Abstract
Objective: To evaluate the benefits of efinaconazole topical solution, 10% on quality of life in onychomycosis patients. Methods: An analysis of 1,655 patients, aged 18 to 70 years, randomized to receive efinaconazole topical solution, 10%, or vehicle from two identical multicenter, double-blind, vehicle-controlled, 48-week studies evaluating safety and efficacy. The primary endpoint was complete cure rate (0% clinical involvement of target toenail, and both negative potassium hydroxide examination and fungal culture); clinical improvement defined as ≤ 10-percent improvement in nail involvement both at Week 52. Quality of life was assessed using a validated OnyCOE-t™ questionnaire. Improvement in quality of life was compared in those patients clinically and not clinically improved. Results: Efinaconazole topical solution, 10%, was significantly more effective than vehicle irrespective of QoL domain. Greatest improvement in mean score was seen in those domains with the lowest baseline scores. All mean scores in the group considered to have clinically improved with efinaconazole exceeded 80.0 at Week 52. Mean treatment satisfaction scores with efinaconazole in those patients who were clinically improved increased from 79.9 (Week 24) to 89.2 (Week 52), compared to a corresponding drop in those patients considered not improved from 65.3 to 58.0. The correlation between change in percent affected nail and change in mean domain scores was significant with efinaconazole for all domains. Limitations: A period of 52 weeks may be too brief to evaluate improvement in quality of life in onychomycosis patients. Some of the questions in the OnyCOE-t questionnaire may be more relevant than others to the study population and the onychomycosis population as a whole. Conclusion: Once-daily efinaconazole topical solution, 10%, provided statistically greater improvement in all aspects of quality of life compared to vehicle. Improvement was most marked in those patients considered clinically improved and correlated with a change in percent affected nail.
Onychomycosis is a common, growing problem in dermatology practice, most frequently seen in toenails and difficult to treat successfully.1,2 It is a progressive disease, not self-healing, and occasionally the source of more widespread infection.3 Many patients suffer long-standing disease affecting several toenails.4
Onychomycosis can have significant impact on the individual and other family members.5-7 The unsightly appearance of the toenail is a real concern, and often the initial reason why patients seek medical help. Indeed, the appearance of the toenail may have a more significant impact on quality of life (QoL) than the severity of the disease.8
It can disrupt daily activities, negatively impact QoL, and occasionally results in significant pain and discomfort.5 The symptoms of onychomycosis and their impact on personal appearance are important determinants of patients’ perceptions of their own health. The effect of onychomycosis is greater on psychosocial than physical functioning and is directly related to the extent of nail involvement.9 Data are conflicting as to whether nail appearance or disease severity are the more important from a patient’s perspective of QoL. Area alone does not necessarily predict disease severity, and a thick nail with limited involvement may have a poor prognosis.10 Commonly reported psychosocial factors include embarrassment, low self-esteem, and social withdrawal.11
The true burden of disease is unknown, but patients’ desire to have their affected nails cured is clear.5,12 Patient surveys have noted a substantial impact in several QoL domains including physical functioning (associated with foot-related activities), social functioning (affected by pain and discomfort restricting social activities and embarrassment associated with the nail appearance), and emotional well-being affected by negative feedback received from their social environment.13-15
A number of disease-targeted questionnaires have been developed to assess the impact of onychomycosis and its treatment on health-related QoL. The instruments differ considerably in the extent to which they have been psychometrically tested. The generic and disease-targeted scales of most of the available questionnaires exhibit poor variability, which may limit their responsiveness to clinically important change.9 As a result; there is a lack of data to demonstrate the true QoL response to onychomycosis treatment.
Improvements in QoL have been reported to correlate with the improvement or cure of infected nails.16-20 As a result, patients satisfied with their treatment had better health-related QoL;21 although patients with recurrent disease had significantly poorer QoL than those with onychomycosis for the first time that had not previously been treated.22 A significant portion of patients feel unattractive and stigmatized by onychomycosis and antimycotic therapy has been reported to result in a significant reduction of stigmatization of about 60 percent from baseline levels.23
A toenail-specific questionnaire, the OnyCOE-t™, and objective clinical measures have been used to assess responsiveness of patients in a prospective onychomycosis trial.24,25 Patients were treated with terbinafine (± aggressive debridement) and comparisons made between those patients who were judged to have clinically improved and those who had not improved.
