Abstract
The promise of cellular therapy lies in healing damaged tissues and organs in vivo as well as generating tissue constructs in vitro for subsequent transplantation. Adult stem cells are ideally suited for cellular therapies due to their pulripotency and the ease with which they can be cultured on novel functionalized substrates. Creating environments to control and successively driving their differentiation toward a lineage of choice is one of the most important challenges of current cell-based engineering strategies. In recent years, a variety of biomedical platforms have been prepared for stem cell cultures, primarily to provide efficient delivery of growth or survival factors to cells and a conducive microenvironment for their growth. Here, we demonstrate that repeating tetralayer structures composed of biocompatible poly(methacrylic acid) (PMAA)/poly(acryl amide) (PAAm)/poly(methacrylic acid) (PMAA)/poly(ethylene oxide)-block-poly(ε-caprolactone) (PEO-b-PCL) micelles arrayed in layer-by-layer (LbL) films can serve as a payload region for dexamethasone (dex) delivery to human mesenchymal stem cells (MSCs). This architecture can induce MSC differentiation into osteoblasts in a dose-dependent manner. The amount of dex loaded in the films is controlled by varying the deposition conditions and the film thickness. Furthermore, release of dex is also controlled by changing the amount of covalent crosslinking of multilayers via thermal treatments. The multilayer architecture including payload and cell-adhesion region introduced here are well suited for extended cell culture thus affording the important and protective effect of both dex release and immobilization. These films may find applications in the local delivery of immobilized therapeutics for biomedical applications, as they can be deposited on a wide range of substrates with different shapes, sizes, and composition.
Keywords: Layer-by-layer, multilayer, controlled release, Human mesenchymal stem cells, differentiation, dexamethasone
1. Introduction
Surface coatings incorporating therapeutic molecules have recently begun to play an important role in the design of biomedical devices, implantable biomaterials, industrial bioprocesses and engineered biological interfaces.1–7 A promising strategy to develop such a biomedical platform is by creating multi-functional polymer coatings that can safely encapsulate and release sensitive biological molecules in an active form within multilayer architectures for controlled release directly from film surfaces.8–14
Layer-by-layer (LbL) deposition has been widely used to fabricate multilayer architectures by complementary interactions between components, such as positively and negatively charged materials15–16 or materials that have hydrogen bond donor and acceptor groups,17–18 where each layer is adsorbed sequentially onto a surface to achieve a film. Due to a growing interest in applying multilayer architectures to biomedical applications, erodible multilayers that degrade in a controlled manner via disassembly are being explored as a potential platform for controlled release drug delivery over extended time periods. Automated LbL deposition methods are advantageous in that the films are prepared under mild aqueous conditions that preserve drug bioactivity. Furthermore, incorporation of various therapeutics into multilayers for systematic release has been studied from small-molecule steroids and antibiotics to macromolecules such as protein therapeutics, active proteins, enzymes, nucleic acids and plasmid DNA.19–27 Multilayers can be conformally coated onto substrates with simple and complex geometries to load therapeutics and to control the release of these over a defined time period. More recently, multilayer thin films have been developed to permit the release of drug dosages with desired periods of time ranging from minutes to days. Thus, there is tremendous potential to use these polyelectrolyte films as cell differentiation platforms. Multilayer thin films are well suited for this application due to the flexibility these structures afford in terms of biocompatibility, material properties, cost of application, and the ability to finely tune surface characteristics as desired. The ability to achieve precise and extended-term drug delivery will enable a broader set of clinical applications where precision dosing must occur over longer time spans.28–32
Human mesenchymal stem cells, also known as multipotent stromal cells (MSCs) are adult progenitors that maintain the potential to differentiate into numerous cell types found within adult connective tissues. A wide range of functionally, morphologically, and transcriptionally distinct phenotypes have been derived from a common MSC precursor. This intrinsic multipotent property, coupled with the ability to isolate large quantities of these cells from the bone marrow, highlights the potential of MSCs as a cell source for regenerative medical applications.33–36 MSCs are influenced by their physical micro-environment (stiffness and geometry), as well as by biochemical cues from small molecules, and exogenous, paracrine, and autocrine signaling. The utility of thin film multilayer surfaces derives from the ability to influence all aspects of the MSC niche. Tuning of surface features, and delivery of biochemical signals in the form of small molecules are both possible using this approach.
