Abstract
Purpose of review
The global incidence of small papillary thyroid carcinoma (PTC) is increasing remarkably, mostly due to the increased use of imaging studies worldwide. The issue of how to manage low-risk small PTC has become urgent. In this review, we focus on how to treat low-risk papillary thyroid microcarcinomas (PMCs; i.e., PTCs measuring ≤10 mm).
Recent findings
Studies of large numbers of patients with low-risk PMC clarified that most of the PMCs did not grow or grew very slowly and were harmless. Active observations of these patients discriminated rare progressive cases from the majority. Surgery performed after the detection of progression signs was not too late, and surgery immediately after the detection and diagnosis of low-risk PMC may be overtreatment for most patients. Interestingly, low-risk PMCs in elderly patients were most unlikely to progress, in sharp contrast to clinical PTC. The reason for this phenomenon remains unknown.
Summary
Active observation without immediate surgery can be a leading alternative to the classical surgical treatment in the majority of the patients with low-risk PMC. It is not too late to perform surgery after the detection of progression signs for these patients.
Video abstract
http://links.lww.com/COON/A10
Keywords: nonsurgical treatment, observation, papillary thyroid carcinoma, papillary thyroid microcarcinoma, prognosis
INTRODUCTION
Papillary thyroid carcinoma (PTC) is the most common malignancy of the thyroid. PTC is generally an indolent disease, unless it has certain clinicopathological features such as gross extrathyroid extension, clinical node metastasis, distant metastasis at diagnosis, and aggressive histological variants. In the past years, most PTCs were treated after the detection of tumors on palpation, and it is well known that many small PTCs have been found during autopsies of patients who died of unrelated diseases. These latent carcinomas were harmless for the patients when they were alive. With the growing use of ultrasound examinations, however, small and impalpable PTCs have become detectable in living individuals, resulting in a greatly increased incidence of PTC worldwide.
In the United States, for example, a 2.4-fold increase in the incidence of thyroid cancer between 1973 and 2002 was reported by Davies and Welch [1], who noted that the increase was mostly due to the increase in the detection of small PTCs, whereas the incidences of other types of thyroid carcinoma were stable and the mortality from thyroid cancer remained stable [1]. In 2014, Davies and Welch [2] reported a 2.9-fold increase in the incidence of thyroid cancer between 1975 and 2009, mostly due to the increased detection of small low-risk PTCs by ultrasound and ultrasound-guided fine needle aspiration biopsy (FNAB). These findings raised an important clinical issue: how to manage small low-risk PTCs, especially papillary thyroid microcarcinomas (PMCs; i.e., PTCs measuring ≤10 mm).
Ultrasound can detect thyroid nodules measuring at least 3 mm, and ultrasound-guided FNABs can diagnose PTCs of this size. In autopsy studies, the incidence of latent PTCs measuring 3–9.9 mm ranged from 0.5 to 5.2% [3]. When these sophisticated technologies were used in a mass screening study for thyroid cancer, Takebe et al.[4] found that 3.5% of otherwise healthy women aged 30 years or older had small thyroid cancers. Of these, 84% measured 15 mm or less. This incidence is close to the incidence of latent thyroid carcinoma found in autopsy studies, and it was about 1000 times the prevalence of clinical PTC at that time (1.9–11.7 per 100 000 women [3]).
These data suggested that most PMCs detected incidentally by ultrasound do not grow or grow very slowly and are harmless throughout the patient's lifetime, which raises the issue of whether all PMCs should be surgically treated immediately after diagnosis. Of course, any advanced PTC started as a small carcinoma. Only a small minority of PMCs seem to grow, but at present it is impossible to discriminate such PMCs from the slow-growing and nongrowing majority except by active observation.
