ABSTRACT
Objective:
Parenteral nutrition in infants with gastrointestinal disorders can be lifesaving, but it is also associated with parenteral nutrition–associated liver disease. We investigated the effects of incorporating ω-3 fish oil in a parenteral nutrition mixture on signs of parenteral nutrition–associated liver disease and explored the mechanism involved in this process.
Methods:
Seven-day-old New Zealand rabbits were divided into 3 groups of 8, and for 1 week they were infused via the right jugular vein with standard total parenteral nutrition with soybean oil (TPN-soy) or TPN with ω-3 fish oil–based lipid emulsion (TPN-FO), or naturally nursed with rabbit milk (control). Serum and liver tissues were analyzed for serological indicators and pathology, respectively. Reverse-transcriptase polymerase chain reaction was used to evaluate the messenger RNA levels of the endoplasmic reticulum stress chaperone protein glucose-regulated protein 94 (GRP94) in liver tissues and GRP94 protein levels were compared through immunohistochemistry and Western blot assays.
Results:
TPN-soy animals had significantly higher serum total bilirubin, direct bilirubin, and γ-glutamyl transpeptidase and lower serum albumin than the controls (P < 0.01, each) or the TPN-FO group, which were similar to the controls (P < 0.01 cf. TPN). Damage to liver tissues of the TPN-FO group was much less than that of the TPN-soy group. GRP94 messenger RNA and protein levels in liver tissues of TPN-soy animals were significantly higher than that of the controls or TPN-FO rabbits, which were similar to the controls.
Conclusions:
Incorporating ω-3 fish oil in parenteral nutrition emulsion greatly prevented liver dysfunction and liver tissue damage in week-old rabbit kits, possibly by preventing endoplasmic reticulum stress.
Keywords: endoplasmic reticulum stress, GRP94, ω-3 fish oil emulsion, parenteral nutrition, total parenteral nutrition–associated liver disease
Parenteral nutrition, or intravenous feeding, provides nutritional support for infants who do not have adequate gastrointestinal function. It can be a lifesaving therapy for newborn patients. Since the first documented report of an infant given parenteral nutrition was published in 1944, >30,000 neonates have survived through its use (1). The treatment is, however, not without its inherent risks, among which parenteral nutrition–associated liver disease (PNALD) is a common complication. Approximately 30% to 60% of the infants who require long-term parenteral nutrition develop PNALD, with abnormalities of liver function and hepatic damage (2). The latter can lead to cholestasis, especially in premature infants, and may result in life-threatening liver cirrhosis (3). Although the pathogenesis of parenteral nutrition–associated cholestasis is not entirely understood, in severe cases bile duct regeneration, portal inflammation, and fibrosis are contributing factors (4).
Soybean oil is composed mainly of ω-6 polyunsaturated fatty acids (PUFAs). Recent evidence suggests that lipid emulsions that consist of soybean oil in parenteral nutrition mixtures may have an essential role in the onset of subsequent liver damage (1,5). On the contrary, fish oil is rich in ω-3 PUFAs, and fish oil–based lipid emulsions may be hepatoprotective and prevent PNALD (5,6). Moreover, the ω-3 PUFAs in fish oil are relatively safe and can be used in neonates (7) and preterm infants (8). Nevertheless, the mechanism associated with putative ω-3 PUFA–mediated hepatoprotection is unclear.
The endoplasmic reticulum (ER) is the site of synthesis and folding of secretory proteins. Disturbances in ER function may cause unfolded protein response and ER stress (ER stress), eventually leading to cell death and many human diseases (9,10). Hepatocytes are secretory cells that are rich in ER, and ER stress in hepatocytes is closely associated with the pathogenesis of liver diseases (11,12). Glucose-regulated protein 94 (GRP94), a member of the heat shock protein 90 family, contributes to the regulation of protein folding in the ER and thus the control of ER stress (13,14).
In the present study, we evaluated the effects of total parenteral nutrition (TPN) containing ω-3fish oil or ω-6 soybean oil on PNALD, by monitoring GRP94 levels in neonate rabbits.
METHODS
Experimental Assignment and Establishment of TPN Model
The animal ethics committee of the Children's Hospital Affiliated to Soochow University granted approval for this study. Seven-day-old full-term New Zealand white rabbits (male and female, n = 24, weighing 100–120 g) were obtained from Wuxi Huishan Jiangnan Experimental Animal Centre (animal license number SCXK [Su] 2009–0005), Jiangsu, China. All of the rabbits were nursed from their mother before arrival. During the experimental period, the rabbits were maintained in an incubator at 26°C to 28°C and 40% to 60% humidity, under a 12 hour/12 hour light/dark cycle.
