Synopsis
The most common pathological manifestation of fear is posttraumatic stress disorder (PTSD). Developing PTSD is closely related with predisposing factors such as genes and early traumatic experiences. In PTSD, enhanced fear learning and poor extinction are common. Fear is manifested through autonomic responses and persistent memories of the traumatic event. These manifestations are related to stress responses modulated by the hypothalamic-pituitary-adrenal axis. The current review evaluates the role of fear and stress in the course of PTSD. Findings on fear learning and extinction are presented in order to guide future treatments for patients with PTSD.
Keywords: fear, PTSD, stress, extinction, conditioning
Exposure to stress can lead to different psychiatric manifestations depending on the individual. One possible pathological manifestation after a stressor is posttraumatic stress disorder (PTSD). Developing PTSD is closely related with predisposing factors such as genes and early traumatic experiences [1]. The Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-V) has included a category of trauma and stressor-related disorders that encompasses the variable clinical expressions of stress [2]. One of the most recognized expressions after a stressful stimulus is fear. Whileear is a natural response that protects us from threats, when this fear is excessive or expressed inappropriately, it can become pathological. Fear elicits natural autonomic responses such as increased heart rate, elevated skin conductance and activation of facial muscles that prepare the body to react to threat [3]. A possible pathological manifestation of excessive fear after a stressor is post-traumatic stress disorder (PTSD).
The diagnosis of PTSD describes the cluster of symptoms that emerge after exposure to actual or threatened death, serious injury or sexual violence. The person then develops intrusion symptoms associated with the trauma such as intrusive memories, distressing dreams, flashbacks or distress or physiological reactions upon exposure to cues of the trauma. There is also the avoidance of the reminders of the trauma, alterations in memories or mood associated with the trauma and marked alterations in physiological arousal and reactivity. Projected lifetime risk for PTSD according to DSM-V is 8.7% in the United States with lower prevalence in other countries. PTSD is a serious problem in certain samples such as war veterans, emergency medical personnel and survivors of rape [2].
Fear expression
In PTSD, a traumatic event causes a fear reaction that is excessively expressed. Based on DSM-V criteria, excessive fear can be seen as the physiological reactions to trauma cues and the alterations in physiological arousal and reactivity. Increased fear can be studied by evaluating the autonomic responses elicited by fear such as increased heart rate or skin conductance and activation of facial muscles, such as the startle response. Initial studies of fear responses in trauma exposed patients showed increases in all of these autonomic responses to non-trauma related stimuli [4, 5]. Increased startle has even been reported in veterans with sub-threshold PTSD symptoms [6]. These heightened responses seemed to be acquired as an effect of trauma exposure as shown by twin studies where the twin exposed to combat developed the elevated physiological responses while the non-combat exposed twin did not [7]. There is also evidence that pre-trauma elevated physiological reactivity is a vulnerability factor in developing PTSD after exposure to trauma [8]. Findings from animal studies as well as functional magnetic resonance imaging studies (fMRI) in humans describe activation of the amygdala during fear expression [9, 10]. Neuroimaging studies support the notion of increased fear in PTSD as amygdala hyperresponsivity has been a consistent finding in such cases [11]..
Fear learning
Another characteristic that has been described in PTSD patients is an increased capacity for conditioning fear responses. In other words, PTSD patients have an elevated facility to associate fear responses with the trauma memory and trauma cues. Conditionability can be related to the DSM-V symptoms of intrusive memories/flashbacks as well as the distortions in the memories of the event.
Fear conditioning is the process by which a neutral or conditioned stimulus (CS) is paired with an aversive, unconditioned stimulus (US) that will now produce a conditioned response(CR) to the CS. The unconditioned response (UR) is the natural response that would have been seen with the US alone, but when paired recurrently with the CS will then result in the CR in the presence of the CS alone. In the laboratory, fear conditioning is usually measured by the skin conductance response (SCR) or startle responses. SCR is the changes in skin conductance that can be elicited when a US (usually an electrical shock to the fingers) is paired to neutral or unconditioned stimuli (UR) (such as images). While many studies of SCRs in PTSD patients have shown enhanced conditionability [12-14], other studies have failed to find this enhanced conditioning [15]. In fact, more recent studies have even found that fear potentiated startle responses discriminated better than SCR between healthy versus PTSD participants [16]. Fear potentiated startle response is another measure of conditioning in which the conditioned fear is measured by an increase in the amplitude of the acoustic startle reflex in the presence of a cue previously paired with a shock [17].
