Abstract
Background
Hailey–Hailey disease (HHD) is a rare, chronic and recurrent blistering disorder, which is characterized clinically by erosions occurring primarily in intertriginous regions, and histologically by suprabasal acantholysis. Oxidative stress plays a specific role in the pathogenesis of HHD, by regulating the expression of factors playing an important role in keratinocyte proliferation and differentiation.
Aim
Given the significance of oxidative stress in HHD, we investigated the potential effects of the antioxidant properties of an α-MSH analogue, Nle4-D-Phe7-α-MSH (afamelanotide), in HHD lesion-derived keratinocytes.
Results
Treatment of HHD-derived keratinocytes with afamelanotide contributed to upregulation of Nrf2 [nuclear factor (erythroid-derived 2)-like 2], a redox-sensitive transcription factor that plays a pivotal role in redox homeostasis during oxidative stress. Additionally, afamelanotide treatment restored the defective proliferative capability of lesion-derived keratinocytes. Our results show that Nrf2 is an important target of the afamelanotide signalling that reduces oxidative stress. Because afamelanotide possesses antioxidant effects, we also assessed the clinical potential of this α-MSH analogue in the treatment of patients with HHD. In a phase II open-label pilot study, afamelanotide 16 mg was administered subcutaneously as a sustained-release resorbable implant formulation to two patients with HHD, who had a number of long-standing skin lesions. For both patients, their scores on the Short Form-36 improved 30 days after the first injection of afamelanotide, and both had 100% clearance of HHD lesions 60 days after the first injection, independently of the lesion location.
Conclusions
Afamelanotide is effective for the treatment of skin lesions in HHD.
Introduction
Familial benign chronic pemphigus or Hailey–Hailey disease (HHD; OMIM 169600) is a rare, autosomal dominant genodermatosis. The prevalence of HHD is unknown. HHD is characterized by development of relapsing and recurrent blisters, erosions and crusts in the intertriginous areas. Although lesions generally first appear after adolescence, with peak onset around the age of 30–40 years, they can develop at any age. Lesions can be complicated by heat, rubbing or superinfection, and they can have a substantial negative effect on patients' quality of life (QOL).1
Using linkage analysis, the HHD candidate region has been localized to chromosome 3q21-q24.2 Within this region resides an expressed sequence tag sequence (EST) that has been identified as the orthologue of a yeast gene, ATP2C1, which encodes a calcium ATPase.2 A complex interplay of genetic and environmental factors is thought to play a crucial role in the pathogenesis of HHD, with keratinocytes and inflammatory mediators most likely playing a cooperative role in the formation of HHD lesions. However, the exact molecular mechanisms regulating the complex interactions between resident skin cells and additional extrinsic signals are still not fully elucidated.
To gain a better understanding of the molecular pathways involved in the initiation and progression of the disease, we previously conducted an exploratory study to identify candidate genes involved in HHD development.3 Our findings clearly support a role for reactive oxygen species (ROS) in HHD symptoms.3,4 We identified ROS accumulation in keratinocytes derived from the cutaneous lesions of patients with HHD.3,4 Interestingly, we also found that Notch1 expression was negatively regulated by ATP2C1-mediated ROS induction in keratinocytes, both in vivo and in vitro.3,4 Our results also implicated a critical cellular factor, p63, in the response to disease progression. These factors participate in and often regulate, a variety of cellular functions, including cell growth, survival and proliferation.3,4 Based on existing evidence, we suggest that disturbances in the oxidant–antioxidant system in the skin may play an important role in the pathogenesis of HHD.