The rabbits were randomly and equally divided into 3 groups of 8, to be sustained for 1 week on total parenteral nutrition with soybean oil (TPN-soy) via infusion, TPN containing fish oil (TPN-FO) via infusion, or naturally nursed with rabbit milk only (control).
In the TPN groups, animals were infused with nutrient mix; the total daily volume of intravenous nutrient solution for each rabbit in the TPN groups was 240 mL/kg, infused within 24 hours. The TPN regimen was sustained for 7 days, as previously described (15) (the components in the mix were purchased from Sino-Swed Pharmaceutical, Beijing, China [Tables 1 and 2].
TABLE 1.
Fat emulsions of the TPN-soy and TPN-FO groups
| Wt/40 mL* | ||
| TPN-soy | ||
| 20% medium- and long-chain fats | Soybean oil | 2 g |
| Medium-chain triglyceride | 2 g | |
| Egg phospholipids | 0.24 g | |
| TPN-FO | ||
| 10% ω-3 fish oil–based lipid emulsion† | Soybean oil | 1.92–2.88 g |
| Medium-chain triglyceride | 1.92–2.88 g | |
| Olive oil | 1.6–2.4 g | |
| Fish oil | 0.96–1.44 g | |
| dl-α-Tocopherol | 4.32–6.48 mg | |
| Egg phosphatide | 0.384–0.576 g | |
| Glycerol | 0.9–1.1 g | |
| Sodium oleate | 9.6–14.4 mg | |
| Sodium hydroxide | 0.72–0.88 mg | |
TPN = total parenteral nutrition; TPN-FO = TPN with ω-3 fish oil; TPN-soy = TPN with soybean oil.
*In each 240-mL portion of TPN, containing 40 mL fat and comprising 210 kcal. Ratio of sugar to lipid was 1.4:1.
†The ratio of ω-6 to ω-3 PUFAs in the ω-3 fish oil-based lipid emulsion was 3.0–2.2 to 1.
TABLE 2.
Vitamins and trace elements per 240 mL TPN in both groups
| Vitamins | |
| Water-soluble | |
| B1 | 0.3 mg |
| B2 | 0.36 mg |
| Nicotinamide | 4 mg |
| B6 | 0.4 mg |
| Pantothenic acid | 1.5 mg |
| C | 10 mg |
| Biotin | 6 μg |
| Folic acid | 40 μg |
| B12 | 0.5 μg |
| Fat-soluble | |
| A | 25 μg (82.5 IU) |
| D | 10.125 μg (5 IU) |
| E | 0.2275 mg (0.25 IU) |
| K1 | 3.75 μg |
| Trace elements | |
| CaCl2·2H2O | 39.25 mg |
| MgCl2·6H2O | 15.21 mg |
| FeCl3·6H2O | 0.675 mg |
| ZnCl2 | 0.135 mg |
| MnCl2·4H2O | 0.395 mg |
| CuCl2·2H2O | 42.5 μg |
| NaF | 0.105 mg |
| KI | 8.5 μg |
TPN = total parenteral nutrition.
Anesthesia was implemented with intraperitoneal injection of chloral hydrate (0.3 g/kg body weight). The rabbit was then placed in a horizontal dorsal decubitus position on the surgical table, and its legs were fixed to the extremities of the table. Skin sterilization was performed with benzalkonium bromide solution. For injection, the jugular vein was located, and a 10-gauge angiocatheter with a 1.2-mm silica gel tube was inserted approximately 1.5 cm into the superior vena cava. The tail end of the silica gel tube led out through a 0.5-cm incision in the dorsal scapular area, which was used as a subcutaneous tunnel exit. To avoid detachment, the end of the silica gel tube was connected to a rotating device.
