional therapies and be aware of practices that may potentially worsen psoriasis (e.g., through Koebner phenomenon) or may contribute to postinflammatory hyperpigmentation.30 Traditional Asian healing practices, such as cupping, coining, moxibustion, and herbal remedies, are examples of factors that may be relevant to the clinical presentation of psoriasis in some patients. Increased lichenification and hyperpigmentation secondary to excessive rubbing or scrubbing of lesions can be observed in some subsets of patients and can be influenced by cultural factors. Furthermore, the use of some herbal medicines can potentially be associated with drug-drug interactions.
While Phase 3 pivotal trials for biological treatment agents have included subjects belonging to a range of racial/ethnic groups, their proportions have not been representative of the US population (Table 4).31 The value of such identification and stratification can lead to recognition of potential differences in safety and therapeutic response within various groups. Such differences have already been seen in therapeutic agents for heart failure and diabetes. The landmark study of the combination drug BiDil (isosorbide dinitrate/hydralazine, [Arbor Pharmaceuticals][LLC][Atlanta][Georgia]) that investigated differences in effectiveness of the drug in congestive heart failure (CHF) patients found survival benefit in black patients with advanced heart failure, leading to United States Food and Drug Administration (FDA) approval of BiDil specifically in this population,32 while a recent analysis of the diabetic medication metformin in the control of HbA1c in African Americans compared to European Americans also found that the drug may be more effective in the African American population.33 Investigating the safety and efficacy of psoriasis therapies in diverse populations is important, given the potential for pharmacogenomic differences that may influence treatment outcomes.
TABLE 4.
Summary of Phase 3 trial demographic data for psoriasis biologics
| DRUG | TRIAL | STUDY PARTICIPANTS | DEMOGRAPHICS |
|---|---|---|---|
| Adalimumab | CHAMPION REVEAL | N = 271 N = 1212 | 257 Caucasian (94.8%), 14 non-Caucasian (5.2%)51 1,101 Caucasian (90.8%), 111 non-Caucasian (9.2%)52 |
| Etanercept | N = 652 N = 583 | 566 Caucasian (86.8%), 86 non-Caucasian (13.2%)53 528 Caucasian (90.6%), 55 non-Caucasian (9.4%)54 | |
| Ustekinumab | ACCEPT | N = 903 | 818 Caucasian (90.6%), 85 non-Caucasian (9.4%)55 |
In the aforementioned Phase 4 etanercept study by Shah et al,25 potential racial/ethnic differences in safety and efficacy were investigated. Evaluating improvements in percentage BSA and PGA following 12 weeks of etanercept, no differences were observed among Caucasians, African Americans, Hispanic/Latinos, and Asians. In addition, no differences in overall adverse events rates were observed between the above racial/ethnic groups studied.25
Conclusion
Understanding clinical and therapeutic nuances in psoriasis patients with skin of color is important given observed disparities in diagnosis and quality-of-life impact in this population. Taking into consideration potential racial/ethnic differences in clinical presentation, cultural factors, and desired treatment outcomes will ultimately improve the care of psoriasis patients in an increasingly diverse global population.
Footnotes
Disclosure:Dr. Alexis is a consultant for Amgen. Mr. Blackcloud reports no relevant conflicts of interest.