There are various experimental factors that can affect the degree of conditioning. For example, when predictability is taken into account in PTSD patients, heightened fear is seen during periods of unpredictable, but not predictable threat [18]. Fear conditioning will also depend on the neutral stimulus that is chosen to be conditioned. Many laboratory fear conditioning studies use non-trauma related CS, but more recent studies using trauma related CS also have shown increased conditionability [12, 19]. Gender also plays a role in conditioning. In those with PTSD, women show larger SCR than men [20]. In women, hormonal levels also affect conditioning with studies showing that low estrogen levels are associated with higher fear-potentiated startle responses in PTSD patients [21]. Another important finding seen in PTSD patients during conditioning is that many cannot verbally report the CS-US contingency [22] pointing to a deficit in their declarative memory of the relationship between the trauma cues and the fear response.
Experimental studies of fear can also assess a safety signal (CS-). This can be done by presenting another neutral stimulus similar to the CS but that will not be paired with the US and thus creates an association between that stimulus and not receiving the US. For example, in a fear conditioning protocol in which a blue light is paired with an electric shock and a red light is not paired with an electric shock, the blue light will be the CS+ (threat signal) and the red light will be the CS- (safety signal). In PTSD, some studies evaluating SCR have described elevated reactions to the CS- which can be described as overgeneralization of the threat signal in comparison to the safety signal [13, 14, 22]. Similar findings have been found with fear potentiated startle in that fear is potentiated to the safety cue in PTSD veterans while not in healthy veterans [23]. Further exploring the processing of safety cues in PTSD has led to studies evaluating fear inhibition during the presence of safety cues, that is, the ability of the person to suppress fear with safety. The severity of the PTSD has been associated with the ability to generalize fear inhibition. For example, patients with more severe symptoms of PTSD will have more difficulty with fear inhibition [24]. These studies have lead to the proposal that safety signal processing might require awareness of the CS-US contingency and if this process is affected, as often happens in PTSD patients, then safety signals will also be faulty [24]. Difficulties with fear inhibition has also been described in patients with acute stress disorder [25].
The neural circuits involved in fear conditioning in humans have been described usingfMRI and include activation of the amygdala, the dorsal anterior cingulate cortex and the hippocampus [10]. These findings also relate to abnormal findings in fMRI of PTSD patients during conditioning, which have found heightened amygdala activity [26, 27], especially the left side of the amygdala [28], and diminished ventromedial prefrontal cortex (vmPFC) activity [27]. Dysfunctions in the connectivity between amygdala and vmPFC may mediate susceptibility to anxiety disorders [29].
Fear regulation
Once unwanted or excessive fear is present, it is important to have mechanisms to regulate this fear. Fear can be regulated by extinction, cognitive emotion regulation, active coping, reversal or reconsolidation [30, 31]. All these mechanism of changes seem to be regulated by a common neural mechanism but evaluating each process can have important treatment implications [31]. Extinction is the process by which a new safety memory is created that can compete with the original conditioned memory. In other words, when a neutral stimulus produces a fear response because the person learned the contingency between CS and US, that fear response can decrease if the person then creates a new memory where that CS is no longer paired to the US;this process is called extinction. Studies with PSTD patients show slower extinction [12, 14, 22]. PTSD patients also show an overestimation of the probability of the US following CS+ during extinction [22].
The neural circuits involved in extinction have been clearly delineated [10] and include interactions between the vmPFC, the amygdala and the hippocampus. The amygdala activates in early extinction and this activation decreases across extinction training [29]. In fMRI of PTSD patients, during fear extinction, there are reports of decreasing vmPFC activity [28] pointing to a possible neurofunctional deficit in the capacity to extinguish. Reduced extinction learning has also been related to risk of development of PTSD in firefighters [32] and Dutch soldiers [33]. The available evidence seems to point to a deficit in extinction that could be a risk factor for the development of PTSD.