The components in 240 mL of the TPN given the TPN-soy group were 40 mL of a 20% medium- and long-chain fat emulsion (72 kcal, 34.3% of total calories), consisting of 2 g soybean oil, 2 g medium-chain triglyceride, and 0.24 g egg phospholipids; 80 mL of 11.4% pediatric compound amino acid injection-18AA-II (36.4 kcal, 17.3% of calories); 36 mL of 50% glucose (72 kcal, 48.4% of calories); 74 mL of 10% glucose (29.6 kcal); 4 mL of 10% NaCl; 3 mL of 10% KCl; 3 mL of 10% calcium gluconate; a half-piece of a water-soluble vitamin (0.3 mg vitamin B1, 0.36 mg vitamin B2, 4 mg nicotinamide, 0.4 mg vitamin B6, 1.5 mg pantothenic acid, 10 mg vitamin C, 6 μg biotin, 40 μg folic acid, and 0.5 μg vitamin B12); a half-piece fat-soluble vitamin (25 μg [82.5 IU] vitamin A, 10.125 μg [5 IU] vitamin D, 0.2275 mg [0.25 IU] vitamin E, and 3.75 μg vitamin K1); and a half-piece of trace elements (CaCl2·2H2O, 39.25 mg; MgCl2·6H2O, 15.21 mg; FeCl3·6H2O, 0.675 mg; ZnCl2, 0.135 mg; MnCl2·4H2O, 0.395 mg; CuCl2·2H2O, 42.5 μg; NaF, 0.105 mg; KI, 8.5 μg; Table 2). The TPN-FO group received the same as the TPN-soy group, but substituting for the 20% medium- and long-chain fat emulsion was 40 mL of 10% ω-3 fish oil–based lipid emulsion (containing 1.92–2.88 g soybean oil, 1.92–2.88 g medium-chain triglycerides, 1.6–2.4 g olive oil, 0.96–1.44 g fish oil, 4.32–6.48 mg dl-α-tocopherol, 0.384–0.576 g egg phosphatide, 0.9–1.1 g glycerol, 9.6–14.4 mg sodium oleate, and 0.72–0.88 mg sodium hydroxide). The ratio of ω-6 to ω-3 PUFAs in the ω-3 fish oil–based lipid emulsion was 3.0 to 2.2 to 1.
Each 240-mL portion of TPN comprised 210 kcal, and the ratio of sugar to lipid was 1.4:1. Fat in each TPN group was given for 40 mL · kg−1 · day−1; components are listed in Table 1.
Serological Evaluation
To evaluate the relevant serological indicators, rabbits were anesthetized by intraperitoneal injection of 10% chloral hydrate. Two milliliters of blood were collected by cardiac puncture into lithium heparin anticoagulant tubes. After centrifuging at 3500 rpm, the serum was carefully separated and stored at −20°C until used. Before analysis, the serum samples were removed from the −20°C refrigerator and incubated overnight at 4°C.
The total bilirubin, direct bilirubin, alanine aminotransferase, aspartate aminotransferase, total protein, albumin, γ-glutamyl transpeptidase (γ-GT), alkaline phosphatase (ALP), triglyceride, total cholesterol, and prealbumin levels were examined using a Hitachi 7600 automated chemistry analyzer (Hitachi, Tokyo, Japan).
Pathological Examination
In addition to collecting blood samples (described above), liver tissues were collected after the animals were anesthetized. The abdomen was opened and the liver tissues were carefully removed. Some of the tissues were stored at −80°C for analysis of GRP94 messenger RNA (mRNA) and GRP94 protein (described below), whereas other portions were used for immunohistochemical (below) or histopathological analysis.
After washing with normal saline tissue, samples were fixed in 10% paraformaldehyde, dehydrated through an alcohol series, cleared in xylene, and embedded in paraffin. Paraffin-embedded tissues were sectioned (5-μm thick) with a microtome. For histopathological comparisons, sections were dried, deparaffinized, and stained with hematoxylin and eosin. A pathologist who is experienced in liver disease reviewed the histology slides.
Reverse-Transcriptase Polymerase Chain Reaction
The mRNA levels of GRP94 were detected via reverse-transcriptase polymerase chain reaction. Liver tissues were removed from the −80°C refrigerator and crushed. Total RNA was extracted using Trizol reagent in accordance with the manufacturer's instructions (Invitrogen, Carlsbad, CA), and the amount and purification were evaluated with an ultraviolet spectrophotometer.
A total of 1 μg RNA was used to synthesize complementary DNA using a Reverse Transcriptase Kit (Promega, Madison, WI). PCR primers were designed using Primer 5.0 software, compared with the GenBank database for identification, and synthesized by Sangon Biotech (Shanghai, China). PCR amplification was performed on the GRP94 gene upstream primer (5′-AGGAAACACTCTGGGACG-3′) and downstream primer (5′-ATTCAGGTACTTAGGCATC-3′), producing an amplified fragment of 583 bp. Amplification of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene upstream primer (5′-GTTTGTGATGGGCGTGAA-3′) and downstream primer (5′-CGAAGGTAGAGGAGTGGGTG-3′) produced an amplified fragment of 497 bp. The PCR reaction included 200 μmol/L dNTP, 2 U Taq DNA polymerase, 0.2 μmol/L of each of the upstream and downstream primers, 5 μL template complementary DNA, and ddH2O to reach a total volume of 50 μL. Reaction conditions were predenaturation at 94°C for 10 minutes; denaturation at 94°C for 45 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 30 seconds, for a total of 35 cycles; and extension at 72°C for 7 minutes.