The objective of this current study was to utilize this validated OnyCOE-t questionnaire to assess differences in QoL response in onychomycosis patients following treatment with efinaconazole topical solution, 10%, or vehicle. In addition, the authors compared results in those patients who were considered clinically improved (≤10% nail involvement) with those who were not clinically improved at Week 52 following active treatment, to assess the clinical meaningfulness of their results.
METHODS
Two multicenter, randomized, double-blind, vehicle-controlled studies designed to evaluate the efficacy, safety, and tolerability of efinaconazole topical solution, 10%, relative to its vehicle in 1,655 male and female patients aged 18 to 70 years with mild-to-moderate toenail onychomycosis.
Patients who presented with 20 to 50 percent clinical involvement of the target toenail were randomized (3:1) to apply blinded study drug once daily to the toenails for 48 weeks. There was a four-week follow-up (Week 52).
Quality-of-life evaluation. Patients self-administered the OnyCOE-t questionnaire at the time of enrollment (baseline), and at Weeks 24 and 52. The OnyCOE-t questionnaire23,24 comprised 33 items (Table 1), grouped into multi- or single-item scales, as follows:
A Toenail Symptom assessment, comprising both Symptom Frequency and Symptom Bothersomeness scales
An Appearance Problems scale
A Physical Activities Problems scale
An Overall Problem scale
A Stigma scale, and
A Treatment Satisfaction scale.
TABLE 1.
| Q1: In the past four weeks, how often have you had the followi (1=never to 5=very often)? How bothered were you (1=not at all to 5=extremely)? |
|
| Q2: During the PAST 4 WEEKS, how much of a problem were the following because of your nail fungal condition (1=very much to 4=not a problem)? |
|
| Q3: How much of the following situations describe you because of your nai fungal condition (0=does not describe me at all to 4=describes me very well)? |
|
| Q4: The following questions ask you to assess your satisfaction with your nail treatment program (1=very satisfied to 5=very dissatisfied)? |
|
All items in the OnyCOE-t questionnaire were transformed to a 0 to 100 scale; higher scores indicated better functioning.24 Each scale score was calculated as an average of all non-missing items if at least half of the items making up the scale were non-missing.
Statistical analyses were performed on the overall patient population to compare QoL data from those patients treated with efinaconazole topical solution, 10%, and those on vehicle, and two subgroups—those patients who were considered clinically improved (i.e., those patients who had ≤10% nail involvement at Week 52) and those who were not considered clinically improved at Week 52.
RESULTS
Baseline QoL domain scores for those patients treated with efinaconazole topical solution, 10%, ranged from 46.7 (Overall Problem) to 70.7 (Stigma). Scores were similar to those recorded by patients subsequently treated with vehicle.
Overall impact of efinaconazole versus vehicle (OC) on QoL domains. Overall, efinaconazole topical solution, 10%, was significantly more effective than vehicle in improving mean scores irrespective of QoL domain (Table 2).
TABLE 2.
Mean change (SD) in Domain Scores from baseline to Week 52: ITT Population Observed Cases
| FINACONAZOLE (N=1,236) | VEHICLE (N=415) | DIFFERENCE (p-VALUE) | |
|---|---|---|---|
| Symptom Frequency | 26.5 (24.0) | 16.2 (25.6) | 10.4 (<0.001) |
| Symptom Bothersomeness | 17.8 (23.2) | 10.9 (25.3) | 6.9 (<0.001) |
| Physical Activities Problems | 17.5 (27.4) | 11.5 (25.9) | 6.0 (0.002) |
| Appearance Problems | 22.6 (24.9) | 14.9 (25.2) | 7.7 (<0.001) |
| Overall Problem | 28.0 (33.5) | 17.1 (33.1) | 10.9 (<0.001) |
| Stigma | 9.2 (21.6) | 4.3 (19.4) | 4.9 (0.002) |
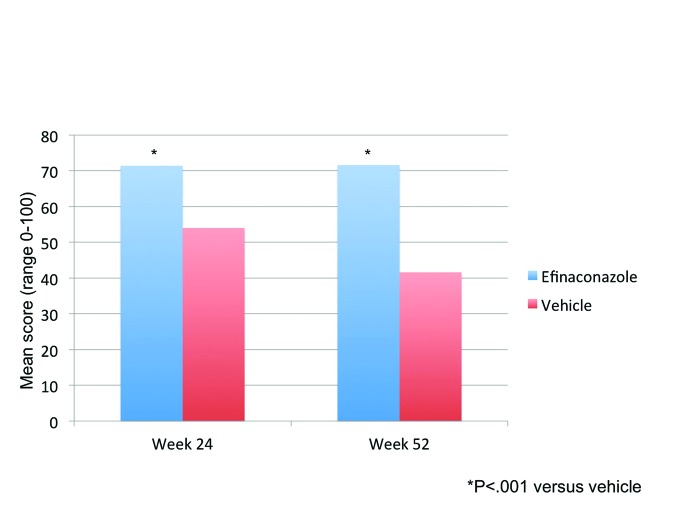
Treatment satisfaction was not assessed at baseline, but was significantly higher than vehicle at Weeks 24 and 52 (four weeks after treatment had stopped). Mean treatment satisfaction scores with efinaconazole topical solution, 10%, were maintained at over 71/100 and at Week 52 were 30 points higher than vehicle (Figure 1). With vehicle, the mean treatment satisfaction score at Week 24 was statistically lower at 54.0 (p<0.001), and reduced further to 41.6 (p<0.001) by Week 52.