In this work, we present a simple strategy to prepare functional surfaces with tunable and durable biological activity by taking advantage of LbL assembled multilayer thin films with embedded small bio-active molecules to guide MSC differentiation. To safely encapsulate drugs, polymeric micelles are introduced into LbL films. Specifically, we have constructed LbL films of repeating tetralayer structures composed of biocompatible poly(acryl amide) (PAAm), poly(methacrylic acid) (PMAA), and poly(ethylene oxide)-block-poly(ε-caprolactone) (PEO-b-PCL) micelles. In this (PMAA/PAAm/PMAA/PEO-b-PCL)n (n = number of tetralayers) architecture, both electrostatic interaction between PMAA/PAAm, and hydrogen bonding between PMAA/PEO-b-PCL were employed to build multilayers. We demonstrate that the resulting films degrade in a repeatable and predictable manner to release drug-loaded micelles into the surrounding cell culture medium under physiological conditions. Furthermore, we are able to tailor the rate of film degradation to control the rate of drug release by varying the degree of covalent anhydride crosslinking between carboxylic acid groups in PMAA thus slowing degradation to the desired rate. Since micelle incorporation into multilayer thin films and encapsulation of drugs into micelles do not require specific chemical modifications during the fabrication process, and introducing further crosslinking permits control over the release profile of drugs, this approach is particularly well suited for incorporating active hydrophobic therapeutics for various biological and biomedical coatings.
2. Experimental Section
Materials
Poly(methacrylic acid) (PMAA, Mw 15,000 30% aqueous solution) and Poly(acryl amide) (PAAm, Mw 5,000,000 1% aqueous solution) were obtained from Polysciences Inc. (Warrington, PA). Amphiphilic block copolymer, poly(ethylene oxide)-block-poly(ε-caprolactone) (PEO-b-PCL, Mn (PEO) 5000, Mn (PCL) 6500, PDI 1.3) was purchased from Polymer Source (Montreal, Canada). poly(diallyldimethyl ammonium chloride) (PDAC, Mw = 200,000–350,000 g/mol), and poly(sodium 4-styrenesulfonate) (PSS, Mw = 70,000) were purchased from Aldrich. poly(diallyldimethyl ammonium chloride) (PDAC, Mw = 200,000–350,000 g/mol), and poly(sodium 4-styrenesulfonate) (PSS, Mw = 70,000) were purchased from Aldrich. Sodium hyaluronate (or hyaluronic acid (HA), Mn = 1.76 MDa) was purchased from Lifecore Biomedical, Inc. (Chaska, MN) Collagen I from rat-tail, Collagen I from calf skin, Collagen IV from human placenta, and Dexamethasone (dex) were obtained from Sigma. All other reagents and solvents were purchased from Aldrich and used as received. Quartz slide, silicon wafer, Teflon, and polypropylene were used as substrates for the LbL assembly and cleaned extensively prior to the deposition.
Block Copolymer Micelle Formation
Block copolymer micelles of PEO-b-PCL were prepared according to a modification of a previously published method. Briefly, a stock solution of PEO-b-PCL was freshly prepared in tetrahydrofuran (THF) at a concentration of 10 mg/mL. Then, 200 mL of stock solution was placed in a vial with gentle stirring. To this solution was gradually added 5.0 mL of Millipore water (18 M·cm) with vigorous stirring. After an additional 1 h of stirring, the resulting suspension was subjected to dialysis against Millipore water for over 24 h (Spectra/Por 4 regenerated cellulose membrane, MWCO 12,000–14,000) to remove any residual solvent. The resulting PEO-b-PCL micelle was filtered prior to use. The pH of the resulting micelle suspension was adjusted with 0.10 M HCl solution right before LbL film formation. For dex loading, we followed a modification of previously published protocol. Briefly, dex solution in THF (concentration 1.0 mg/mL) was dropwise added to 5.0 mL of the micelle suspension prepared above (0.40 mg/mL). The emulsion was vigorously stirred overnight with a loose cap to evaporate the organic solvent. The solution obtained was centrifuged (4500 rpm, 10 min) and filtered through a 0.45 m syringe filter. The drug content in a micelle was evaluated by measuring the characteristic absorbance of dex in a solvent mixture of MeOH and H2O (9:1 v/v), employing a calibration curve with a known concentration of Dexamethasone using an Agilent 8453 UV–Visible spectrometer.
Layer-by-Layer (LbL) Film Assembly
All LbL films were assembled with a modified programmable Carl Zeiss HMS slide stainer. Typically, films were constructed on various substrates with approximate size of 1 ~ 2 in2. The O2-plasma-treated substrate was first dipped into PMAA aqueous solution (10 mM, pH 3) for 10 min and rinsed three times with water (pH 3) for 1 min each. Subsequently, the substrate was introduced into aqueous solution of PAAm (10 mM, pH 3), PMAA aqueous solution (10 mM, pH 3) and PEO-b-PCL micelle solution (0.40 mg/mL, pH 3) for 10 min and washed again three times with water (pH 3) for 1 min each. This cycle provides one tetralayer of PMAA, PAAm, PMAA and PEO-b-PCL micelle, denoted (PMAA/PAAm/PMAA/PEO-b-PCL). The dipping process was repeated until the desired number of bilayers was obtained. Cell adhesion region was prepared by spin-LbL deposition (2500 rpm, 30 sec).
Surface Morphology
The surface morphology of BCM multilayer films was investigated by field emission scanning electron microscopy (XL30FEG, Philips)
Measurement of Film Thickness
Following deposition, films were immediately removed from the final rinsing bath and nitrogen-dried. Film thickness was determined either by ellipsometry at ten different predetermined locations on the film surface or by profilometry at three different scratch sites.