On the basis of the evidence summarized above, Akira Miyauchi proposed nonsurgical treatment for low-risk PMCs at a physicians’ meeting at Kuma Hospital in 1993, and an active observation trial was initiated the same year at the hospital. Miyauchi suspected that it would not be too late to treat PMCs surgically after progression signs (such as an increase in size or the novel appearance of cervical node metastasis) were detected. In 1995, Cancer Institute Hospital in Tokyo also started an observation trial, and the two institutes have published promising results [5,6,7▪▪,8]. The primary goal of the observation of low-risk PMCs is to avoid unnecessary surgery, as surgery is sometimes accompanied by complications – especially for surgeries performed by less-experienced surgeons. As a secondary effect, societal medical costs are reduced by avoiding numerous unnecessary surgeries.
Box 1.
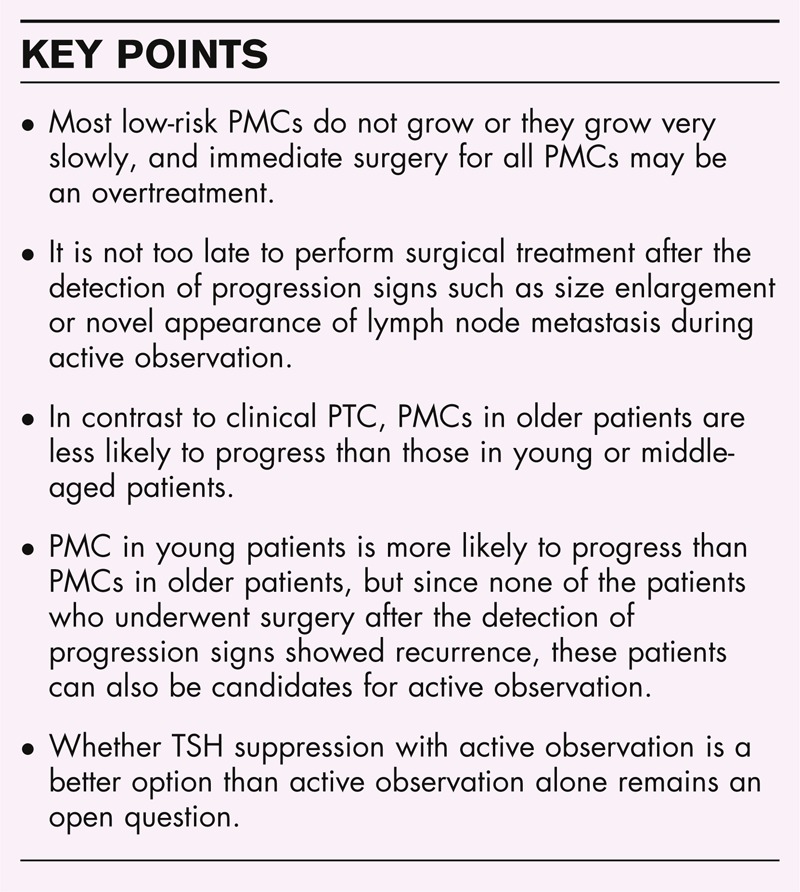
no caption available
CLASSIFICATION AND BIOLOGICAL CHARACTERISTICS OF PAPILLARY THYROID MICROCARCINOMA
PMC is defined as a PTC measuring 1.0 cm or less. This includes a wide range of clinical and biological features. In this section, we describe the classification of PMCs and their biological characteristics.
Papillary thyroid microcarcinoma classification based on how it was detected
PMCs are classified in the following five categories based on the momentum of detection: first, PMCs causing clinical symptoms such as vocal cord paralysis, although rare (clinically symptomatic PMC); second, PMCs discovered as the origin of metastases of regional lymph nodes or distant organs (occult PMCs, also a form of clinically symptomatic PMC); third, PMCs found incidentally in thyroids resected for other benign diseases by pathological examination (pathologically incidental PMCs); fourth, PMCs detected by imaging studies conducted not for the PMC itself (clinically incidental PMCs); and fifth, PMCs revealed during an autopsy (latent PMCs). The recent increase in the incidence of thyroid carcinoma is due mainly to the increase of clinically incidental PMCs, usually detected by ultrasound for thyroid screening and atherosclerosis evaluations of the carotid artery.
