Abstract
Purpose of review
Proline metabolism impacts a number of regulatory targets in both animals and plants and is especially important in cancer. Glutamine, a related amino acid, is considered second in importance only to glucose as a substrate for tumors. But proline and glutamine are interconvertible and linked in their metabolism. In animals, proline and glutamine have specific regulatory functions and their respective physiologic sources. A comparison of the metabolism of proline and glutamine would help us understand the importance of these two nonessential amino acids in cancer metabolism.
Recent findings
The regulatory functions of proline metabolism proposed 3 decades ago have found relevance in many areas. For cancer, these functions play a role in apoptosis, autophagy and in response to nutrient and oxygen deprivation. Importantly, proline-derived reactive oxygen species served as a driving signal for reprogramming. This model has been applied by others to metabolic regulation for the insulin-prosurvival axis, induction of adipose triglyceride lipase for lipid metabolism and regulation of embryonic stem cell development. Of special interest, modulatory proteins such as parkinson protein 7 and oral cancer overexpressed 1 interact with pyrroline-5-carboxylate reductase, a critical component of the proline regulatory axis. Although the interconvertibility of proline and glutamine has been long established, recent findings showed that the proto-oncogene, cellular myelocytomatosis oncogene, upregulates glutamine utilization (glutaminase) and routes glutamate to proline biosynthesis (pyrroline-5-carboxylate synthase, pyrroline-5-carboxylate reductases). Additionally, collagen, which contains large amounts of proline, may be metabolized to serve as a reservoir for proline. This metabolic relationship as well as the new regulatory targets of proline metabolism invites an elucidation of the differential effects of these nonessential amino acids and their production, storage and mobilization.
Summary
Mechanisms by which the proline regulatory axis modulates the cancer phenotype are being revealed. Proline can be synthesized from glutamine as well as derived from collagen degradation. The metabolism of proline serves as a source of energy during stress, provides signaling reactive oxygen species for epigenetic reprogramming and regulates redox homeostasis.
Keywords: apoptosis, autophagy, collagen, glutamine, metabolic stress
INTRODUCTION
Metabolism in tumors plays a key role in cellular logistics for rapid proliferation, maintenance of redox homeostasis and in epigenetics and reprogramming [1,2]. Studies on metabolism have focused on core pathways, that is, oxidative glycolysis [3]. Enzymes mediating these pathways are reprogrammed by oncogenes and dysfunctional or nonfunctional suppressor genes [4]. Recently, attention has turned to the nonessential amino acids and their parametabolic regulation of epigenetics [5▪]. This chapter will briefly review some of these regulatory mechanisms emphasizing proline and its relationships to nutrition.
Box 1.
no caption available
DIET AND NUTRITION
Classically, nutrition implies dietary intake. However, recent research at the cellular, tissue and organism level has introduced new paradigms in which nutrition transcends diet. For example, at the cellular level, prosurvival autophagy is based on mechanisms in which cellular components are cannibalized for their own or a sibling cell's survival [6,7]. Storage of energy or precursor substrates in the cell's microenvironment can be mobilized for stress nutrition [8–10]. A surprising finding is that substrates mobilized from stores may have a metabolic route that differs from substances delivered by the circulation [11]. It is the goal of this chapter to present novel paradigms of nutrition in cancer.
AMINO ACIDS: ESSENTIAL AND NONESSENTIAL
The concept of essential and nonessential amino acids is based on classical dietary nutrition. But it is the metabolism of nonessential amino acids, especially their biosynthesis, and not the endogenous supply of the amino acid per se that has been evolutionarily conserved. The metabolic pathways for nonessential amino acids perform a number of necessary tasks including redox regulation, generation of methyl donors, nitrogen transfer for nucleotide synthesis and interorganellar shuttle mechanisms [1,5▪]. In this context, proline plays a special role. It is the only proteinogenic secondary ‘imino acid’ and the initial step of its degradative metabolism is distinct from that for other amino acids. Thus, it has special features enabling its regulatory role. However, once oxidized to pyrroline-5-carboxylate (P5C) or its tautomer glutamic-γ-semialdehyde, proline can be interconverted to many other substrates and is the source for carbon exchange between the tricarboxylic acid cycle and urea cycle [8]. Furthermore, proline can be stored in collagen, the most abundant protein by weight in the body. As nearly 25% of the residues in collagen are incorporated as proline, collagen can be a dump as well as a reservoir for proline [9].
