α-Synuclein is an abundant presynaptic protein that binds to negatively charged phospholipids1,2, functions as a SNARE-complex chaperone3, and contributes to Parkinson’s Disease pathogenesis4,5. Recombinant α-synuclein in solution is largely unfolded and devoid of tertiary structure6–11, but Bartels et al. (2011) suggested that native α-synuclein purified from human erythrocytes forms a stably folded, soluble tetramer that resists aggregation12. In contrast, we here show that native α-synuclein purified from mouse brain consists of a largely unstructured monomer, exhibits no stable tetramer formation, and is prone to aggregation. The native state of α-synuclein is important for understanding its pathological effects since a stably folded protein would be much less prone to aggregation than a conformationally labile protein.
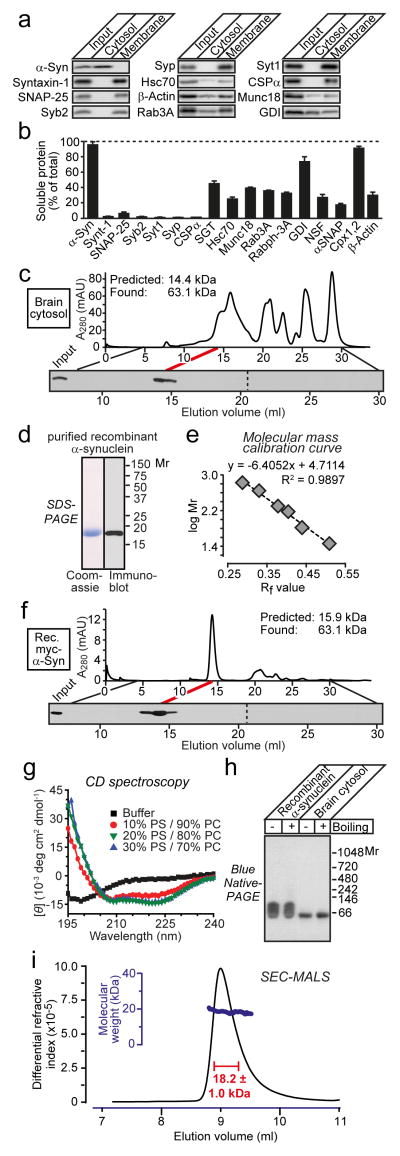
We examined native α-synuclein from brain, the most relevant organ for understanding neurodegeneration. Separation of mouse brain homogenates into soluble and membrane fractions revealed that during ultracentrifuation, most α-synuclein partitioned into cytosol fractions similar to complexins, but different from membrane proteins such as CSPα and SNAP-25 (Figs. 1a and 1b). Using gel filtration, we analyzed the size of native α-synuclein in brain cytosol and of recombinant myc-epitope tagged human α-synuclein, purified without boiling or detergents3. Both α-synucleins eluted in a single peak with an apparent molecular mass of ~63 kDa (Figs. 1c–1f), close to that predicted for a folded tetramer12.
Figure 1.
a & b, Immunoblotting analysis of mouse brain homogenate (input), cytosol, and membranes (a), and quantification of protein levels (b; means ± SEMs; n=3)3.
c, Native mouse brain α-synuclein (375 μg) elutes as an apparent tetramer during gel filtration on a Superdex 200 column (top), as analyzed by α-synuclein immunoblotting (bottom).
d, Analysis of purified recombinant myc-epitope-tagged α-synuclein by SDS-PAGE and immunoblotting.
e, Molecular mass calibration curve for gel filtration (Rf = migration distance of proteins versus total running distance).
f, Recombinant myc-tagged human α-synuclein (16 μg) also elutes as an apparent tetramer during gel filtration.
g, Circular dichroism spectroscopy shows that recombinant α-synuclein (10 μg) is unstructured in solution and becomes α-helical upon liposome binding (PS = phosphatidylserine; PC = phosphatidylcholine; molar protein-to-lipid ratio = 1:530).
h, Recombinant (0.5 μg) and α-synuclein in brain cytosol (12 μg total protein) run as apparent tetramers on blue-native gels without boiling or after boiling for 5 min.
i, Size-exclusion chromatography coupled with multi-angle light scattering (SEC-MALS) reveals that recombinant α-synuclein (0.5 mg) is monomeric.