There is no known cure for HHD, and existing treatments do not provide a long-lasting positive therapeutic result. Therapies for HHD often attempt to control the underlying inflammatory immune response associated with the disease to induce symptomatic remission. The most commonly used therapies include steroids, antifungals and antibiotics, administered either topically or systemically. α-MSH is a peptide hormone member of a family of peptides known as the melanocortins, and can bind to five known melanocortin receptors: MC1R, MC2R, MC3R, MC4R and MC5R.5–7 Many studies have provided evidence that α-MSH has potent protective and antioxidative effects.8–13 At the molecular level, α-MSH affects various pathways implicated in the regulation of inflammation and protection. In particular, in primary keratinocytes, α-MSH increased expression of Nrf2 [Nuclear factor (erythroid-derived 2)-like 2], a key transcription factor involved in orchestrating the expression of antioxidative enzymes.10 Nrf2 has emerged as a master regulator of an intracellular antioxidant response operating through transcriptional activation of an array of genes, including phase II detoxifying enzymes, antioxidants and transporters, which protect cells from toxic and carcinogenic chemicals.14–16 Additionally, reports have shown that α-MSH is capable of inducing expression of both Nrf2 and Nrf-dependent phase II detoxifying enzymes in keratinocytes, suggesting the potential role of α-MSH not only as a pigment inducer but also as a guardian of epidermal homeostasis and oxidative stress balance.10
Nle4-D-Phe7-α-melanocyte-stimulating hormone (afamelanotide) is an α-MSH analogue.17 Deficiency of ATP2C1 in HHD-derived keratinocytes is associated with alterations in proliferation and differentiation, and increased oxidative stress.3,4 In the current study, we aimed to find evidence to support a strategy for the use of afamelanotide as a therapeutic approach for HHD.
Methods
Ethics approval
The study was approved by the ethics committee of the S. Gallicano Institute, and conducted according to the principles of the Declaration of Helsinki. Informed consent was obtained from all participants.
Patients
Five patients with familial HHD (two men, three women; mean ± SD age 46.6 ± 11.5 years, range 35–61) were included in the study. All patients were of Italian origin. Diagnosis of HHD was confirmed by a dermatologist, based on clinical features and the personal and family history for each patient.
Genomic DNA
Genomic DNA was extracted from whole blood (QIAamp Blood Mini Kit; Qiagen, Milan, Italy). All translated ATP2C1 exons and exon–intron boundaries were amplified using 28 primer pairs. Amplification products were then sequenced in both directions using an automatic sequencing system (310 Genetic Analyzer; Applied Biosystems, Foster City, CA, USA). Sequencing results were analysed with reference to cDNA and gDNAATP2C1 sequences (HGMD cDNA sequence and GenBank accession number NM_000003.9, respectively).
Primary human keratinocytes
We extracted keratinocytes from 4-mm punch biopsies derived from healthy donors undergoing cosmetic surgery and from patients with HHD (patients 1–3; patient details have been described previously).3 Briefly, biopsies were taken from both normal-appearing and lesioned skin of patients, and were washed in a solution of phosphate-buffered saline (PBS) containing 100 μg/mL streptomycin, 100 μg/mL penicillin and 10 μg/mL gentamicin. The dermal surface of the biopsy was then brushed gently with forceps to remove the dermis. The epidermis was subsequently incubated for 1 h at 37 °C in 0.3% trypsin and 0.02% EDTA, and then centrifuged and transferred to a T-25 flask coated with collagen. Cells were maintained in modified low-calcium medium (Clonetics; Cambrex Bio Science, Walkersville, MD, USA). The medium was changed the day after seeding, and on every other day thereafter. Cultures were passaged at 70–80% confluence, and seeded at 10 000 cells/cm2 (passage 1). When cells again reached 70–80% confluence, they were again seeded at 10 000 cells/cm2 (passage 2), and allowed to reach 70–80% confluence, then used for experiments. One confluent T-25 flask of keratinocytes could be obtained routinely from each 4-mm punch biopsy.
Cells were either left untreated or were treated with afamelanotide. For the latter, afamelanotide 10 nmol/L was added to the medium and left for 14 days, then the medium was removed and replaced with fresh medium containing afamelanotide 10 nmol/L every 2 days.
Real-time reverse-transcriptase PCR
Expression of Nrf2 was verified by measuring the mRNA level in HHD keratinocytes. Real-time reverse-transcriptase (RT)-PCR was performed, using a commercial kit (TaqMan Assay Kit; no. HS00975961-g1; Applied Biosystems). The kit uses a two-step protocol requiring reverse transcription with a mRNA specific primer, followed by real-time PCR with TaqMan probes. The fold change of the mRNA gene in the HHD samples relative to the control samples was calculated using the ΔΔCt method.19 Real-time PCR was performed using a sequence detection system (ABI PRISM 700; Applied Biosystems).