UV-Vis Spectroscopy
The UV-Vis spectra were obtained using a Perkin Elmer Lambda UV-vis spectrometer. Dexamethasone showed the absorbance peak centered at 238 nm.
Cell Culture
All MSCs used in this work were passage three human bone marrow derived mesenchymal stromal cells. Passage two human MSCs were obtained from the Texas A&M Health Sciences Center and expanded one additional passage before each experiment. Cellular differentiation assays to confirm osteogenic, chondrogenic, and adipogenic potential are performed at Texas A&M Health Science Center. Cells were expanded in Dulbecco’s Modified Eagle’s Medium supplemented with 16.5% fetal calf serum, 4.5 g/L glucose, L-glutamine, sodium pyruvate, non-essential amino acids, and penicillin/streptomycin at 37 °C and 5% CO2. All experiments were conducted using cells derived from the same patient lot.
Alkaline phosphatase Activity Assay
Alkaline phosphatase activity was measured using the colorimetric substrate para-nitrophenylphosphate which develops a yellow end product readable at 405 nm (pNPP ALP Kit, Sigma Aldrich). Samples were incubated at room temperature for 60 minutes then stopped with 2 N NaOH. Standard curves were generated using dilutions of p-nitrophenol. Activity was normalized to total protein content as measured by BCA assay (Pierce).
Quantitative Real Time PCR (qRT-PCR)
RNA extracts were prepared using the Qiagen RNeasy kit. Samples were normalized to 500 ng total RNA content prior to qRT-PCR cycling. All biological replicates were aliquoted into three technical replicates. Primers and OneStep qRT-PCR kit were from Qiagen. Realtime optical readings were performed using the Chromas 4 system. Abundance levels for each gene (A) of interest were calculated from the take-off cycle (Ct) and efficiency (E) for each gene using: A=1/(1+E)Ct. All sample abundances were normalized to GAPDH abundance for each experimental condition.
3. Results and Discussion
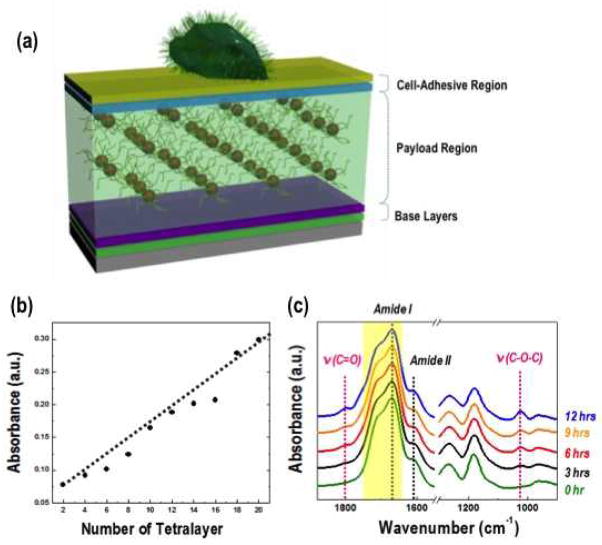
To develop a polyelectrolyte multilayer film with controllable drug loading and release properties, we created three functional regions (Figure 1a). First, an base layer was prepared directly onto the substrate to mitigate substrate effects on binding by precoating a planar silicon wafer or glass slide with ten and a half bilayers of poly(diallyldimethyl ammonium chloride) (PDAC) and poly(sodium 4-styrenesulfonate) (PSS) as a nondegradable layers (ca. 67 nm thick) to ensure the presence of uniform surface charge for the deposition. Next, the payload region holding the nano-container with drugs was fabricated. Dexamethasone (dex) is a synthetic glucocorticoid that can induce early commitment along an osteogenic lineage when applied to MSCs.37–39 Dex was encapsulted within PEO-b-PCL micelles and assembled into multilayer thin films. The dex containing PEO-b-PCL (PEO-b-PCLdex) micelles had a hydrodynamic diameter of 98.8 ± 13.4 nm in solution, measured by dynamic light scattering (DLS). The payload region consisted of twenty tetralayers of electrostatically and hydrogen-bonded PMAA/PAAm/PMAA/PEO-b-PCLdex and was terminated with a three and half PMAA/PAAm layers. The advantages of this tetralayer (i.e., (PMAA/PAAm/PMAA/PEO-b-PCL) are: (1) even distribution of dex in payload region, (2) ability to generate amide and anhydride bonds with thermal treatment to control the film crosslinking density of the tetralayer, and (3) the ability to easily control the amount of dex incorporated. The topmost region of the film was cell adhesive prepared by collagen, polysaccharide and disaccharide to promote cell adhesion at top of multilayer film (Figure 1a).
Figure 1.
