Abstract
Background
In the Mediterranean fruit fly (medfly), Ceratitis capitata, a highly invasive agricultural pest species, polyandry, associated with sperm precedence, is a recurrent behaviour in the wild. The absence of tools for the unambiguous discrimination between competing sperm from different males in the complex female reproductive tract has strongly limited the understanding of mechanisms controlling sperm dynamics and use.
Results
Here we use transgenic medfly lines expressing green or red fluorescent proteins in the spermatozoa, which can be easily observed and unambiguously differentiated within the female fertilization chamber. In twice-mated females, one day after the second mating, sperm from the first male appeared to be homogenously distributed all over the distal portion of each alveolus within the fertilization chamber, whereas sperm from the second male were clearly concentrated in the central portion of each alveolus. This distinct stratified sperm distribution was not maintained over time, as green and red sperm appeared homogeneously mixed seven days after the second mating. This dynamic sperm storage pattern is mirrored by the paternal contribution in the progeny of twice-mated females.
Conclusions
Polyandrous medfly females, unlike Drosophila, conserve sperm from two different mates to fertilize their eggs. From an evolutionary point of view, the storage of sperm in a stratified pattern by medfly females may initially favour the fresher ejaculate from the second male. However, as the second male's sperm gradually becomes depleted, the sperm from the first male becomes increasingly available for fertilization. The accumulation of sperm from different males will increase the overall genetic variability of the offspring and will ultimately affect the effective population size. From an applicative point of view, the dynamics of sperm storage and their temporal use by a polyandrous female may have an impact on the Sterile Insect Technique (SIT). Indeed, even if the female's last mate is sterile, an increasing proportion of sperm from a previous mating with a fertile male may contribute to sire viable progeny.
Keywords: Medfly, polyandry, sperm stratification, transgenic sperm, fertilization chamber
Background
The Mediterranean fruit fly (medfly), Ceratitis capitata, is a notorious pest with a worldwide distribution and a history of rapid expansion and devastating invasions [1,2]. The high reproductive capacity of this species is a key factor in its ability to rapidly colonize new areas. In the wild, polyandry associated with sperm precedence is a recurrent behaviour [3-6] that may represent an important adaptive trait. Remating by females sets the stage for post-copulatory sexual selection, which can shape both male and female biochemistry, physiology, morphology, and behaviour. As in many other species, a highly specialised female reproductive tract has evolved to deal efficiently with the sperm resources received from the males [7-11]. The medfly female's reproductive apparatus consists of a pair of ovaries, two short lateral oviducts joined by a short common oviduct to a long vagina, a pair of spermathecae and a pair of accessory glands. The anterior part of the vagina, that receives the sperm during copulation, contains the openings to the ducts that lead to the spermathecae and accessory glands. The spheroidal-shaped fertilization chamber lies ventrally in the anterior vagina and is characterized by the presence of about 70 small cavities called spermiophore alveoli, where the sperm are well placed to fertilize passing eggs [12,13]. The presence of multiple sperm storage organs potentially permits polyandrous females to manipulate ejaculates by segregating them into different locations and hence control the paternity of their progeny [14]. A number of studies have investigated the dynamics of sperm storage in the medfly in terms of relative amount of sperm stored in each organ and priority in fertilization. From a functional point of view, ablation experiments have established that the two spermathecae are long-term storage organs, whereas the fertilization chamber acts as staging point for sperm prior to their use in fertilization and is periodically replenished with sperm from the spermathecae [14].
Males have an intromittent organ (aedeagus) with three ejaculatory openings (gonopores). These three gonopores are thought to permit sperm emission towards the two spermathecae and the fertilization chamber, suggesting that sperm are injected into the three female sperm storage compartments simultaneously [13,15]. Both types of sperm storage organs are able to maintain sperm viability, as more than 80% of the stored sperm transferred to females can survive in both organs for at least 18 days following insemination [14]. In addition, several studies have focused on the pattern of sperm precedence, in terms of the proportion of progeny sired by the second of two males (P2) following female remating [3-6,16]. Recently, we correlated estimates from sperm counts in the female storage organs with paternity in twice-mated females [15]. We found a significant advantage of the second male's sperm in siring progeny [15]. Strikingly however, we found that patterns of paternity in multiply mated females are dynamic - second male sperm precedence (P2) decreases in favour of the first male (P1) as a function of time. This temporal increase in the proportion of offspring sired by the first male may be the result of the way the sperm of successive males are stored in the female reproductive tract. We thus proposed a model for which sperm from the first and the second male are stored in a somehow stratified fashion, without immediate mixing of the two ejaculates, so that those that enter later are used earlier in fertilization [15].
To date, understanding the mechanisms involved in medfly sperm use has been constrained by the technical challenges of directly observing sperm dynamics within the female reproductive tract and our limited ability to discriminate between sperm of different males.
Thus, here we use transgenic medfly lines expressing green or red fluorescent proteins in the spermatozoa [17], which can be easily observed and unambiguously differentiated within the female fertilization chamber. We tested and validated the hypothesis that it is the order and time-line of sperm storage to determine its subsequent use by twice-mated females.
