Abstract
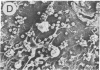
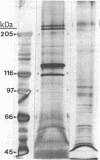
We have developed a long-term culture system in which murine marrow cells are cultured on a complex extracellular matrix (ECM) that is derived from marrow and extracted with guanidine hydrochloride and dithiothreitol. Marrow cultures were established with fresh murine marrow cells and recharged at 2 wk (week 0). Phase microscopy showed a dramatically increased adherent cell layer development on ECM compared with controls within a week after recharge. By electron microscopy, this adherent layer was composed of numerous reticular cells apparently attached to the ECM which extended cytoplasmic projections to the surrounding hematopoietic cells. Adherent cellularity on ECM-coated dishes increased to 30 times the control values by week 2. Cumulative suspension cells on ECM dishes were eight times controls. ECM influenced both hematopoietic progenitor cell proliferation and differentiation. Adherent colony-forming unit-granulocyte/macrophage and colony-forming unit-megakaryocyte were greater than 30 and 15 times the control values, respectively, by week 2 (P less than or equal to 0.05). There were more mature granulocytic and megakaryocytic cells in ECM-coated dishes than in controls at all time points. This new culture system directly demonstrates that ECM is an important component of the hematopoietic microenvironment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. D., Dexter T. M. The essential cells of the hemopoietic microenvironment. Exp Hematol. 1984 Aug;12(7):517–521. [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Moore M. A., Sheridan A. P. Maintenance of hemopoietic stem cells and production of differentiated progeny in allogeneic and semiallogeneic bone marrow chimeras in vitro. J Exp Med. 1977 Jun 1;145(6):1612–1616. doi: 10.1084/jem.145.6.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M. Stromal cell associated haemopoiesis. J Cell Physiol Suppl. 1982;1:87–94. doi: 10.1002/jcp.1041130414. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Testa N. G., Allen T. D., Rutherford T., Scolnick E. Molecular and cell biologic aspects of erythropoiesis in long-term bone marrow cultures. Blood. 1981 Oct;58(4):699–707. [PubMed] [Google Scholar]
- Eliason J. F., Dexter T. M., Testa N. G. The regulation of hemopoiesis in long-term bone marrow cultures. III. The role of burst forming activity. Exp Hematol. 1982 May;10(5):444–450. [PubMed] [Google Scholar]
- Jackson C. W. Cholinesterase as a possible marker for early cells of the megakaryocytic series. Blood. 1973 Sep;42(3):413–421. [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982 Nov 23;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Dexter T. M. Stem cell regulation in continuous hematopoietic cell culture. Transplant Proc. 1978 Mar;10(1):83–90. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Pelus L. M., Broxmeyer H. E., Kurland J. I., Moore M. A. Regulation of macrophage and granulocyte proliferation. Specificities of prostaglandin E and lactoferrin. J Exp Med. 1979 Aug 1;150(2):277–292. doi: 10.1084/jem.150.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Moore M. A., Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. J Exp Med. 1976 Jun 1;143(6):1528–1533. doi: 10.1084/jem.143.6.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath T. K., Reddi A. H. Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7599–7603. doi: 10.1073/pnas.78.12.7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z. X., Quesenberry P. J. Radioresistant murine marrow stromal cells: a morphologic and functional characterization. Exp Hematol. 1984 Aug;12(7):523–533. [PubMed] [Google Scholar]
- Spooncer E., Gallagher J. T., Krizsa F., Dexter T. M. Regulation of haemopoiesis in long-term bone marrow cultures. IV. Glycosaminoglycan synthesis and the stimulation of haemopoiesis by beta-D-xylosides. J Cell Biol. 1983 Feb;96(2):510–514. doi: 10.1083/jcb.96.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha M. S., Lowrie G., Kohn E., Bagavandoss P., Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc Natl Acad Sci U S A. 1982 May;79(10):3213–3217. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N., Eger R. R., Jackson H. M., Nelson D. J. Two-factor requirement for murine megakaryocyte colony formation. J Cell Physiol. 1982 Jan;110(1):101–104. doi: 10.1002/jcp.1041100116. [DOI] [PubMed] [Google Scholar]
- Williams N., Jackson H., Ralph P., Nakoinz I. Cell interactions influencing murine marrow megakaryocytes: nature of the potentiator cell in bone marrow. Blood. 1981 Jan;57(1):157–163. [PubMed] [Google Scholar]
- Zuckerman K. S., Rhodes R. K., Goodrum D. D., Patel V. R., Sparks B., Wells J., Wicha M. S., Mayo L. A. Inhibition of collagen deposition in the extracellular matrix prevents the establishment of a stroma supportive of hematopoiesis in long-term murine bone marrow cultures. J Clin Invest. 1985 Mar;75(3):970–975. doi: 10.1172/JCI111798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman K. S., Wicha M. S. Extracellular matrix production by the adherent cells of long-term murine bone marrow cultures. Blood. 1983 Mar;61(3):540–547. [PubMed] [Google Scholar]