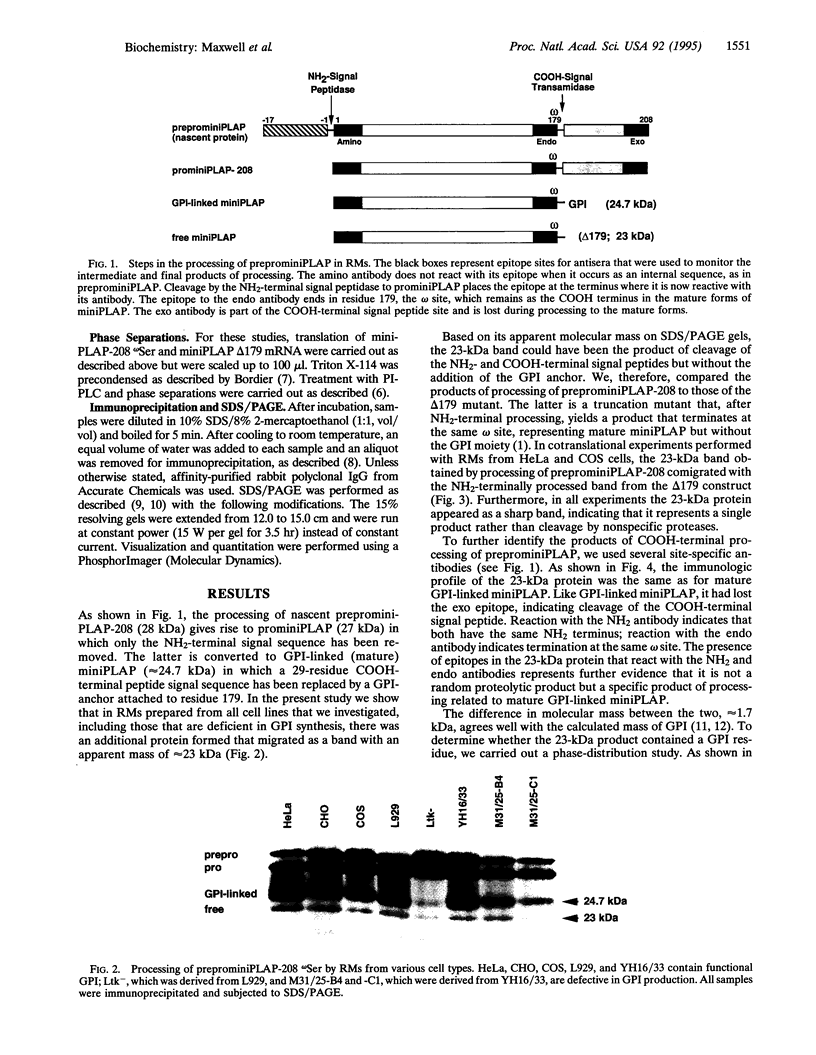
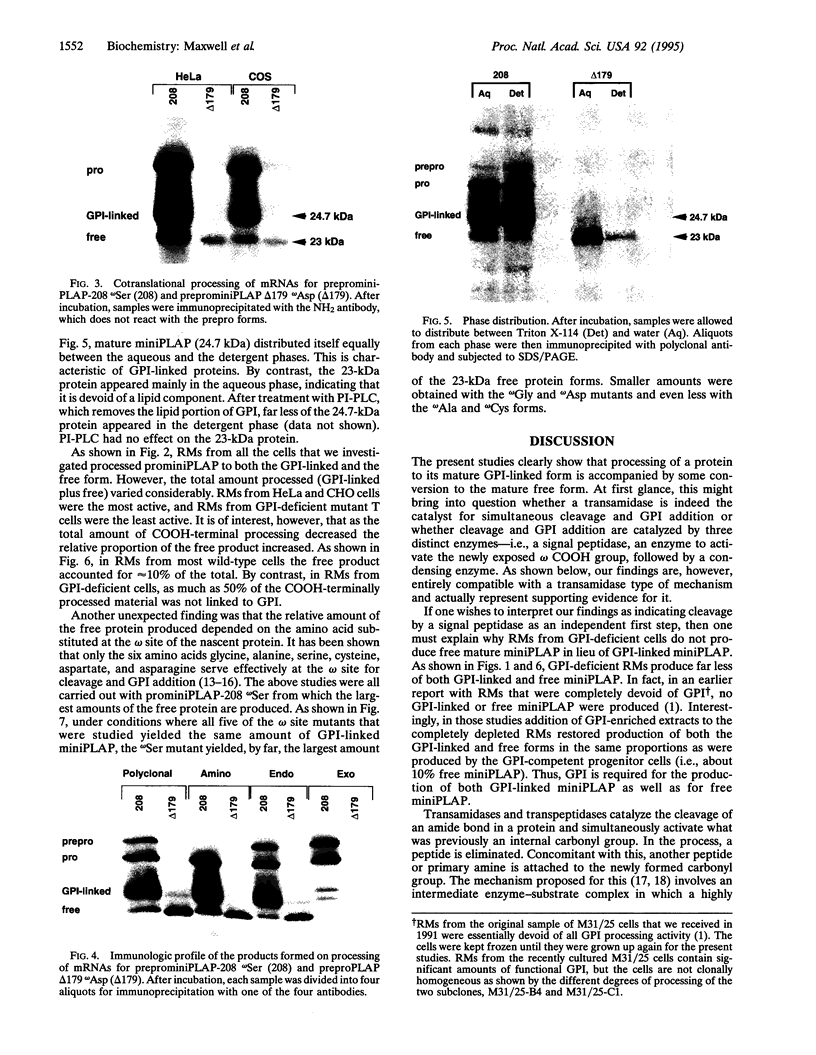
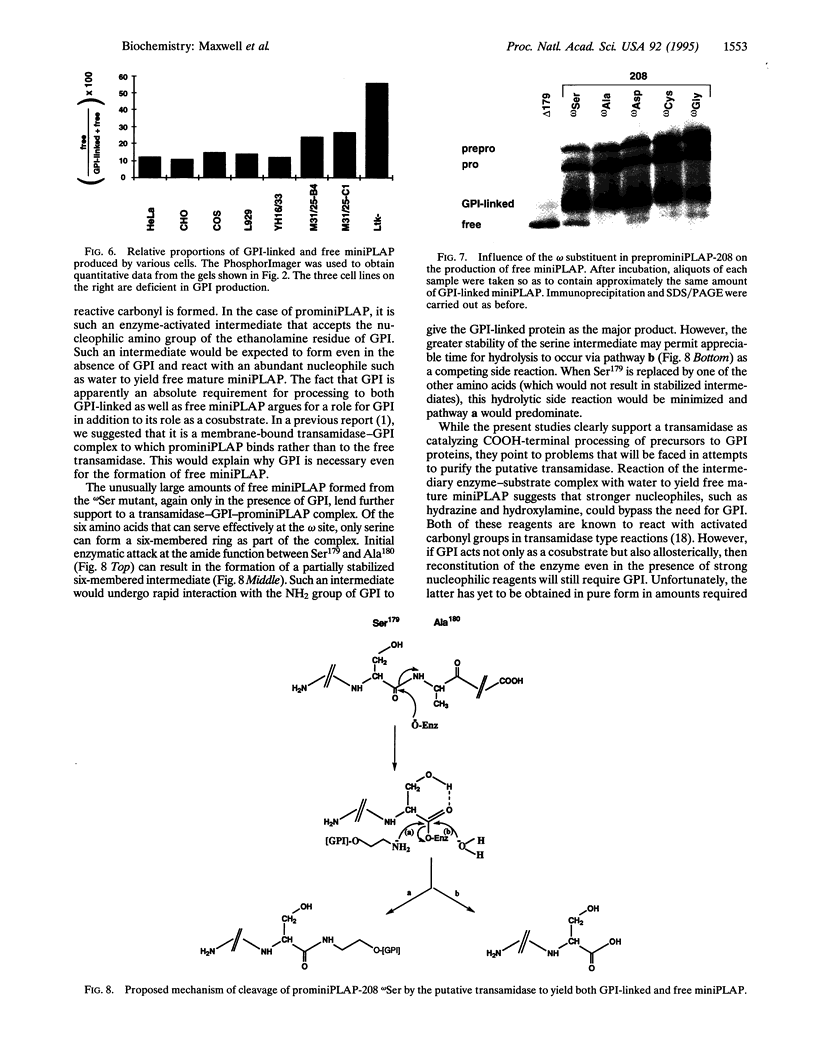
Abstract
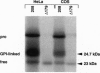
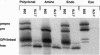
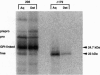
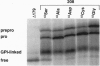
Rough microsomal membranes from most mammalian cells, in the presence of a translation system, process nascent proteins with appropriate COOH-terminal signal peptides to their mature glycosylphosphatidylinositol (GPI)-linked forms. The present study, using preprominiplacental alkaline phosphatase as substrate, shows that as much as 10% of the mature product is cleaved correctly but is not linked to GPI. Some of the factors that influence the relative proportions of GPI linked to free mini-placental alkaline phosphatase are the amounts of GPI in the cells and the amino acid substituent at the omega site of the nascent protein. A mechanism for explaining cleavage both with and without GPI addition is presented, which supports a transamidase type of enzyme as the catalyst.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amthauer R., Kodukula K., Gerber L., Udenfriend S. Evidence that the putative COOH-terminal signal transamidase involved in glycosylphosphatidylinositol protein synthesis is present in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3973–3977. doi: 10.1073/pnas.90.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson N. N., Jr, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;31:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- Bailey C. A., Gerber L., Howard A. D., Udenfriend S. Processing at the carboxyl terminus of nascent placental alkaline phosphatase in a cell-free system: evidence for specific cleavage of a signal peptide. Proc Natl Acad Sci U S A. 1989 Jan;86(1):22–26. doi: 10.1073/pnas.86.