(a) The schematic illustration of the thin film multilayer platform. (b) UV-Vis spectra of (PMAA/PAAm/PMAA/PEO-b-PCLdex)20 multilayer film as a function of tetralayer number. The absorption peak at 238 nm originates from dex in PEO-b-PCL micelles. (c) FT-IR absorbance spectra of multilayer films with different heat treatment times.
The dex absorbance peak at 238 nm was monitored as the (PMAA/PAAm/PMAA/PEO-b-PCLdex)n films were assembled. The absorbance at 238 nm grows linearly with increasing tetralayer numbers of (PMAA/PAAm/PMAA/PEO-b-PCLdex) (Figure 1(b)) demonstrating the linear film growth. The average thickness per tetralayer was 67.1 ± 6.8 nm. Covalent crosslinking in the LbL films was achieved through heat treatment at 150 °C under vacuum for up to 12 hours. Figure 1c is the FT-IR spectrum of the heat-treated (PMAA/PAAm/PMAA/PEO-b-PCLdex)20(PMAA/PAAm)3.5 payload region with respect to time. New peaks at 1042 cm−1 and 1804 cm−1 appeared after heat treatment which are attributed to anhydride bond formation by PMAA-PAAm (Amide), PMAA-PMAA (anhydride) and PAAm-PAAm (imide bonds) form through a condensation process. However the peaks for amide, anhydride and imide bonds overlap as indicated by the asymmetric and symmetric signatures of C=O in PEO-b-PCL (1780−1 to 1660m−1). Therefore covalent bonded crosslinking derived from heat treatment were indirectly confirmed through the formation of anhydride peaks (i.e., 1042 cm−1 and 1804 cm−1). The average film thickness of the payload region before heat treatment was 1432 ± 87 nm while after treatment it decreased to 1227 ± 15 nm, a reduction of about 7%. It is well known that hydrogen-bonded self-assembled multilayers can be easily disrupted by thermal means. However, heating the multilayer film at 150 °C for 12 hour introduces chemical crosslinks that stabilize the multilayer assembly even at pH 7.4 in phosphate buffer saline (PBS) buffer. The heated multilayer film experiences an 8.5% film thickness reduction after heat treatment after 60 hours of immersion in PBS solution. Cross linking the film for 3 hours, on the other hand, does not enhance the film resistance to the PBS buffer to a large extent, possibly due to the low degree of an-hydride linkage formation. Films that are not crosslinked by heat treatment dissolve immediately when immersed in PBS.
A cell adhesive region was introduced onto the payload region to control MSCs adhesion For which various materials were examined. Positively charged Collagen I (Most abundant form of collagen) from rat tail, Collagen III from calf skin, and Collagen IV (Most interstitial tissues) from human placenta, as well as negative charged hyaluronic acid (HA), heparin (HEP), Poly(methacrylic acid) (PMAA) and Poly(acrylic acid) (PAA). Triple helix-rich fragments of collagen are well known adhesion substrate to cells and HA is a highly attractive natural biomaterial due to its role in the extracellular matrix,40 and its influence on cell behavior.41 Heparin, a highly-sulfated glycosaminoglycan, is widely used as an injectable anticoagulant, and has the highest negative charge density of any known biological molecule.42–43 PMAA and PAA are also well known biodegradable, biocompatible polyelectrolytes.44–45 Multilayer films with each of these biomaterials were used to fabricate the four and half bilayer top surface for cell adhesion and were screened for suitability (see supporting information S1). We examined the effect of the various top-level materials on initial cell adhesion. Phase-contrast micrographs were taken 24h after MSC seeding in MSC growth media at densities optimum for differentiation. After 24h, the cell adhesion region appeared to have a negligible effect on initial cell adhesion and spreading on each combination. Among the combinations, multilayers prepared with PMAA and PAA spontaneously peeled off in PBS buffer, which is not suitable for cell adhesion. Cell adhesive layers of other combinations of materials (i.e., Collagen I and III multilayered with HA and Heparin) exhibited higher cell density and better stability in solution. In this manuscript, 112 ± 19 nm of the 4.5 bilayer of collagen I/HA cell adhesive region was selected for further experiments.
The surface morphology of both payload and cell-adhesion region were observed by SEM (Figure 2). Dex encapsulated PEO-b-PCL micelles remain distinguishable on the surface with high roughness resulting from the swell and coalescence that occur during the LbL assembly process as shown in Figure 2(a). After coating the 4.5 bilayers of collagen I/HA multilayer onto the payload region, we observed uniform and dense morphology consistent with micro/nano structures (Figure 2(b)).
Figure 2.
