Introduction
Acute Kidney Injury (AKI) is common, expensive to manage, prolongs hospitalization and is associated with increased mortality. In 2009 the National Confidential Enquiry into Patient Outcome and Death (NCEPOD) published a report into the care of patients who died with a diagnosis of AKI1. Only 50% of patients were deemed to have had a ‘good” standard of care. The NCEPOD report identified inadequate assessment of patients at risk of AKI and deemed 60% of post-admission AKI was predictable and 21% was avoidable. Two thirds of patients had a significant level of AKI before a diagnosis was made and there was inadequate senior review of these patients. More severe AKI is associated with higher mortality. The in-hospital mortality rate for AKI has been reported at 24% and increases with more severe AKI2. In Northern Ireland, an audit of patients with severe AKI requiring dialysis demonstrated a 90 day mortality rate of up to 40% (personal communication) In 2013, the National Institute for Health and Care Excellence (NICE) published guidance on AKI3. NICE have calculated that if AKI was recognized and treated with attention to hydration and medication, 100,000 cases could be prevented and up to 42,000 deaths avoided annually. The National Clinical Director for Kidney4 care has stated that management of AKI can provide a barometer by which we can measure and improve the care of the acutely unwell patient whatever the underlying cause. There is clearly a need for all clinicians to be competent in managing this common condition. The aim of this Grand Rounds article is to provide practical information on prevention and management of AKI.
Definition of Aki
The term AKI has replaced that of Acute Renal Failure.
This is to recognize that there are varying degrees of kidney injury severity and to encourage early identification and management of AKI.
AKI is defined by an acute reduction in kidney function as identified by an increase in the serum creatinine and reduction in urine output.
The severity of AKI is reflected by the AKI stage5 AKI 1 - 3 (Table 1), with Stage 1 defined as a rise of serum creatinine of >26 umol/L or 1.5 to 1.9 times the baseline serum creatinine. Such minor serum creatinine elevations are associated with increasing mortality6 providing a rationale for the inclusion of this apparently small rise in serum creatinine in the AKI staging scheme.
Table 1.
Kidney Disease Improving Global Outcomes (KDIGO) staging classification for AKI
| Kidney Disease Improving Global Outcomes (KDIGO) staging classification for AKI | ||
|---|---|---|
| Stage | Serum creatinine (Scr) criteria | Urine output criteria |
| 1 | Rise in Scr of 26 umol/L within 48 hrs Increase of 1.5 – 1.9 x baseline Scr within past 7 days | < 0.5 mL/Kg/hr for > 6 consecutive hours |
| 2 | Increase of 2 - 2.9 x baseline Scr | < 0.5 mL/Kg/hr for > 12 consecutive hours |
| 3 | Increase of 3 x baseline Scr or Scr > 354 umol/L or Commenced on dialysis | |
| Additional RIFLE Criteria reflecting outcome of AKI | ||
| Loss | Need for ongoing dialysis for > 4 weeks | |
| Failure | Need for ongoing dialysis for > 3 months | |
There are also two outcome stages - Loss and End Stage Renal Disease (ESRD)7. These stages recognize that AKI can subsequently lead to chronic kidney disease (CKD) and ESRD requiring long term dialysis (Figure 1). Studies in the diabetic population8 have shown that episodes of AKI double the risk of patients developing Stage 4 CKD (estimated GFR 15 – 29 mL/min/1.73m2). Recent NICE Guidelines on CKD recommend monitoring of all patients who recover renal function following an episode of AKI for a minimum of 2 years to ensure early detection and management of CKD3.
Fig 1.

Potential outcomes following episode of Acute Kidney Injury
It is important to appreciate that the serum creatinine does not accurately reflect the Glomerular Filtration Rate (GFR) in a patient who is not in steady state. The serum creatinine is influenced by creatinine generation, volume of distribution and excretion. Thus a sudden fall in GFR is accompanied by a slow rise in serum creatinine which plateaus at between 7 and 10 days when creatinine production equals creatinine excretion. Large changes in GFR are initially manifested as small changes in serum creatinine. This may lead to a delay in recognizing the degree of AKI in a patient demonstrating an initial “minor” rise in serum creatinine. Similarly, during recovery from AKI, the serum creatinine may lag behind renal recovery.