Biological characteristics of papillary thyroid microcarcinomas
Symptomatic PMCs display a rather poor prognosis. Hotomi et al.[9] showed that the 10-year disease-free survival and cause-specific survival (CSS) for PMCs with preoperative vocal cord paralysis were 50 and 89.5%, respectively. PMCs with clinical node metastasis also showed a poor prognosis, especially when the node metastasis was large in size [10]. The 10-year lymph node recurrence-free survival rate, distant recurrence-free survival rate, and CSS rate of PMCs with node metastasis at least 3 cm were 70, 88, and 90%, respectively [10].
In contrast, pathologically incidental PMCs were reported to have an excellent prognosis and only 2.2% showed a recurrence, although no additional surgery such as completion total thyroidectomy or lymph node dissection was performed [11]. Incidentally detected PMC on ultrasound without high-risk features also showed an excellent prognosis postoperatively: the 10-year lymph node recurrence-free and distant recurrence-free survival rates and CSS were 99, 100, and 100%, respectively [10].
NONOPERATIVE MANAGEMENT (ACTIVE OBSERVATION) OF LOW-RISK PAPILLARY THYROID MICROCARCINOMA
Here we describe how patients with PMCs are selected and observed, and their outcomes.
Selection of papillary thyroid microcarcinoma patients suitable for active observation
Following the 1993 physicians’ meeting at Kuma Hospital mentioned above, we decided to perform ultrasound-guided FNABs for the nodules of patients referred for a nodule evaluation and to then explain the diagnoses to the patients. At that time, there were no guidelines regarding the indications for FNABs for thyroid nodules. If we did not perform an FNAB for a given patient, the patient may have sought treatment from another facility, where an FNAB might be performed, resulting in the possibility of the patient being told that he or she has thyroid cancer, that Kuma Hospital missed the diagnosis, and that he/she should undergo surgery immediately. This would be an unhappy situation for the patients and for us.
After the diagnosis of PTC by FNAB, we propose two options to patients (unless their PMCs have high-risk features): active observation versus surgical treatment. High-risk features include the presence of clinical lymph node metastasis (N) or distant metastasis (M) (although very rare), signs of invasion to the recurrent laryngeal nerve (i.e., vocal cord paralysis) or trachea, suspicion of high-grade malignancy such as tall cell variant or poorly differentiated carcinoma on FNAB cytology (although very rare in PMC), and progression signs such as size enlargement and novel N appearance during observation. We have cautiously included tumor location adjacent to the recurrent laryngeal nerve as a feature unsuitable for observation. PMCs without these features can be candidates for observation. Tumor multiplicity, family history, sex, and age are not considered risk factors.
How low-risk papillary thyroid microcarcinoma can be observed
For the observation of low-risk PMCs, the patients who choose active observation after being fully informed of all options and potential outcomes undergo examinations by ultrasound once or twice per year. Progression signs detected by ultrasound are carefully checked and may provide the basis for further action.
Progression signs consist mainly of two factors: tumor size enlargement and the novel appearance of node metastasis. We define tumor enlargement as when the size of the tumor has increased by 3 mm or more compared with its original size. For suspicious lymph nodes, an FNAB and thyroglobulin measurement of the washout of the needles used for FNAB are indicated [12].
Ultrasound has several limitations. It is difficult to evaluate the dorsal side of tumors with strong echoes. We add a computed tomography scan in such cases. Another limitation is the variation in observers’ interpretations of ultrasound images. This is more frequently problematic for tumors with unclear borders due to the coexistence of chronic thyroiditis. As noted above, we set the cut-off of size enlargement at 3 mm because we recognize that ±2 mm might be accounted for by simply observers’ variation in our experience.