References
- 1.Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 2.Farber EM, Nall L. Psoriasis in the tropics. Epidemiologic, genetic, clinical, and therapeutic aspects. Dermatol Clin. 1994;12:805–816. [PubMed] [Google Scholar]
- 3.Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512–516. doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23–26. doi: 10.1016/j.jaad.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 5.Wu JJ, Black MH, Smith N, et al. Low prevalence of psoriasis among children and adolescents in a large multiethnic cohort in southern California. J Am Acad Dermatol. 2011;65:957–964. doi: 10.1016/j.jaad.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Adams PF, Hendershot GE, Marano MA, Centers for Disease C, Prevention/National Center for Health S. Current estimates from the National Health Interview Survey, 1996. Vital and health statistics Series 10, Data from the National Health Survey. 1999;200:1–203. [PubMed] [Google Scholar]
- 7.Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60:218–224. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Namazi MR. Why is psoriasis uncommon in Africans? The influence of dietary factors on the expression of psoriasis. Int J Dermatol. 2004;43:391–392. doi: 10.1111/j.1365-4632.2004.02126.x. [DOI] [PubMed] [Google Scholar]
- 9.Ryan C, Korman NJ, Gelfand JM, et al. Research gaps in psoriasis: opportunities for future studies. J Am Acad Dermatol. 2013 doi: 10.1016/j.jaad.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 10.Nair RP, Stuart PE, Nistor I, et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet. 2006;78:827–851. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandran V, Raychaudhuri SP. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun. 2010;34:J314–J321. doi: 10.1016/j.jaut.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Marsh S, Parham P, Barber LD. London: Academic Press; 2000. Cw*06 - Cw6. In: The HLA FactsBook. pp. 255–256. [Google Scholar]
- 13.Raychaudhuri SP, Farber EM. The prevalence of psoriasis in the world. J Eur Acad Dermatol Venereal. 2001;15:16–17. doi: 10.1046/j.1468-3083.2001.00192.x. [DOI] [PubMed] [Google Scholar]
- 14.Al-Mamari F, Al-Shirawi A, Banodkar D, et al. HLA antigens in Omani psoriasis vulgaris patients. Oman Med J. 2009;24:27–29. doi: 10.5001/omj.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YH, Choi SJ, Ji JD, Song GG. Associations between interleukin-10 polymorphisms and susceptibility to psoriasis: a metaanalysis. Inflammation Research: Official Journal of the European Histamine Research Society [et al]. 2012;6:657–663. doi: 10.1007/s00011-012-0458-2. [DOI] [PubMed] [Google Scholar]
- 16.Leder RO, Farber EM. The variable incidence of psoriasis in sub-Saharan Africa. Int J Dermatol. 1997;36:911–919. doi: 10.1046/j.1365-4362.1997.00251.x. [DOI] [PubMed] [Google Scholar]
- 17.Barlow RJ, Schulz EJ. Chronic subcorneal pustulosis with vasculitis: a variant of generalized pustular psoriasis in black South Africans. Br J Dermatol. 1991;124:470–474. doi: 10.1111/j.1365-2133.1991.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 18.Curtis AR, Yosipovitch G. Erythrodermic verrucous psoriasis. J Dermatolog Treat. 2012;23:215–218. doi: 10.3109/09546634.2010.550912. [DOI] [PubMed] [Google Scholar]
- 19.Delaney TA, Smith NP. Lichen planus mimicking and coexisting with psoriasis in a black patient. Australas J Dermatol. 1993;34:59–62. doi: 10.1111/j.1440-0960.1993.tb00859.x. [DOI] [PubMed] [Google Scholar]
- 20.McMichael AJ, Vachiramon V, Guzman-Sanchez DA, Camacho F. Psoriasis in African-Americans: a caregivers’ survey. J Drugs Dermatol. 2012;11:478–482. [PubMed] [Google Scholar]
- 21.Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387–394. [PubMed] [Google Scholar]
- 22.Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466–473. [PubMed] [Google Scholar]
- 23.Wang CQ, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741–2752. doi: 10.1038/jid.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petit A, Tailor A. Skin semiology and grading scales. In: Dadzie OE, Petit A, Alexis AF, editors. Ethnic Dermatology Principles and Practice. Chichester, West Sussex: Wiley-Blackwell; 2013. pp. 5–17. [Google Scholar]
- 25.Shah SK, Arthur A, Yang YC, Stevens S, Alexis AF. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866–872. [PubMed] [Google Scholar]
- 26.Adegbidi H, Atadokpede F, do Ango-Padonou F, Yedomon H. Keloid acne of the neck: epidemiological studies over 10 years. Int J Dermatol. 2005;44(Suppl l):49–50. doi: 10.1111/j.1365-4632.2005.02815.x. [DOI] [PubMed] [Google Scholar]
- 27.Talakoub L, Wesley NO. Differences in perceptions of beauty and cosmetic procedures performed in ethnic patients. Semin Gutan Med Surg. 2009;28:115–129. doi: 10.1016/j.sder.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 28.El-Essawi D, Musial JL, Hammad A, Lim HW. A survey of skin disease and skin-related issues in Arab Americans. J Am Acad Dermatol. 2007;56:933–938. doi: 10.1016/j.jaad.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Syed ZU, Hamzavi IH. Role of phototherapy in patients with skin of color. Semin Gutan Med Surg. 2011;30:184–189. doi: 10.1016/j.sder.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Petit A, Diallo M. Common skin conditions and ethnicity. In: Dadzie OE, Petit A, Alexis AF, editors. Ethnic Dermatology Principles and Practice. Chichester, West Sussex: Wiley-Blackwell; 2013. pp. 19–61. [Google Scholar]
- 31.Baron ED. Light-Based Therapies for Skin of Color. Dordrecht, New York: Springer; 2009. [Google Scholar]
- 32.Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 33.Keoki Williams L, Padhukasahasram B, Ahmedani BK, et al. Differing effects of metformin on glycemic control by race-ethnicity. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2014-1539. jc20141539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Convit J. Investigation of the incidence of psoriasis among Latin American Indians. Proceedings of the 13th Congress on Dermatology. Amsterdam. 1962:196.