E-Alert for Acute Kidney Injury
A national algorithm, standardizing the definition of AKI, has now been agreed for use in Northern Ireland. This has been integrated into the Regional Laboratory system Audit Implementation Network (GAIN www.gain-ni. org) Acute Kidney Injury Algorithm (Figure 3). This alert will automatically identify patients with AKI and enhance clinicians’ ability to recognize AKI and instigate early treatment.
Fig 3.
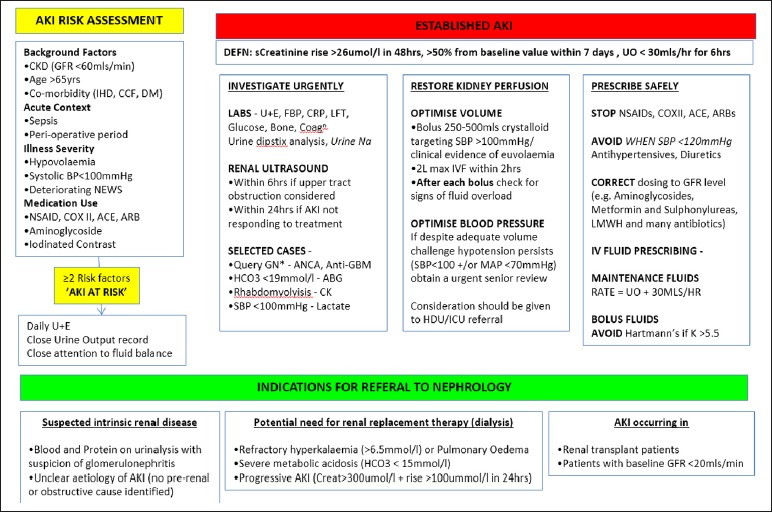
Northern Ireland GAIN AKI Algorithm
Fig 2.
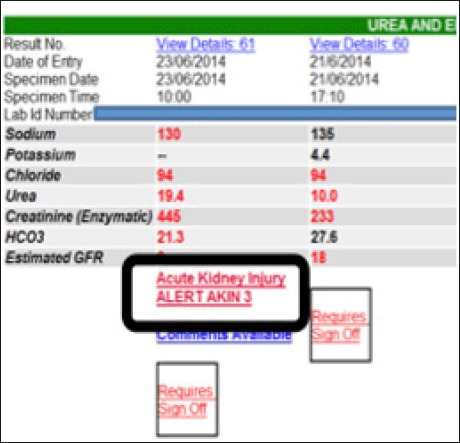
Example of AKI E-Alert
Causes of Aki
AKI is common with a recent study reporting an incidence of 25% in unselected medical admissions9. It is important to recognize that two thirds of episodes of AKI develop prior to the hospital admission. This affords an important opportunity to identify and prevent AKI in at risk patients.
For clarity the causes of AKI have been divided into three groups; Pre-renal, Intrinsic-renal and Post-renal. Table 2 lists some common causes.
Table 2.
Causes of AKI
| Pre-renal (hypoperfusion) | Intrinsic-renal | Post-renal Volume depletion |
|---|---|---|
| Volume depletion | Acute Tubular Injury | Bladder outlet obstruction |
| • Dehydration | Interstitial nephritis | Bilateral ureteric obstruction. |
| • Blood loss | Glomerulonephritis | Obstruction of a single functioning kidney. |
| Hypotension | Vasculitis | |
| • Sepsis | ||
| • Medications | ||
| • Cardiac failure |
In reality the majority of causes of AKI are not renal specific. The kidneys receive 25% of the cardiac output at rest are therefore sensitive to any systemic upset. In the largest trial of patients admitted to ICU with severe AKI10 the most common causes (often in combination) were septic shock (47%), post major surgery (34%), cardiogenic shock (27%), and hypovolaemia (26%). Deterioration in kidney function should provoke careful inspection for the common causes of haemodynamic compromise and sepsis throughout the body. Only when pre-renal causes have been excluded should specific attention return to consideration of intrinsic renal disease and renal tract obstruction.