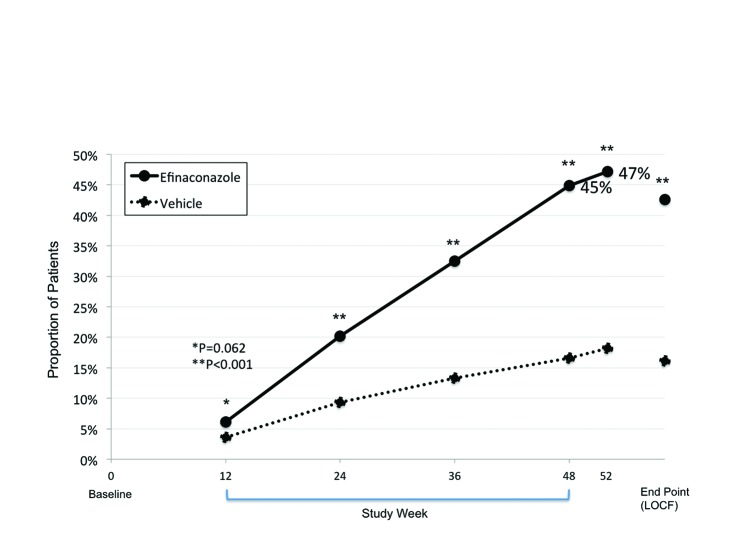
Figure 1.
Secondary efficacy endpoint: Patients considered clinically improved (≤10% clinical involvement of the target toenail) Weeks 12-52 (OC) and at End Point (LOCF), ITT pooled data
By Week 52, mean change from baseline in domain scores ranged from 9.2 (Stigma) to 28.0 (Overall Problem) following treatment with efinaconazole topical solution, 10%. AH changes were significant compared to vehicle (p≤0.002). Improvement over vehicle in domain scores ranged from 4.9 (Stigma) to 10.9 (Overall Problem), see Table 2.
Impact of efinaconazole versus vehicle (OC) on QoL domains: Patients considered clinically improved. Mean treatment satisfaction scores with efinaconazole in those patients who were considered clinically improved at the end of the study increased from 79.9 at Week 24 to 89.2 at Week 52, compared to a corresponding drop in scores for those patients considered not improved (from 65.3 to 58.0, respectively), see Figure 2.
Figure 2.
Treatment satisfaction: Comparison between patients treated with efinaconazole and vehicle (Weeks 24 and 52)
Improvement in treatment satisfaction was statistically significant (efinaconazole vs. vehicle) at Week 52 in those patients who were clinically improved (p<0.001).
All mean domain scores in patients considered to be clinically improved following efinaconazole treatment exceeded 80.0 (where 100 was the maximum score achievable). Greatest improvement was seen in those domains with lower scores at baseline. At Week 52, mean change in scores from baseline ranged from 9.3 (Stigma) to 33.7 (Overall Problem). These data contrasted with a range of 9.1 (Stigma) to 23.5 (Overall Problem) in those patients deemed clinically not improved (Table 3). Biggest differences were seen in Overall Problem and Symptom Frequency domains.
TABLE 3.
