Abstract
Background
After perinatal transmission of hepatitis B virus, infants of anti-HBe positive HBsAg carrier mothers may develop fulminant hepatitis B. Previously it has been suggested, that fulminant hepatitis B in adults was associated with specific mutations in the HBV-genome. The aim of this study was to investigate, whether specific viral variants are associated with fulminant hepatitis B in young infants.
Methods
The complete HBV-genomes of five mothers and their infants with fulminant hepatitis were isolated from the sera, amplified and directly sequenced.
Results
Between 6 and 43 base pair exchanges between the HBV genomes of the infants and their mothers were identified. The mutations spread over the entire virus genome. Nucleotide exchanges in the basic core promotor and precore region were identified in all cases. A heterogeneous virus population was detected in four mothers.
Conclusions
Many new mutations were proved to emerge during fulminant hepatitis B in infants, who had been perinatally infected. HBeAg negative variants were the predominant population in all children, whereas these mutants could only be detected as subpopulations in four mothers. The data suggest that the selection of a specific HBeAg negative viral strain may be associated with the development of fulminant hepatitis B in children.
Background
To date, the pathogenic mechanism leading to the different clinical courses of hepatitis B in early childhood is largely unknown. Without immunization, 90% of the babies born to HBeAg-positive mothers develop a chronic carrier status. On the other side children of anti-HBe positive mothers become less frequently infected, but are at considerable risk to develop fulminant hepatitis B (FHB). The clinical outcome is poor and without liver transplantation most of these patients die at the age of 3–5 months. Both, host and virus specific factors are considered to have an important impact on the clinical course.
FHB in adults has been associated with mutations in the basic core promotor BCP (1762 A to T and 1764 G to A) [1] and the precore region (1896 G to A) [2,3]. A number of additional changes have been identified in cis acting regulatory elements and the four open reading frames including mutations of the pre-S2 start codon. It was suggested that these mutations may influence viral replication and alter the HBV-protein expression [4-6]. In a study from Sterneck et al. the full length genome analysis of one mother-child pair with fulminant disease did not show the presence of a particular HBV-strain in both individuals [7]. However, in a previous study we were able to demonstrate that a mixed virus pool was present in 80% of the chronically infected mothers and that certain variants emerged in the infants regarding the BCP and precore region [8].
Since in most studies only specific regions of the virus had been analyzed, it is yet unclear, if sequence differences do exist in other parts of the genome. In this context neonatal hepatitis represents an interesting "in vivo" model for two reasons: First, newborns have a partly immature immune system with the possibility of tolerance and may have maternal antibodies against HBV, so that an exaggerated immune response concerning the hepatitis B virus infection rarely occurs in the first months of live. Second, the viral population isolated in mothers and newborns can be directly compared with each other.
The aim of our study was to identify mutations in the entire hepatitis B virus genome, which might play a role in fulminant hepatitis in infants. Since only rare data are currently available in children, we analysed the complete viral nucleotide sequences of five chronic HBsAg carrier mothers and their infants who died of fulminant hepatitis B.
Methods
Patients
The mothers were clinically asymptomatic HBsAg carriers from caucasian origin in the age of 20–24 years and had normal liver function parameters. With one exception (mother M4) all of them had seroconverted to anti-HBe and no HBV-DNA was detectable with a commercial hybridization assay. All patients were negative for antibodies against hepatitis C, hepatitis D and human immunodeficiency virus. Since the carrier status of the mothers was unknown at the date of delivery and serological investigations during pregnancy were refused, the infants happened not to be immunized against HBV postnatally. Diagnosis of chronic HBV infection in the mothers was established after discovering the disease in their children. The perinatally infected infants developed fulminant hepatitis B with progressive liver failure at the age of 3–4 months and died 4–6 weeks after the onset of symptoms. The clinical course was characterized by increasing jaundice and decreasing liver function parameters, particularly clotting factors. At the time of diagnosis the children were HBsAg and anti-HBe seropositive and had moderately elevated transaminases (ALAT 60 – 120 U/l). HBV DNA in serum was not detectable by the commercial liquid hybridization assay, indicating low viremia with a viral load below 50.000 virions per ml. However, HBV DNA ascertainment by PCR revealed a positive result. In the phase of progressive liver failure HBV DNA determination remained then negative.
Methods
The serological markers of viral infection were tested using commercial radioimmunoassays (Abbott Laboratories, Chicago, IL). HBV-DNA was determined quantatively by a liquid hybridization assay (Hybride Capture Systems, Digene Diagnostics, Beltsville, MD).