Methods
The experimental strategy involves: (1) the choice of appropriate transgenic medfly lines. (2) The set-up of mating and remating tests. (3) The assessment of paternity in females mated successively to two males. (4) Statistical analyses. (5) Confocal and stereomicroscopic visualization of dissected fertilization chambers from twice-mated females to directly observe transgenic sperm distribution at different times following insemination.
Medfly lines
Three different medfly lines were used: i) the long-established laboratory strain Egypt-II (EgII), obtained from the FAO/IAEA Insect Pest Control Laboratory (Seibersdorf, Austria) and two molecularly and functionally characterized and critically evaluated homozygous transgenic strains generated by piggyBac-mediated germline transformation, namely ii) #1260_F-3_m-1 (tGFP1) and iii) #1261_F-5_m-5 (DsRedEx1) [17]. Both transgenic strains carry a body- and a sperm-specific marking system. The sperm-specific marker systems were generated by fusing the medfly β2-tubulin promoter (Ccβ2t) to turboGFP (tGFP) or DsRedExpress (DsRedEx) genes which were then used to engineer constructs based on piggyBac vectors carrying polyubiquitin (PUb)-driven EGFP or DsRed germline transformation markers, respectively. Specifically, males from the tGFP1 line express Ccβ2t promoter-driven tGFP in the testes/sperm and the Pub-DsRed marker produces red fluorescence in the body. Conversely, males from the DsRedEx1 line express Ccβ2t promoter-driven DsRedEx in the testes/sperm and green fluorescence in the body because of the presence of the Pub-EGFP marker. In both transgenic lines, transgene insertions are single transposon integrations as proven by Southern hybridisation and inverse PCR [17] and fluorescence of all markers has remained stable for more than 90 generations in our insectary. The two transgenic lines display very similar hatching rates [17].
Wild-type and transgenic medfly lines were maintained under standard rearing conditions [18].
Mating experiments
EgII, tGFP1, and DsRedEx1 adult flies of similar size were sexed by chilling at emergence and maintained under the same nutritional and environmental conditions. Mating and remating tests were performed in the ratio 1:3 (1 wild-type female : 3 transgenic males). In total, about 100 females and 300 males for first mating assays and 70 females plus 200 males for remating assays were used. Virgin females and males (48 h old) were used for the first mating tests. To assess any effects attributable to differential sperm storage and use, some wild-type EgII females were mated to tGFP1 and then to DsRedEx1 males, and others first to DsRedEx1 and then to tGFP1 males. The remating tests were performed 24 hours after the first mating, by exposing the females to virgin males of the same age [15]. For both mating and remating tests, pair formation was monitored for four hours and copulating pairs were coaxed into a test tube. Only females that remained in copula for at least 100 min were used in the following assays [19]. Single-mated females were dissected to assess the fluorescent sperm distribution within their fertilization chamber. For twice-mated females, flies were either dissected to observe sperm distribution in the fertilization chamber or transferred immediately after the remating to single cages to allow for oviposition. Daily ovipositions from these females (24 h collections) were collected separately and reared to adults for paternity assignment.
Paternity assay
The proportion of progeny sired by the first (P1) and second (P2) male was determined on the offspring of 102 twice-mated EgII females, 52 of which were mated first to tGFP1 and then to DsRedEx1 males (overall progeny n = 7530) and 50 mated first to DsRedEx1 and then to tGFP1 males (overall progeny n = 4882). For each twice-mated female, seven daily ovipositions were analysed to monitor the paternity trend over time. This time frame corresponds to the medfly steady state period of constant rate of egg-laying [20]. We considered only ovipositions that resulted in at least ten adult progeny. For each oviposition day, the total progeny sired by each female were screened by epifluorescence for the expression of PUb-DsRed, PUb-EGFP, Ccβ2t-tGFP and Ccβ2t-DsRedEx using an Olympus SZX7 fluorescence stereomicroscope with the filter set SZX-MGFP for the detection of green (cod. 33775; EX 460-490; DM 505; BA 510IF) and SZX-MIY for red fluorescence (cod. 33778; EX 540-580; DM 600; BA 610IF). The proportion of progeny that were sired by the first (P1) or second male (P2) was then calculated.
Statistical analysis
We analysed the paternity data using a logistic regression (generalized linear model with binomial errors) in the two reciprocal mating/remating crosses separately. We thus fitted the following model for p as a function of x:
p represents the proportion of progeny attributable to the first male (P1), x corresponds to the oviposition day, a and b to the intercept and the slope of the graph of P1 against oviposition day, respectively. Statistical analyses were performed using R project software, v2.2.1 (http://www.r-project.org/).
Light and fluorescence microscopy
The fertilization chambers of once- and twice-mated females were dissected in PBS one day and seven days after the last copula. Fresh samples were mounted on glass slides in PBS and analysed with an Axioplan (Zeiss) epifluorescent microscope. Filter sets used for the screening of tGFP and DsRedEx were the Zeiss filter set 13 (Ex. 470/20; Em. 505-530) and filter set 20 (Ex. 546/12; Em. 575-640), respectively. Images were captured with a 40x objective using an Olympus DP70 digital camera.