Mean change (SD) in scores from baseline to Week 52: Responsiveness based on clinical improvement (≤10% nail involvement)—ITT Population Observed Cases
| TREATMENT WITH EFINACONAZOLE 10% SOLUTION | CLINIC IMPROVED (N=527) | CLINICALLY NOT IMPROVED (N=709) | DIFFERENCE |
|---|---|---|---|
| Symptom Frequency | 32.2 (22.5) | 22.1 (24.2) | 10.1 |
| Symptom Bothersomeness | 22.1 (23.4) | 14.9 (22.7) | 7.2 |
| Physical Activities Problems | 21.2 (28.0) | 14.6 (26.6) | 6.6 |
| Appearance Problems | 26.8 (25.0) | 19.3 (24.4) | 7.5 |
| Overall Problem | 33.7 (35.0) | 23.5 (31.7) | 10.2 |
| Stigma | 9.3 (23.4) | 9.1 (20.1) | 0.2 |
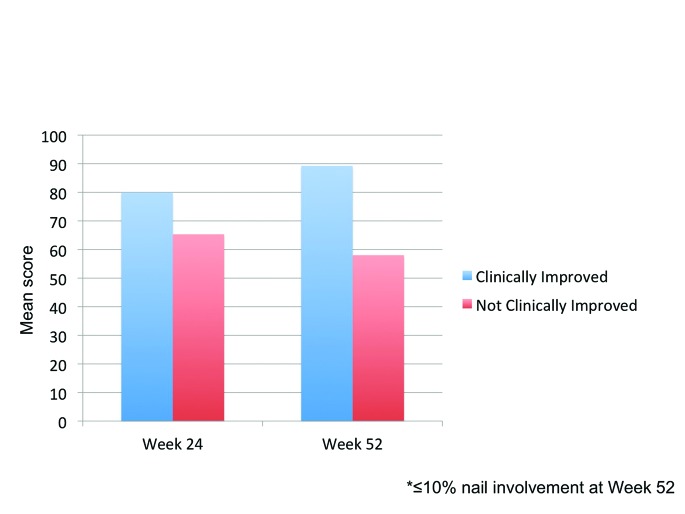
Percent improvement in mean domain scores with efinaconazole topical solution, 10% in those patients considered clinically improved ranged from 13.2 (Stigma) to 72.1 percent (Overall Problem), see Figure 3.
Figure 3.
Treatment satisfaction: Comparison between patients defined as clinically improved* and those not clinically improved following efinaconazole treatment (Weeks 24 and 52)
Although a number of patients were not clinically improved at Week 52, those treated with efinaconazole still showed statistically significant improvements in a number of domains compared to vehicle— Symptom Frequency (p<0.001), Appearance Problems (p=0.010), Overall Problem (p=0.006), and Stigma (p=0.002).
Correlation between improvements in affected nail and QoL. The correlation between change in percent affected nail and change in mean domain scores was significant with efinaconazole for all domains. The correlation was greatest for Symptom Frequency, Symptom Bothersomeness, Appearance Problems, Overall Problem, and Treatment Satisfaction (all p<0.001).
DISCUSSION
The burden of illness from onychomycosis is well documented. However, studies where validated QoL instruments have shown the impact of successful treatment are limited.25 The authors’ goal was to present data following incorporation of the OnyCOE-t questionnaire into a clinical trial program of onychomycosis patients expected to have different clinical outcomes (e.g., active versus vehicle treatment groups), as well as providing for sharper distinctions between improved and not improved active treatment groups based on a clinically meaningful outcome.
It has been reported that treatment satisfaction correlates with better health-related QoL.21 In the authors’ study, treatment satisfaction scores with efinaconazole topical solution, 10%, were maintained at high levels throughout, whereas scores dropped markedly in patients treated with vehicle. Efinaconazole topical solution, 10%, was significantly more effective than vehicle in improving QoL, irrespective of domain. Symptom Frequency and Overall Problem showed the biggest difference between active and vehicle, with similar results to those reported previously with terbinafine using the same questionnaire.25
Improvements in QoL have been reported to correlate with improvement or cure of the infected nails.16-20 The authors considered those patients who had ≤10 percent nail involvement at Week 52 clinically improved. This represented almost half of the patients treated with efinaconazole. There was a significant divergence in the treatment satisfaction scores between those patients considered clinically improved and those not improved.
Again, Symptom Frequency and Overall Problem were the domains showing the biggest score increases, and also the greatest difference between the two efinaconazole-treated groups. Although most clinicians would consider a ≤ 10-percent nail involvement at Week 52 as clinically relevant, the authors were able to show a significant correlation between percent affected nail and change in score for each domain. In addition, a number of patients who were considered not improved at Week 52 had significant improvements in many QoL parameters. This finding suggests that any improvement in nail appearance is important and can have a positive impact on QoL.
A significant reduction of stigma-tization following treatment has been previously reported.23 This was not seen in the authors’ study (in terms of an improving score), nor was there any difference between patients clinically improved or not improved. This could be due to higher baseline scores for Stigma compared to other domains, differences in questionnaire design or study demographics (for example, fingernail involvement was a major variable negatively influencing the stigmatization level; and younger, female patients were more stigmatized).