An aliquot of 200 μl of each patient's serum was obtained to isolate viral DNA with the QuiaAmp blood kit (Quiagen, Chatsworth, CA). The DNA was eluted with 50 μl distilled water according to the manufacturer's recommendations. The HBV DNA isolated was amplified by PCR in 50 μl of buffer containing 50 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 200 mM deoxynucleoside triphosphates (dNTPs), 1 U of Taq DNA-Pwo Polymerase (Expand High Fidelity assay, Boehringer Mannheim) and 30 pmol of primers P1–P8. The PCR was performed for 35 cycles at 94°C for 1 min, 58°C for 1 min and 72°C for 1 min in a thermal cycler (Perkin Elmer Cetus, Norwalk, CT). The PCR products were visualized by 2% agarose electrophoresis and stained with ethidium bromide. A second PCR was carried out for samples not detectable after the first amplification. To minimize artificial mutations introduced by PCR, all fragments were amplified twice with a proofreading DNA polymerase.
Primers for amplification of 4 HBV fragments
Fragment 1:
P1: 5'-TTT TTC ACC TCT GCC TAA TCA-3' (1821-1841)
P2: 5'-TTG GGA TTG AAG TCC CAA TCT GG-3' (2957-2935)
Fragment 2:
P3: 5'-GGG TCA CCT TAT TCT TGG-3' (2813-2831)
P4: 5'-ATA ACT GAA AGC CAA ACA GTG GG-3' (738-716)
Fragment 3:
P5: 5'-GTC TTC TTG GTT GTT CTT CTA C-3' (427-448)
P6: 5'-GCA GCA CAG CCT AGC AGC CAT GG-3' (1394-1372)
Fragment 4:
P7: 5'-CCA TAC TGC GGA ACT CCT AGC-3' (1266-1286)
P8: 5'-CAA TGC TCA GGA GAC TCT AAG GC-3' (2043-2021)
The four overlapping HBV fragments (plus and minus strands) were directly sequenced twice with 16 different sense and antisense primers using a Dye Terminator Cycle Sequencing Kit (ABI PRISM, Foster City, CA) in an automated sequencer model 377 (ABI). According to Leitner et al. [9] very small populations of sequence variants can be detected with this method. Additionally in all cases parts of the genome were subcloned and sequenced.
Sequence alignments were performed using the Sequencher 3.0 software program. The HBV-DNA sequences were assigned to the appropriate genotype according to Li et al. [10]. Additionally, sequences were genotyped by alignment to published HBV sequences of genotypes A to E including the two strains HT and FH which were isolated from patients with FHB (Genbank/EMBL no. M57663, X02763, V00866, E00010, X65257-59, M32138, X02496, V01460, I08805, I27106; L08805 and L27106). The sequences were aligned according to Galibert [11]. The first base pair is the first T residue in the EcoRI recognition site. Nucleotide differences between mother and infant were defined as mutations. The reference genome [11] is depicted for better comparison (Figure 1).
Figure 1.
Nucleotide Exchanges between mothers (M1–M5) and their infants (1–5) in comparison with the Reference Sequence. Nucleotide positions are according to the nomenclature of Galibert et al. 1979. Mutations occurring in the HBV genomes of both, mother and child-compared to the reference sequence, are not listed. Nucleotide positions with a heterogeneous HBV population are shown in italics.
Results
Analysis of the complete HBV DNA sequences
The nucleotide sequences of the HBV genomes analysed from mothers and infants were most closely related to the HBV genome of genotype D, which was therefore used as the reference genome [11] for comparative analysis (Figure 1). In comparison with this strain, multiple mutations were found in all isolates, which were distributed over the entire genome. Mutations occurring in the HBV genomes of both, mother and child compared to the reference genome are not listed. In our analysis we particularly focused on the sequence differences between the HBV populations obtained from the mother-child pairs (Figure 1 and Table 1).
Table 1.
Distribution of amino acid (aa) changes in B- and T-cell epitopes of the Core protein in the infants compared to the HBV genomes of the mother.