Outcomes of active observation for low-risk papillary thyroid microcarcinoma
The first report of active observation for low-risk PMCs was published in 2003 from our institution, which showed that the tumor size of more than 70% of the 162 patients was stable (we set the cut-off at 2 mm in that study) in every follow-up period, and node metastasis appeared only in 1.2% of the patients [5]. In our second report in 2007, we included a longitudinal data analysis by the Kaplan–Meier method and demonstrated that the 5-year size enlargement and appearance of novel node metastasis rates were 6.7 and 1.7%, respectively [3].
In 2010, Sugitani et al.[8] reported that the tumor size enlargement and appearance of lymph node metastasis were detected in 7.3 and 1.3% of their 230 patients, respectively, although no longitudinal data were demonstrated. In the same year, we showed that the 5-year and 10-year size enlargement rates were 6.4 and 15.9% and those of novel lymph node metastasis appearance were 1.4 and 3.4%, respectively [6]. The incidence of tumor enlargement in a 10-year follow-up decreased to 8.0% in our most recent study of 1235 patients [7▪▪]. Sugitani et al.[13] recently updated their data and showed that after 2–22 years of observation, the PMCs in only 16 and three of their 322 patients (5 and 1%) showed a size increase and clinical node metastasis, respectively.
Is the novel appearance of clinical node metastasis a failure of active observation? If patients in whom the novel appearance of clinical node metastasis had undergone surgical treatment at their presentation, the most likely procedure would have been hemithyroidectomy with or without paratracheal node dissection. This procedure cannot prevent the appearance of node metastasis, however, and thus these patients would need a second surgery. For patients with node metastasis, we perform a total thyroidectomy and neck dissection. We think that one surgery is better than two surgeries, since the final outcomes seem similarly excellent. We note that both of the studies from Kuma Hospital and the Cancer Institute Hospital demonstrated that none of the PMC patients under observation showed distant metastasis or died of PTC.
A SIGNIFICANT RELATIONSHIP BETWEEN PATIENT AGE AND THE PROGRESSION OF PAPILLARY THYROID MICROCARCINOMA
Age is a significant prognostic factor of PTC. Mazzaferri and Jhiang [14] demonstrated in 1994 that the recurrence rate of PTC and follicular thyroid cancer was high in patients younger than 20 years and older than 59 years, and that the mortality rates increased with patients’ age. In 2013, Miyauchi et al.[15▪▪] showed that the rate of persistent disease (thyroglobulin detectable after total thyroidectomy) was higher in older (≥60 years) and young (<40 years) patients compared with middle-aged patients (40–59 years). Importantly, Miyauchi et al.[16] also demonstrated that the incidence of a short thyroglobulin-doubling time (<2 years) – a strong dynamic prognostic factor of the CSS of thyroglobulin-antibody-negative PTC patients after total thyroidectomy [16] – was significantly higher in older patients. These findings suggest that, in clinical PTC, the CSS is poorer in old patients but disease-free survival shows a triphasic pattern, that is, poorer in young and old patients than in middle-aged patients.
However, we obtained findings of low-risk PMC progression during active observation that differed from the prognoses of clinical PTC. Size enlargement was observed in 5.9 and 5.7% in young and middle-aged patients, both values which were significantly higher than that in older patients, 2.2%. The incidence of the novel appearance of lymph node metastasis decreased with patients’ age; they were 5.3, 1.4, and 0.4% in young, middle-aged, and older patients, respectively [7▪▪]. These findings suggested that low-risk PMCs in older patients were less likely to progress than those in the other two age groups, which is in sharp contrast to clinical PTC progression. Thus, older patients with low-risk PMCs are the best candidates for observation.
Young age (<40 years) was an independent risk factor for PMC progression. However, in clinical PTC, the CSS rate of young patients is known to be excellent, and none of the 191 patients in our study who underwent surgery after observation showed distant recurrence or died of PTC [7▪▪]. Thus, we can conclude that young PMC patients are also candidates for observation because it would not be too late to surgically treat them after detecting progression signs.