Immunoblotting
We used protein extracts prepared from the blood of patients 1 and 2 to identify changes at the protein level. All cell extracts were prepared according to the manufacturer's instructions for detection of phospho-extracellular signal-regulated kinase (Cell Signalling Technology, Beverly, MA, USA) as previously described.18,20 Briefly, after removing media from cultures; cells were washed with 1 × PBS. Cell extracts were prepared by adding 1 × sodium dodecyl sulfate (SDS) sample buffer [62.5 mmol/L Tris–HCl (pH 6.8 at 25 °C), 2% w/v SDS, 10% glycerol, 50 mmol/L dithiothreitol, 0.01% w/v bromophenol blue] and then placing 100 μL of the solution into each well of a six-well plate. Cells were immediately scraped off, and the extract transferred to a microcentrifuge tube. Cells were sonicated for 10–15 s to complete cell lysis and shear the DNA to reduce sample viscosity. After heating 20 μL of each sample to 95–100 °C for 5 min, the extracts were separated using SDS-PAGE (30 μL μg per well), and the proteins transferred to nitrocellulose membranes (PROTRAN; Schleicher and Schuell, Keene, NH, USA). The membranes were probed with antibodies against the NRF2 protein (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and photographed. The blot was then stripped and reprobed with an anti-tubulin antibody as a loading control (Santa Cruz Biotechnology).
In vitro proliferation assay
Primary keratinocytes derived from either normal-appearing skin or cutaneous lesions of patients 1 and 2 with HHD were cultured in collagen-treated dishes, and first-passage cells were used for 3H-thymidine assay. Cells either treated or untreated with afamelanotide were pulse-labelled by adding 3H-thymidine (PerkinElmer Life And Analytical Sciences, Inc., Waltham, MA, USA) 1 Ci to each well, and cells cultured for another 12 h, before determination of 3H thymidine incorporation, as described previously.3
Treatment of patients with Nle4-D-Phe7-α-melanocyte-stimulating hormone
We carried out a phase II open-label pilot study of afamelanotide on two female patients. The diagnosis was based on clinical examination and family history, and was confirmed by histological and genetic analysis. At the time of the study, patient 4 was aged 53 years and patient 5 was aged 61 years, and both patients had lesions on their skin. The symptoms of HHD had first appeared at the age of 21 and 25 years, respectively, and neither patient had experienced any periods of complete remission since that time. The patients' medical history showed that neither of them had received any form of systemic therapy in the 2 years before the study, and they were required to avoid any systemic and topical therapy for at least 1 month before enrolment.
Afamelanotide was administered as a sustained-release resorbable implant formulation with 16 mg per implant (the drug is detectable in plasma for up to 10 days after implantation). Each implant was injected subcutaneously into the fat above the anterior portion of the iliac crest. The two patients each received one implant on day 0, and a second implant on day 30. The primary endpoints were assessment of overall lesions, and patient-reported outcome as assessed by the Medical Outcome Survey Short Form-36 (SF-36). This form contains 36 questions and yields profile of eight functional health and well-being scores, as well as psychometrically based physical and mental health summary measures, and a preference-based health utility index. Safety and tolerability of the drug were recorded and monitored by laboratory data analysis, including blood counts, electrolytes, urine analysis, and liver and kidney function tests.
Results
Expression pattern of Nrf2 in primary keratinocytes from patients with Hailey–Hailey disease
Real-time RT-PCR analysis showed that Nrf2 mRNA was significantly downregulated in keratinocytes derived from cutaneous lesions of patients with HHD (Fig.1a). These results were confirmed by the western blotting of keratinocyte lysates from patients 1 and 2 (Fig.1b).
Figure 1.