FUNCTION OF GLUTAMINE
Of nonessential amino acids, glutamine is especially important in metabolism. It is the indispensable source of imido groups for synthesis of purines and pyrimidines. In a physiologic setting, glutamine is synthesized in muscle and transferred to gastrointestinal tract as fuel and to central organs for metabolic processing (Fig. 1)[12]. Citrulline and proline are the two main amino acid products. In tissue culture, the supraphysiologic concentrations of glutamine are combined with diabetic levels of glucose (450 mg/dl) to maximize proliferation, especially of tumor cells. Thus, it is not surprising that cultured tumor cells are ‘addicted’ to glutamine and glucose. The dysregulation in cancer cells includes an increase in glutamine transporters and increased activity of glutaminase [13]. Additionally, glutamine synthetase is downregulated by oncogenes, for example, cellular myelocytomatosis oncogene (c-MYC) [14]. Thus, high levels of glutaminase and low levels of glutamine synthetase make exogenous sources of glutamine essential.
FIGURE 1.
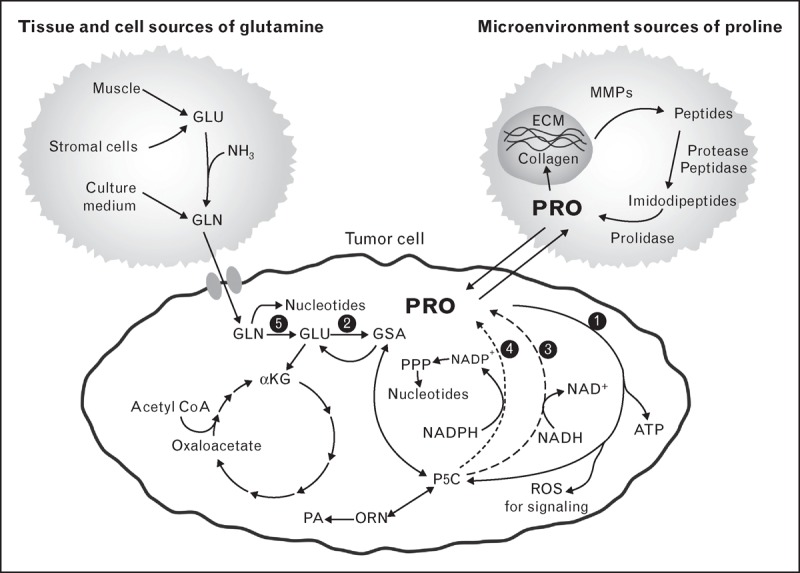
A proposed metabolic model involving glutamine, proline and collagen. The depicted tumor cell is also applicable to nonmalignant proliferating cells. The sources for glutamine are shown on the upper left and the source for proline is shown in the upper right. These are not necessarily specific for cellular or other anatomic sites. GSA and P5C are tautomers. Their interconversions are spontaneous. Overall, the proposed pathway is that glutamine synthesized from adequate protein intake is used for energy and de-novo synthesis of purines and pyrimidines. Proline is an important amino acid product that is used for signaling as well as an alternate source of ATP. During nutritional plenty, proline is stored in collagen, the main component of ECM. This reservoir of proline can be mobilized during conditions of nutritional stress. ECM, extracellular matrix; GSA, glutamic-γ- semialdehyde; P5C, pyrroline-5-carboxylate; ORN, ornithine; PA, polyamines; PPP, pentose phosphate pathway. The black circles designate specific enzymes numbered as follows: 1, proline dehydrogenase a.k.a. proline oxidise; 2, P5C synthase; 3, P5C reductase 1 and 2; 4, P5C reductase L; 5, glutaminase.
REGULATORY FUNCTIONS OF PROLINE METABOLISM
The interrelatedness of glutamine, glutamate and proline has been well recognized, even though they may serve different functions. The first step in glutamine synthesis is the activation of the gamma carbon of glutamate by ATP to form γ-glutamyl phosphate. This is also the first step catalyzed by P5C synthase, the first enzyme in proline synthesis from glutamate [12]. The classic studies showing that proline is an important product of glutamine in the splanchnic circulation were emphasized by recent studies using 13C, 15N-labeled glutamine [14]. The proto-oncogene c-MYC, which is expressed at high levels in a variety of human tumors, markedly upregulated the enzymes of proline synthesis from glutamine [14]. Unlike glutamine, the free amino acid pools of proline are relatively low. Instead, protein-bound proline comprises a major amino acid reservoir in the body [8]. Collagen can serve as a controlled source or dump for proline. This reservoir is also a source of amino acids converted from proline. Recent findings suggest that in cells in the quiescent state, core metabolism changes little but products formed during quiescence may be deposited as extracellular matrix (ECM), that is, collagen [10]. Thus, when cells are not proliferating, they store their metabolic substrates in the ECM.