Pathophysiology of Pre-Renal Aki and Acute Tubular Necrosis (ATN)
Well perfused, healthy kidneys will produce on average 180 L of glomerular filtrate per day, the majority of which is reabsorbed leading to a usual excretion of 1.5 - 2 L of urine. Production of this volume of filtrate is dependent on an adequate glomerular capillary pressure which is the driving force for filtration. Normal glomerular capillary pressure is maintained by afferent arteriole vasodilation and efferent vasoconstriction. This mechanism is known as renal autoregulation. The ability to maintain renal haemodynamics becomes impaired at a renal arterial pressure below 70 mmHg11. When this occurs the GFR will fall in proportion to further reduction in blood pressure. The GFR will cease when the renal arterial blood pressure is < 50 mmHg.
A reduction in renal perfusion due to hypotension results in prostaglandin mediated dilation of the afferent renal arteriole and constriction of the efferent glomerular capillary mediated by angiotensin II. However in patients with impaired autoregulation the GFR will fall even if the mean arterial pressure remains within the normal range.
Factors which increase susceptibility to renal hypoperfusion are listed in Table 3.
Table 3.
Factors increasing susceptibility to renal hypoperfusion
| Failure to decrease arteriolar resistance |
|
| Failure to increase efferent arteriolar resistance |
|
| Renal artery stenosis |
With prompt restoration of intravascular volume and blood pressure, normal renal haemodynamics can be restored resulting in complete recovery of renal function.
However, in the face of persisting hypoperfusion, endogenous vasoconstrictors increase afferent arteriolar resistance. Causes of a low perfusion state are shown in Table 4.
Table 4.
Causes of Renal Hypoperfusion
Hypovolaemia
|
Cardiac causes
|
Reduced peripheral vascular resistance
|
Local renal hypoperfusion
|
Renal hypoperfusion reduces glomerular capillary pressure and the GFR. The post-glomerular capillary bed which perfuses the tubules will also have diminished blood flow leading to a subsequent ischaemic structural injury to the renal tubules often referred to as ischaemic Acute Tubular Necrosis (ATN)12. This state is characterized by a rising serum creatinine and a reduced urine volume refractory to further increases in intravascular volume and renal perfusion pressure. Management of this state includes avoidance of fluid overload, maintenance of an adequate mean arterial pressure (> 65 mmHg), correction of electrolyte disorders (potassium) and treatment of the underlying precipitating condition. Such patients may require a temporary period of dialysis support.
AKI in hospitalized patients can be thought off as a two hit process. Susceptibility factors create an admission patient group vulnerable to a subsequent 'second hit’ - hypoperfusion events13. This can be seen in studies of AKI in critically ill patients10 where the median length of inpatient stay prior to the development of severe AKI is 5 days. This delay is an opportunity to both prevent and reduce the severity of AKI in hospitalized patients.
Prevention of Aki
Community Acquired
Patients in the community with CKD (eGFR < 60 mL/ min/1.73m2) and patients with normal renal function who are treated with an ACE inhibitor (ACEi) or angiotensin receptor blocker (ARB) are at increased risk of AKI if they develop an illness associated with hypovolaemia and hypertension. Such patients can be identified, educated and issued with a Kidney Care Card (Figure 4). This provides instructions for temporary cessation of certain medications, which may in this setting, induce, exacerbate and complicate AKI. These drugs can be remembered by the mnemonic DAMN (diuretics, ACEi/ ARBs, metformin, NSAIDs).
Fig 4.

Community Kidney Care Card
Hospitalised patients
Prevention of AKI should follow the following principles;
Risk Assessment
All patients, both on admission and during their hospital stay should be assessed regularly for their risk of developing AKI. Figure 5 gives an example of a risk assessment tool used in the Southern Health and Social Care Trust. NICE have identified the following patient groups (Table 5) who require special attention.
Fig 5.

Example of an admission AKI risk assessment tool
Table 5.