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Gerber L. D., Kodukula K., Udenfriend S. Phosphatidylinositol glycan (PI-G) anchored membrane proteins. Amino acid requirements adjacent to the site of cleavage and PI-G attachment in the COOH-terminal signal peptide. J Biol Chem. 1992 Jun 15;267(17):12168–12173. [PubMed] [Google Scholar]
- Kamitani T., Chang H. M., Rollins C., Waneck G. L., Yeh E. T. Correction of the class H defect in glycosylphosphatidylinositol anchor biosynthesis in Ltk- cells by a human cDNA clone. J Biol Chem. 1993 Oct 5;268(28):20733–20736. [PubMed] [Google Scholar]
- Kodukula K., Amthauer R., Cines D., Yeh E. T., Brink L., Thomas L. J., Udenfriend S. Biosynthesis of phosphatidylinositol-glycan (PI-G)-anchored membrane proteins in cell-free systems: PI-G is an obligatory cosubstrate for COOH-terminal processing of nascent proteins. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4982–4985. doi: 10.1073/pnas.89.11.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodukula K., Cines D., Amthauer R., Gerber L., Udenfriend S. Biosynthesis of phosphatidylinositol-glycan (PI-G)-anchored membrane proteins in cell-free systems: cleavage of the nascent protein and addition of the PI-G moiety depend on the size of the COOH-terminal signal peptide. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1350–1353. doi: 10.1073/pnas.89.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodukula K., Micanovic R., Gerber L., Tamburrini M., Brink L., Udenfriend S. Biosynthesis of phosphatidylinositol glycan-anchored membrane proteins. Design of a simple protein substrate to characterize the enzyme that cleaves the COOH-terminal signal peptide. J Biol Chem. 1991 Mar 5;266(7):4464–4470. [PubMed] [Google Scholar]
- McConville M. J., Ferguson M. A. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993 Sep 1;294(Pt 2):305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A. K. Structural analysis of glycosylphosphatidylinositol anchors. Methods Enzymol. 1994;230:418–442. doi: 10.1016/0076-6879(94)30027-5. [DOI] [PubMed] [Google Scholar]