PROLINE DEGRADATION
The metabolism of proline occurs only through proline dehydrogenase a.k.a. proline oxidase (PRODH/POX) (Fig. 1). Because the alpha-nitrogen of proline is locked within a pyrrolidine ring, proline cannot be a substrate for the generic amino acid enzymes, that is, transaminases, decarboxylases and racemases. Instead, the first step of proline degradation is catalyzed by a special enzyme that not only opens the proline ring but also is in a strategic location associated with proteins of the electron transport chain [8]. The formation of reactive oxygen species (ROS) accompanying the oxidation of proline has been linked to a number of downstream events. Others have suggested that the generation of ROS through complex III is the source of mitochondrial ROS used for signaling because ROS is released into the intermembranous space [15]. From this site, ROS can be transferred out of the mitochondria to regulate various targets. With its special link to ROS generation, it is not surprising that PRODH/POX is under close regulation that occurs at the genetic as well as the catalytic level [8].
PROLINE DEHYDROGENASE/PROLINE OXIDASE IN CANCER
The regulatory function of proline metabolism was proposed some 3 decades ago, but the key role of proline degradation in cancer was suggested by the rapid and robust increase in the transcription of PRODH/POX by the tumor suppressor P53. Several recent reviews [8,9,16,17▪] have described the regulation of PRODH/POX, its generation of mitochondrial ROS and the cellular targets of this regulatory ROS. Although PRODH/POX was identified as one of the early and robust responses to P53, the mechanism of this effect was more fully elucidated by the finding that intronic P53 recognition sites of PRODH/POX played a role more important than the PRODH/POX promoter [18▪]. Additionally, a human-specific endogenous retrovirus may control PRODH/POX expression by methylation-dependent and independent mechanisms [19▪▪]. The ligand-dependent transcriptional factor, peroxisome proliferator-activated receptor gamma (PPAR-γ), activated PRODH/POX expression and was confirmed by electrophoretic mobility shift assay and chromatin immunoprecipitation. With nutritional and hypoxic stress, increased PRODH/POX mRNA and enzyme protein resulted in autophagy rather than apoptosis [20]. The mechanism was dependent on AMP-activated protein kinase (AMPK) activation rather than P53. The transcriptional mechanism for AMPK has not been shown, although it is likely that it is through peroxisome proliferator-activated receptor gamma coactivator 1. With P53 or PPARγ induction, the endpoint appears to be blockade of the cell cycle and initiation of apoptosis by activating or suppressing a number of genes [21]. AMPK activation stimulated by hypoxia results in autophagy [20]. The coupling of P53 to PRODH/POX and the generation of proline-dependent ROS constitute a potential link to metabolic epigenetics. Specific genes regulated by PRODH/POX include enzymes of cell cycle checkpoints (cell division cycle 25c, Geminin, growth arrest and DNA damage family members) [21], mediators of autophagy (conversion of LC3-I to LC3-II, induction of Beclin 1) [20]. Furthermore, a number of gene products are downregulated such as cyclooxygenase 2 [22]. The details of these effects on cancer have been recently reviewed [23,24] and will be mentioned only briefly here.