After electrophoresis in a 1.5% agarose gel, ethidium bromide–stained bands were visualized by ultraviolet transillumination, and the fluorescence intensity was semiquantified using a Bio2239 gel analysis system (Bio-Print, Chicago, IL).
Immunohistochemistry
Some of the paraffin-embedded tissue sections (described above) were used for immunohistochemical analysis. The glass slides used for immunohistochemistry were precoated with poly-l-lysine (WuHanBoster Biological Technology, Wuhan, China). Immunostaining was carried out with a streptavidin-peroxidase kit obtained from Suzhou Enmaike Bio-Tech (Suzhou, China) in accordance with the manufacturer's instructions. Three nonoverlapping fields were randomly selected under ×400 magnification.
The cells positively stained with anti-GRP94 primary antibody appeared with brown–yellow granules in the cytoplasm of hepatocytes. The intensity of immunohistochemical staining was analyzed using Image-Pro-Plus image analysis software. An average gray value supplied by the software was used to reference the intensity of GRP94 staining (ie, normalized to an internal control).
Western Blot
Total protein was extracted from cells using lysis buffer. Protein concentrations were measured and equal amounts of protein extracts were resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which were then transferred to a polyvinylidene fluoride membrane (Millipore, Temecula, CA). Membranes were blocked with blocking buffer for 2 hours, and then incubated with primary antibody against GRP94 or β-actin (1:1000 dilution) at 4°C overnight. After washing, membranes were incubated with ALP-conjugated goat anti-rabbit secondary antibody (1:600 dilution) at room temperature. Immunobands were visualized using an ALP kit (WesternBreeze; Invitrogen). To quantify protein levels, the expression bands of target proteins were analyzed, and the densitometric values were used to conduct statistical analysis. The housekeeping protein β-actin was used as an internal control.
Statistical Analyses
Data were analyzed using SPSS 17.0 software (SPSS Inc, Chicago, IL) and are presented as mean ± standard deviation. Statistical significance was determined using 1-way analysis of variance. P < 0.05 was recognized as significantly different.
RESULTS
Serological Indicators
No statistical significance was found in the levels of total protein, alanine aminotransferase, ALP, aspartate aminotransferase, triglyceride, total cholesterol, or prealbumin among the 3 groups (P > 0.05, Table 3). Compared with control animals, TPN-soy animals had significantly higher serum total bilirubin, direct bilirubin, and γ-GT levels, but lower albumin (P < 0.01). No statistical differences were detected in the levels of these indicators in TPN-FO animals compared with the controls (P > 0.05, each). Compared with the TPN-soy group, serum total bilirubin, direct bilirubin, and γ-GT were significantly lower in the TPN-FO group (F = 1247.40, 1037.94, 971.09, respectively; P < 0.01 each), and albumin was significantly higher (F = 70.31, P < 0.01).
TABLE 3.
Serological indicator levels of the 3 experimental groups
| TPN-FO | TPN-soy | Control | F | P | |
| Total bilirubin, μmol/L | 3.28 ± 1.69* | 50.2 ± 3.06† | 2.28 ± 1.04 | 1247.40 | <0.01 |
| Direct bilirubin, μmol/L | 1.76 ± 0.86* | 47.2 ± 3.91† | 1.05 ± 0.57 | 1037.94 | <0.01 |
| Total protein, g/L | 25.6 ± 3.01 | 23.0 ± 1.94 | 24.2 ± 3.12 | 1.48 | 0.25 |
| Albumin, g/L | 26.3 ± 3.12* | 11.7 ± 1.05† | 26.8 ± 2.83 | 70.31 | <0.01 |
| AST, IU/L | 77.1 ± 4.41 | 78.8 ± 2.70 | 72.7 ± 10.3 | 1.47 | 0.25 |
| ALT, IU/L | 24.9 ± 2.98 | 26.8 ± 6.57 | 21.9 ± 1.49 | 2.79 | 0.08 |
| γ-GT, IU/L | 14.3 ± 5.46* | 130 ± 5.61† | 11.2 ± 5.68 | 971.09 | <0.01 |
| ALP, IU/L | 74.0 ± 4.30 | 72.3 ± 6.69 | 72.3 ± 13.6 | 0.08 | 0.92 |
| Triglyceride, mmol/L | 2.18 ± 0.89 | 2.86 ± 1.49 | 1.74 ± 1.29 | 0.14 | 0.87 |
| Total cholesterol, mmol/L | 0.69 ± 0.53 | 0.92 ± 0.62 | 0.41 ± 0.21 | 0.42 | 0.66 |
| Prealbumin, mg/L | 88.8 ± 27.2 | 86.5 ± 13.8 | 80.8 ± 29.9 | 0.64 | 0.54 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GT, γ-glutamyl transpeptidase; TPN = total parenteral nutrition; TPN-FO = TPN with ω-3 fish oil; TPN-soy = TPN with soybean oil.