In order for extinction to continue regulating fear, a person will need to be able to recall the extinction memory when exposed to trauma cues. Studies with PTSD patients have shown that even if the subjects did not show increased conditioning or extinction, they still present difficulty using the extinction memory to control fear when exposed to the CS in the extinction context [15, 34]. In fMRI studies, PTSD patients show reduced activity in vmPFC and hippocampus but increased activity in dorsolateral (dl) PFC during extinction recall [34]. Reduced vmPFC activity and increased dlPFC may mediate an inability to use contextual cues to predict safety [29]. Further studies have found that these impaired recall extinction findings are seen in participants with PTSD but not in their non-traumatized twins which could mean this deficit is acquired and not a risk factor for PTSD [15].
Fear can also be modulated in humans by cognitively regulating fear in which thoughts are used to diminish fear. In contrast with extinction, these techniques are thought to require active participation of the person. The available studies on how fear can be modulated cognitively could have important implications in the treatment for PTSD. For example, telling subjects that they will be shocked will elicit a fear response and amygdala activation even if the shock is not given [35]. Instructions to regulate conditioned fear leads to reduced conditioned responses [36], increased activation of the dlPFC, decreased activation of amygdala and activation of the vmPFC [37]. Similar patterns of activation in amygdala and vmPFC are seen if fear is diminished by extinction or by cognitive regulation; only cognitive regulation activates dlPFC. One study has also found that the effect of cognitive reappraisal on fear is impaired by acute stress [38]. Data on cognitive regulation of fear in PTSD is limited, but given that PTSD patients show abnormalities in the same anatomical areas used for cognitive regulation, future studies could be used to better control fear.
Another way to modulate fear is through the disruption of reconsolidation. Every time a memory is retrieved the underlying memory trace is again labile and needs to reconsolidate; this reconsolidation period allows disruption of memory [39]. Since beta-receptors regulate long-term memory storage, propranolol has been shown to decrease fear expression after activating a traumatic memory in PTSD patients [40]. High levels of glucocorticosteroids can also interrupt the consolidation of the traumatic memory [41]. Reconsolidation manipulations might actually change the original conditioned memory and thus might be immune to return of fear [42].
Factors modulating fear
The process of extinction can be affected by several factors such as how much time occurs between extinction trials, contextual cues, how related to the person's fear is the CS, the presence of other excitatory CS during extinction or the introduction of inhibitory CS- (safety signals) during extinction [43]. In terms of inhibitory CS- or safety signals, one study showed that the use of a button press to avoid shock prevented extinction in healthy subjects [44]. This is of relevance for PTSD patients as they tend to show avoidance of trauma cues to prevent physiological arousal at the moment, but given the studies with inhibitory CS-, this might worsen the fear responses in the long run.
There are several other factors that have been related to the return of fear even after adequate extinction such as renewal, reinstatement, spontaneous recovery or reacquisition [45]. Extinction is highly specific to the precise circumstances (context) in which the extinction occurs [43]. Presenting the CS in the context where conditioning took place or in a new context can lead to renewal of fear. Renewal is greater when the conditioning context is encountered versuswhen exposed to a novel context [46]. Renewal is of particular relevance for PTSD as they may learn to extinguish the conditioned fear responses to trauma cues in a safe environment but might have a return of symptoms if they encounter the same context but in this case, where the trauma occurred.
Extinguished fear can also re-appear if the US is presented again in the conditioning context, a process called reinstatement [43]. Extinction is also specific to time [45] causing spontaneous recovery to occur with the passage of time.
Interaction of stress and fear in the development of PTSD
The stress response of the hypothalamic-pituitary-adrenal (HPA) axis is closely related to fear expression and regulation. The amygdala has glucocorticoid receptors, which modulate fear memory consolidation [47]. One of the areas of convergence is the effect of early trauma on the circuits involved in fear. Early life trauma has been shown to sensitize the HPA axis producing an ineffective stress response system. For example, when exposed to stressful situation as adults, women with early life trauma have a greater adrenal cortico-tropin hormone release and increases in heart rates [48]. Stress in humans also may lead to hippocampal damage [49-51] thus affecting the memory processing of fear. In fact, childhood abuse is associated with increased startle reactivity in adults that cannot be accounted for by PTSD or depressive symptoms suggesting that an increased startle may be a biomarker of stress responsiveness that is a persevering consequence of childhood trauma exposure [1]. The number of traumas a person has experienced also has implications on fear expression and PTSD symptoms. In a study of PTSD patients who had multiple traumas, it was found that they did not present increased fear expression.Instead these patients had lower skin conductance and startle responses than the patients who had only one traumatic event. Multiple traumas also had an effect on PTSD symptomatology, as these patients also presented higher levels of avoidance and numbing than the single trauma patients [52].