| Child No | CTL-epitopes [aa] | CD4+ epitopes [aa] | B cell-epitopes [aa] | |||
| 18–27 | 88–96 | 1–20 | 50–69 | 74–89 | 107–118 | |
| 1 | - | - | - | - | - | - |
| 2 | - | - | 14 | - | - | - |
| 3 | - | 92 | - | - | - | - |
| 4 | - | - | - | 65 | - | - |
| 5 | 21 | 92 | 5,14 | 60 | 80,85 | 116 |
Nucleotide exchanges between mothers and infants
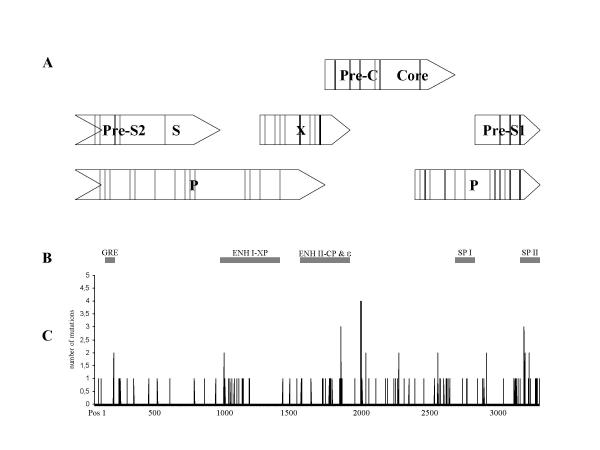
27, 6, 43, 22 and 33 nucleotide exchanges were identified between the HBV isolates from mothers and infants. The mutations were distributed over the entire HBV genome and a few hot spots (Figure 2). Many mutations were rare or unique compared with the other genomes investigated. A specific mutation that occurred "de novo" in the HBV genomes of all 5 infants could not be identified. Several base pair exchanges occurred in at least 2 of the 5 infant HBV genomes investigated (nucleotides 144, 930, 1764, 1940, 2174, 2452, 2797, 3072, 3102). However, 4 mutations (nucleotides 1762, 3067 and 1896, 1899) were overrepresented (60–100%) in the cases of fulminant hepatitis compared to the chronic carriers (Figure 1).
Figure 2.
Mutations identified in the HBV genomes from mothers and infants are illustrated. (C)The horizontal line represents the HBV nucleotide sequence of the reference genome (11). (A) The four open reading frames (ORF) are depicted in open bars. Lines within these bars represent mutations resulting in amino acid changes in the infant with fulminant disease. (B) Black bars in the middle indicate enhancer and promotor regions (GRE: Glucocorticoid response element, ENH I-XP: enhancer I and X promotor, ENH II-CP: enhancer II and core promotor, ε: pregenome RNA encapsidation signal epsilon, SP I: surface promotor I, SP II: surface promotor II).
Since mutations in promotor-enhancer regions may be of particular functional relevance, these regions were analyzed in detail. 71 mutations were located within the 5 cis-acting regulatory elements (Figure 2).
A total of 10 mutations were located in the predicted stem-loop structure of the encapsidation signal ε, whereas nucleotides 1896 and 1899 were overrepresented (in 5 of 5 and 4 of 5 infants). The mutation at position 1896-A is responsible for the generation of an in-frame translation stop codon in the precore region.
Six mutations occurred in the surface promotor I (SP I) and 23 base pair exchanges were observed in the Surface Promotor II (SPII).
14 and 18 mutations occurred in the enhancer I-X promotor (ENH I-X) and in the enhancer II-core promotor region (ENH II-CP). The glucocorticoid response element (GRE) was conserved in all HBV isolates.
Analysis of the predicted amino acid sequences
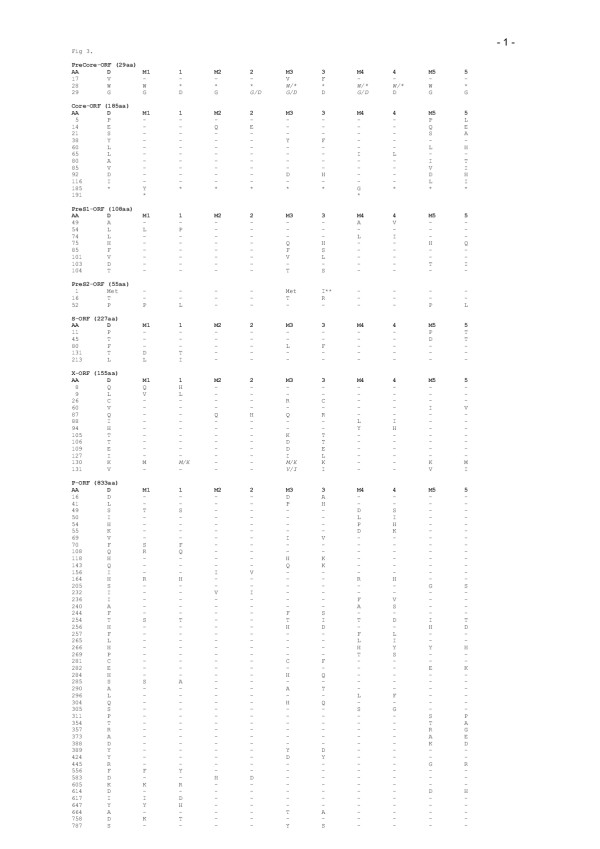
Many of the mutations resulted in amino acid changes in one or two open reading frames (ORF) (Figure 3). A total of 114 amino acid subsitutions were observed (Figure 3). The pre-C /C gene had 9 and 14 amino acid changes. The HBeAg synthesis was abrogated in all infants due to a precore stop codon, whereas this stop codon was detected as a major HBV-population in only one mother. 11 of 14 core mutations were detected in known B- T-cell epitopes (Table 1). The arginin-rich region (aa 155–171) that is important for DNA binding was highly conserved in all 5 cases. Furthermore, the cystein residues in the pre-C/ C gene (aa 23 of pre-C and aa 48, 61, 107, 183 of C gene), which are important for secretion and antigenicity of the core antigen were also conserved.