The QoL benefits of efinaconazole topical solution, 10%, the authors noted may be different to those seen in a real-world dermatology and podiatry setting due to study demographics. Consistent with other onychomycosis studies, the authors’ included more men (77.2%) and the mean age was 51.5 years. However, onychomycosis has been shown to more likely cause embarrassment in women than in men, with nail polish frequently used to mask the problem.14 Furthermore, younger people seem to be more affected emotionally by their nail’s appearance.22 The burden of illness is greatest in patients with long-standing disease affecting multiple toenails. It has been observed that patients with onychomycosis for ≥10 years have more psychosocial than physically impaired health-related QoL; the psychosocial ramifications directly related to the number of toenails involved.13 These patients were well represented in the authors’ study; 46.5 percent of patients having onychomycosis for more than five years and 42.7 percent with more than two affected non-target toenails.
The authors’ results in toenail onychomycosis cannot be generalized to all onychomycosis. Fingernail involvement (on its own or coexisting with toenail onychomycosis) has been reported to have a greater impact on QoL.27
Clinical assessments in the authors’ studies were confined to the target great toenail. The OnyCOE-t questionnaire captures patient’s perception of the effects of disease and treatment on all affected toenails. In a real-world scenario, this research artifact would not exist.
This study is the first to use a validated instrument specific to toenail onychomycosis within a clinical program. The authors were able to show that once-daily topical efinaconazole solution, 10%, provided statistically greater improvement in all aspects of QoL compared to vehicle. Improvement was most marked in those patients considered clinically improved and correlated with a change in percent affected nail.
ACKNOWLEDGMENT
The authors acknowledge Brian Bulley, MSc, of Inergy Limited for medical writing support. Valeant Pharmaceuticals North America LLC funded Inergy’s activities pertaining to this manuscript.
Footnotes
DISCLOSURE:Dr. Tosti is a consultant for Valeant. Dr. Elewski is a consultant for Valeant and Anacor and an investigator for Anacor and Dow.
REFERENCES
- 1.Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244–248. doi: 10.1067/mjd.2000.104794. [DOI] [PubMed] [Google Scholar]
- 2.Szepietowski JC, Reich A, Garlowska E, et al. Factors influencing coexistence of toenail onychomycosis with tinea pedis and other dermatomycoses: a survey of 2761 patients. Arch Dermatol. 2006;142:1279–1284. doi: 10.1001/archderm.142.10.1279. [DOI] [PubMed] [Google Scholar]
- 3.Seebacher C, Brasch J, Abeck D, et al. Onychomycosis. Mycoses. 2007;50:321–327. doi: 10.1111/j.1439-0507.2006.01351.x. [DOI] [PubMed] [Google Scholar]
- 4.Assaf RR, Elewski BE. Intermittent fluconazole dosing in patients with onychomycosis: results of a pilot study. J Am Acad Dermatol. 1996;35:216–219. doi: 10.1016/s0190-9622(96)90327-8. (2 Pt 1) [DOI] [PubMed] [Google Scholar]
- 5.Schein JR, Gause D, Stier DM, et al. Onychomycosis: baseline results of an observational study. J Am Pod Med Ass. 1997;87:512–519. doi: 10.7547/87507315-87-11-512. [DOI] [PubMed] [Google Scholar]
- 6.Scher RK. Onychomycosis: a significant medical disorder. J Am Acad Dermatol. 1996;35:S2–S5. doi: 10.1016/s0190-9622(96)90061-4. (3 Pt 2) [DOI] [PubMed] [Google Scholar]
- 7.Milobratovic D, Jankovic S, Vukicevic J, et al. Quality of life in patients with toenail onychomycosis. Mycoses. doi: 10.1111/myc.12072. 2013 Mar 18. [DOI] [PubMed] [Google Scholar]
- 8.Beleyayeva E, Gregoriou S, Chalikias J, et al. The impact of nail disorders on quality of life. Eur J Dermatol. 2013;23:336–371. doi: 10.1684/ejd.2013.2048. [DOI] [PubMed] [Google Scholar]