The reason why PMCs in older patients often remain stable but clinical PTCs in older patients are generally aggressive remains to be elucidated. At present, we can hypothesize that certain gene mutations as a second hit make PMC become clinical and aggressive, especially in older patients.
SURGICAL TREATMENT FOR PAPILLARY THYROID MICROCARCINOMA
Sugitani et al.[8] divided PMC into three distinctive types: type I, incidentally detected, asymptomatic PMC; type II, the early stage of the usual low-risk PMC; and type III, clinically symptomatic PMC. They recommended thyroid-conserving surgery for type II, an aggressive treatment for type III, and nonsurgical observation for type I. Type I accounted for about 95% of the PMCs in their study. Pelizzo et al. and Cappelli et al. recommended aggressive treatment for PMCs, including total thyroidectomy, level VI dissection, and radioactive iodine (RAI) ablation [17,18] because PMC patients often display an unfavorable clinical course. However, these two studies analyzed low-risk and high-risk (corresponding to type III by Sugitani et al.[8]) PMCs as a single group. Indeed, the prognosis of the incidentally detected PMCs was excellent [19].
Hay et al.[20] showed that total thyroidectomy and RAI ablation did not reduce recurrence, and Lang and Wong [21] demonstrated that RAI ablation increased the risk of second primary carcinoma. Other groups showed that BRAFV600E mutation can be a marker of aggressive characteristics and may require aggressive treatment [22,23], but there is no evidence that BRAF mutation affects the prognosis except for high-risk PTC patients in a Japanese population [24,25]. These data suggest that aggressive treatment, including surgery, is not necessary for low-risk PMC patients.
ALTERNATIVE TREATMENTS FOR LOW-RISK PAPILLARY THYROID MICROCARCINOMA: ARE THEY APPROPRIATE?
Percutaneous ethanol injection therapy and radiofrequency ablation were used to treat local recurrences of thyroid carcinoma that were not resectable [26,27]. One might attempt to ablate PMC foci with these techniques. However, a pathology examination of low-risk PMCs revealed lymph node metastasis in the central compartment and lateral compartment in 43% (258 of 594 patients) and 44% (141 of 317 patients), respectively [9]. The incidence of multiplicity is also high, at 22–44% [3]. We think that ablating only the primary lesion results in the loss of an important biomarker of the progressiveness of the tumor.
OUR TREATMENT STRATEGY FOR PAPILLARY THYROID MICROCARCINOMA AT KUMA HOSPITAL
Our current treatment strategy for PMC at Kuma Hospital is summarized in Table 1.
Table 1.
Our treatment strategies for papillary thyroid microcarcinoma at Kuma Hospital, Kobe, Japan
| Low-risk PMC | Active observation. |
| If patients prefer surgery, hemithyroidectomy (if solitarya) with ipsilateral level VI dissection without RAI ablation. | |
| PMC with unsuitable features for observation | Hemithyroidectomy (if solitarya) with ipsilateral level VI dissection without RAI ablation. |
| PMC with high-risk features | Total thyroidectomy with level VI lymph node dissection.b RAI ablation may be considered. |
PMC, papillary thyroid microcarcinoma; RAI, radioactive iodine ablation.
aIf bilateral and multiple, total thyroidectomy.
bIf clinical node metastasis is present in the lateral neck, systematic node dissection in the lateral compartment is done.
ISSUES TO ADDRESS
On observation, there are two prominent issues to be addressed by future studies.
Thyroid-stimulating hormone suppression
Thyroid-stimulating hormone (TSH) suppression therapy is a classical postoperative management of PTC to prevent recurrence. In our most recent study [7▪▪], we showed that although only 51 patients with PMCs underwent observation with TSH suppression, all patients except one did not show carcinoma progression. In contrast, Sugitani et al.[13] showed that serum TSH levels were not related to PMC progression on observation. Whether TSH suppression to around the lower limit of the normal range prevents PMC progression, especially in young patients, is an interesting question.