Treatment implications
The findings on fear processing in PTSD patients can lead to better screening methods and treatments. In terms of using fear for screening, the genetic heritability of conditioning and extinction is 35-45% [53]. There is some evidence that genetic variation in the serotonin transporter gene affects conditionability, the catechol-O-methyltransferase gene affects fear memory consolidation and the brain-derived neurotrophic factor (BDNF) val66met genotype has been found to have various effects on conditioning and extinction processes [54]. Thus the capacity for fear conditioning and/or extinction might be an inheritable risk factor for the development of PTSD and might be used as a way to identify people at risk before being exposed to trauma. In fact, the capacity to extinguish fear has been identified as a risk factor for the development of PTSD before trauma exposure in high-risk samples [8, 32, 33]. Longitudinal studies that measure fear conditioning or extinction capacity before trauma are needed in order to be able to classify these fear processes as biological risk factors (endophenotypes) for PTSD symptoms. There is also a need to evaluate how the factors affecting fear acquisition and extinction, such as consolidation and context, could also be used to prevent PTSD emergence when a person encounters the traumatic experience. For example, the production of the traumatic memory might be impaired if propranolol [55] or systemic glucocorticoids [41] are given before the initial consolidation of the fear memory.
Research on fear has also provided insight into the mechanisms of exposure therapy. Theoretically, exposure therapy is thought to improve anxiety through emotional processing in which a fear network is activated and new incompatible information is added which causes decreases in these fear responses within the therapy session (within-session habituation) and between therapy sessions (between-session habituation) [56]. This theory could be described with fear terminology by stating that fear is elicited and then extinguished. Thus research on fear expression and extinction can lead to better understanding of exposure therapy and improvements for those patients who do not respond to therapy. In fact, laboratory measures of extinction retention have been shown to predict degree of improvement with exposure therapy in social anxiety disorder but to our knowledge no studies of this kind have been done in PTSD [57]. Clinical measures of fear (self-report arousal symptoms) have also been related to cognitive behavior therapy (CBT) outcomes in general. For example, higher ratings of anxiety at the start of the first exposure were related to no improvement, while higher within session habituation was related to improvement of anxiety symptoms [58]. Differential pre-treatment conditioned responses were related to better responses of PTSD symptoms to the serotonin-norepinephrine reuptake inhibitor duloxetine [59]. Changes in symptom severity with trauma focused CBT has also been associated with a decrease in physiological reactivity [60].
Studies on the factors that affect extinction learning can also improve exposure therapy. Studies with fMRIs have found that areas related to fear learning and extinction, such as greater activation of amygdala and ventral anterior cingulate region when viewing fearful faces prior to CBT, can predict who will have a better response to the therapy [61]. Also, one study has shown that theBDNF val66met polymorphism predicts response to exposure therapy in PTSD [62].
Research on inhibitory CS during extinction has been done on the use of safety signals in exposure therapy, which in fact has been shown to impair positive outcomes in therapy [43]. Relapse or return of fear can affect as many as 33-50% of successfully treated individuals [63]. Return of fear has been seen when changing therapists or rooms in which exposure takes place [64]. Contextual stimuli might include external stimuli (room, place, background stimuli) or interoceptive stimuli (drug states, hormonal states, deprivation, expectation of events, time) [45]. Drug states, as a contextual cue, are particularly relevant in the treatment of PTSD as exposure therapy is frequently combined with pharmacotherapy. If the patient learns to extinguish fear under the effect of a medication, and after some time this medication is discontinued, that patient might be at higher risk of relapse because of a renewal effect [65] possibly due to the the fact that the success was based on the neural pathways being dependent on the presence of medication, an example of state dependent memory . To prevent relapses, these studies have provided information that using multiple context exposures or booster sessions (different contextual times) can improve outcomes of exposure therapy. It has also been recommended that when using medications in combination with CBT, the medications should allow for the full activation of the fear structure with the least possible side effects to minimize this aforementioned state-dependent learning [65].