Confocal microscopy
Fluorescent confocal microscopy was used to examine and to resolve the details of the fertilization chambers dissected from once- and twice-mated females. Images were obtained from a Leica TCS-SP5 II system mounted on a Leica DMIRBE inverted microscope. Spaced optical sections were recorded using a 63x oil immersion objective. Images were collected in the 1024 × 1024 pixel format and processed by the Leica Confocal Software. Green and red fluorescent images were captured simultaneously and merged into a new image to visualize the localisation of red and green sperm.
Results
Sperm of the first male are under-represented in the initial progeny of twice-mated females, independently of the transgenic male order
The overall number of progeny produced by the 52 females mated first to the tGFP1 and then to the DsRedEx1 males (n = 7530), was significantly greater than those produced by the 50 females in the reciprocal mating cross (n = 4882)(Wilcoxon rank sum test with continuity correction P = 3.06e-6). Moreover, in the females first mated to DsRedEx1 and then to tGFP1 males, the mean number of progeny per female, in the first two oviposition days, was significantly lower compared to that in the following five oviposition days (Wilcoxon rank sum test with continuity correction, P = 8.76e-10). However, in the reciprocal cross there was no significant difference in these two oviposition intervals (Wilcoxon rank sum test with continuity correction, P = 0.07).
In both remating tests, the proportion of progeny sired by the first male (P1) is under-represented in the cumulative progeny of the first seven days. Indeed, the combined data from the 52 females mated first to tGFP1 and then to DsRedEx1 males indicated that, during the first seven days following the second copulation, the first male (tGFP1) sired 33% (P1) and the second male (DsRedEx1) sired 67% (P2) of the total progeny. In the reciprocal cross, P1 (attributable to DsRedEx1) was 31%, while P2 (tGFP1) was 69% of the total progeny (Table 1).
Table 1.
Proportion of progeny sired by the first male (P1) in relation to oviposition day.
| Oviposition day | ||||||||
|---|---|---|---|---|---|---|---|---|
| Male parental combination | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Overall |
| tGFP1, DsRedEx1 | ||||||||
| No. of ovipositing females | 50 | 34 | 50 | 39 | 43 | 50 | 40 | |
| No. of progeny | 1192 | 782 | 1198 | 1151 | 1099 | 1145 | 963 | 7530 |
| Mean progeny/female ± SE | 23.8 ± 1.83 | 23.0 ± 1.85 | 24.0 ± 1.32 | 29.5 ± 2.37 | 25.6 ± 1.53 | 22.9 ± 1.06 | 24.1 ± 1.56 | |
| Mean P1 ± SE | 0.31 ± 0.03 | 0.29 ± 0.04 | 0.32 ± 0.04 | 0.30 ± 0.05 | 0.33 ± 0.05 | 0.36 ± 0.05 | 0.42 ± 0.06 | 0.33 ± 0.02 |
| DsRedEx1,tGFP1 | ||||||||
| No. of ovipositing females | 41 | 23 | 27 | 40 | 36 | 36 | 36 | |
| No. of progeny | 611 | 367 | 482 | 1077 | 746 | 875 | 724 | 4882 |
| Mean progeny/female ± SE | 14.9 ± 0.46 | 15.9 ± 1.10 | 17.9 ± 1.44 | 26.9 ± 1.95 | 20.7 ± 1.15 | 24.3 ± 1.21 | 20.1 ± 1.45 | |
| Mean P1 ± SE | 0.16 ± 0.03 | 0.13 ± 0.02 | 0.32 ± 0.05 | 0.35 ± 0.05 | 0.31 ± 0.05 | 0.46 ± 0.06 | 0.43 ± 0.06 | 0.31 ± 0.05 |
First male sperm use increases over time
Twice-mated females were also used to obtain a distinct record of paternity for each of the first seven oviposition days (Table 1 and Figure 1). In both reciprocal crosses, the average first male paternity (P1) showed a tendency to increase as more eggs were laid, from 31% on the first day, up to 42% on the seventh oviposition day when tGFP1 males were the first mate, and from 16% to 43% when DsRedEx1 males acted as first mate. To determine whether this apparent trend is statistically significant, a logistic regression analysis was performed using a generalized linear model with binomial errors. The overall regression model slope, corresponding to the oviposition day, was significantly different from zero when the DsRedEx1 males acted as first mate (z = 11.87, P < 2e-16), whereas marginally non-significant when the tGFP1 males acted as first mate (z = 1.798, P = 0.0722). Indeed, in the DsRedEx1,tGFP1 remating parental combination, the P1 values in the first two oviposition days were significantly lower than in the following five days (Wilcoxon rank sum test with continuity correction, P = 4.9e-7), whereas no significant difference was detected in the reciprocal cross (P = 0.80).
Figure 1.