*P < 0.01 compared with TPN-soy.
†P < 0.01 compared with the control.
Liver Pathology
Histological examination of the liver tissues obtained from the 3 experimental groups revealed that those of the control rabbits appeared normal, with intact hepatocytes (Fig. 1A) and without any signs of hepatocyte degeneration, necrosis, inflammatory cell infiltration, cholangiectasis, bile duct epithelial hyperplasia, or cholestasis. In the liver tissues of the TPN-soy group, inflammatory cell infiltration, diffuse hepatic steatosis, and disrupted hepatic cord structure were, however, evident (Fig. 1B), but there was no cholestasis or liver fibrosis, and the hepatic lobule was still visible. In the TPN-FO group, only mild hepatic steatosis and inflammatory cell infiltration were found (Fig. 1C); the morphology of hepatocytes was normal, and there was no cholangiectasis, bile duct epithelial hyperplasia, or cholestasis.
FIGURE 1.
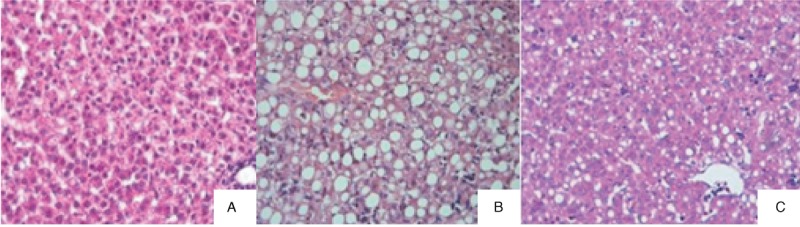
Representative histologic images of liver tissues. A, Control; B, TPN-soy group; C, TPN-FO group. Original magnification ×400. TPN = total parenteral nutrition; TPN-FO = TPN with ω-3 fish oil; TPN-soy = TPN with soybean oil.
GRP94 mRNA Levels in Liver Tissues
Hematoxylin and eosin staining of liver tissues revealed that rabbits given TPN containing fish oil sustained only mild hepatic steatosis and inflammatory cell infiltration compared with the animals infused with TPN containing soybean oil. Considering that ER stress is associated with liver pathology and GRP94 participates in the regulation of ER stress, we then assessed the mRNA levels of GRP94 in the liver tissues of the different groups to investigate the molecular mechanism underlying the seeming hepatic protection of TPN-FO against TPN-soy–induced liver damage.
We found that the GRP94 mRNA levels in liver tissues of the TPN-soy group (1.217 ± 0.113, referenced to the gray value standard) were significantly higher than these levels in the control (0.614 ± 0.034, P < 0.01; Fig. 2) and also significantly higher than the GRP94 mRNA levels of the TPN-FO group (0.661 ± 0.117). The GRP94 mRNA levels of the TPN-FO and controls were similar.
FIGURE 2.
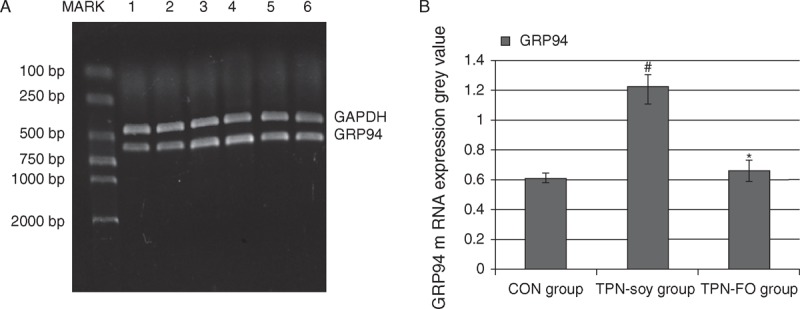
GRP94 mRNA levels in liver tissues determined by RT-PCR. A, Representative RT-PCR results. MARK, DNA marker; lanes 1, 2, control; lanes 3, 4, TPN-soy; lanes 5, 6, TPN-FO. B, Quantitative data. #P < 0.01, compared with control; ∗P < 0.01, compared with TPN-soy. Glyceraldehyde 3-phosphate dehydrogenase was used as internal control. GRP94 = glucose-regulated protein 94; mRNA = messenger RNA; RT-PCR = reverse-transcriptase polymerase chain reaction; TPN = total parenteral nutrition; TPN-FO = TPN with ω-3 fish oil; TPN-soy = TPN with soybean oil.