Figure 3.
Amino acid exchanges between mothers and their infants in comparison to the Reference Sequence. Amino acid positions are numbered from the start codon of each protein. Only amino acid exchanges from mothers (M1–M5) to their infants (1–5) are depicted, the reference sequence according to Galibert et al.(1979) is listed for comparison; a heterogeneous population according to the nucleotide sequence is shown in italics.* = stop ; met = start codon, **aa exchange prevents preS2 production.
In the pre-S /S gene 13 and 5 amino acid changes were observed. The HBV population of patient 3 was defective in pre-S2 protein expression due to a mutation in the pre-S2 start codon. One (aa 131) of five amino acid substitutions in the S gene was located within the major antigenic determinant, which is the target of neutralizing anti-HBs antibodies.
In the X gene 17 amino acid changes were identified, with a cluster of exchanges between codons 127–131 (Figure 3).
The P gene had a total of 56 amino acid changes including 13 in the terminal protein (TP), 20 in the spacer domain (SP), 14 in the polymerase-reverse transcriptase domain (Pol RT) and 2 in the ribonuclease H (RnaseH).
Discussion
The aim of this study was to investigate, whether specific HBV variants emerge after perinatal transmission and whether these variants may be associated with fulminant hepatitis.
Due to methodological reasons sequence analyses of the entire HBV genome were performed by direct sequencing. The disadvantage of this method is that viral quasispecies harboured in very small amounts may escape the detection. For this reason this method is not suitable to perform phylogenetic studies or to precisely follow the selective pressure occurring on those quasispecies during the course of fulminant hepatitis. On the other hand cloning studies regarding the complete HBV genome would have not been feasable in this setting.
Numerous nucleotide and amino acid substitutions at conserved segments were present in all of the four open reading frames (Figure 2). Interestingly all HBV genomes belonged to genotype D. This is in accordance with Li et al. [10], who demonstrated a prevalence of genotype D in HBeAg negative patients.
Since the core antigen has been postulated as target of cell mediated and humoral immunity, it is tempting to speculate, that core mutations contribute to a more severe course of the disease [12-15]. The precore stop codon 1896-A was present in all infants but only in 3 mothers. Nucleotide 1899-A could be observed in 4 infants but only in 2 mothers as a subpopulation. These nucleotide changes had previously been attributed to fulminant hepatitis B in adults [3,16,17]. However, wild type (HBeAg positive) virus has also been implicated in FH [18,19] and our study shows that HBeAg-negative virus strains were already present in 3 chronic carriers. Thus, it remains unclear if mutations 1896 and 1899 play a major role in infants with FHB although it is conceivable that the de novo infection of the new host with HBeAg defective variants contributes to the development of fulminant hepatitis [17,20-22].
Interestingly, 11 out of 12 mutations in the core protein resulted in changes of known B or T-cell epitopes (Table 1) affecting the immune response of the host [12]. The frequency of these mutations varied from 0 to 8 in the mother child pairs. This is in contrast to a previous study of a mother-child pair, in which none of the observed mutations was located in immunodominant epitopes [7]. A changed core protein due to a 6 aa prolongation in the dominant virus population of mothers M1 and M4 changed back to the wild type in the infants (Figure 3). Additionally the cysteine residues at positions 61 and 183, which are important for dimerization and particle assembly [23] were conserved in all cases. The amino acid changes in CTL epitopes have been associated with liver damage and it has been shown that frequent mutations occur during active or fulminant hepatitis [12,15,24]. It is conceivable that mutations in this region could induce a more intense reaction of CTL, followed by hepatocellular damage and severe hepatitis. In two mother child pairs (M3-3, M5-5) 3 changes could be observed in these epitopes (Table 1). Considering the different results of the five studied mother child pairs it remains speculative, if changes in B- T-cell epitopes play a significant role in FHB.