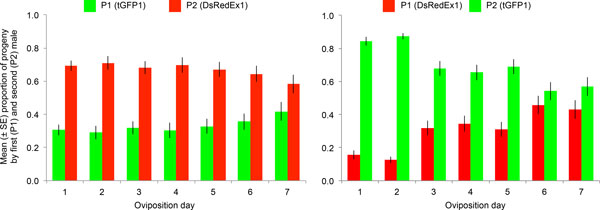
Changes in the proportion of offspring sired by the first (P1) and second (P2) male over time from twice-mated females. The mean proportion of progeny sired by 52 wild-type females mated first to tGFP1 males and then to DsRedEx1 males is shown on the left, whereas the mean proportion of progeny sired by 50 females mated according to the reciprocal male order is shown on the right. Green bars represent the progeny attributable to tGFP1, whereas red bars represent the progeny attributable to DsRedEx1 males. Vertical bars indicate standard errors.
Direct visualization of sperm dynamics in the fertilization chamber of twice-mated females
We used both an epifluorescence and a confocal microscope-based approach to visualize the dynamics of the transgenic green and red sperm within the fertilization chamber of either once- and twice-mated wild-type females. In the fertilization chamber of once-mated females, either tGFP1 or DsRedEx1 sperm appeared to be clearly distributed in all the alveoli, where numerous bundles of coiled spermatozoa were visible (Figure 2). In the fertilization chambers of twice-mated females 24 h after the second copulation, the sperm from the first male appeared to be homogenously distributed all over the distal portion of each alveolus, whereas sperm from the second male were clearly concentrated in the central portion of each alveolus (Figure 3A). This distribution was evident in both the reciprocal parental male combinations. Seven days after remating, this distinct stratified sperm distribution was no longer evident. The green and the red sperm were homogeneously mixed, occupying the complete cavity of each alveolus (Figure 3B).
Figure 2.
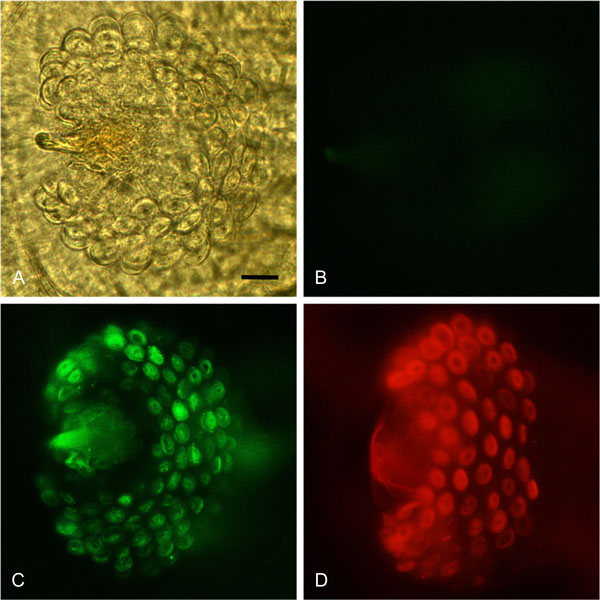
Fertilization chambers of once-mated females. The fertilization chambers were dissected from wild-type females once-mated to wild-type (2A-B), tGFP1 (2C), and DsRedEx1 (2D) males, respectively. Picture 2A was captured using phase contrast, whereas Pictures 2B, 2C and 2D are the result of merging of phase contrast and epifluorescence microscopy captured with the Zeiss filters sets 13 and 20. Scale bar = 15 µm.
Figure 3.
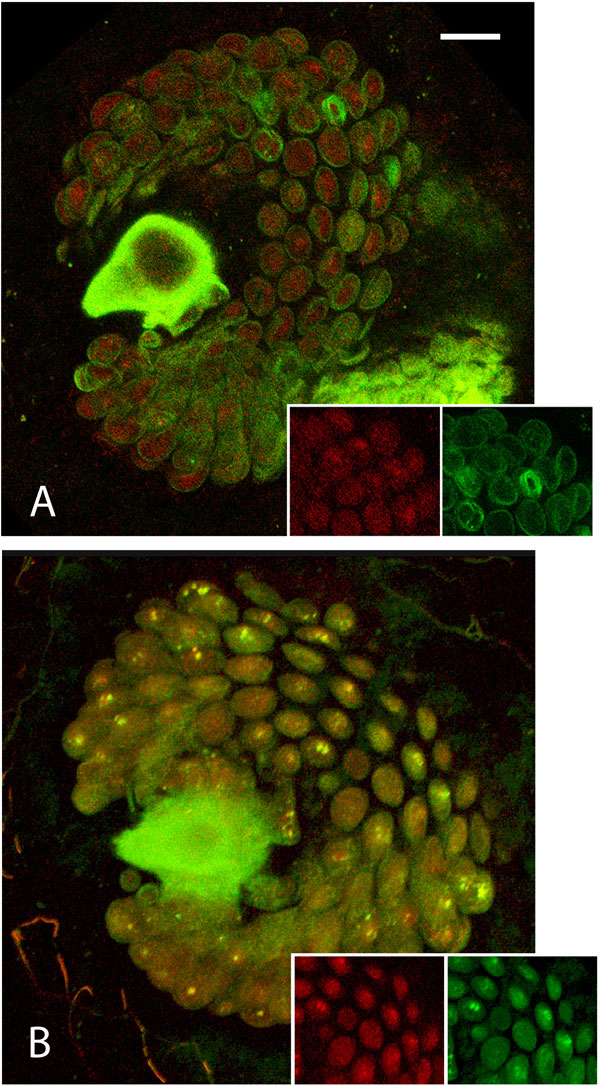
Confocal merged images of fertilization chambers of twice-mated females. Green sperm was transferred by the first male (tGFP1 line) and the red sperm (DsRedEx1 line) by the second male. In 3A, the fertilization chamber was dissected 24h after the remating and immediately observed. In 3B, the fertilization chamber was dissected seven days after the remating. The two squares at the bottom of each picture show, from the left, the single red and green unmerged images, respectively. Scale bar = 15 µm.