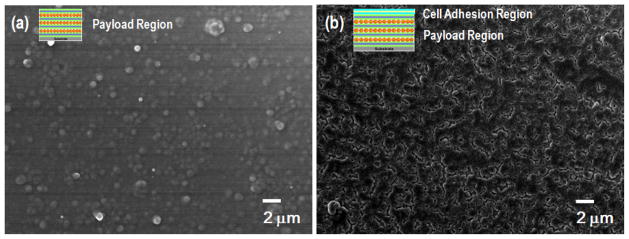
SEM images of (a) substrate/(PDAC/PSS)10.5(PMAA/PAAm/PMAA/PEO-b-PCLdex)20(PMAA/PAAm)3.5 and (b) substrate/(PDAC/PSS)10.5(PMAA/PAAm/PMAA/PEO-b-PCLdex)20 (PMAA/PAAm)3.5 (Collagen I/ HA)4.5
Dex release experiments were performed by immersing a substrate in a sealed vial containing 3 ml of buffered solution. At indicated time points, films were removed and re-immersed into appropriate buffer solution. Figure 3(a) shows the change in payload/cell adhesive region multilayer film thickness with time following immersion in PBS solution. The thickness of the portions of film remaining on the surface was determined to decrease linearly with respect to time of exposure to PBS over a 3 min time period. All films with different crosslinking densities demonstrate both dissolving and swelling during the submersion. Following a brief swelling period, hydrolysis emerges as the dominant factor affecting film thickness. In all cases, film thickness decreases at a constant rate: approximately 438, 433.6, 7.47, 3.63 and 1.88 nm/hour for 0, 3, 6, 9 and 12 hour heat treatment-based conditions, respectively.
Figure 3.
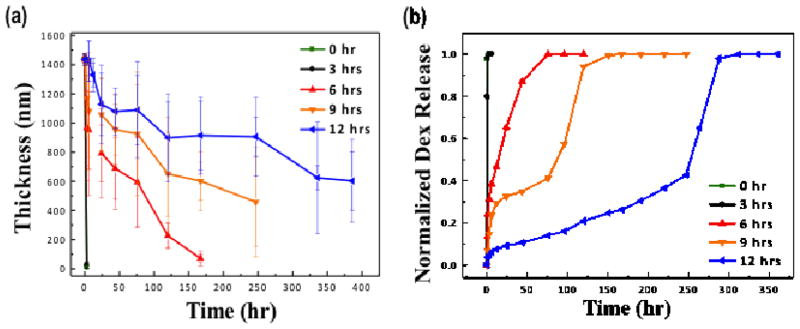
(a) Film thickness as a function of release time following immersion in PBS at pH 7.4. Error bars represent the standard deviation of measured thickness values. (b) Cumulative normalized kinetics of dex release from platform immersed in PBS (pH 7.4 at 37 °C), for films prepared with different crosslinking density by heat treatment. In both (a) and (b) platforms include payload and cell adhesive regions.
Finally, to examine the potential of a drug payload region as a platform for hydrophobic therapeutic delivery the release profiles of dex were evaluated by measuring the dex release from the payload region after incubation in a model physiological solution (PBS buffer, 37 °C, 5% CO2). Dex was released over a course of two hours from untreated multilayer films, whereas the cross-linked film exhibited a significantly longer release up to 72 hours for a 6 hour thermally cross-linked film and 290 hours for 12 hours for thermally cross-linked films (Figure 3). This is a unique feature of the hydrogen bonded system, for which simple post-modification to introduce internal cross-links within the LbL film enables the control of the release kinetics of active small, hydrophobic therapeutics from 3 hours up to 290 hours. We expect that this fine control over the release profile will be highly advantageous for potential use in biological applications that require a controllable release of hydrophobic active therapeutics. For example, untreated, non-cross-linked films would be interesting for wound dressings and therapeutic surface coatings, where an immediate delivery of therapeutics is necessary upon contact with the film. In contrast, cross-linked film would be useful as a carrier for active therapeutics on biomaterial surfaces for an extended period of time such as with surgical implant materials.
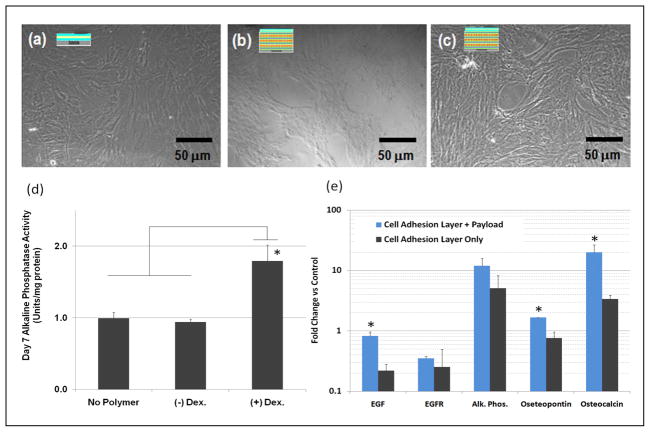
To assess the ability of dex loaded thin film multilayers to exert a biological effect we examined the expression of early osteogenic markers in MSCs cultured on multilayers which produce a controlled dex release over extended times. Cells were cultured for 14 days on three different kinds of multilayer films including cell adhesive region only (Figure 4(a)), payload-without dex/cell adhesive region (Figure 4(b)) and payload-with dex/cell adhesive region (Figure 4(c)) with a dex release profile matching the longest release time. As shown in figure 4(d), MSCs cultured on dex releasing surfaces exhibited a two-fold increase in alkaline phosphatase activity when compared with MSCs cultured on control surfaces with no dex payload. Likewise, mRNA for later markers of osteogenic differentiation such as osteopontin and osteocalcin were upregulated by 1.5 fold and 20 fold, respectively, when compared with control cultures, as indicated by quantitative real time PCR in figure 4(e). The ability of thin films with a dex payload to induce early and mid-point osteoenic markers in MSCs is indicative of the utility of these surfaces to bring about real biological effects over time spans that are therapeutically relevant. The induction of osteogenic markers in MSCs using only expansion medium and under conditions which derive osteogenic simtuli only from the payload release and cell adhesive properties of the thin film substrate is a clear demonstration of the potential of this approach.