These results seem to confirm that α-synuclein forms a stable tetramer in solution. However, dynamic or unstructured states of a protein may increase its hydrodynamic radius and apparent molecular mass during gel filtration. Indeed, circular dichroism (CD) spectroscopy showed that recombinant α-synuclein lacked detectable secondary structure, but became α-helical upon membrane binding (Fig. 1g). Consistent with the gel-filtration analysis, both native and recombinant α-synuclein migrated as a single band of ~65 kDa on blue-native gels. Strikingly, however, both recombinant and native α-synuclein still migrated at that apparent size after boiling, which disrupts secondary and tertiary structures, arguing against a folded multimer (Fig. 1h). Furthermore, size-exclusion chromatography coupled with multi-angle laser-light scattering (SEC-MALS) revealed that recombinant α-synuclein was monomeric (Fig. 1i). Since native α-synuclein in brain cytosol and recombinant α-synuclein behave identically in gel filtration and blue-native gel-electrophoresis experiments, the SEC-MALS demonstration that recombinant α-synuclein is monomeric suggests that native brain α-synuclein in cytosol is also monomeric.
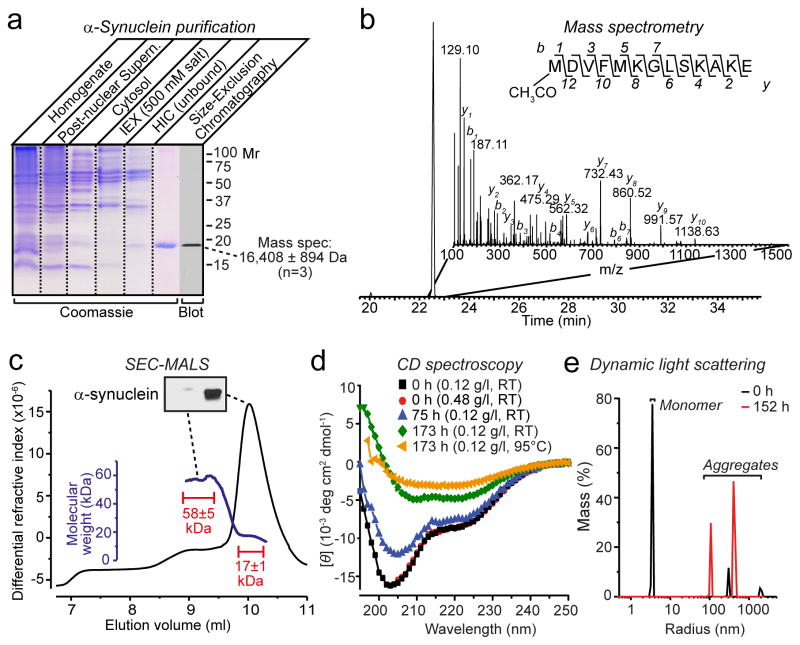
We next tested whether native brain α-synuclein is still monomeric even when purified. We purified α-synuclein from mouse brain without detergents or denaturing conditions (purity >90%; Fig. 2a). Mass spectrometry showed that native brain α-synuclein was significantly larger than predicted (measured mass, 16,408±894 Da [n=3]; predicted, 14460 Da). The increased mass is partly due to N-terminal acetylation of brain α-synuclein (Fig. 2b)12,13. SEC-MALS revealed that freshly purified native α-synuclein was again predominantly monomeric (Fig. 2c). We additionally observed a plateau along the left shoulder of the main SEC-MALS peak with a mass of ~58 kDa that contained little detectable α-synuclein (<5% by immunoblotting). CD spectroscopy showed a largely random-coil conformation (34–59%) with α-helical contributions (21–24%; Fig. 2d). Purified α-synuclein aggregated, in a time-dependent manner, with a relative increase in overall secondary structure as observed by CD spectroscopy (Fig. 2d), and the appearance of larger particles as uncovered by dynamic light scattering (Fig. 2e).
Figure 2.
a, SDS-PAGE analysis of five stages of α-synuclein purification from mouse brain (IEX, anion exchange chromatography, HIC, hydrophobic interaction chromatography). Purified α-synuclein was also analyzed by immunoblotting and mass spectrometry as shown.
b, Mass spectrometry analysis reveals N-terminal acetylation of native α-synuclein. Shown is an extracted ion chromatogram of the N-terminally acetylated α-synuclein peptide (inset: MSMS spectrum containing the sequence of the N-terminal peptide and identified b and y ions).
c, SEC-MALS shows that purified brain α-synuclein (150 μg) is largely monomeric (main peak with a mass of 17±1 kDa), but includes a minor component (plateau along the left shoulder with a mass of 58±5 kDa) that contains little detectable α-synuclein (see immunoblot in insert). Calculated masses were extracted from marked areas.
d, CD spectroscopy of freshly purified brain α-synuclein (7.5 μM) shows mainly disordered conformations that progressively acquire structured conformations as a result of time- and temperature-dependent aggregation.
e, Purified brain α-synuclein (0.12 mg/ml) rapidly aggregates as measured by dynamic light scattering immediately (0 h) or 152 h after purification.