Patients at risk of AKI
| Age over 65 years |
| Existing CKD (eGFR < 60 mL/min/1.73m2), Previous episode(s) of AKI |
| Co - Morbidity (Cardiac / Liver failure, Diabetes Mellitus |
| Use of nephrotoxic drugs (Diuretics, ACEi/ARBs, NSAIDs) |
| Diagnosis of sepsis |
| Hypovolaemia / Hypotension / Oliguria (< 0.5 mL/kg/hr) |
| Deteriorating Early Warning Scores |
| Symptoms / history or condition that may lead to urinary tract obstruction |
| Use of iodinated contrast agents within the previous week |
Perioperative AKI is common. Recognition of patients who are at risk will allow measures to be undertaken to reduce exposure to renal insults and maximize renal recovery should AKI occur14. An similar risk assessment tool for use in Surgical patients is shown in Figure 6.
Fig 6.

Example of a Surgical AKI risk assessment tool
Optimisation of fluid balance
Fluid volume status should be carefully assessed with respect to both fluid depletion and fluid overload. Patients at risk of dehydration due to prohibited or poor oral intake should be prescribed maintenance IV fluids.
Optimisation of blood pressure
Hypotension systolic blood pressure (SBP) <110 mmHg / mean arterial pressure (MAP) < 65 mmHg) needs urgent assessment and treatment with IV fluid challenges and vasopressor agents where indicated.
Medication review
Temporary cessation of ACEi and ARBs is appropriate in patients with dehydration, hypotension (systolic blood pressure < 110 mmHg) and / or deteriorating renal function. In patients who continue to be prescribed ACEi or ARBs, alteration of timing of drug prescription to 6 pm will allow adequate time to assess clinical state and review their renal function in case there is a need to temporarily hold these medications.
Where clinically indicated aminoglycosides can continue to be used paying careful attention to renal function and drug levels.
Reducing the risk of contrast induced AKI
AKI secondary to radiological contrast media typically occurs within 72 hrs of receiving such agents. The risk of contrast nephropathy can be reduced by temporary cessation of potentially nephrotoxic medication and adequate volume expansion (Table 6)
Table 6.
Prevention of Contrast Nephropathy
| Identify risk | • eGFR < 30 mL/min/1.73m2 • eGFR 30 - 60 mL/min/1.73m2 and risk factors (Table 5) |
| Manage risk | • Hydration - IV 1.4% sodium bicarbonate / 0.9% saline at 3 mL/kg/hr 1hr pre and 1 mL/kg/hr for 6hrs post procedure • Omit potentially nephrotoxic medications (ACEi/ARBs / NSAIDs / metformin on day of procedure and do not restart until renal function stable at 48 - 72 hrs • Use low osmolar agents in the lowest dose. • Recheck renal function 48 - 72 hrs following the procedure |
Assessment of Aki
Clinical assessment
This should begin with a search for the cause of AKI focusing on clinical evidence of hypoperfusion states (volume depletion and hypotension) and urinary tract obstruction. AKI in the setting of resistant hypotension suggests significant underlying sepsis, the source of which may not be immediately apparent especially in cases of intra-abdominal sepsis.
Intrinsic renal disease in the form of vasculitis may present with a typical rash, uveitis and / or arthropathy. Intrinsic renal disease should always be considered where AKI is occurring in the absence of significant dehydration, hypotension and obstruction.
The effect of AKI should also be assessed, paying particular attention to evidence of volume overload - often manifest by peripheral and pulmonary oedema and increasing oxygen requirements. In severe AKI (Stage 3) pericarditis and encephalopathy may be present.
Dipstick urinalysis is part of the clinical assessment and should be done as soon as urine is available for testing. Hypoperfusion states are suggested by a raised urine specific gravity (> 1.020). In the absence of haemodynamic upset and sepsis, the presence of blood and protein in the urine should prompt consideration of a vasculitis / glomerulonephritis rather than a urinary tract infection.
Laboratory and Radiological Investigations
The GAIN AKI Algorithm (Figure 3) lists the required investigations.