Understanding the molecular mechanisms behind fear extinction can lead to novel treatments that enhance extinction learning or recall. One of the areas that have received the most attention is the N-Methyl-D-aspartic acid receptor (NMDAR), as NMDAR antagonists block both fear and extinction learning with the opposite being true of NMDAR agonists. This has led to the discovery that D-cycloserine, a partial agonist of NMDARs, facilitates fear extinction and has been tried with contradictory results during exposure therapy to enhance fear reduction [65]. Other areas of interest are the endocannabinoid system with anandamide showing facilitation of fear extinction [66]. Although no adequate molecules have been found, the BDNF-tyrosine kinase B pathway and the pituitary adenylate cyclase-activating polypeptide are also areas of interest for the development of treatments as they have been shown to be implicated in fear extinction and in PTSD development [67]. Several other neurotransmitters, such as gamma-aminobutyric acid (GABA), dopamine, acetylcholine, norepinephrine and opioids have shown to be involved in the consolidation and extinction of fear but the clinical implications of these findings are still unclear [68]. Direct stimulation of the circuits involved in fear learning and extinction with devices such as deep brain stimulation, vagal nerve stimulators and transcranial magnetic stimulation are also other avenues of treatment that need further study [69].
Conclusion
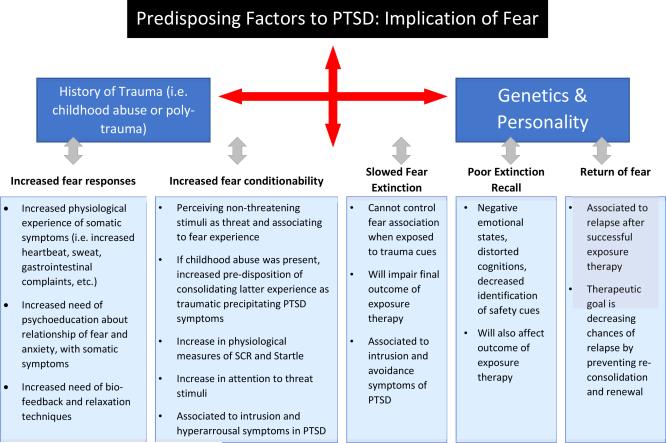
The expression, conditioning and regulation of fear have been implicated in the development of all of the symptoms of PTSD (Figure 1). These fear processes have a direct relationship with stress responses as the traumatic experience starts a cascade in which fear circuits and the HPA axis are affected. Experimental and clinical measures of fear can be used to help in the understanding of how a stressful situation can lead to pathology and also serve in the development of better screening and treatment for PTSD patients.
Figure 1. Predisposing factors related to increased experience of fear in PTSD and their implications for treatment.
Predisposing factors are a key to altering an individual's resiliency. Not only are genetic variances accountable for psychopathology, but environmental experiences can either awaken or alter genetic behavior leading to psychopathology. In the development of PTSD, fear plays a crucial role.
Key-points.
Stress and fear, in response to actual or possible threat, enhances the possibility of forming trauma-related memories leading to post-traumatic stress disorder (PTSD).
Excessive fear responses in PTSD can be seen as physiological reactions to trauma cues and alterations in arousal and reactivity increasing fear conditioning capacity.
Predisposing factors such as suffering childhood abuse increases risk of fear conditioning, renewal, and reconsolidation.
Studies on fear learning, extinction, and neuroimaging support the notion that increased fear is related to amygdala hyperresponsivity and dysfunctions in neural circuitry, specifically the areas of the ventromedial prefrontal cortex (vmPFC) which regulate fear.
Severity of PTSD is associated with the inability to inhibit fear generalization, poor cognitive emotion regulation, and hippocampal damage affecting the memory processing of fear.