Figure 4.
Brightfield micrographs of MSCs after 14 days on (a) Glass/(PDAC/PSS)10.5(Collagen I/HA)4.5 (b) Glass/(PDAC/PSS)10.5(PMAA/PAAm/PMAA/PEO-b-PCL)20(PMAA/PAAm)3.5 (Collagen I/HA)4.5 and (c) Glass/(PDAC/PSS)10.5(PMAA/PAAm/PMAA/PEO-b-PCLdex)20(PMAA/PAAm)3.5(Collagen I/ HA)4.5. (d) Alkaline Phosphatase activity on day 7 under the three culture conditions shown (n = 3, replicates, +/− s.d., *p<0.01) (e) Fold change in gene expression levels of the genes shown on day 10 (n = 3, replicates, +/− s.d., *p<0.05).
4. Conclusion
We have demonstrated a simple, tunable drug release thin film multilayer platform that is capable of producing significant biological effects over extended time periods. Using our approach we demonstrated early osteogenic differentiation of mesesenchymal stem cells induced through controlled release of dex from a payload region as well as from surface adhesion cues. The simplicity of this design is attributable to the few functional layers required: a substrate base layer, a payload region and a cell adhesion region. The best performing construct incorporating these layers consisted of (PMAA/PAAm/PMAA/PEO-b-PCLdex)20(PMAA/PAAm)3.5 and (Collagen I/HA)4.5 which exhibited highly tunable release profiles to complement surface cues from Collagen I and HA. The ability to tune drug release and surface properties to this extent opens additional applications of LbL technology to challenging problems in regenerative medicine.
Supplementary Material
Acknowledgments
This research is funded by Singapore-MIT Alliance for Research & Technology (SMART) in Massachusetts Institute of Technology (MIT). Additionally, this work was also financially supported by the National Research Foundation of Korea Grant funded by the Korean Government (MEST) (The National Creative Research Initiative Program for “Intelligent Hybrids Research Center” (2010–0018290), (NRF-2009-0093282) in Seoul National University. Additionally, LMA acknowledges support from the Hertz Foundation (Livermore, CA).
References
- 1.Langer R, Folkman J. Polymers for Sustained-Release of Proteins and Other Macromolecules. Nature. 1976;263:797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- 2.Pekarek KJ, Jacob JS, Mathiowitz E. Double-Walled Polymer Microspheres for Controlled Drug Release. Nature. 1991;367:258–260. doi: 10.1038/367258a0. [DOI] [PubMed] [Google Scholar]
- 3.Langer R. Drug Delivery and Targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 4.Klugherz BD, Jones PL, Cui XM, Chen WL, Meneveau NF, DeFelice S, Connolly J, Wilensky RL, Levy RJ. Gene Delivery from a DNA Controlled-Release Stent in Porcine Coronary Arteries. Nat Biotechnol. 2000;18:1181–1184. doi: 10.1038/81176. [DOI] [PubMed] [Google Scholar]
- 5.Qiu Y, Park K. Environment-Sensitive Hydrogels for Drug Delivery. Adv Drug Deliver Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishna S, Mayerb J, Wintermantelc E, Leongd KW. Biomedical applications of polymer-composite materials. Compos Sci Technol. 2001;61:1189–1224. [Google Scholar]
- 7.Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound healing dressings and drug delivery systems. J Pharm Sci. 2008;97:2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 8.Wood KC, Chuang HF, Batten RD, Lynn DM, Hammond PT. Controlling interlayer diffusion to achieve sustained, multiagent delivery from layer-by-layer thin films. Proc Natl Acad Sci U S A. 2006;103:10207–10212. doi: 10.1073/pnas.0602884103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macdonald M, Rodriguez NM, Smith R, Hammond PT. Release of a model protein from biodegradable self assembled films for surface delivery applications. J Controlled Release. 2008;131:228–234. doi: 10.1016/j.jconrel.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su XF, Kim BS, Kim SR, Hammond PT, Irvine DJ. Layer-by-Layer-Assembled Multilayer Films for Transcutaneous Drug and Vaccine Delivery. ACS Nano. 2009;3:3719–3729. doi: 10.1021/nn900928u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun B, Lynn DM. Release of DNA from polyelectrolyte multilayers fabricated using ‘charge-shifting’ cationic polymers: Tunable temporal control and sequential multi-agent release. J Controlled Release. 2010;148:91–100. doi: 10.1016/j.jconrel.2010.07.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shukla A, Fleming KE, Chuang HF, Chau TM, Loose CR, Stephanopoulos GN, Hammond PT. Controlling the Release of Peptide Antimicrobial Agents from Surfaces. Biomaterials. 2010;31:2348–2357. doi: 10.1016/j.biomaterials.2009.11.082. [DOI] [PubMed] [Google Scholar]