PROLINE SYNTHESIS
The pathway for proline synthesis has P5C as an intermediate produced from glutamate by P5C synthase or from ornithine by ornithine aminotransferase (OAT) (Fig. 1). P5C can then be converted to proline by P5C reductases (PYCR) [8]. Three isozymes of PYCR are known, and their localization and function have been characterized [25]. PYCR1 and 2 are localized to mitochondria. PYCR2 appears to be in the mitochondrial matrix, PYCR1 may be on the surface of mitochondria. It can be easily dissociated from mitochondria by exposure to phosphate buffer. Both PYCR1/2 prefer reduced nicotinamide adenine dinucleotide (NADH) as cofactor. PYCRL, on the other hand, localizes to the cytosol and prefers reduced nicotinamide adenine dinucleotide phosphate (NADPH) for cofactor [25]. Crystallographic studies of PYCR1 showed that the catalytic activity is the property of PYCR1 dimers, but the physiologic structure is a decamer forming a torus (donut-like structure), which is often seen in proteins with membrane associations or with chaperone functions. How this structure relates to the functions of the PYCRs has yet to be elucidated, but it is tempting to speculate that it may mediate redox channeling or transmembrane shuttling. De Ingeniis et al. [25] proposed that P5C produced by OAT is channeled to PYCRL, which utilizes NADPH as cofactor. Thus, the pathway from arginine to proline catalyzed sequentially by arginase, OAT and PYCRL would produce NADP+[25]. This NADP+ could be used by the oxidative arm of the pentose shunt to produce PP-ribose-P for nucleotide synthesis. The biosynthetic pathway is regulated by proto-oncogenes, that is, c-MYC, which increases the levels of P5C synthase, PYCR1/2/L and OAT and decreases the levels of catabolic enzymes, PRODH/POX and P5C dehydrogenase, the enzyme which converts P5C back to glutamate.
REGULATORY FUNCTION IN METABOLIC AND DEVELOPMENTAL MODELS
The aforementioned models have focused on stress signaling in cancer, that is, those activated by or associated with P53, PPARγ, AMPK, c-MYC, hypoxia and nutrient starvation. Metabolomic studies in humans with cancer have shown derangements of proline metabolism. Recently, a number of interesting applications of this model have been discovered. In Caenorhabditis elegans lacking signaling with the insulin analog and corroborated by studies in mouse embryonic fibroblasts from mice with knockout of insulin/insulin growth factor (I/IGF) receptor, Zarse et al.[26] showed that the long-term prosurvival effect of dysfunctional I/IGF signaling requires the functional linkage of PRODH/POX. They showed that the reprogramming mediated by PRODH/POX involves genes that subsequently provide antioxidant protection and prolongation of lifespan. In another laboratory's metabolic system involving adipose tissue, aging results in decreased blood flow and compromised nutrition [27▪▪]. The induction of adipose triglyceride lipase provides supplemental nutrients and prevents apoptosis. The necessary link is the induction of PRODH/POX by AMPK and induction of adipose triglyceride lipase through the nuclear translocation of forkhead box protein O1 (FOXO1) by PRODH/POX-derived ROS [27▪▪]. The specificity of this linkage was shown by inability to blockade the signaling by inhibition of either NADPH oxidase or nitric oxide synthase [27▪▪]. A third model involves reprogramming in development of embryonic stem cells (ESCs). The first observation was reported by Washington et al.[28] in J. Rathjen's lab in Melbourne and this was confirmed by Casalino et al.[29] in Naples. Both laboratories showed that the addition of proline to Dulbecco's minimum essential media, which is usually proline-free, enabled ESC to maintain their pluripotency or to be stably transformed to an early stage. A subsequent study [30▪▪] from the Naples group showed that the addition of proline induced the transformation of ESC to a mesenchymal-like state as well as the induction of global histone methylation involving H3K9 and H3K36. The recent demonstration that regulatory proteins, parkinson protein 7 (PARK7) in Parkinson's disease and oral cancer overexpressed 1 (ORAOV1) in tumors of the head and neck, esophagus and pancreas, specifically bind to P5C reductase suggests that their effects are due to modulation of the proline metabolic axis [31▪,32▪▪]. These are interesting findings and not only corroborate the role of the proline axis in metabolic epigenetics but also that activation of transcriptional factors may occur through PRODH/POX-generated ROS.
COLLAGEN AND PROLINE METABOLISM
The effect of stroma on tumor development is well recognized. However, mechanisms were proposed predating the rigorous re-examination of tumor metabolism and its implications. Nevertheless, emphasis was placed on the dynamic nature of the ECM and on the major role played by collagen. Collagen I comprises 80–90% of all collagenous proteins, and it is the most abundant protein, nearly 25%, in the human body. Thus, collagen in the ECM is a microenvironmental reservoir of proline (Fig. 1), analogous to glycogen for glucose and adipose tissue for fatty acids. Furthermore, various tumors have special features relative to collagen. For example, breast cancer is known to produce large amounts of collagen in which the tumor cells are encased. Ovarian cancer cells released into the abdominal cavity are wrapped in collagen, a feature sheltering these cells from chemotherapy [33]. Melanomas, arising in a microenvironment of ECM, also produce large amounts of collagen [34]. The preferential metastasis to bone for a variety of tumor types suggests that something in that microenvironment is conducive to tumor growth.