Our data show that native brain α-synuclein primarily consists of an unstructured monomer, but readily aggregates in a time-dependent manner. This conclusion was demonstrated both for unpurified α-synuclein as a component of brain cytosol (Fig. 1), and for purified α-synuclein in solution (Fig. 2c). Purified brain α-synuclein – analyzed here for the first time – carries significant posttranslational modifications (Fig. 2b), which however do not appear to significantly alter its folding since the biophysical properties of recombinant unmodified α-synuclein and native modified α-synuclein were similar (Figs. 1 and 2). The differences between our results with brain α-synuclein and those obtained with erythrocyte α-synuclein12 may be due to erythrocyte-specific posttranslational modifications, or to time-dependent multimerization/aggregation of erythrocyte α-synuclein that may have been overlooked. Indeed, the CD spectrum of erythrocyte α-synuclein12 is similar to that of purified brain α-synuclein after 75 hour incubation (Fig. 2d). Independent of which explanation will explain our differences in results, the conformationally labile state of native brain α-synuclein documented here provides a potential explanation for why α-synuclein is susceptible to pathological aggregation as observed in multiple neurodegenerative disorders4,5.
METHODS
α-Synuclein was purified from mouse brain cytosol (obtained from brain homogenates by ultracentrifugation at 280,000gav) by sequential chromatography on Q-Sepharose (elution at 0.3–0.5 M NaCl, 20 mM Tris-HCl pH7.4), phenyl-Sepharose (flow-through in 1 M (NH4)2SO4), and Superdex-200 10/300GL. SEC-MALS was performed on a WTC-030S5 column (Heleos OptiLab instruments, Wyatt Technology). Circular dichroism spectra were measured in 25% PBS on an Aviv CD Spectrometer (Model 202-01) and deconvolved (http://dichroweb.cryst.bbk.ac.uk/html/home.shtml) with Contin-4 and -7 reference sets. Mass spectrometry was performed on purified α-synuclein or α-synuclein-containing gel pieces digested with Glu-C and Protease Max (Promega using standard procedures14. All other methods were described previously3.
Footnotes
AUTHOR CONTRIBUTIONS
Jacqueline Burré, Sandro Vivona, Jiajie Diao, and Manu Sharma performed the experiments; all authors planned and analyzed the experiments and wrote the paper.
References
- 1.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 2.Jo E, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE. α-Synuclein membrane interactions and lipid specificity. J Biol Chem. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 3.Burré J, et al. α-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin I, Dawson VL, Dawson TM. Recent advances in the genetics of Parkinson’s disease. Annu Rev Genomics Hum Genet. 2011;12:301–25. doi: 10.1146/annurev-genom-082410-101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devine MJ, Gwinn K, Singleton A, Hardy J. Parkinson’s disease and α-synuclein expression. Mov Disord. 2011;26:2160–2168. doi: 10.1002/mds.23948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 7.Kim J. Evidence that the precursor protein of non-A beta component of Alzheimer’s disease amyloid (NACP) has an extended structure primarily composed of random-coil. Mol Cells. 1997;7:78–83. [PubMed] [Google Scholar]
- 8.Uversky VN, Lee HJ, Li J, Fink AL, Lee SJ. Stabilization of partially folded conformation during α-synuclein oligomerization in both purified and cytosolic preparations. J Biol Chem. 2001;276:43495–43498. doi: 10.1074/jbc.C100551200. [DOI] [PubMed] [Google Scholar]
- 9.Chandra S, Chen X, Rizo J, Jahn R, Südhof TC. A broken α-helix in folded α-synuclein. J Biol Chem. 2003;278:15313–15318. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- 10.Lokappa SB, Ulmer TS. α-Synuclein populates both elongated and broken helix states on small unilamellar vesicles. J Biol Chem. 2011;286:21450–21457. doi: 10.1074/jbc.M111.224055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fauvet B, et al. α-synuclein in the central nervous system and from erythrocytes, mammalian cells and E. coli exists predominantly as a disordered monomer. J Biol Chem. 2012;287:15345–15364. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maltsev AS, Ying J, Bax A. Impact of N-terminal acetylation of α-synuclein on its random coil and lipid binding properties. Biochem. 2012;51:5004–5013. doi: 10.1021/bi300642h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson H, Eyers CE. Analysis of post-translational modifications by LC-MS/MS. Methods Mol Biol. 2010;658:93–108. doi: 10.1007/978-1-60761-780-8_5. [DOI] [PubMed] [Google Scholar]