GRP94 Protein Levels in Liver Tissues
To further our investigation of the mechanism underlying hepatic protection associated with TPN-FO, the protein levels of GRP94 in liver tissues were determined via immunohistochemistry and Western blot assays.
Immunostaining of these tissues showed that GRP94 protein levels in the liver tissues of the TPN-soy group (133.84 ± 13.66, referenced to the gray value standard) were significantly higher than those of the controls (78.14 ± 8.17, P < 0.01; Fig. 3) and also significantly higher than the GRP94 protein levels of the TPN-FO group (80.73 ± 9.36, P < 0.01), whereas the GRP94 protein levels of the TPN-FO and controls were similar.
FIGURE 3.
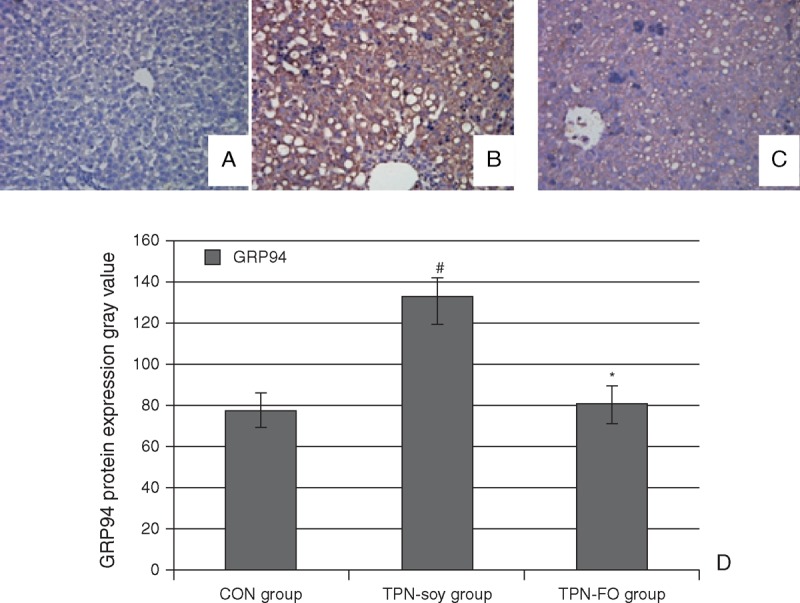
Immunohistological analysis of GRP94 protein in liver tissues. Representative results of GRP94 staining in samples derived from (A) control, (B) TPN-soy group, and (C) TPN-FO group. Quantitative data (D). Original magnification ×400. #P < 0.01, compared with control; ∗P < 0.01, compared with TPN-soy. GRP94 = glucose-regulated protein 94; TPN = total parenteral nutrition; TPN-FO = TPN with ω-3 fish oil; TPN-soy = TPN with soybean oil.
The results obtained by Western blot were in accord with those of the immunostaining (Fig. 4). That is, there was no associated upregulation in the GRP94 protein levels in the TPN-FO group (0.29 ± 0.03, relative optical density) as there was in the TPN-soy–treated animals (0.63 ± 0.04, P < 0.05), and GRP94 protein levels of the TPN-FO and controls (0.22 ± 0.01) did not differ significantly (P > 0.05). These data suggest that TPN-FO may prevent liver damage induced by TPN-soy, possibly by suppressing GRP94 upregulation and ER stress.
FIGURE 4.
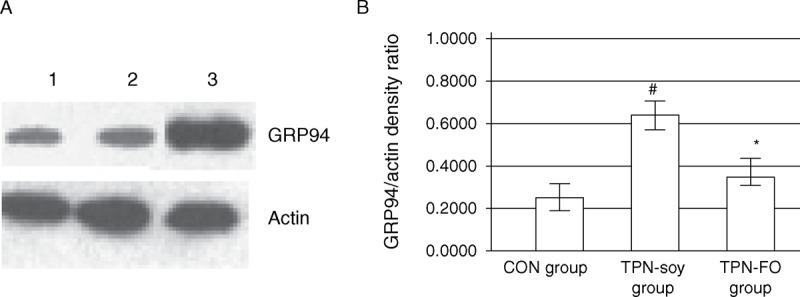
Western blot of the GRP94 protein in liver tissues. A, Representative Western blot results. Lane 1, control; lane 2, TPN-FO; lane 3, TPN-soy. B, Quantitative data. #P < 0.05, compared with control; ∗P < 0.05, compared with TPN-soy. Actin was used as internal control. GRP94 = glucose-regulated protein 94; TPN = total parenteral nutrition; TPN-FO = TPN with ω-3 fish oil; TPN-soy = TPN with soybean oil.