In patients suspected of underlying sepsis, a serum lactate and arterial blood gas are essential in defining the severity of the metabolic upset.
Where there is no obvious precipitating factor for AKI, a renal immunology screen (including antineutrophil cytoplasmic antibodies (ANCA), anti-glomerular basement membrane antibodies (anti-GBM), serum electrophoresis and serum free light chains) should be sent urgently as the clinical management of such cases requires prompt specialist treatment.
In addition the finding of AKI, anaemia and thrombocytopenia with a normal coagulation profile should prompt a search for haemolysis (blood film, haptoglobulins) which occurs in Haemolytic Uraemic Syndrome, malignant hypertension, scleroderma and pre-eclampsia.
A chest x-ray can provide both evidence of a cause (pneumonia, pulmonary shadowing in vasculitis) and also help evaluate potential volume overload.
Renal tract ultrasound should be done within 6 hours where obstruction is considered. The presence of bilateral hydronephrosis and an empty bladder will require an urgent non-contrast CT scan to identify retroperitoneal obstruction to the ureters.
The presence of AKI should not prevent the use of contrast enhanced CT scanning to diagnose the source of sepsis, especially if a surgically remediable condition (intraabdominal abscess, ischaemic bowel) is being considered.
Management of Established Aki
Restore Renal Perfusion
As the majority of cases of AKI occur in association with volume depletion and sepsis, it is essential to restore effective renal perfusion as soon as possible. This will allow early recovery of renal function and help to avoid the development of acute tubular necrosis.
Optimise intra-vascular fluid volume
Volume status should be carefully assessed and an attempt should be made to categorise the patient into one of three states; hypovolaemic, euvolaemic or hypervolaemic.
Hypovolaemic patients may have clinical signs of dehydration, are oliguric (urine output < 30 mL/hr) often with a concentrated urine (Specific Gravity > 1.020)
There is no gold standard to definitively identify dehydration. However, hypotension (SBP < 110 mmHg), a postural fall in blood pressure with increase in heart rate, reduction in peripheral perfusion / skin turgor and dry mucous membranes are indicative signs.
Hypovolaemia should be promptly corrected with repeated boluses of 250 - 500 mL of crystalloid up to an initial total of 2 litres over 2 hours.
Hartmann's solution or 0.9% sodium chloride solution should be used. Hartmann's solution contains a small amount of potassium (5 mmol/L) and should be avoided in patients with significant hyperkalaemia (Potassium > 6 mmol/L). Large volumes of 0.9% sodium chloride can provoke a hyperchloraemic metabolic acidosis.
Failure of the patient to maintain an effective blood pressure following this regime should raise the possibility of underlying sepsis or significant ongoing losses. Both these latter scenarios require senior assessment rather than continuing to prescribe increasing large volumes of fluid in the face of poor urine output. Fluid accumulation resulting in a positive fluid balance is a frequent event in critically ill patients with AKI. Such fluid accumulation (> 10% fluid weight gain) is independently associated with increased mortality15 fails to improve renal function and is associated with worsening respiratory function.
Euvolaemia is characterised by an absence of clinical signs of dehydration, haemodynamic stability and an absence of volume overload. Oliguria in this context often reflects established ATN and will not respond to increasing fluid challenges, which put the patient at risk of fluid overload. In this phase, recovery of an adequate urine output is impossible to predict. Fluid intake should be restricted to a match daily output / losses. For patients who require ongoing maintenance IV fluids, one regime is to prescribe hourly crystalloid at a rate of the previous hours urine output + 30 mL.
Patients should be carefully assessed for signs of hypervolaemia. Features may include a raised JVP, peripheral and pulmonary oedema (clinically and radiologically). Calculation of total fluid balance since admission should alert clinicians to the potential of fluid overload. In the presence of AKI, hypervolaemic patients are vulnerable to pulmonary oedema and should be fluid restricted. There is no definitive evidence that the use of loop diuretics alters outcome in such patients. However, it is appropriate to consider a short trial of loop diuretics in patients with features suggestive of pulmonary oedema provided the patient has a reasonable perfusion pressure (MAP > 65 mmHg, SBP > 110 mmHg). Failure to respond is an indication for urgent haemodialysis and ultrafiltration.