Discussion
Here we provide clear support that polyandrous medfly females, unlike Drosophila [21-23], conserve sperm from two different mates to fertilize their eggs. The female initially stores the sperm from the first and second male in the fertilization chamber in a stratified fashion, with subsequent sperm mixing. The presence of evident sperm stratification in the fertilization chamber one day after remating explains the initially low P1 values. These results exclude a role of sperm displacement or dumping mechanisms that are present in Drosophila [21-23], yet seem be due to strategic insemination by males and the sperm dynamics within the female storage organs. When the egg enters the anterior vagina with its micropyle towards the fertilization chamber entrance, the sperm from the chamber are the first to be used for fertilization and, given the clear stratification that we documented (Figure 3), the last sperm to enter the chamber are the first to be used for fertilization, accounting for a P2 value of roughly 70% of the total progeny. The gradual increase in P1 in the following days may be explained by the progressive exhaustion of the sperm in the fertilization chamber and the contemporary arrival of other sperm from the spermathecae, re-filling the spermiophore alveoli [15]. The microscope images are consistent with our paternity results, since we show that in the first days after remating P1 increases, suggesting the arrival of spermathecal sperm to replenish the chamber, resulting in an increase in sperm mixing. In addition, these results are consistent with the sperm dynamics recorded by Twig and Yuval [14]. They noted that although there is a significant drop in sperm numbers in the fertilization chamber between one and three days after mating, subsequently the level of sperm in the chamber remains quite constant, suggesting replenishment from the spermathecae. As further support, in other tephritids similar patterns have been observed [24-26].
In terms of fertilization success, the sperm of two transgenic lines used in this study displayed a similar efficiency in siring their overall P1 and P2 offspring (P1 = 33 and 31%; P2 = 67 and 69%, in the two reciprocal crosses, respectively). This data, together with the results from a previous study [17], suggest the absence of major dysfunctions of the transformed sperm.
However, in the remating tests we observed that, when tGFP1 was the first mate, the increase in P1 was less evident than in the reciprocal cross. This may be due to differences in the amount of sperm transferred by tGFP1 with respect to DsRedEx1 males (1854 ± 232 (SE) and 1164 ± 176 (SE), respectively), as previously assessed [17]. We suggest that this difference in sperm transfer rates may have a stronger effect in the first days after the remating. The more abundant tGFP1 first male sperm may indeed reflect the high P1 values, which are twice the P1 attributable to DsRedEx1 males in the reciprocal cross, both at day 1 and day 2 after remating.
Conclusions
Our findings represent an advance in understanding the complex reproductive biology of this highly invasive pest [2,27]. We confirm that the sperm load from a single mating does not exploit the storage capacity of the female sperm storage organs, mirroring the strategic partitioning of the male's sperm reserves among different females to optimize his reproductive success [28-31]. As a reproductive strategy, the sperm stratification we observed may initially favour the fresher ejaculate from the second male. However, as his sperm gradually becomes depleted, the sperm from the first male becomes increasingly available for fertilization. Despite the relatively high longevity of medfly sperm [14,17], the freshest sperm will most probably have a higher viability compared to that from the first male partner. The utilisation of sperm from different male partners in the medfly represents an adaptive strategy to maintain genetic variability in populations arising from new invasions or bottlenecks [2,15]. Given that medfly adults disperse over large distances in search of suitable hosts, young females need to fill their sperm reserves [3,5,6,32,33] to ensure egg fertilization long after dispersal. As a consequence, colonizing polyandrous females can benefit their descendants through a reduction in the cost of inbreeding since matings among their progeny will tend to be between half siblings as well as between full siblings. This favors a rapid increase in the effective population size and provides selective advantages in limiting the erosion of genetic diversity [15].
As in other insect species, male competition for fertilization success may be a multivariate process involving ejaculate-female and ejaculate-ejaculate interactions, as well as complex sperm behaviour in vivo [21,34,35]. Studies on the effects of male accessory secretions on female physiology and fertilization dynamics have been initiated [36], and may provide the key to understanding the subtle differences between strains in paternity, and how sperm mobilization from the spermathecae to fertilization chamber is regulated.