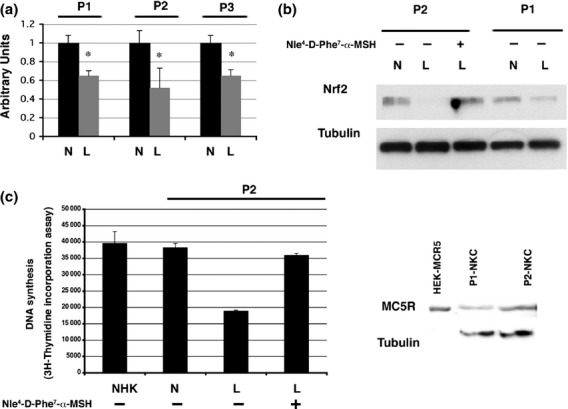
(a–d) Experiments carried out in primary keratinocytes derived from normal-appearing (N) or lesioned (L) skin of one or more of three patients (P1, P2 and P3) with Hailey–Hailey disease (HHD). (a) Total RNA (arbitrary units). *P < 0.001 compared with control. (b) The top bands show the reduction in protein expression of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) in lesioned skin compared with normal-appearing skin. The same blot was then stripped and reprobed with anti-tubulin antibodies. Lane 3 shows the keratinocytes that had been treated with the α-melanocyte-stimulating hormone analogue afamelanotide; the reduction in Nrf2 was lessened. (c) DNA synthesis in keratinocytes derived from healthy donors (normal human keratinocytes; NHK), and from normal-appearing and lesioned skin from patients with HHD, with or without afamelanotide treatment. Cells were pulse-labelled with 3H-thymidine for 12 h. All samples were tested in triplicate. Error bars indicate standard deviation. (d) Protein expression of the melanocortin receptor MC5R in total cell extracts (60 microgram) prepared from normal keratinocytes (NKC) derived from the normal-appearing skin biopsies of P1 and P2. The same blot was then stripped and reprobed with anti-tubulin antibodies. Lane 1 shows the control whole cell extract 10 microgram (HEK293 cells transfected with MC5R cDNA).
Addition of afamelanotide to keratinocytes derived from cutaneous lesions of patients with HHD partially restored Nrf2 expression (Fig.1b), and also restored the defective proliferative capability of the lesion-derived keratinocytes (Fig.1c).
Western blotting of MC5R expression in total cell extracts derived from HHD keratinocytes showed a single specific immunoreactive band of approximately 45 kDa in size (Fig.1d). However, immunoblotting with the two commercial anti-MC1R antibodies failed to generate specific signals.
Efficacy of Nle4-D-Phe7-α-melanocyte-stimulating hormone treatment of patients with Hailey–Hailey disease
Sequencing of the entire ATP2C1 coding region and exon–intron boundaries identified a heterozygous mutation (c.457C > T in patient 4 and c.2395C > T in patient 5), resulting in a premature termination codon in both cases. Patient 4 had erythematous and crusted lesions located on her subclavicular, periscapular and substernal regions (Fig.2), while patient had scaly erythematous plaques on her umbilical area, right flank and inguinal fold. Previous topical treatment had given poor results.
Figure 2.
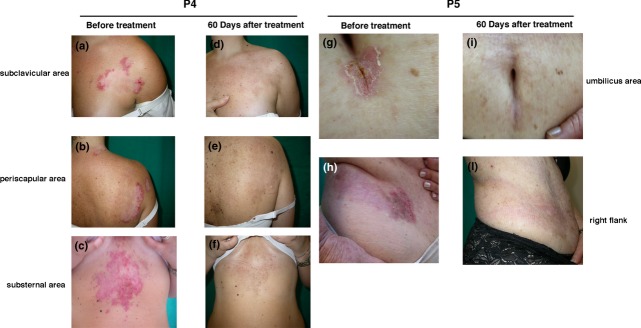
Clinical presentation of (a–c) patient 4 and (g,h) patient 5 before treatment. Clinical remission was seen after 60 days of treatment with the α-melanocyte-stimulating hormone analogue afamelanotide: (d–f) patient 4; (i,l) patient 5.
During the initial 30-day treatment period with afamelanotide, the lesions began to reduce in size, and during the following 30-day period, they reduced further, until by day 60, the lesions had disappeared completely, restoring the skin's natural appearance (Fig.2). The clinical remission was reflected in the improved QOL as measured by the SF-36.