DISCUSSION
PNALD is a serious complication of patients, especially infants, who require long-term parenteral nutrition therapy. Here, we sustained 7-day-old rabbit kits for 1 week with TPN containing either soybean oil or ω-3 fish oil, to examine the role of ω-3 fish oil in preventing parenteral nutrition–associated liver injury. The effects of both of these were compared with normally nursed controls with regard to signs of PNALD. We found that, compared with the control group, usual soybean oil parenteral nutrition was associated with significant liver dysfunction, as indicated by higher serum total bilirubin, direct bilirubin, and γ-GT levels and lower serum albumin. These effects were not observed in the TPN-FO group, which was similar to the control group. Moreover, histological examination of liver tissues revealed hepatic damage in the TPN-soy group not seen in the TPN-FO, including inflammatory cell infiltration, diffuse hepatic steatosis, and disrupted hepatic cord structure. These observations are consistent with previous reports (15).
Rats given TPN were found to have higher levels of the ER stress marker protein CHOP (C/EBP homologous protein), and also higher levels of molecules that are proapoptotic under ER stress, c-Jun NH2-terminal kinase (JNK1/2), and p38 MAPK (16). This suggests that ER stress is induced by TPN therapy. GRP94 is also a marker for ER stress, and in the present study we detected higher levels of GRP94 mRNA and GRP94 protein in the liver tissues of the rabbits given TPN-soy. Therefore, ER stress may participate in TPN-mediated liver damage in both rats and rabbits. Furthermore, it was reported that in normal liver L02 cells cultured in vitro, ER stress contributed to the progression of PNALD (17). In that report, ERS was induced with palmitate, which led to the upregulation of tribbles homolog 3 (TRB3), a pseudokinase that is known to be involved in the pathogenesis of PNALD. Therefore, the ER stress seems be an important contributing factor in the pathogenesis and progression of PNALD.
Although the etiology of PNALD is poorly understood, the soybean or combined soybean and safflower oils that are included in TPN are accepted as contributing factors (18). Both of these oils are rich in ω-6 fatty acids. It has been reported that ω-6 PUFAs generate proinflammatory mediators, which may contribute to the onset of liver diseases, whereas mediators derived from ω-3 PUFAs are largely anti-inflammatory (19). A randomized controlled trial conducted by Puder et al (20) showed that fish oil–based intravenous lipid emulsion was safe for infants with PNALD, and could reduce mortality and organ transplantation rates in children with short bowel syndrome. Consistent with their observations, Diamond et al (21) reported that ω-3 fatty acids may prevent PNALD by improving bile flow, inhibiting steatosis, and exerting immunomodulatory effects, although the molecular mechanism involved in this process remains unclear. In the present study, we found that substitution of ω-3 fish oil for soybean fat emulsion was associated with prevention of liver dysfunction indicated by serology results, and liver tissue damage observed through histology. This implies that ω-3 fish oil may protect against PNALD. Moreover, levels of GRP94 mRNA and GRP94 protein in the kits given TPN with ω-3 fish oil were comparable with the control rabbits, and significantly lower than those given TPN with soybean fat emulsion. These data indicate that TPN-induced liver injury was reduced in those given ω-3 fish oil, unlike those given soybean fat emulsion, and its mechanism may be associated with a reduction in ER stress by reducing the GRP94 expression associated with soybean fat emulsion. Our findings are consistent with a previous report that glycyrrhizin, an active component of licorice root that has been used to treat chronic hepatitis, represses TPN-associated acute liver injury in rats by suppressing ER stress (16).
There was a difference in the amount of α-tocopherol between the 2 TPN solutions. α-Tocopherol is a well-known lipophilic antioxidant that has the ability to scavenge peroxyl radicals (22). Nandivada et al (19) reported that the risk factors of cholestasis and hepatic injury observed in PNALD included elevated serum concentrations of phytosterols, an abundance of ω-6 PUFAs, and a relative paucity of α-tocopherol. Moreover, previous research indicated that α-tocopherol protected against CCl4-induced liver damage (22). Unfortunately, we did not investigate whether the α-tocopherols play a role in the prevention of PNALD in the TPN-FO group.
In summary, our present study showed that substitution of ω-3 fish oil for soybean fat emulsion in TPN greatly prevented liver dysfunction and liver tissue damage in week-old rabbit kits, possibly by preventing ER stress. Our study may provide valuable evidence for the use of ω-3 fish oil for preventing PNALD in infants.
Acknowledgments
The authors gratefully acknowledge all members of the laboratory for sharing reagents and advice and thank the pathologist for reviewing the histology slides. The authors also thank Medjaden Bioscience for assisting in the preparation of this manuscript.
Footnotes
Drs Zhu and Wang contributed equally to the article.