- 35.Abdel-Hafez K, Abdel-Aty MA, Hofny ER. Prevalence of skin diseases in rural areas of Assiut Governorate, Upper Egypt. Int J Dermatol. 2003;42:887–892. doi: 10.1046/j.1365-4362.2003.01936.x. [DOI] [PubMed] [Google Scholar]
- 36.Verhagen AR, Koten JW. Psoriasis in Kenya. Arch Dermatol. 1967;96:39–41. [PubMed] [Google Scholar]
- 37.Lomholt G. Psoriasis in Uganda: a comparative study with other parts of Africa. In: Farber E, Cox AJ, editors. Psoriasis: Proceedings of an International Symposium 1971. Stanford, CA: Stanford University Press; 1971. pp. 41–48. [Google Scholar]
- 38.Masawe A. Psoriasis in Tanzania. Int Ps Bull. 1973;1:1. [Google Scholar]
- 39.Emokpare N. Psoriasis in Nigeria. Int Ps Bull. 1976;3:1. [Google Scholar]
- 40.Saint-Andre P, Louvet M. [Psoriasis in blacks of Bamako. Therapeutic notes] Ann Dermatol Syphiligr (Paris). 1976;103:666. [PubMed] [Google Scholar]
- 41.Oliveira AD. Notes on dermatology and venereology in two towns of Angola (Lobito and Benguela: 1954-1957) J Med (Lisbon). 1957;34:597. [PubMed] [Google Scholar]
- 42.Perera A, Atukorale DN, Sivayogan S, Ariyaratne VS, Karunaratne LA. Prevalence of skin diseases in suburban Sri Lanka. Ceylon Med J. 2000;45:123–128. doi: 10.4038/cmj.v45i3.8112. [DOI] [PubMed] [Google Scholar]
- 43.Tsai TF, Wang TS, Hung ST, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci. 2011;63:40–46. doi: 10.1016/j.jdermsci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Yip SY. The prevalence of psoriasis in the Mongoloid race. J Am Acad Dermatol. 1984;10:965–968. doi: 10.1016/s0190-9622(84)80314-x. [DOI] [PubMed] [Google Scholar]
- 45.Nair RP, Duffin KC, Helms C, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riveira-Munoz E, He SM, Escaramis G, et al. Meta-analysis confirms the LCE3C_LCE3B deletion as a risk factor for psoriasis in several ethnic groups and finds interaction with HLA-Cw6. J Invest Dermatol. 2011;131:1105–1109. doi: 10.1038/jid.2010.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellinghaus E, Ellinghaus D, Stuart PE, et al. Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat Genet. 2010;42:991–995. doi: 10.1038/ng.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stuart PE, Nair RP, Ellinghaus E, et al. Genome-wide association analysis identifies three psoriasis susceptibility loci. Nat Genet. 2010;42:1000–1004. doi: 10.1038/ng.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elder JT, Bruce AT, Gudjonsson JE, et al. Molecular dissection of psoriasis: integrating genetics and biology. J Invest Dermatol. 2010;130:1213–1226. doi: 10.1038/jid.2009.319. [DOI] [PubMed] [Google Scholar]
- 50.Genetic Analysis of Psoriasis C the Wellcome Trust Case Control C. Strange A, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985–990. doi: 10.1038/ng.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saurat JH, Stingl G, Dubertret L, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) Br J Dermatol. 2008;158:558–566. doi: 10.1111/j.1365-2133.2007.08315.x. [DOI] [PubMed] [Google Scholar]
- 52.Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58:106–115. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 53.Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–2022. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 54.Papp KA, Tyring S, Lahfa M, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152:1304–1312. doi: 10.1111/j.1365-2133.2005.06688.x. [DOI] [PubMed] [Google Scholar]
- 55.Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118–128. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]