Knowledge on the dynamics of sperm storage and use will have a major impact on the application of Sterile Insect Technique (SIT) for pest control [37]. It is widely known that sterile males display lower mating competitiveness than wild males [38,39]. In addition, it has been repeatedly observed that females mated first to sterile males display higher remating frequencies than those mated to normal males [6,40-44]. According to our findings on the mechanisms of sperm storage and use, approaches based on the SIT could be less efficient in the presence of polyandry, since, even if the last male to mate a female is sterile, an increasing proportion of sperm from a previous mating with a fertile male may contribute to sire viable progeny. For this reason, further experiments to clarify the outcome of matings between wild-type females and sterile/fertile males as first/second mates are needed. In any case, the efficiency of the SIT can be improved by increasing the ratio of sterile males to wild fertile males, although accurate modelling will be needed to determine the optimal balance between the wild population and the sterile males released. As a last consideration, the use of sterile strains with easily recognizable, labelled, sperm will be invaluable for monitoring their competitiveness and hence the success of the release programme.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
Conceived and designed the experiments: FS, BY, MFS, EAW, ARM, GG. Performed the experiments: FS, PG. Analysed the data: FS, LMG, MFS, FB. Wrote the paper: FS, LMG, BY, MFS, ARM, GG. All authors read and approved the final manuscript.
Contributor Information
Francesca Scolari, Email: francesca.scolari@unipv.it.
Boaz Yuval, Email: yuval@agri.huji.ac.il.
Ludvik M Gomulski, Email: gomulski@unipv.it.
Marc F Schetelig, Email: marc.schetelig@agrar.uni-giessen.de.
Paolo Gabrieli, Email: paolo.gab@gmail.com.
Federico Bassetti, Email: federico.bassetti@unipv.it.
Ernst A Wimmer, Email: ewimmer@gwdg.de.
Anna R Malacrida, Email: malacrid@unipv.it.
Giuliano Gasperi, Email: gasperi@unipv.it.
Acknowledgements
We thank Geoffrey M. Attardo for critical reading of the manuscript. We also thank Eugenio Regazzini for the statistical support, Sabrina Bertin, Angelica Bonomi, Magda de Eguileor, Marzia Giagnacovo, Carlo Pellicciari, Giovanni Bottiroli and Anna Cleta Croce for technical support, and Maria Elisa Perotti for her valuable advice. The work was carried out within the FAO/IAEA Coordinated Research Programme (CRP) on ''Development and evaluation of improved strains of insect pests for SIT'' (GG, EAW) and the IAEA Technical Contract No:16966 (GG). This work was partially funded by Italian Ministry of education, University and Research PRIN grant 2008FLZZ37_02 (ARM) and the Emmy Noether Fellowship 1833/1 of the German Research Foundation (MFS). The authors would like to thank the two anonymous referees for their valuable comments which helped to improve the manuscript.
This article has been published as part of BMC Genetics Volume 15 Supplement 2, 2014: Development and evaluation of improved strains of insect pests for SIT. The full contents of the supplement are available online at http://www.biomedcentral.com/bmcgenet/supplements/15/S2.
Publication of this supplement was funded by the International Atomic Energy Agency. The peer review process for articles published in this supplement was overseen by the Supplement Editors in accordance with BioMed Central's peer review guidelines for supplements. The Supplement Editors declare that they have no competing interests.
References
- Harris EJ. In: Fruit Flies: Their Biology, Natural Enemies and Control. Robinson AS, Hooper GH, editor. Amsterdam: Elsevier; 1989. Pest status of fruit flies; pp. 73–81. [Google Scholar]
- Malacrida AR, Gomulski LM, Bonizzoni M, Bertin S, Gasperi G, Guglielmino CR. Globalization and fruitfly invasion and expansion: the medfly paradigm. Genetica. 2007;131(1):1–9. doi: 10.1007/s10709-006-9117-2. [DOI] [PubMed] [Google Scholar]
- Bonizzoni M, Katsoyannos BI, Marguerie R, Guglielmino CR, Gasperi G, Malacrida A, Chapman T. Microsatellite analysis reveals remating by wild Mediterranean fruit fly females, Ceratitis capitata. Mol Ecol. 2002;11(10):1915–1921. doi: 10.1046/j.1365-294X.2002.01602.x. [DOI] [PubMed] [Google Scholar]
- Bonizzoni M, Gomulski L, Bertin S, Scolari F, Guglielmino C, Yuval B, Gasperi G, Malacrida A. In: Area-Wide Control of Insect Pests. Vreysen M, Robinson A, Hendrichs J, editor. Dordrecht: Springer; 2007. Unfaithful Mediterranean fruit fly Ceratitis capitata: impact on the SIT? pp. 175–182. [Google Scholar]
- Kraaijeveld K, Katsoyannos B, Stavrinides M, Kouloussis N, Chapman T. Remating in wild females of the Mediterranean fruit fly, Ceratitis capitata. Animal Behaviour. 2005;69:771–776. doi: 10.1016/j.anbehav.2004.06.015. [DOI] [Google Scholar]