This research was supported by grants from the Suzhou Science and Technology Development Project (No. SYS201136, SYS201440), Natural Science Foundation Project of Jiangsu Province (No. BK20141183), Research Project of Department of Health of Jiangsu Province (No. H201316), 135 Project of Department of Health of Jiangsu Province (No. RC2007076), and the Research Project of the Suzhou Key Laboratory of Children's Developmental Brain Injury Prevention and Care (No. SZS201108).
The authors report no conflicts of interest.
REFERENCES
- 1.Rangel SJ, Calkins CM, Cowles RA, et al. Parenteral nutrition-associated cholestasis: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J Pediatr Surg 2012; 47:225–240. [DOI] [PubMed] [Google Scholar]
- 2.Buchman A. Total parenteral nutrition-associated liver disease. JPEN J Parenter Enteral Nutr 2002; 26:S43–S48. [DOI] [PubMed] [Google Scholar]
- 3.Klein CJ, Havranek TG, Revenis ME, et al. Plasma fatty acids in premature infants with hyperbilirubinemia: before-and-after nutrition support with fish oil emulsion. Nutr Clin Pract 2013; 28:87–94. [DOI] [PubMed] [Google Scholar]
- 4.Guglielmi FW, Regano N, Mazzuoli S, et al. Cholestasis induced by total parenteral nutrition. Clin Liver Dis 2008; 12:97–110viii. [DOI] [PubMed] [Google Scholar]
- 5.de Meijer VE, Gura KM, Le HD, et al. Fish oil-based lipid emulsions prevent and reverse parenteral nutrition-associated liver disease: the Boston experience. JPEN J Parenter Enteral Nutr 2009; 33:541–547. [DOI] [PubMed] [Google Scholar]
- 6.Fallon EM, Le HD, Puder M. Prevention of parenteral nutrition-associated liver disease: role of omega-3 fish oil. Curr Opin Organ Transplant 2010; 15:334–340. [DOI] [PubMed] [Google Scholar]
- 7.Deshpande G, Simmer K. Lipids for parenteral nutrition in neonates. Curr Opin Clin Nutr Metab Care 2011; 14:145–150. [DOI] [PubMed] [Google Scholar]
- 8.D’Ascenzo R, D’Egidio S, Angelini L, et al. Parenteral nutrition of preterm infants with a lipid emulsion containing 10% fish oil: effect on plasma lipids and long-chain polyunsaturated fatty acids. J Pediatr 2011; 159:33–38e1. [DOI] [PubMed] [Google Scholar]
- 9.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev 2006; 86:1133–1149. [DOI] [PubMed] [Google Scholar]
- 10.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ 2004; 11:372–380. [DOI] [PubMed] [Google Scholar]
- 11.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol 2011; 54:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dara L, Ji C, Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology 2011; 53:1752–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eletto D, Dersh D, Argon Y. GRP94 in ER quality control and stress responses. Semin Cell Dev Biol 2010; 21:479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marzec M, Eletto D, Argon Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim Biophys Acta 2012; 1823:774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hata S, Kamata S, Nezu R, et al. A newborn rabbit model for total parenteral nutrition: effects of nutritional components on cholestasis. JPEN J Parenter Enteral Nutr 1989; 13:265–271. [DOI] [PubMed] [Google Scholar]
- 16.Tsai JJ, Kuo HC, Lee KF, et al. Glycyrrhizin represses total parenteral nutrition-associated acute liver injury in rats by suppressing endoplasmic reticulum stress. Int J Mol Sci 2013; 14:12563–12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan W, Wang Y, Xiao Y, et al. Palmitate induces TRB3 expression and promotes apoptosis in human liver cells. Cell Physiol Biochem 2014; 33:823–834. [DOI] [PubMed] [Google Scholar]
- 18.Gura KM, Lee S, Valim C, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics 2008; 121:e678–e686. [DOI] [PubMed] [Google Scholar]
- 19.Nandivada P, Carlson SJ, Chang MI, et al. Treatment of parenteral nutrition-associated liver disease: the role of lipid emulsions. Adv Nutr 2013; 4:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puder M, Valim C, Meisel JA, et al. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann Surg 2009; 250:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond IR, Sterescu A, Pencharz PB, et al. The rationale for the use of parenteral omega-3 lipids in children with short bowel syndrome and liver disease. Pediatr Surg Int 2008; 24:773–778. [DOI] [PubMed] [Google Scholar]
- 22.Kataoka T, Nishiyama Y, Yamato K, et al. Comparative study on the inhibitory effects of antioxidant vitamins and radon on carbon tetrachloride-induced hepatopathy. J Radiat Res 2012; 53:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]