- 9.Shaw JW, Joish VN, Coons SJ. Onychomycosis: health-related quality of life considerations. Pharmacoeconomics. 2002;20:23–36. doi: 10.2165/00019053-200220010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Carney C, Tosti A, Daniel R, et al. A new classification system for grading the severity of onychomycosis: onychomycosis severity index. Arch Dermatol. 2011;147:1277–1282. doi: 10.1001/archdermatol.2011.267. [DOI] [PubMed] [Google Scholar]
- 11.Chacon A, Franca K, Fernandez A, et al. Psychosocial impact of onychomycosis: a review. Int J Dermatol. 2013;52:1300–1307. doi: 10.1111/ijd.12122. [DOI] [PubMed] [Google Scholar]
- 12.Drake LA. Quality of life issues for patients with fungal nail infections. AIDS Patient Care. 1995;9(suppl 1):S15. [PubMed] [Google Scholar]
- 13.Elewski BE. The effect of toenail onychomycosis on patient quality of life. Int J Dermatol. 1997;36:754–756. doi: 10.1046/j.1365-4362.1997.00163.x. [DOI] [PubMed] [Google Scholar]
- 14.Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5 Pt 1):702–704. doi: 10.1016/s0190-9622(98)70199-9. [DOI] [PubMed] [Google Scholar]
- 15.Lubeck DP. Measuring health-related quality of life in onychomycosis. J Am Acad Dermatol. 1998;38:S64–S68. doi: 10.1016/s0190-9622(98)70487-6. [DOI] [PubMed] [Google Scholar]
- 16.Warshaw EM, Foster JK, Cham PM, et al. NailQoL: a quality-of-life instrument for onychomycosis. Int J Dermatol. 2007;46:1279–1286. doi: 10.1111/j.1365-4632.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 17.Lubeck DP, Cause D, Schein JR, et al. A health-related quality of life measure for use in patients with onychomycosis: a validation study. Qual Life Res. 1999;8:121–129. doi: 10.1023/a:1026429012353. [DOI] [PubMed] [Google Scholar]
- 18.Lubeck DP, Schein JR, Cause D, et al. Health-related quality of life in patients with toenail onychomycosis: data from 9-month observational study. J Clin Outcomes Manage. 1999;6:37–42. [Google Scholar]
- 19.Turner RR, Testa MA. Measuring the impact of onychomycosis on patient quality of life. Qual Life Res. 2000;9:39–53. doi: 10.1023/a:1008986826756. [DOI] [PubMed] [Google Scholar]
- 20.Firooz A, Khamesipour A, Dowlati Y. Itraconazole pulse therapy improves the quality of life of patients with toenail onychomycosis. J Dermatol Treat. 2003;14:95–98. doi: 10.1080/09546630310012109. [DOI] [PubMed] [Google Scholar]
- 21.Drake LA, Patrick DL, Fleckman P, et al. The impact of onychomycosis on quality of life: development of an international onychomycosis-specific questionnaire to measure patient quality of life. J Am Acad Dermatol. 1999;41(2Pt 1):189–196. doi: 10.1016/s0190-9622(99)70047-2. [DOI] [PubMed] [Google Scholar]
- 22.Szepietowski JC, Reich A, Paean P, et al. Evaluation of quality of life in patients with toenail onychomycosis by Polish version of an international onychomycosis-specific questionnaire. J Eur Acad Dermatol Venerol. 2007;21:491–496. doi: 10.1111/j.1468-3083.2006.02004.x. [DOI] [PubMed] [Google Scholar]
- 23.Szepietowski JC. Reich A; for the National Quality of Life in Dermatology Group. Stigmatisation in onychomycosis patients: a population-based study. Mycoses. 2008;52:343–349. doi: 10.1111/j.1439-0507.2008.01618.x. [DOI] [PubMed] [Google Scholar]
- 24.Potter LP, Mathias SD, Raut M, et al. The OnyCOE-t™ questionnaire: responsiveness and meaningfulness of patient-reported outcomes questionnaire for toenail onychomycosis. Health Qual Life Outcomes. 2006;4:50. doi: 10.1186/1477-7525-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potter LP, Mathias SD, Raut M, et al. The impact of aggressive debridement used as an adjunct therapy with terbinafine on perceptions of patients undergoing treatment for toenail onychomycosis. J Dermatol Treat. 2007;18:46–52. doi: 10.1080/09546630600965004. [DOI] [PubMed] [Google Scholar]
- 26.Elewski BE, Rich P, PoUak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase 3 multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600–608. doi: 10.1016/j.jaad.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Szepietowski JC, Reich A, Wozniak M, et al. Evaluation of quality of life in patients with onychomycosis using the Polish version of Dermatology Life Quality Index. Mikol Lek. 2006;13:193–198. [Google Scholar]