REGULATED DEGRADATION OF COLLAGEN
Collagen in ECM is dynamic and under the control of diverse influences to play a regulatory role. Cleavage of collagen into cryptic peptides, release of bound preformed cytokines, breakdown of physical constraints, physico-regulatory interaction with tumor cells – all have been proposed previously as exerting regulatory effects. However, the release of proline as a unique metabolic substrate and the regulatory functions of this metabolism has not been considered as a function of collagen metabolism. Proline released from collagen degradation can be a source of energy, a precursor for other amino acids, and a regulated source of redox signaling. In the process of remodeling during wound healing, collagen is degraded. Similarly, the induction of metalloproteinases has been associated with decreased blood supply, hypoxia and the concomitant deprivation of nutrients. Studies using second harmonic generation microscopy have shown that collagen content in tumor xenografts is decreased in hypoxic regions [35]. Metalloproteinases have also been used as markers for malignant transformation, specifically matrix metalloproteinase 9 [36]. Recent studies suggest that tumor endothelial marker 8 (TEM8), a product of a gene expressed in tumors but not in normal endothelial cells, is involved in collagen turnover [37]. In TEM8 knockout mice, there is markedly increased collagen in a variety of tissues. The expression of TEM8 is markedly increased in cells starved of amino acids. Thus, TEM8 may be a mechanism to tap the collagen reservoir when cells are amino acid deficient [38].
THE METABOLIC FUNCTION OF COLLAGEN SYNTHESIS
Collagen formation as well as collagen degradation may be important in metabolic regulation. The transfer of reducing potential from NADH or NADPH to P5C to form proline provides a mechanism for redox homeostasis. In tissue culture, in which cells are metabolizing glucose by oxidative glycolysis, lactate is released into the medium to regenerate NAD+ as cofactor for glyceraldehyde dehydrogenase. To a lesser extent tissue culture cells produce and release free proline. Similarly, collagen synthesis is upregulated under conditions of excess metabolic reducing potential such as with ethanol intake. The incorporation of proline into collagen removes it from the metabolic pool. Thus, by using collagen as a ‘dump’ for reducing equivalents in the form of proline, tumors can optimize conditions for metabolism and growth.
NUTRITIONAL COMPARISON OF PROLINE AND GLUTAMINE
The nonessential amino acids, proline and glutamine, are closely related metabolically, but they serve different physiologic functions. Glutamine is the most abundant free amino acid in the body. It is synthesized in muscle during the postabsorptive state from amino acids derived from the degradation of dietary proteins. Endogenous proline is synthesized mainly from glutamine and can play an important role in cell signaling. Under normal metabolic regulation, proline is incorporated into collagen, the most abundant protein in the body. This reservoir of proline is a source of amino acids and energy during conditions of metabolic stress.
CONCLUSION
Studies of the proline regulatory axis initially emphasized mechanisms of apoptosis and autophagy. Both ATP formation and ROS generation are facilitated by the linkage of PRODH/POX to the mitochondrial electron transport chain. The upregulation of the enzymes of proline biosynthesis by c-MYC increased proline to affect this regulatory function as well as substrate for protein synthesis. The regulatory axis is also applicable to a number of other metabolic systems including the prosurvival effects of dysfunctional insulin/IGF signaling, the induction of adipose triglyceride lipase with metabolic stress, the regulation of stem cell identity and the function of PARK 7 in Parkinson's disease and of ORAOV1 in aggressive tumors of head/neck, esophagus and pancreas. Recent work reviewed here suggests that the importance of glutamine to tumor cells is due, at least in part, to its conversion of proline and activation of the proline regulatory axis. Glutamine is a transfer vehicle in protein metabolism. Proline, on the other hand, is not only a regulatory substrate but also these regulatory functions can be derived from collagen, which serves as a reservoir and/or dump for proline in the extracellular matrix.
Acknowledgements
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project also has been funded in part with federal funds from the National Cancer Institute, NIH, under contract no. HHSN27612080001.