- 13.Yan Y, Such GK, Johnston APR, Lomas H, Caruso F. Toward Therapeutic Delivery with Layer-by-Layer Engineered Particles. ACS Nano. 2011;5:4252–4257. doi: 10.1021/nn201793f. [DOI] [PubMed] [Google Scholar]
- 14.Shah NJ, Macdonald ML, Beben YM, Padera RF, Samuel RE, Hammond PT. Tunable dual growth factor delivery from polyelectrolyte multilayer films. Biomaterials. 2011;32:6183–6193. doi: 10.1016/j.biomaterials.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pei R, Cui X, Yang X, Wang E. Assembly of Alternating Polycation and DNA Multilayer Films by Electrostatic Layer-by-Layer Adsorption. Biomacromolecules. 2001;2:463–468. doi: 10.1021/bm0001289. [DOI] [PubMed] [Google Scholar]
- 16.Ai H, Jones SA, Lvov YM. Biomedical applications of electrostatic layer-by-layer nano-assembly of polymers, enzymes, and nanoparticles. Cell Biochem Biophys. 2003;39:23–43. doi: 10.1385/CBB:39:1:23. [DOI] [PubMed] [Google Scholar]
- 17.Kozlovskaya V, Ok S, Sousa A, Libera M, Sukhishvili SA. Hydrogen-Bonded Polymer Capsules Formed by Layer-by-Layer Self-Assembly. Macromolecules. 2003;36:8590–8592. [Google Scholar]
- 18.Kim BS, Park SW, Hammond PT. Hydrogen-Bonding Layer-by-Layer-Assembled Biodegradable Polymeric Micelles as Drug Delivery Vehicles from Surfaces. ACS Nano. 2008;2:386–392. doi: 10.1021/nn700408z. [DOI] [PubMed] [Google Scholar]
- 19.Ai H, Jones SA, Lvov YM. Biomedical applications of electrostatic layer-by-layer nano-assembly of polymers, enzymes, and nanoparticles. Cell Biochem Biophys. 2003;39:23–43. doi: 10.1385/CBB:39:1:23. [DOI] [PubMed] [Google Scholar]
- 20.Richert L, Lavalle P, Payan E, Shu XZ, Prestwich GD, Stoltz JF, Schaaf P, Voegel JC, Picart C. Layer by layer buildup of polysaccharide films: Physical chemistry and cellular adhesion aspects. Langmuir. 2004;20:448–458. doi: 10.1021/la035415n. [DOI] [PubMed] [Google Scholar]
- 21.Jewell CM, Zhang JT, Fredin NJ, Lynn DM. Multilayered polyelectrolyte films promote the direct and localized delivery of DNA to cells. J Controlled Release. 2005;106:214–223. doi: 10.1016/j.jconrel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Ren KF, Ji J, Shen JC. Tunable DNA release from cross-linked ultrathin DNA/PLL multilayered films. Bioconjugate Chem. 2006;17:77–83. doi: 10.1021/bc050264g. [DOI] [PubMed] [Google Scholar]
- 23.Jayant RD, Srivastava R. Dexamethasone release from uniform sized nanoengineered alginate microspheres. J Biomed Nanotechnol. 2007;3:245–253. [Google Scholar]
- 24.Zhao QH, Li BY. pH-controlled drug loading and release from biodegradable microcapsules. Nanomedicine (N Y, NY, U S) 2008;4:302–310. doi: 10.1016/j.nano.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimitrova M, Affolter C, Meyer F, Nguyen I, Richard DG, Schuster C, Bartenschlager R, Voegel JC, Ogier J, Baumert TF. Sustained delivery of siRNAs targeting viral infection by cell-degradable multilayered polyelectrolyte films. Proc Natl Acad Sci U S A. 2008;105:16320–16325. doi: 10.1073/pnas.0800156105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith RC, Riollano M, Leung A, Hammond PT. Layer-by-Layer Platform Technology for Small-Molecule Delivery. Angew Chem Int Ed. 2009;48:8974–8977. doi: 10.1002/anie.200902782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macdonald ML, Samuel RE, Shah NJ, Padera RF, Beben YM, Hammond PT. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials. 2011;32:1446–1453. doi: 10.1016/j.biomaterials.2010.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang JT, Chua LS, Lynn DM. Multilayered thin films that sustain the release of functional DNA under physiological conditions. Langmuir. 2004;20:8015–8021. doi: 10.1021/la048888i. [DOI] [PubMed] [Google Scholar]