Pregnancy
Because of the homology between beta-subunits of human chorionic gonadotropin and TSH, human chorionic gonadotropin has a weak ability to stimulate the thyroid gland, possibly promoting the growth of thyroid nodules and carcinomas. Shindo et al.[28] at Kuma Hospital demonstrated that the incidence of PMC enlargement was higher in pregnant patients compared with controls. However, none of the patients in either group, including those who underwent surgery after delivery, showed postoperative tumor recurrence or died of PTC. This is the only study regarding the observation of low-risk PMC during pregnancy, and the number of patients in this series was only nine. It thus remains unknown whether pregnancy specifically affects the progression of low-risk PMC.
CONCLUSION
Active observation for low-risk PMC is a well tolerated therapeutic strategy, and this strategy was adopted in Japanese guidelines for the management of thyroid tumors in 2010 [29]. The periodic observation of low-risk PMC might become a standard strategy if the PMCs do not have any of the exclusion criteria. Observation is better for patients because they can avoid unnecessary surgery and possible surgical complications. In contrast, for PMC with high-risk features, extensive treatment including total thyroidectomy with prophylactic lymph node dissection of the central neck compartment and therapeutic lymph node dissection of the lateral neck compartment when indicated is recommended.
Acknowledgements
None.
Conflicts of interest
The authors have no conflicts of interest to declare.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Supplementary Material
REFERENCES
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006; 295:2164–2167. [DOI] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014; 140:317–322. [DOI] [PubMed] [Google Scholar]
- 3.Ito Y, Miyauchi A. A therapeutic strategy for incidentally detected papillary microcarcinoma of the thyroid. Nat Clin Pract Endocrin Metab 2007; 3:240–248. [DOI] [PubMed] [Google Scholar]
- 4.Takebe K, Date M, Yamamoto Y, et al. Mass screening for thyroid cancer with ultrasonography [in Japanese]. KARKINOS 1994; 7:309–317. [Google Scholar]
- 5.Ito Y, Uruno R, Nakano K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 2003; 13:381–388. [DOI] [PubMed] [Google Scholar]
- 6.Ito Y, Miyauchi A, Inoue H, et al. An observation trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 2010; 34:28–35. [DOI] [PubMed] [Google Scholar]
- 7▪▪.Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 2014; 24:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study analyzed a large number of low-risk PMC patients and showed that, in contrast to clinical PTC, low-risk PMC is less likely to progress in old patients.
- 8.Sugitani I, Koda K, Yamada K, et al. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg 2010; 34:1222–1231. [DOI] [PubMed] [Google Scholar]
- 9.Hotomi M, Sugitani I, Toda K, et al. A novel definition of extrathyroid invasion for patients with papillary thyroid carcinoma for predicting prognosis. World J Surg 2012; 36:1230–1240. [DOI] [PubMed] [Google Scholar]
- 10.Ito Y, Fukushima M, Kihara M, et al. Investigation of the prognosis of patients with papillary thyroid carcinoma by tumor size. Endocr J 2012; 59:457–464. [DOI] [PubMed] [Google Scholar]
- 11.Ito Y, Higashiyama T, Takamura Y, et al. Prognosis of patients with benign thyroid diseases accompanied by incidental papillary carcinoma undetectable on preoperative imaging tests. World J Surg 2007; 31:1872–1876. [DOI] [PubMed] [Google Scholar]
- 12.Uruno T, Miyauchi A, Shimizu K, et al. Usefulness of thyroglobulin measurement in fine-needle aspiration biopsy specimens for diagnosing cervical lymph node metastasis in patients with papillary thyroid cancer. World J Surg 2005; 29:483–485. [DOI] [PubMed] [Google Scholar]
- 13.Sugitani I, Fujimoto Y, Yamada K. Association between serum thyrotropin concentration and growth of asymptomatic papillary thyroid microcarcinoma. World J Surg 2014; 38:673–678. [DOI] [PubMed] [Google Scholar]
- 14.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 1994; 97:418–428. [DOI] [PubMed] [Google Scholar]
- 15▪▪.Miyauchi A, Kudo T, Kihara M, et al. Relationship of biochemically persistent disease and thyroglobulin-doubling time to age at surgery in patients with papillary thyroid carcinoma. Endocr J 2013; 60:415–421. [PubMed] [Google Scholar]; This study showed that persistent disease after total thyroidectomy is likely to be seen in young and old patients, but thyroglobulin elevation during follow-up is frequently detected in old, but not young, patients.