There were no side-effects noted. Both patients experienced moderate skin tanning, but no clinically important laboratory abnormalities were detected. After afamelanotide withdrawal, there was no recurrence of clinical disease for 8 months, but signs of recurrence were seen after this point.
Discussion
α-MSH is known to have protective and antioxidative effects, thus in this study, we aimed to assess the clinical potential of an α-MSH analogue, afamelanotide in patients with HHD. Our previously published results had implicated oxidative stress and the response to it as contributing factors to the presentation of HHD.3,4 We hypothesized that afamelanotide might be an effective treatment for HHD by decreasing the level of oxidative stress. Our hypothesis was based on previous observations suggesting that α-MSH defends cells against the detrimental effects of oxidative stress, and may also abate the consequences of this stress.8–13
To test this hypothesis, we first analysed whether there was evidence of afamelanotide activity in primary keratinocytes derived from patients with HHD. To determine a possible functional link between Nrf2 (a master regulator of the balance of oxidative stress in cells) and the presence of oxidative stress in HHD primary keratinocytes, we performed a series of experiments in primary keratinocytes derived from either normal-appearing skin or cutaneous lesions of patients with HHD. We found that Nrf2 was significantly downregulated in keratinocytes derived from cutaneouslesions of patients with HHD. Our RT-PCR and western blotting experiments provided an initial insight into the molecular mechanism of increased oxidative stress in HHD keratinocytes, and indicated that the high level of oxidative stress seen in these keratinocytes might be the consequence of Nrf2 downmodulation. Thus, we hypothesized that afamelanotide might induce expression of both Nrf2 and Nrf-dependent genes.
The results showed that the impaired oxidative-stress balance in HHD keratinocytes leading to defects in differentiation and proliferation is associated with downregulated expression of Nrf2 (Fig.1) Interestingly, when these HHD keratinocytes were treated with afamelanotide, Nrf2 expression was partially restored. Consistent with a positive effect of afamelanotide, we found that after treatment, the proliferation of keratinocytes derived from lesional skin resembled that of keratinocytes derived from nonlesional skin from the same patients and from healthy control cells (Fig.1). Together, these results indicate that afamelanotide can act directly on keratinocytes to protect them from HHD defects, consistent with previous observation suggesting that α-MSH increases Nrf2 and Nrf2-dependent gene expression in keratinocytes, and in turn increases defence against oxidative stress.10 This mechanism seems to be completely independent of the role of α-MSH on melanocytes.
We then investigated whether afamelanotide rescues the defects in the HHD-derived keratinocytes via the canonical melanocortin receptor pathway or via a different signalling cascade. α-MSH is a peptide hormone member of a family of peptides known as the melanocortins, and binds with varying affinity to five known melanocortin receptors, MC1R, MC2R, MC3R, MC4R and MC5R.5,7 To gain insight into the mechanism of the afamelanotide-mediated effects, protein extracts prepared from HHD keratinocytes were used for western blotting to evaluate the melanocortin receptors involved in mediating its effects. We focused our analysis on MC1R and MC5R, because MC1R has been shown to be expressed in primary keratinocytes, and MCR5 is ubiquitously expressed,5–7 whereas MC2R binds to adrenocorticotropic hormone, and has no affinity for the other melanocortins. There is no evidence that MC3R and MC4R are expressed by keratinocytes. We found that the MC5R protein was expressed at detectable levels in HHD-derived keratinocytes, but no band was seen with the MC1R antibodies. These results suggest that the MC5R pathway might be involved in the observed effect of afamelanotide. It is plausible that the α-MSH–mediated effect we saw in the treated HHD keratinocytes is entirely dependent on the activation of MC5R.
These experiments provided preclinical proof of principle for the use of afamelanotide in HHD, and defined the biologically plausible mechanisms of action of afamelanotide. Together, these results provided a rationale for the use of afamelanotide for the treatment of HHD.