Conflicts of interest
The content of this review does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsements by the US Government.
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Yun J, Johnson JL, Hanigan CL, et al. Interactions between epigenetics and metabolism in cancers. Front Oncol 2012; 2:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu C, Thompson CB. Metabolic regulation of epigenetics. Cell Metab 2012; 16:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med 2004; 10:789–799. [DOI] [PubMed] [Google Scholar]
- 5▪.Phang JM, Liu W, Hancock C. Bridging epigenetics and metabolism: role of nonessential amino acids. Epigenetics 2013; 8:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article introduces the link between the metabolism of nonessential amino acids and mechanisms for epigenetics. Parametabolic regulatory mechanisms are proposed for the proline regulatory axis. This model has been invoked by others in studies of the prosurvival effects of insulin/IGF dysfunction and in the regulated development of ESCs.
- 6.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 2010; 22:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell 2010; 40:280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phang JM, Donald SP, Pandhare J, et al. The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids 2008; 35:681–690. [DOI] [PubMed] [Google Scholar]
- 9.Phang JM, Pandhare J, Liu Y. The metabolism of proline as microenvironmental stress substrate. J Nutr 2008; 138:2008S–2015S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valcourt JR, Lemons JM, Haley EM, et al. Staying alive: metabolic adaptations to quiescence. Cell Cycle 2012; 11:1680–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favaro E, Bensaad K, Chong MG, et al. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells. Cell Metab 2012; 16:751–764. [DOI] [PubMed] [Google Scholar]
- 12.Watford M. Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. J Nutr 2008; 138:2003S–2007S. [DOI] [PubMed] [Google Scholar]
- 13.Gao P, Tchernyshyov I, Chang TC, et al. C-myc suppression of mir-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009; 458:762–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Le A, Hancock C, et al. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-myc. Proc Natl Acad Sci U S A 2012; 109:8983–8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandel NS. Mitochondrial complex iii: an essential component of universal oxygen sensing machinery? Respir Physiol Neurobiol 2010; 174:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phang JM, Liu W, Zabirnyk O. Proline metabolism and microenvironmental stress. Annu Rev Nutr 2010; 30:441–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪.Liang X, Zhang L, Natarajan SK, et al. Proline mechanisms of stress survival. Antioxid Redox Signal 2013; 19:998–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]; This comprehensive review covers the regulatory functions of proline metabolism in plants as well as in animals.
- 18▪.Raimondi I, Ciribilli Y, Monti P, et al. P53 family members modulate the expression of prodh, but not prodh2, via intronic p53 response elements. PLoS One 2013; 8:e69152. [DOI] [PMC free article] [PubMed] [Google Scholar]; Although p53 is known to induce the expression of prodh, molecular interaction between the suppressor protein and the promoter region of prodh has not been shown. This study shows that intronic p53 recognition sites are more important than the promoter region in inducing prodh expression. These findings establish prodh as an important target gene for p53.
- 19▪▪.Suntsova M, Gogvadze EV, Salozhin S, et al. Human-specific endogenous retroviral insert serves as an enhancer for the schizophrenia-linked gene prodh. Proc Natl Acad Sci U S A 2013; 110:19472–19477. [DOI] [PMC free article] [PubMed] [Google Scholar]; The regulation of PRODH in the human brain can be regulated by a methylation-sensitive human-specific endogenous retrovirus and this may be related to the susceptibility for schizophrenia.
- 20.Liu W, Glunde K, Bhujwalla ZM, et al. Proline oxidase promotes tumor cell survival in hypoxic tumor microenvironments. Cancer Res 2012; 72:3677–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Borchert GL, Donald SP, et al. Proline oxidase functions as a mitochondrial tumor suppressor in human cancers. Cancer Res 2009; 69:6414–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Borchert GL, Surazynski A, et al. Proline oxidase, a p53-induced gene, targets cox-2/pge2 signaling to induce apoptosis and inhibit tumor growth in colorectal cancers. Oncogene 2008; 27:6729–6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phang JM, Liu W. Proline metabolism and cancer. Front Biosci (Landmark Ed) 2012; 17:1835–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phang JM, Liu W, Hancock C, et al. The proline regulatory axis and cancer. Front Oncol 2012; 2:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Ingeniis J, Kazanov MD, Shatalin K, et al. Glutamine versus ammonia utilization in the nad synthetase family. PLoS One 2012; 7:e39115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zarse K, Schmeisser S, Groth M, et al. Impaired insulin/igf1 signaling extends life span by promoting mitochondrial l-proline catabolism to induce a transient ros signal. Cell Metab 2012; 15:451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27▪▪.Lettieri Barbato D, Aquilano K, Baldelli S, et al. Proline oxidase-adipose triglyceride lipase pathway restrains adipose cell death and tissue inflammation. Cell Death Differ 2014; 21:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using the model of adipose tissue with decreased blood supply in aging mice, the authors show that the shift to the use of fat as energy source requires the expression of adipose triglyceride lipase. This activation requires ROS generated by PRODH, which causes the nuclear translocation of FOXO1.