Optimise Blood Pressure
Blood pressure is key to driving ultrafiltration at the glomerulus. Within the glomerulus the systemic blood pressure creates a hydrostatic pressure of 70 mmHg. This is opposed by the colloid oncotic pressure of 30 mmHg and hydrostatic back pressure from the tubules (20 mmHg). The net filtration pressure which drives the production of up to 180 litres of glomerular filtrate is only 20 mmHg.
Absolute hypotension (defined as a SBP < 90 mmHg) has been shown to be associated with the development of AKI following sepsis and major surgery16. However, relative hypotension (where there is a decrease in BP from pre-morbid levels in the absence of overt hypotension) has been shown to be an independent contributor to the development of AKI in elderly patients17. Maintenance of SBP according to pre- morbid values may play an important role in preventing kidney injury in hospitalised patients.
In patients with AKI and hypotension, blood pressure should be targeted to a MAP of > 65 mmHg. This can be achieved by three interventions.
Withholding drugs that interfere with renal autoregulation (ACEi / ARBs). Temporary cessation of all drugs that induce hypotension. This includes antihypertensives, diuretics, and agents such as nicorandil and opiates.
Correction of hypovolaemia as described above.
Consideration of vasopressor therapy (i.e. noradrenaline) in patients refractory to adequate correction of hypovolaemia. Vasopressors should be considered early in such patients to avoid needless fluid overload. Patients should be clearly identified as being suitable for vasopressor therapy and referred to Critical Care Teams. It should be stressed that there is no evidence for a role for “renal dose” dopamine in the management of AKI. In addition, dobutamine has significant vasodilatory effects which can aggravate hypotension and worsen renal perfusion in patients with sepsis and AKI.
Prescribe Medicines Safely
Patients who develop AKI require revision of all prescribed medications.
Drugs interfering with renal perfusion
These include those medications interfering with renal autoregulation (ACEi/ARBs, NSAIDs) and those medications with the potential to reduce blood pressure. Antihypertensive medications (including diuretics) should be withheld in patients with both absolute (SBP < 90 mmHg) and relative (SBP < 120 mmHg) hypotension. Patients treated with beta blockers need careful consideration of the risk / benefit of temporary cessation.
Drugs requiring dose reduction or cessation
All medications that are metabolized and excreted by the kidneys should be dose adjusted for an assumed eGFR of < 10 mL/min/1.73m2. Such drugs include fractionated heparins, opiates, penicillin-based antibiotics, sulphonylurea-based hypoglycaemic agents, and aciclovir. Although metformin is not specifically nephrotoxic, it will accumulate in renal failure and is associated with life threatening lactic acidosis.
Drugs requiring close monitoring
These include warfarin and aminoglycosides. Gentamicin in particular demands careful consideration. It should not be withheld where there is a clear benefit to its use (life threatening sepsis). If used, the daily trough level should be < 1 mg/L.
Drugs aggravating hyperkalemia
All drugs which block renal excretion of potassium (trimethoprin and potassium sparing diuretics (spironolactone, amiloride) should be stopped. In addition, both beta-blockers and digoxin can inhibit the sodium / potassium ATPase pumps which move potassium inside cells. The presence of these drugs can render the patient resistant to insulin/glucose treatment of hyperkalaemia.
Referral criteria to a Nephrology Team
Whilst AKI is common, less that 10% of such patients require direct care by Nephrologists2. Studies show that less than 4% of hospitalized patients with AKI are treated with dialysis2.
The indication for dialysis is based on the complications of AKI rather than an absolute value for serum urea, creatinine or GFR. There is no clear benefit for undertaking dialysis solely on the basis of a low GFR. Instigation of dialysis carries with it risk (access issues, haemodynamic instability) and can delay the identification of recovery of independent renal function.
The Northern Ireland GAIN guidelines18 recommend referral of the following groups of patients (Table 7).
Table 7.