The analysis of the 5 regulatory elements, which might change viral pathogenicity due to an altered protein expression and virus replication revealed nucleotide substitutions clustering in all five cis acting elements (Figure 2). These mutations were of particular relevance, because it has been demonstrated that intracellular accumulation of HBV-proteins may have a direct cytotoxic effect [25-27].
In the ENH II-CP the nucleotide exchanges at positions 1762/ 1764 of the Basic Core Promotor (BCP) have been associated with FHB [1,4,6,28]. Nucleotide changes between mothers and infants were observed in 3 (1762) and 2 (1764) cases. These mutations have been shown to affect viral antigen expression and replication capacity [29,30]. Since a reduced HBeAg production may enhance viral replication [31-33] and modulate the immune response of the patient [34] it might contribute to the pathogenesis of fulminant hepatitis. However, the role of these mutations according to in vitro experiments is currently unclear [30,35,36].
While only few mutations were detected in the SP I region, we frequently found mutations in the other cis acting regulatory elements, clustering in ENH I XP, the stem loop structure of the encapsidation signal ε and SP II. In these regions a number of binding sites of several trancription factors are located, which are important for viral gene expression. Point mutations may alter the binding of transcription factors, enhance viral replication and influence protein synthesis. Mutations in the CCAAT-box of the SP II have been shown to promote expression of the large S-protein [37,38]. It has been shown in vitro by Xu et al. [27], that an increased expression and retention of the large surface protein may be cytopathic for cells [26].
The mutations observed in the S-Gene were distributed over the entire gene (preS1, preS2 and S) without hot spots. In only one infant a point mutation in the preS2 start codon prevents the production of the preS2 protein. Pre-S2 defective strains are rare in HBeAg positive hepatitis but emerge during seroconversion to anti-HBe [39,40]. An association with fulminant hepatitis has previously been shown and further supports the hypothesis that strains of the anti-HBe positive phase of infection preferentially induce fulminant hepatitis [5,7,41].
Only few mutations were observed in the S protein and only one aa-substitution was located in the immunodominant a-determinant. Base pair exchanges in this region seem to be uncommon in fulminant hepatitis [41].
49 amino acid substitutions were found in the polymerase gene, especially in the 5' terminal binding protein and the DNA polymerase-reverse transcriptase domains, which is in accordance with published data from the fulminant HBV strains HT and FH [4,6]. According to Asahina et al. [42] these mutations were rarely observed in patients with chronic hepatitis B. The binding of DNA polymerase-reverse transcriptase to the encapsidation signal ε is important for packaging of pregenomic RNA and the synthesis of viral DNA [43]. Therefore, the polymerase gene is thought to play a critical role in viral replication.
In summary we could demonstrate that many mutations emerged throughout the HBV genome of the dominant HBV-population in the infants compared to their mothers. Many of the mutations occurred in regulatory and immunodominant elements. Although HBV is not thought to be cytopathic, it is conceivable that enhanced viral replication and altered protein expression with different immunodominant epitopes may contribute to a more severe form of liver disease [25,26]. Although the mother's and infant's virus pools differed significantly, it is still unclear if all these variants might have evolved de novo or if they have already been present as a minor subpopulation in the mother. The viral strains which have been analyzed at the state of fulminant liver disease were not obligatory those which had been present at the time of perinatal infection. Due to the nature of the natural course of unknown vertical transmission HBV strains at an earlier phase were not available. However, either possibility implies that neonatal FHB is associated with the selection of variant genomes.
Conclusions
In our study we showed that after vertical HBV infection many mutations emerged throughout the HBV genome of the dominant HBV-population in the infants compared to their mothers, quite frequently occurring in regulatory and immunodominant elements. The results suggest that the selection of specific virus variants might be one factor in FHB of newborns, although further structural and functional analysis of the virus strains and the individual immune system of the host is required.
Competing interests
None declared.
List of abbreviations
HBV- Hepatitis B Virus
FHB- fulminant hepatitis B
SP- Surface promotor
Enh-CP -enhancer core promotor
GRE- Glucocorticoid response element
ORF- open reading frame
TP- terminal protein
SP- spacer domain
Pol-RT – polymerase reverse transciptase
aa- amino acid
Authors' contributions
MF and PG carried out the molecular genetic studies and participated in the sequence alignment. PW participated in the sequence alignment and drafted the manuscript. SW designed and coordinated the study and drafted the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This study was supported by Deutsche Forschungs Gemeinschaft (project WI 991/3-1).