- 28.Washington JM, Rathjen J, Felquer F, et al. L-proline induces differentiation of es cells: a novel role for an amino acid in the regulation of pluripotent cells in culture. Am J Physiol Cell Physiol 2010; 298:C982–C992. [DOI] [PubMed] [Google Scholar]
- 29.Casalino L, Comes S, Lambazzi G, et al. Control of embryonic stem cell metastability by l-proline catabolism. J Mol Cell Biol 2011; 3:108–122. [DOI] [PubMed] [Google Scholar]
- 30▪▪.Comes S, Gagliardi M, Laprano N, et al. L-proline induces a mesenchymal-like invasive program in embryonic stem cells by remodeling h3k9 and h3k36 methylation. Stem Cell Reports 2013; 1:307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]; The work is an extension of their previous work showing that added proline regulates the properties of ESCs. The authors show that l-proline induces a mesenchymal-like invasive program that has implications in the metastatic properties of cancer cells and cancer stem cells. Furthermore, they show that the addition of l-proline results in increased histone methylation of h3k9 and h3k36 that may be the basis for the reprogramming. This is the first demonstration that that proline metabolism can influence epigenetics.
- 31▪.Yasuda T, Kaji Y, Agatsuma T, et al. Dj-1 cooperates with pycr1 in cell protection against oxidative stress. Biochem Biophys Res Commun 2013; 436:289–294. [DOI] [PubMed] [Google Scholar]; DJ-1, a gene involved in the development of Parkinson's disease, was shown to interact with the proline regulatory axis. DJ-1 encodes a protein that binds to and inhibits the activity of PYCR1, the enzyme converting pyrroline-5-carboxylate to proline. The mechanism is related to redox dysregulation.
- 32▪▪.Togashi Y, Arao T, Kato H, et al. Frequent amplification of oraov1 gene in esophageal squamous cell cancer promotes an aggressive phenotype via proline metabolism and ros production. Oncotarget 2014; 5:2962–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]; The work of others suggests that PYCR1 has a structural configuration favoring its role as a chaperone protein. Previous work showed that oraov1 gene is amplified in esophageal squamous cancer and promotes an aggressive phenotype. They showed that this protein binds to PYCR1 and increases ROS generation. This is the first demonstration that the proline regulatory axis may be modulated to alter the cancer phenotype.
- 33.Choi J, Credit K, Henderson K, et al. Intraperitoneal immunotherapy for metastatic ovarian carcinoma: resistance of intratumoral collagen to antibody penetration. Clin Cancer Res 2006; 12:1906–1912. [DOI] [PubMed] [Google Scholar]
- 34.van Kempen LC, Rijntjes J, Mamor-Cornelissen I, et al. Type i collagen expression contributes to angiogenesis and the development of deeply invasive cutaneous melanoma. Int J Cancer 2008; 122:1019–1029. [DOI] [PubMed] [Google Scholar]
- 35.Kakkad SM, Solaiyappan M, O’Rourke B, et al. Hypoxic tumor microenvironments reduce collagen i fiber density. Neoplasia 2010; 12:608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouchet S, Bauvois B. Neutrophil gelatinase-associated lipocalin (ngal), pro-matrix metalloproteinase-9 (pro-mmp-9) and their complex pro-mmp-9/ngal in leukaemias. Cancers 2014; 6:796–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nanda A, Carson-Walter EB, Seaman S, et al. Tem8 interacts with the cleaved c5 domain of collagen alpha 3(vi). Cancer Res 2004; 64:817–820. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhary A, St Croix B. Selective blockade of tumor angiogenesis. Cell Cycle 2012; 11:2253–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]