- Gavriel S, Gazit Y, Yuval B. Remating by female Mediterranean fruit flies (Ceratitis capitata, Diptera: Tephritidae): temporal patterns and modulation by male condition. J Insect Physiol. 2009;55(7):637–642. doi: 10.1016/j.jinsphys.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Winterton S, Merritt D, O'Toole A, Yeates D, Irwin M. Morphology and histology of the spermathecal sac, a novel structure in the female reproductive system of Therevidae (Diptera: Asiloidea) International Journal of Insect Morphology & Embryology. 1999;28:273–279. doi: 10.1016/S0020-7322(99)00030-6. [DOI] [Google Scholar]
- Pitnick S, Markow T, Spicer G. Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution. 1999;53(6):1804–1822. doi: 10.2307/2640442. [DOI] [PubMed] [Google Scholar]
- Demary KC. Sperm storage and viability in Photinus fireflies. J Insect Physiol. 2005;51(7):837–841. doi: 10.1016/j.jinsphys.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Puniamoorthy N, Kotrba M, Meier R. Unlocking the "Black box": internal female genitalia in Sepsidae (Diptera) evolve fast and are species-specific. BMC Evol Biol. 2010;10:275. doi: 10.1186/1471-2148-10-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons L. Sperm Competition and its Evolutionary Consequences in the Insects. Princeton: Princeton University Press; 2001. [Google Scholar]
- Solinas M, Nuzzaci G. Functional anatomy of Dacus oleae Gmel. Female genitalia in relation to insemination and fertilization processes. Entomologica. 1984;19:135–165. [Google Scholar]
- Marchini D, Del Bene G, Falso LF, Dallai R. Structural organization of the copulation site in the medfly Ceratitis capitata (Diptera: Tephritidae) and observations on sperm transfer and storage. Arth Struct & Dev. 2001;30:39–54. doi: 10.1016/S1467-8039(01)00018-4. [DOI] [PubMed] [Google Scholar]
- Twig E, Yuval B. Function of multiple sperm storage organs in female Mediterranean fruit flies (Ceratitis capitata, Diptera: Tephritidae) J Insect Physiol. 2005;51(1):67–74. doi: 10.1016/j.jinsphys.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Bertin S, Scolari F, Guglielmino CR, Bonizzoni M, Bonomi A, Marchini D, Gomulski LM, Gasperi G, Malacrida AR, Matessi C. Sperm storage and use in polyandrous females of the globally invasive fruitfly, Ceratitis capitata. J Insect Physiol. 2010;56(11):1542–1551. doi: 10.1016/j.jinsphys.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Whittier T, Shelly T. Productivity of singly vs multiply mated female Mediterranean fruit-flies, Ceratitis capitata (Diptera: Tephritidae) Journal of the Kansas Entomological Society. 1993;66:200–209. [Google Scholar]
- Scolari F, Schetelig MF, Bertin S, Malacrida AR, Gasperi G, Wimmer EA. Fluorescent sperm marking to improve the fight against the pest insect Ceratitis capitata (Wiedemann; Diptera: Tephritidae) N Biotechnol. 2008;25(1):76–84. doi: 10.1016/j.nbt.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Saul S. Rearing methods for the medfly, Ceratitis capitata. Ann Entomol Soc Am. 1982;75:480–483. [Google Scholar]
- Taylor PW, Yuval B. Postcopulatory sexual selection in Mediterranean fruit flies: advantages for large and protein-fed males. Anim Behav. 1999;58(2):247–254. doi: 10.1006/anbe.1999.1137. [DOI] [PubMed] [Google Scholar]
- Novoseltsev VN, Carey RJ, Novoseltseva JA, Papadopoulos NT, Blay S, Yashin AI. Systemic mechanisms of individual reproductive life history in female medflies. Mechanisms of Ageing and Development. 2004;125:77–87. doi: 10.1016/j.mad.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Manier MK, Belote JM, Berben KS, Novikov D, Stuart WT, Pitnick S. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science. 2010;328(5976):354–357. doi: 10.1126/science.1187096. [DOI] [PubMed] [Google Scholar]
- Price CS, Dyer KA, Coyne JA. Sperm competition between Drosophila males involves both displacement and incapacitation. Nature. 1999;400(6743):449–452. doi: 10.1038/22755. [DOI] [PubMed] [Google Scholar]
- Snook RR, Hosken DJ. Sperm death and dumping in Drosophila. Nature. 2004;428(6986):939–941. doi: 10.1038/nature02455. [DOI] [PubMed] [Google Scholar]
- Abraham S, Goane L, Cladera J, Vera MT. Effects of male nutrition on sperm storage and remating behavior in wild and laboratory Anastrepha fraterculus (Diptera: Tephritidae) females. J Insect Physiol. 2011;57(11):1501–1509. doi: 10.1016/j.jinsphys.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Perez-Staples D, Harmer A, Taylor P. Sperm storage and utilization in female Queensland fruit flies (Bactrocera tryoni) Physiological Entomology. 2007;32(2):127–135. doi: 10.1111/j.1365-3032.2006.00554.x. [DOI] [Google Scholar]
- Radhakrishnan P, Perez-Staples D, Weldon C, Taylor P. Multiple mating and sperm depletion in male Queensland fruit flies: effects on female remating behaviour. Animal Behaviour. 2009;78(4):839–846. doi: 10.1016/j.anbehav.2009.07.002. [DOI] [Google Scholar]
- Diamantidis AD, Carey JR, Nakas CT, Papadopoulos NT. Population-specific demography and invasion potential in medfly. Ecol Evol. 2011;1(4):479–488. doi: 10.1002/ece3.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrio G, Cavalloro R. Influenza dell'accoppiamento sulla recettività sessuale e sull'ovideposizione in femmine di Ceratitis capitata Wiedemann. Entomologica. 1979. pp. 127–143.