- Micanovic R., Gerber L. D., Berger J., Kodukula K., Udenfriend S. Selectivity of the cleavage/attachment site of phosphatidylinositol-glycan-anchored membrane proteins determined by site-specific mutagenesis at Asp-484 of placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1990 Jan;87(1):157–161. doi: 10.1073/pnas.87.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micanovic R., Kodukula K., Gerber L. D., Udenfriend S. Selectivity at the cleavage/attachment site of phosphatidylinositol-glycan anchored membrane proteins is enzymatically determined. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7939–7943. doi: 10.1073/pnas.87.20.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P., Raab H., Kohr W. J., Caras I. W. Glycophospholipid membrane anchor attachment. Molecular analysis of the cleavage/attachment site. J Biol Chem. 1991 Jan 15;266(2):1250–1257. [PubMed] [Google Scholar]
- Taguchi R., Asahi Y., Ikezawa H. Purification and properties of phosphatidylinositol-specific phospholipase C of Bacillus thuringiensis. Biochim Biophys Acta. 1980 Jul 14;619(1):48–57. [PubMed] [Google Scholar]
- Tate S. S., Meister A. gamma-Glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem. 1981 Sep 25;39:357–368. doi: 10.1007/BF00232585. [DOI] [PubMed] [Google Scholar]
- Thomas L. J., DeGasperi R., Sugiyama E., Chang H. M., Beck P. J., Orlean P., Urakaze M., Kamitani T., Sambrook J. F., Warren C. D. Functional analysis of T-cell mutants defective in the biosynthesis of glycosylphosphatidylinositol anchor. Relative importance of glycosylphosphatidylinositol anchor versus N-linked glycosylation in T-cell activation. J Biol Chem. 1991 Dec 5;266(34):23175–23184. [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]