Therefore, as proof of principle, we tested the in vivo efficacy of afamelanotide in two patients with long-lasting/refractory HHD lesions. In previous trials that we conducted in our clinical facility in patients with erythropoietic protoporphyria, we used a dose of 16 mg of afamelanotide (Biolcati et al., unplublished observation). Based on the pharmacokinetics of the slow-release formulation of afamelanotide we used, Nle4-D-Phe7-a-MSH is detectable in peripheral plasma for up to 10 days after a single implant containing 20 mg.6,21 However, in patients with HHD, such a treatment schedule may not be sufficient. Therefore, we decided empirically to give afamelanotide to our patients at shorter intervals, i.e. every 4 weeks, and to monitor the evolution of the skin lesions over time. The extent of the lesions declined from day 0 to day 30 in both patients, and by day 60 the lesions had disappeared completely, independently of lesion location, and the skin's natural appearance was restored. The clinical remission was also accompanied by an improved QOL as measured by the SF-36.
The precise mechanism of how afamelanotide affects the course of HHD lesions remains to be determined. Our findings suggest that afamelanotide acts directly on keratinocytes to increase Nrf2 expression, and this may be relevant to its mechanism of action in HHD. However, we cannot exclude the possibility that the anti-inflammatory property of afamelanotide may be the mechanism of action that produces the in vivo effect of afamelanotide in our treated patients. Thus, it will be interesting to measure in future studies if afamelanotide affects the level of inflammatory mediators, e.g. cytokines and regulatory T cells, in patients with HHD.
Conclusion
In summary, this pilot study suggests that afamelanotide has a therapeutic efficacy in patients with chronic and treatment-resistant HHD. Despite the fact that HHD is a rare disease and it is therefore difficult to enrol sufficient patients in a placebo-controlled trial, a much larger number of patients with HHD receiving afamelanotide will be needed to confirm the preliminary data of this small pilot study. An interesting possibility would be use of a therapeutic protocol in which patients are scheduled for treatment with afamelanotide suspension every 6–7 months. Based on the responses of our two patients with HHD to a sustained resorbable implant formulation of afamelanotide in an open-label pilot study, afamelanotide treatment may be able to induce long-term disease remission.
Acknowledgments
We would like to thank Clinuvel Pharmaceuticals for providing afamelanotide. The financial support of Telethon – Italy (grant no. GGP12264) is gratefully acknowledged.
What's already known about this topic?
Benign chronic familial pemphigus or HHD is a rare, autosomal dominant genodermatosis, characterized by development of recurrent blisters, erosions, and crusts in the intertrigenous areas.
Genetic and environmental factors are both thought to play a role in the pathogenesis of this disease, but the exact molecular mechanisms are still not fully elucidated.
Treatments for HHD usually attempt to control the underlying inflammatory immune response associated with the disease.
What does this study add?
Given the significance of oxidative stress in HHD, we investigated the potential effects of an α-MSH analogue, Nle4-D-Phe7-α-MSH, to assess its antioxidant properties in keratinocytes derived from HHD lesions.
Nle4-D-Phe7-α-MSH treatment may be able to induce long-term disease remission.