- Wedell N, Gage MJG, Parker GA. Sperm competition, male prudence and sperm-limited females. Trends in Ecology and Evolution. 2002;17:313–320. doi: 10.1016/S0169-5347(02)02533-8. [DOI] [Google Scholar]
- Perez-Staples D, Aluja M. Sperm allocation and cost of mating in a tropical tephritid fruit fly. Journal of Insect Physiology. 2006;52:839–845. doi: 10.1016/j.jinsphys.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Aluja M, Perez-Staples D, Sivinski J, Sanchez A, Pinero J. Effects of male condition on fitness in two tropical tephritid flies with contrasting life histories. Animal Behaviour. 2008;76:1997–2009. doi: 10.1016/j.anbehav.2008.08.020. [DOI] [Google Scholar]
- Bonizzoni M, Gomulski LM, Mossinson S, Guglielmino CR, Malacrida AR, Yuval B, Gasperi G. Is polyandry a common event among wild populations of the pest Ceratitis capitata? J Econ Entomol. 2006;99(4):1420–1429. doi: 10.1603/0022-0493-99.4.1420. [DOI] [PubMed] [Google Scholar]
- Chapman T, Miyatake T, Smith HK, Partridge L. Interactions of mating, egg production and death rates in females of the Mediterranean fruit fly, Ceratitis capitata. Proceedings of Biological Sciences/The Royal Society. 1998;265:1879–1894. doi: 10.1098/rspb.1998.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalez F, Simmons LW. Male-induced costs of mating for females compensated by offspring viability benefits in an insect. J Evol Biol. 2010;23(10):2066–2075. doi: 10.1111/j.1420-9101.2010.02065.x. [DOI] [PubMed] [Google Scholar]
- Aluja M, Rull J, Sivinski J, Trujillo G, Pérez-Staples D. Male and female condition influence mating performance and sexual receptivity in two tropical fruit flies (Diptera: Tephritidae) with contrasting life histories. J Insect Physiol. 2009;55(12):1091–1098. doi: 10.1016/j.jinsphys.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Scolari F, Gomulski LM, Ribeiro JM, Siciliano P, Meraldi A, Falchetto M, Bonomi A, Manni M, Gabrieli P, Malovini A. et al. Transcriptional profiles of mating-responsive genes from testes and male accessory glands of the Mediterranean fruit fly, Ceratitis capitata. PLoS One. 2012;7(10):e46812. doi: 10.1371/journal.pone.0046812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck VA, Hendrichs J, Robinson AS. Sterile Insect Technique: Principles and practice in Area-wide Integrated Pest Management. Dordrecht The Netherlands: Springer; 2005. [Google Scholar]
- McInnis DO, Lance DR, Jackson CG. Behavioral resistance to the sterile insect release technique by the Mediterranean fruit fly (Diptera: Tephritidae) in Hawaii. Annals of the Entomological Society of America. 1996;89:739–744. [Google Scholar]
- Lance DR, McInnis DO, Rendon P, Jackson CG. Courtship among sterile and wild Ceratitis capitata (Diptera: Tephritidae) in field cages in Hawaii and Guatemala. Annals of the Entomological Society of America. 2000;93:1179–1185. doi: 10.1603/0013-8746(2000)093[1179:CASAWC]2.0.CO;2. [DOI] [Google Scholar]
- Cavalloro R, Delrio G. Studi sulla radiosterilizzazione di Ceratitis capitata Wiedemann e sul comportamento dell'insetto normale e sterile. Redia. 1970. pp. 511–547.
- Bloem K, Bloem S, Rizzo N, Chambers D. In: Fruit Flies: Biology and Management. Aluja M, Liedo P, editor. New York: Springer; 1993. Female medfly refractory period: effect of male reproductive status; pp. 189–190. [Google Scholar]
- Vera MT, Cladera JL, Calcagno G, Vilardi JC, McInnis DO. Remating of wild Ceratitis capitata (Diptera: Tephritidae) females in field cages. Annals of the Entomological Society of America. 2003;96:563–570. doi: 10.1603/0013-8746(2003)096[0563:ROWCCD]2.0.CO;2. [DOI] [Google Scholar]
- Kraaijeveld K, Chapman T. Effects of male sterility on female remating in the mediterranean fruitfly, Ceratitis capitata. Proc Biol Sci. 2004;271(Suppl 4):S209–211. doi: 10.1098/rsbl.2003.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera MT, Wood R, Cladera JL, Gilburn A. Remating frequency in the Mediterranean fruit fly (Diptera: Tephritidae) under laboratory conditions. Florida Entomologist. 2002;85:156–164. doi: 10.1653/0015-4040(2002)085[0156:FAFRFI]2.0.CO;2. [DOI] [Google Scholar]