- 16.Miyauchi A, Kudo T, Miya A, et al. Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid 2011; 21:707–716. [DOI] [PubMed] [Google Scholar]
- 17.Cappelli C, Castellano M, Braga M, et al. Aggressiveness and outcome of papillary thyroid carcinoma (PTC) versus microcarcinoma (PMC): a mono-institutional experience. J Surg Oncol 2007; 95:555–560. [DOI] [PubMed] [Google Scholar]
- 18.Pelizzo MR, Boschin LM, Toniato A, et al. Papillary thyroid microcarcinoma (PTMC): Prognostic factors, management and outcome in 403 patients. Eur J Sur Oncol 2006; 32:1144–1148. [DOI] [PubMed] [Google Scholar]
- 19.Neuhold N, Schulthels A, Hermann M, et al. Incidental papillary microcarcinoma of the thyroid–further evidence of a very low malignant potential: a retrospective clinicopathological study with up to 30 years of follow-up. Ann Surg Oncol 2011; 18:3430–3436. [DOI] [PubMed] [Google Scholar]
- 20.Hay ID, Hutchinson ME, Gonzalez-losada T, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery 2008; 144:980–987. [DOI] [PubMed] [Google Scholar]
- 21.Lang BHH, Wong KP. Risk factors for nonsynchronous second primary malignancy and related death in patients with differentiated thyroid carcinoma. Ann Surg Oncol 2011; 11:3559–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee X, Gao M, Ji Y, et al. Analysis of differential BRAFV600E mutational status in high aggressive papillary thyroid microcarcinoma. Ann Surg Oncol 2009; 16:240–245. [DOI] [PubMed] [Google Scholar]
- 23.Rossi ED, Martini M, Capodimonti S, et al. BRAF (V600E) mutation analysis on liquid-based cytology-processed aspiration biopsies predicts bilaterality and lymph node involvement in papillary thyroid carcinoma. Cancer Cytopathol 2013; 121:291–297. [DOI] [PubMed] [Google Scholar]
- 24.Ito Y, Yoshida H, Maruo R, et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival patients. Endocr J 2009; 56:89–97. [DOI] [PubMed] [Google Scholar]
- 25.Ito Y, Yoshida H, Kihara M, et al. BRAF (V600E) mutation analysis in papillary thyroid carcinoma: is it useful for all patients? World J Surg 2014; 38:679–687. [DOI] [PubMed] [Google Scholar]
- 26.Lim CY, Yun JS, Lee J, et al. Percutaneous ethanol injection for locally recurrent papillary thyroid carcinoma. Thyroid 2007; 17:347–350. [DOI] [PubMed] [Google Scholar]
- 27.Baek JH, Kim YS, Sung JY, et al. Locoregional control of metastatic well differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol 2011; 197:W331–W336. [DOI] [PubMed] [Google Scholar]
- 28.Shindo H, Amino N, Ito Y, et al. Papillary thyroid microcarcinoma might progress during pregnancy. Thyroid 2014; 24:840–844. [DOI] [PubMed] [Google Scholar]
- 29.Takami H, Ito Y, Okamoto T, Yoshida A. Therapeutic strategy for differentiated thyroid carcinoma in Japan based on a newly established guideline managed by Japanese Society of Thyroid Surgeons and Japanese Association of Endocrine Surgeons. World J Surg 2011; 35:111–121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