- 29.Pargaonkar N, Lvov YM, Li N, Steenekamp JH, de Villiers MM. Controlled release of dexamethasone from microcapsules produced by polyelectrolyte layer-by-layer nanoassembly. Pharm Res. 2005;22:826–835. doi: 10.1007/s11095-005-2600-0. [DOI] [PubMed] [Google Scholar]
- 30.Kim TG, Lee H, Jang Y, Park TG. Controlled Release of Paclitaxel from Heparinized Metal Stent Fabricated by Layer-by-Layer Assembly of Polylysine and Hyaluronic Acid-g-Poly(lactic-co-glycolic acid) Micelles Encapsulating Paclitaxel. Biomacromolecules. 2009;10:1532–1539. doi: 10.1021/bm900116r. [DOI] [PubMed] [Google Scholar]
- 31.Mehrotra S, Lynam D, Maloney R, Pawelec KM, Tuszynski MH, Lee I, Chan C, Sakamoto J. Time Controlled Protein Release from Layer-by-Layer Assembled Multilayer Functionalized Agarose Hydrogels. Adv Funct Mater. 2010;20:247–258. doi: 10.1002/adfm.200901172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen XY, Wu W, Guo ZZ, Xin JY, Li JS. Controlled insulin release from glucose-sensitive self-assembled multilayer films based on 21-arm star polymer. Biomaterials. 2011;32:1759–1766. doi: 10.1016/j.biomaterials.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Griffith LG, Naughton G. Tissue Engineering--Current Challenges and Expanding Opportunities. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 34.Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, Kaplan D, Langer R, Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: Effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- 35.Nuttelman CR, Tripodi MC, Anseth KS. Dexamethasone-functionalized gels induce osteogenic differentiation of encapsulated hMSCs. J Biomed Mater Res Part A. 2006;76:183–195. doi: 10.1002/jbm.a.30537. [DOI] [PubMed] [Google Scholar]
- 36.Hannouche D, Terai H, Fuchs JR, Terada S, Zand S, Nasseri BA, Petite H, Sedel L, Vacanti JP. Engineering of implantable cartilaginous structures from bone marrow-derived mesenchymal stem cells. Tissue Eng. 2007;13:87–99. doi: 10.1089/ten.2006.0067. [DOI] [PubMed] [Google Scholar]
- 37.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 38.Derfoul A, Perkins GL, Hall DJ, Tuan RS. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells. 2006;24:1487–1495. doi: 10.1634/stemcells.2005-0415. [DOI] [PubMed] [Google Scholar]
- 39.Xiao YL, Peperzak V, van Rijn L, Borst J, de Bruijn J. Dexamethasone treatment during the expansion phase sustains stemness of mesenchymal stem cells from human bone marrow. Cell Res. 2008;18:116. doi: 10.1002/term.250. [DOI] [PubMed] [Google Scholar]
- 40.Khademhosseini A, Suh KY, Yang JM, Eng G, Yeh J, Levenberg S, Langer R. Layer-by-layer deposition of hyaluronic acid and poly-l-lysine for patterned cell co-cultures. Biomaterials. 2004;25:3583–3592. doi: 10.1016/j.biomaterials.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 41.Wu ZR, Ma J, Liu BF, Xu QY, Cui FZ. Layer-by-layer assembly of polyelectrolyte films improving cytocompatibility to neural cells. J Biomed Mater Res Part A. 2006;81A:355–362. doi: 10.1002/jbm.a.30993. [DOI] [PubMed] [Google Scholar]
- 42.Thierry B, Winnik FM, Merhi Y, Tabrizian M. Nanocoatings onto Arteries via Layer-by-Layer Deposition: Toward the in Vivo Repair of Damaged Blood Vessels. J Am Chem Soc. 2003;125:7494–7495. doi: 10.1021/ja034321x. [DOI] [PubMed] [Google Scholar]
- 43.Menga S, Liub Z, Shenc L, Guoa Z, Choud LL, Zhonga W, Dua Q, Ge J. The effect of a layer-by-layer chitosan–heparin coating on the endothelialization and coagulation properties of a coronary stent system. Biomaterials. 2009;30:2276–2283. doi: 10.1016/j.biomaterials.2008.12.075. [DOI] [PubMed] [Google Scholar]
- 44.Yang SY, Mendelsohn JD, Rubner MF. New Class of Ultrathin, Highly Cell-Adhesion-Resistant Polyelectrolyte Multilayers with Micropatterning Capabilities. Biomacromolecules. 2003;4:987–994. doi: 10.1021/bm034035d. [DOI] [PubMed] [Google Scholar]
- 45.Mendelsohn JD, Yang SY, Hiller JA, Hochbaum AI, Rubner MF. Rational Design of Cytophilic and Cytophobic Polyelectrolyte Multilayer Thin Films. Biomacromolecules. 2003;4:96–106. doi: 10.1021/bm0256101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.