Contributor Information
Michael Friedt, Email: mfriedt@web.de.
Patrick Gerner, Email: patrick.gerner@web.de.
Philip Wintermeyer, Email: ph.wint@gmx.de.
Stefan Wirth, Email: s-k-wirth@t-online.de.
References
- Sato S, Suzuki K, Akahane Y, Akamatsu K, Akiyama K, Yunomura K, Tsuda F, Tanaka T, Okamoto H, Miyakawa Y. Hepatitis B virus strains with mutations in the core promoter in patients with fulminant hepatitis. Ann Intern Med. 1995;122:241–248. doi: 10.7326/0003-4819-122-4-199502150-00001. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Huang JK, Wands JR, Obata H, Liang TJ. Association of hepatitis B viral precore mutations with fulminant hepatitis B in Japan. Virology. 1991;185:460–463. doi: 10.1016/0042-6822(91)90799-H. [DOI] [PubMed] [Google Scholar]
- Liang TJ, Hasegawa K, Rimon N, Wands JR, Ben Porath E. A hepatitis B virus mutant associated with an epidemic of fulminant hepatitis. N Engl J Med. 1991;324:1705–1709. doi: 10.1056/NEJM199106133242405. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Huang J, Rogers SA, Blum HE, Liang TJ. Enhanced replication of a hepatitis B virus mutant associated with an epidemic of fulminant hepatitis. J Virol. 1994;68:1651–1659. doi: 10.1128/jvi.68.3.1651-1659.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollicino T, Zanetti AR, Cacciola I, Petit MA, Smedile A, Campo S, Sagliocca L, Pasquali M, Tanzi E, Longo G, Raimundo G. Pre-S2 defective hepatitis B virus infection in patients with fulminant hepatitis. Hepatology. 1997;26:495–499. doi: 10.1002/hep.510260235. [DOI] [PubMed] [Google Scholar]
- Ogata N, Miller RH, Ishak KG, Purcell RH. The complete nucleotide sequence of a pre-core mutant of hepatitis B virus implicated in fulminant hepatitis and its biological characterization in chimpanzees. Virology. 1993;194:263–276. doi: 10.1006/viro.1993.1257. [DOI] [PubMed] [Google Scholar]
- Sterneck M, Kalinina T, Otto S, Günther S, Fischer L, Burdelski M, Greten H, Broelsch CE, Will H. Neonatal fulminant hepatitis B: structural and functional analysis of complete hepatitis B virus genomes from mother and infant. J Infect Dis. 1998;177:1378–1381. doi: 10.1086/515269. [DOI] [PubMed] [Google Scholar]
- Friedt M, Gerner P, Lausch E, Trübel H, Zabel B, Wirth S. Mutations in the Basic Core Promotor and the Precore Region of Hepatitis B Virus and Their Selection in Children With Fulminant and Chronic Hepatitis B. Hepatology. 1999;29:1252–1258. doi: 10.1002/hep.510290418. [DOI] [PubMed] [Google Scholar]
- Leitner T, Halapi E, Scarlatti G, Rossi P, Albert J, Fenyo EM, Uhlne M. Analysis of heterogeneous viral populations by direct DNA sequencing. Biotechniques. 1993;15:120–127. [PubMed] [Google Scholar]
- Li JS, Tong SP, Wen YM, Vitvitski L, Zhang Q, Trepo C. Hepatitis B virus genotype A rarely circulates as an HBe-minus mutant: possible contribution of a single nucleotide in the precore region. J Virol. 1993;67:5402–5410. doi: 10.1128/jvi.67.9.5402-5410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F, Mandart E, Fitoussi F, Tiollais P, Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979;281:646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Lee YI, Hur GM, Suh DJ, Kim SH. Novel pre-C/C gene mutants of hepatitis B virus in chronic active hepatitis: naturally occurring escape mutants. J Gen Virol. 1996;77:1129–1138. doi: 10.1099/0022-1317-77-6-1129. [DOI] [PubMed] [Google Scholar]
- Naoumov NV, Schneider R, Grotzinger T, Jung MC, Miska S, Pape GR, Will H. Precore mutant hepatitis B virus infection and liver disease. Gastroenterology. 1992;102:538–543. doi: 10.1016/0016-5085(92)90101-4. [DOI] [PubMed] [Google Scholar]