References
- 1.Kellermayer R. Hailey-Hailey disease from a clinical perspective. Cell Calcium. 2008;43:105–6. doi: 10.1016/j.ceca.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Hu Z, Bonifas JM, Beech J, et al. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000;24:61–5. doi: 10.1038/71701. [DOI] [PubMed] [Google Scholar]
- 3.Cialfi S, Oliviero C, Ceccarelli S, et al. Complex multipathways alterations and oxidative stress are associated with Hailey-Hailey disease. Br J Dermatol. 2010;162:518–26. doi: 10.1111/j.1365-2133.2009.09500.x. [DOI] [PubMed] [Google Scholar]
- 4.Manca S, Magrelli A, Cialfi S, et al. Oxidative stress activation of miR-125b is part of the molecular switch for Hailey-Hailey disease manifestation. Exp Dermatol. 2011;20:932–7. doi: 10.1111/j.1600-0625.2011.01359.x. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Malek ZA. Melanocortin receptors: their functions and regulation by physiological agonists and antagonists. Cell Mol Life Sci. 2001;58:434–41. doi: 10.1007/PL00000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohm M, Luger TA. Alpha-melanocyte-stimulating hormone. From bench to bedside. Hautarzt. 2010;61:497–504. doi: 10.1007/s00105-009-1891-1. [DOI] [PubMed] [Google Scholar]
- 7.Hoogduijn MJ, McGurk S, Smit NP, et al. Ligand-dependent activation of the melanocortin 5 receptor: cAMP production and ryanodine receptor-dependent elevations of [Ca (2+)] (I) Biochem Biophys Res Commun. 2002;290:844–50. doi: 10.1006/bbrc.2001.6283. [DOI] [PubMed] [Google Scholar]
- 8.Bohm M, Luger TA. Alpha-melanocyte-stimulating hormone. Its current significance for dermatology. Hautarzt. 2004;55:436–45. doi: 10.1007/s00105-004-0729-0. [DOI] [PubMed] [Google Scholar]
- 9.Haycock JW, Rowe SJ, Cartledge S, et al. Alpha-melanocyte-stimulating hormone reduces impact of proinflammatory cytokine and peroxide-generated oxidative stress on keratinocyte and melanoma cell lines. J Biol Chem. 2000;275:15629–36. doi: 10.1074/jbc.275.21.15629. [DOI] [PubMed] [Google Scholar]
- 10.Kokot A, Metze D, Mouchet N, et al. Alpha-melanocyte-stimulating hormone counteracts the suppressive effect of UVB on Nrf2 and Nrf-dependent gene expression in human skin. Endocrinology. 2009;150:3197–206. doi: 10.1210/en.2008-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song X, Mosby N, Yang J, et al. Alpha-MSH activates immediate defense responses to UV-induced oxidative stress in human melanocytes. Pigment Cell Melanoma Res. 2009;22:809–18. doi: 10.1111/j.1755-148X.2009.00615.x. [DOI] [PubMed] [Google Scholar]
- 12.Henri P, Beaumel S, Guezennec A, et al. MC1R expression in HaCaT keratinocytes inhibits UVA-induced ROS production via NADPH oxidase- and cAMP-dependent mechanisms. J Cell Physiol. 2012;227:2578–85. doi: 10.1002/jcp.22996. [DOI] [PubMed] [Google Scholar]
- 13.Kadekaro AL, Chen J, Yang J, et al. Alpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes. Mol Cancer Res. 2012;10:778–86. doi: 10.1158/1541-7786.MCR-11-0436. [DOI] [PubMed] [Google Scholar]
- 14.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 15.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–89. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–94. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 17.Harms J, Lautenschlager S, Minder CE, Minder EI. An alpha-melanocyte-stimulating hormone analogue in erythropoietic protoporphyria. N Engl J Med. 2009;360:306–7. doi: 10.1056/NEJMc0805682. [DOI] [PubMed] [Google Scholar]
- 18.Cialfi S, Palermo R, Manca S, et al. Glucocorticoid sensitivity of T-cell lymphoblastic leukemia/lymphoma is associated with glucocorticoid receptor-mediated inhibition of Notch1 expression. Leukemia. 2013;27:485–8. doi: 10.1038/leu.2012.192. [DOI] [PubMed] [Google Scholar]
- 19.Applied Biosystems. ABI PRISM 7700 Sequence Detection System: User Bulletin 2: Rev B. User Bulletin Relative Quantification of Gene Expression.
- 20.Talora C, Cialfi S, Oliviero C, et al. Cross talk among Notch3, pre-TCR, and Tal1 in T-cell development and leukemogenesis. Blood. 2006;107:3313–20. doi: 10.1182/blood-2005-07-2823. [DOI] [PubMed] [Google Scholar]
- 21.Bohm M, Ehrchen J, Luger TA. Beneficial effects of the melanocortin analogue Nle4-D-Phe7-alpha-MSH in acne vulgaris. J Eur Acad Dermatol Venereol. 2012 doi: 10.1111/j.1468-3083.2012.04658.x. doi: 10.1111/j.1468–3083.2012.04658.x. [DOI] [PubMed] [Google Scholar]