Findings on fear have lead to the identification of effective treatment modalities to reduce fear alterations in PTSD such as effective pharmacotherapy includingpropranolol and selective norepinephrine reuptake inhibitors (SNRI's), cognitive behavior therapy, and exposure therapy.
| PTSD | posttraumatic stress disorder |
| DSM-V | Diagnostic and Statistical Manual of Mental Disorders, 5th ed. |
| fMRI | functional magnetic resonance imaging studies |
| US | unconditioned stimulus |
| UR | unconditioned response |
| CS | safety signal |
| vmPFC | ventromedial prefrontal cortex |
| HPA | hypothalamic-pituitary-adrenal |
| CBT | cognitive behavior therapy |
| GABA | gamma-aminobutyric acid |
| vmPFC | the ventromedial prefrontal cortex |
| DSM-IV-TR | Diagnostic and statistical manual of mental disorders, 4th ed. text revision |
| CS+ | conditioned stimulus |
| CR | conditioned response |
| SCR | skin conductance response |
| CS+ | threat signal |
| dl | dorsolateral |
| BDNF | brain-derived neurotrophic factor |
| NMDAR | N-Methyl-D-aspartic acid receptor |
Acknowledgments
This work was funded by NCRR U54RR026139-01A1 and NIMHHD 8U54MD007587-03 (K. Martínez)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Jovanovic T, Blanding NQ, Norrholm SD, et al. Childhood abuse is associated with increased startle reactivity in adulthood. Depress Anxiety. 2009;26(11):1018–26. doi: 10.1002/da.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association . Diagnostic and statistical manial of mental disorders. Fifth Edition American Psychiatric Association; Arlington, VA: 2013. [Google Scholar]
- 3.Lang PJ, McTeague LM. The anxiety disorder spectrum: Fear imagery, physiological reactivity, and differential diagnosis. Anxiety Stress Coping. 2009;22(1):5–25. doi: 10.1080/10615800802478247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orr SP, Lasko NB, Shalev AY, Pitman RK. Physiological responses to loud tones in Vietnam Veterans with Posttraumatic Stress Disorder. J Abnorm Psychol. 1995;104(1):75–82. doi: 10.1037//0021-843x.104.1.75. [DOI] [PubMed] [Google Scholar]
- 5.Orr SP, Lasko NB, Metzger LJ, Pitman RK. Physiological responses to non-startling tones in Vietnam veterans with post-traumatic stress disorder. Psychiatry Res. 1997;73(1-2):103–7. doi: 10.1016/s0165-1781(97)00110-8. [DOI] [PubMed] [Google Scholar]
- 6.Roy M, Costanzo M, Leaman S. Psychophysiological identification of subthreshold PTSD in combat veterans. Stud Health Technol Inform. 2012;181:149–55. [PubMed] [Google Scholar]
- 7.Orr SP, Metzger LJ, Lasko NB, et al. Physiological responses to sudden loud tones in monozygotic twins discordant for combat exposure. Arch Gen Psychiatry. 2003;60:283–8. doi: 10.1001/archpsyc.60.3.283. [DOI] [PubMed] [Google Scholar]
- 8.Guthrie RM, Bryant RA. Auditory startle response in firefighters before and after trauma exposure. Am J Psychiatry. 2005;162(2):283–90. doi: 10.1176/appi.ajp.162.2.283. [DOI] [PubMed] [Google Scholar]
- 9.Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychiatry. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Sehlmeyer C, Schoning S, Zwitserlood P, et al. Human fear conditioning and extinction in neuroimaging: a systemic review. PLoS One. 2009;4(6):e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am J Psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wessa M, Flor H. Failure of Extinction of Fear Responses in Posttraumatic Stress Disorder: Evidence from second-order conditioning. Am J Psychiatry. 2007;164(11):1684–92. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- 13.Orr SP, Metzger LJ, Lasko NB, et al. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109(2):290–98. [PubMed] [Google Scholar]
- 14.Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiological assessment of aversive conditioning in Posttraumatic Stress Disorder. Biol Psychiatry. 2000;47:512–19. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- 15.Milad MR, Orr SP, Lasko NB, et al. Presence and Acquired Origin of Reduced Recall for Fear Extinction in PTSD: Results of a Twin Study. J Psychiatr Res. 2008;42(7):515–20. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glover EM, Phifer JE, Crain DF, et al. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depress Anxiety. 2011;28:1058–66. doi: 10.1002/da.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58(1-2):175–98. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- 18.Grillon C, Pine DS, Lissek S, et al. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wegener M, Blechert J, Kerschbaum H, Wilhelm FH. Relationship between fear conditionability and aversive memories: evidence from a novel conditioned-intrusion paradigm. PLoS One. 2013;8(11):1–13. doi: 10.1371/journal.pone.0079025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inslicht SS, Metzler TJ, Garcia NM, et al. Sex differences in fear conditioning in posttraumatic stress disorder. J Psychiatr Res. 2013;47(1):64–71. doi: 10.1016/j.jpsychires.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glover EM, Jovanovic T, Mercer KB, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry. 2012;72(1):19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blechert J, Michael T, Vriends N, et al. Fear conditioning in posttraumatic stress disorder: Evidence for delayed extinction of autonomic, experiential and behavioural responses. Behav Res Ther. 2007;45:2019–33. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Grillon C, Morgan CA. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol. 1999;108:134–42. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- 24.Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167(6):648–62. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jovanovic T, Sakoman AJ, Kozaric D, et al. Acute stress disorder versus chronic posttraumatic stress disorder: inhibition of fear as a function of time since trauma. Depress anxiety. 2013;30(3):217–24. doi: 10.1002/da.21991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47(9):769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 27.Shin LM, Wright CI, Cannistrano PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 28.Bremner J, Vermetten E, Schmahl C, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Pychol Med. 2005;35(6):791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham BM, Milad MR. The study of fear extinction: implications for anxiety disorders. Am J Psychiatry. 2011;168:1255–65. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–46. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends in Cogn Sci. 2010;14:268–76. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guthrie RM, Bryant RA. Extinction learning before trauma and subsequent posttraumatic stress. Psychosom Med. 2006;68:307–11. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- 33.Lommen MJ, Engelhard IM, Sijbrandij M, et al. Pre-trauma individual differences in extinction learning predict posttraumatic stress. Behav Res Ther. 2013;51:63–7. doi: 10.1016/j.brat.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phelps EA, O'Connor KJ, Gatenby JC, et al. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4(4):437–41. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- 36.Shurick AA, Hamilton JR, Harris LT, et al. Durable effects of cognitive restructuring on conditioned fear. Emotion. 2012;12(6):1393–7. doi: 10.1037/a0029143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–38. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raio CM, Orederu TA, Palazzolo L, et al. Cognitive emotion regulation fails the stress test. PNAS. 2013;110(37):15139–44. doi: 10.1073/pnas.1305706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10(9):1116–24. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunet A, Orr SP, Tremblay J, et al. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42(6):503–6. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Schelling G. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry. 2001;50:978–985. doi: 10.1016/s0006-3223(01)01270-7. [DOI] [PubMed] [Google Scholar]
- 42.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324(5929):951–5. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hermans D, Craske MG, Mineka S, Lovibond PF. Extinction in human fear conditioning. Biol Psychiatry. 2006;60:361–8. doi: 10.1016/j.biopsych.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Lovibond PF, Mitchell CJ, Minard E, et al. Safety behaviours preserve threat beliefs: protection from extinction of human fear conditioning by an avoidance response. Behav Res Ther. 2009;47:716–20. doi: 10.1016/j.brat.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Bouton ME. Context, ambiguity and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–86. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 46.Neuman DL, Kitlertsirivatana E. Exposure to a novel context after extinction causes a renewal of extinguished conditioned responses: Implications for the treatment of fear. Behav Res Ther. 2010;48(6):565–70. doi: 10.1016/j.brat.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Ursano RJ, Goldenberg M, Zhang L, et al. Posttraumatic stress disorder and traumatic stress: from bench to bedside, from war to disaster. Ann N Y Acad Sci. 2010;1208:72–81. doi: 10.1111/j.1749-6632.2010.05721.x. [DOI] [PubMed] [Google Scholar]