- Ehata T, Omata M, Yokosuka O, Hosoda K, Ohto M. Variations in codons 84–101 in the core nucleotide sequence correlate with hepatocellular injury in chronic hepatitis B virus infection. J Clin Invest. 1992;89:332–338. doi: 10.1172/JCI115581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehata T, Omata M, Chuang WJ, Yokosuka O, Ito Y, Hosoda K, Ohto M. Mutations in core nucleotide sequence of hepatitis B virus correlate with fulminant and severe hepatitis. J Clin Invest. 1993;91:1206–1213. doi: 10.1172/JCI116281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman WF, Fagan EA, Hadziyannis S, Karayiannis P, Tassopoulos NC, Williams R, Thomas HC. Association of a precore genomic variant of hepatitis B virus with fulminant hepatitis. Hepatology. 1991;14:219–222. doi: 10.1016/0270-9139(91)91406-Q. [DOI] [PubMed] [Google Scholar]
- Kosaka Y, Takase K, Kojima M, Shimizu M, Inoue K, Yoshiba M, Tanaka S, Akahane Y, Okamoto H, Tsuda F. Fulminant hepatitis B: induction by hepatitis B virus mutants defective in the precore region and incapable of encoding e antigen. Gastroenterology. 1991;100:1087–1094. doi: 10.1016/0016-5085(91)90286-t. [DOI] [PubMed] [Google Scholar]
- Feray C, Gigou M, Samuel D, Bernuau J, Bismuth H, Brechot C. Low prevalence of precore mutations in hepatitis B virus DNA in fulminant hepatitis type B in France [see comments] J Hepatol. 1993;18:119–122. doi: 10.1016/s0168-8278(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Aye TT, Uchida T, Becker SO, Hirahima M. Variations of hepatitis B virus precore/core gene sequence in acute and fulminant hepatitis B. Dig Dis Sci. 1994;39:1281–1287. doi: 10.1007/BF02093794. [DOI] [PubMed] [Google Scholar]
- Beath S, Boxall E, Watson R, Tarlow M, Kelly D. Fulminant hepatitis B in infants born to anti-HBe hepatitis B carrier mothers. BMJ. 1992;304:1169–1170. doi: 10.1136/bmj.304.6835.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterneck M, Gunther S, Gerlach J, Naoumov NV, Santantonio T, Fischer L, Rogiers X, Greten H, Williams R, Will H. Hepatitis B virus sequence changes evolving in liver transplant recipients with fulminant hepatitis. J Hepatol. 1997;26:754–764. doi: 10.1016/S0168-8278(97)80239-5. [DOI] [PubMed] [Google Scholar]
- Fagan E, Smith P, Davison P, Williams R. Fulminant hepatitis B in successive female partners of two anti-HBe-positive males. Lancet. 1986;2:538–540. doi: 10.1016/S0140-6736(86)90112-1. [DOI] [PubMed] [Google Scholar]
- König S, Beterams G, Nassal M. Mapping of homologous interaction sites in the hepatitis B virus core protein. J Virol. 1998;72:4997–5005. doi: 10.1128/jvi.72.6.4997-5005.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SL, Chen MH, Yeh CT, Chu CM, Lin AN, Chiou FH, Chang TH, Liaw YF. Purification and characterization of a naturally processed hepatitis B virus peptide recognized by CD8+ cytotoxic T lymphocytes. J Clin Invest. 1996;97:577–584. doi: 10.1172/JCI118450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roingeard P, Romet Lemonne JL, Leturcq D, Goudeau A, Essex M. Hepatitis B virus core antigen (HBcAg) accumulation in an HBV nonproducer clone of HepG2-transfected cells is associated with cytopathic effect. Virology. 1990;179:113–120. doi: 10.1016/0042-6822(90)90280-5. [DOI] [PubMed] [Google Scholar]
- Chisari FV, Filippi P, Buras J, McLachlan A, Popper H, Pinkert CA, Palmiter RD, Brinster RL. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc Natl Acad Sci. 1987;84:6909–6913. doi: 10.1073/pnas.84.19.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Yen TS. Intracellular retention of surface protein by a hepatitis B virus mutant that releases virion particles. J Virol. 1996;70:133–140. doi: 10.1128/jvi.70.1.133-140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulou A, Karayiannis P, Hadziyannis SJ, Hou J, Pickering J, Luo K, Thomas HC. Whole genome analysis of hepatitis B virus from four cases of fulminant hepatitis: genetic variability and its potential role in disease pathogenicity. J Viral Hepat. 1996;3:173–181. doi: 10.1111/j.1365-2893.1996.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Buckwold VE, Xu Z, Chen M, Yen TS, Ou JH. Effects of a naturally occurring mutation in the hepatitis B virus basal core promoter on precore gene expression and viral replication. J Virol. 1996;70:5845–5851. doi: 10.1128/jvi.70.9.5845-5851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K, Okamoto H, Tsuda F, Mayumi M. Reduced precore transcription and enhanced core-pregenome transcription of hepatitis B virus DNA after replacement of the precore-core promoter with sequences associated with e antigen-seronegative persistent infections. Virology. 1996;226:269–280. doi: 10.1006/viro.1996.0655. [DOI] [PubMed] [Google Scholar]
- Scaglioni PP, Melegari M, Wands JR. Posttranscriptional regulation of hepatitis B virus replication by the precore protein. J Virol. 1997;71:345–353. doi: 10.1128/jvi.71.1.345-353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts C, Nassal M, Velhagen I, Zentgraf H, Schroder CH. Precore-mediated inhibition of hepatitis B virus progeny DNA synthesis. J Virol. 1993;67:3756–3762. doi: 10.1128/jvi.67.7.3756-3762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti LG, Matzke B, Pasquinelli C, Schoenberger JM, Rogler CE, Chisari FV. The hepatitis B virus (HBV) precore protein inhibits HBV replication in transgenic mice. J Virol. 1996;70:7056–7061. doi: 10.1128/jvi.70.10.7056-7061.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich DR, Chen MK, Hughes JL, Jones JE. The secreted hepatitis B precore antigen can modulate the immune response to the nucleocapsid: a mechanism for persistence. J Immunol. 1998;160:2013–2021. [PubMed] [Google Scholar]
- Buckwold VE, Xu Z, Yen TS, Ou JH. Effects of a frequent double-nucleotide basal core promoter mutation and its putative single-nucleotide precursor mutations on hepatitis B virus gene expression and replication. J Gen Virol. 1997;78:2055–2065. doi: 10.1099/0022-1317-78-8-2055. [DOI] [PubMed] [Google Scholar]
- Günther S, Piwon N, Will H. Wild-type levels of pregenomic RNA and replication but reduced pre-C RNA and e-antigen synthesis of hepatitis B virus with C(1653)-T, A(1762)-T and G(1764)-A mutations in the core promotor. J Gen Virol. 1998;79:375–380. doi: 10.1099/0022-1317-79-2-375. [DOI] [PubMed] [Google Scholar]
- Lu CC, Chen , Ou JH, Yen TS. Key role of a CCAAT element in regulating hepatitis B virus surface protein expression. Virology. 1995;206:1155–1158. doi: 10.1006/viro.1995.1042. [DOI] [PubMed] [Google Scholar]
- Trautwein C, Schrem H, Tillmann HL, Kubicka S, Walker D, Boker KH, Maschek HJ, Pichlmayr R, Manns MP. Hepatitis B virus mutations in the pre-S genome before and after liver transplantation. Hepatology. 1996;24:482–488. doi: 10.1053/jhep.1996.v24.pm0008781311. [DOI] [PubMed] [Google Scholar]
- Santantonio T, Jung MC, Schneider R, Fernholz D, Milella M, Monno L, Pastore G, Pape GR, Will H. Hepatitis B virus genomes that cannot synthesize pre-S2 proteins occur frequently and as dominant virus populations in chronic carriers in Italy. Virology. 1992;188:948–952. doi: 10.1016/0042-6822(92)90559-8. [DOI] [PubMed] [Google Scholar]
- Gerner RP, Friedt M, Oettinger R, Lausch E, Wirth S. The hepatitis B virus seroconversion to anti-HBe is frequently associated with HBV genotype changes and selection of preS2-defective particles in chronically infected children. Virology. 1998;245:163–172. doi: 10.1006/viro.1998.9126. [DOI] [PubMed] [Google Scholar]
- Günther S, Fischer L, Pult I, Sterneck M, Will H. Naturally occurring variants of hepatitis B virus. Adv Virus Res. 1999;52:25–137. doi: 10.1016/s0065-3527(08)60298-5. [DOI] [PubMed] [Google Scholar]
- Asahina Y, Enomoto N, Ogura Y, Kurosaki M, Sakuma I, Izumi N, Marumo F, Sato C. Sequential changes in full-length genomes of hepatitis B virus accompanying acute exacerbation of chronic hepatitis B. J Hepatol. 1996;25:787–794. doi: 10.1016/S0168-8278(96)80280-7. [DOI] [PubMed] [Google Scholar]
- Pollack JR, Ganem D. Site-specific RNA binding by a hepatitis B virus reverse transcriptase initiates two distinct reactions: RNA packaging and DNA synthesis. J Virol. 1994;68:5579–5587. doi: 10.1128/jvi.68.9.5579-5587.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]