- 48.Heim C, Newport JD, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–7. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 49.Bremner JD, Narayan M. The effects of stress on memory and hippocampus throughout the life cycle: implications for childhood development and aging. Dev Psychopathol. 1998;10(4):871–85. doi: 10.1017/s0954579498001916. [DOI] [PubMed] [Google Scholar]
- 50.Pitman RK, Shin LM, Rauch SL. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry. 2001;62(Suppl 17):47–54. [PubMed] [Google Scholar]
- 51.Bremner JD. Neuroimaging of childhood trauma. Semin Clin Neuropsychiatry. 2002;7(2):104–12. doi: 10.1053/scnp.2002.31787. [DOI] [PubMed] [Google Scholar]
- 52.McTeague LM, Lang PJ. The anxiety spectrum and the reflex physiology of defense: from circumscribed fear to broad distress. Depress Anxiety. 2012;29(4):264–81. doi: 10.1002/da.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hettema JM, Annas P, Neale MC, et al. A twin study of the genetics of fear conditioning. Arch Gen Psychiatry. 2003;60(7):702–8. doi: 10.1001/archpsyc.60.7.702. [DOI] [PubMed] [Google Scholar]
- 54.Londsorf TB, Kalisch R. A review on experimental and clinical genetic associations studies on fear conditioning, extinction and cognitive-behavioral treatment. Transl Psychiatry. 2011;1:e41. doi: 10.1038/tp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pitman RK, Delahanty DL. Conceptually driven pharmacologic approaches to acute trauma. CNS Spectr. 2005;10(2):99–106. doi: 10.1017/s109285290001943x. [DOI] [PubMed] [Google Scholar]
- 56.Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychol Bull. 1986;99:20–35. [PubMed] [Google Scholar]
- 57.Berry AC, Rosenfield D, Smits JA. Extinction retention predicts improvement in social anxiety symptoms following exposure therapy. Depress Anxiety. 2009;26:22–7. doi: 10.1002/da.20511. [DOI] [PubMed] [Google Scholar]
- 58.van Minnen A, Hagenaars M. Fear activation and habituation patterns as early process predictors of response to prolonged exposure treatment in PTSD. J Traum Stress. 2002;15(5):359–67. doi: 10.1023/A:1020177023209. [DOI] [PubMed] [Google Scholar]
- 59.Aikins DE, Jackson ED, Christensen A, et al. Differential conditioned fear response predicts duloxetine treatment outcome in male veterans with PTSD: A pilot study. Psychiatry Res. 2011;188:453–5. doi: 10.1016/j.psychres.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Zantvoord JB, Diehle J, Lindauer RJ. Using neurobiological measures to predict and assess treatment outcome of psychotherapy in posttraumatic stress disorder: systematic review. Psychother Psychosom. 2013;82(3):142–51. doi: 10.1159/000343258. [DOI] [PubMed] [Google Scholar]
- 61.Bryant RA, Felmingham K, Whitford TJ, et al. Rostral anterior cingulate volume predicts treatment response to cognitive-behavioural therapy for posttraumatic stress disorder. Rev Psychiatr Neurosci. 2008;33(2):142–6. [PMC free article] [PubMed] [Google Scholar]
- 62.Felmingham KL, Dobson-Stone C, Schofiel PR, et al. The brain-derived neurotrophic factor Val66Met polymorphism predicts response to exposure therapy in posttraumatic stress disorder. Biol Psychiatry. 2012;73(11):1059–63. doi: 10.1016/j.biopsych.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Craske MG, Rachman SJ. Return of fear: perceived skill and heart-rate responsivity. Br J Clin Psychol. 1987;26(Pt 3):187–99. doi: 10.1111/j.2044-8260.1987.tb01346.x. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez BI, Craske MG, Mineka S, Hladek D. Context-specificity of relapse: effects of therapist and environmental context on return of fear. Behav Res Ther. 1999;37:845–62. doi: 10.1016/s0005-7967(98)00106-5. [DOI] [PubMed] [Google Scholar]
- 65.Hofmann SG. Enhancing exposure-based therapy from a tranlational research perspective. Behav Res Ther. 2007;45(9):1987–2001. doi: 10.1016/j.brat.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gunduz-Cinar O, MacPherson KP, Cinar R, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Molecular Psychiatry. 2013;18:813–23. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implication for posttraumatic stress disorder. Trends neurosci. 2012;35(1):24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morrison FG, Ressler KJ. From the neurobiology of extinction to improved clinical treatments. Depress anxiety. 2014;31:279–90. doi: 10.1002/da.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marin MF, Camprodon JA, Dougherty DD, Milad MR. Device-based brain stimulation to augment fear extinction: implications for PTSD treatment and beyond. Depress and anxiety. 2014;31:269–278. doi: 10.1002/da.22252. [DOI] [PubMed] [Google Scholar]