GAIN AKI referral guidelines.
| Referral Indication | Comments |
|---|---|
| Complications of AKI requiring dialysis | Refractory hyperkalaemia, pulmonary oedema. Severe metabolic acidosis due to kidney failure (pH < 7.2). Uraemic pericarditis and encephalopathy. |
| Suspicion of a diagnosis that may require specialty Nephrology treatment | For example; vasculitis, myeloma, interstitial nephritis or glomerulonephritis. |
| AKI occurring in patients with CKD | Stage 4 or 5 CKD (eGFR ≤ 30 mL/min/1.73m2) |
| AKI occurring in renal transplant patients | Complex interactions with immunosuppressive medications. Infection can provoke acute rejection. |
Nephrology - Critical Care interface
Many patients develop AKI in the context of multiple organ failure. They often manifest hypotension, severe metabolic acidosis and hypoxia due to pulmonary oedema. Such patients may require mechanical ventilation and vasopressor therapy to support renal replacement. This level of care is best delivered in an Intensive Care Unit rather than on a Renal ward and early involvement of the Critical Care team should be sought.
Nephrology - Conservative care interface
It should also be recognized that AKI may reflect a terminal event in a hospitalised patient. Such patients usually have suffered a very severe episode of illness complicating the progression of advanced, untreatable co-morbidity.
In such patients, provision of dialysis therapy is futile, is likely to prolong suffering and lead to false hopes of survival.
Senior medical staff should identify these patients early in the course of their deterioration an if necessary discuss with the Nephrology team the ceiling of care for renal support.
Two Scenarios
Case 1:
A 78 year old woman is admitted to the surgical ward with a left iliac fossa pain and a clinical suspicion of acute diverticulitis. She has a background of CKD (eGFR 35 mL/ min/1.73m2), hypertension treated with perindopril and type 2 diabetes treated with metformin. One week before admission she developed dysuria and was empirically prescribed trimethoprim. Despite a poor oral intake during the week she continued to take all of her medication. On admission she was febrile (38oC), hypotensive (BP 80/50 mmHg), and heart rate 120 bpm. Oxygen saturation was 96% on room air. Urine output was 5 - 10 mL/hr. Investigations reveal; stage 3 AKI (creatinine 480 umol/L), hyperkalaemia - Potassium 7.1mmol/L, acidaemia (pH 7.25) with a high anion gap metabolic acidosis, elevated plasma lactate (8 mmol/L), CRP 235. She was treated with IV Tazocin® / gentamicin and insulin/glucose for her hyperkalaemia. Over the next 24 hr despite 6 L of Hartmann's solution she remained hypotensive and oliguric. Her serum creatinine rose to 650 umol/L. She became increasingly hypoxic with radiological evidence of pulmonary oedema and was transferred to ICU for mechanical ventilation, vasopressor support and dialysis. A CT scan of abdomen demonstrated a perforated sigmoid diverticulum with generalized peritonitis. She underwent a left hemicolectomy but 2 days later whilst still vasopressor dependent in ICU suffered a cardiac arrest from which she did not recover.
Learning points:
Prevention: This lady was at extremely high risk of developing AKI in the community. She has pre-existing CKD, is elderly with significant co-morbidity and is treated with an ACEi. Such patients should be identified with from the GP register, provided with a Kidney Care Card and counseled to temporarily stop potentially nephrotoxic drugs (including metformin) during periods of poor oral intake. In addition, trimethoprim should be used with caution in patients with Stage 4/5 CKD due to its potential to cause hyperkalaemia.
Treatment: It is essential to restore an effective blood pressure within the first 4 hr of hospital admission. Failure to achieve an adequate BP (MAP > 65 mmHg) despite an initial rapid infusion of up to 2 L of crystalloid is a marker of illness severity. In the absence of obvious fluid / blood loss or cardiogenic shock, such patients should be regarded as being in septic shock and should be referred to the Critical Care team for consideration of vasopressor therapy. In this case additional fluids failed to restore an effective perfusion pressure, failed to improve renal function and contributed to the development of pulmonary oedema. Finally this case demonstrates the high mortality associated with AKI where the renal insult is simply a talisman for serious underlying pathology.
Case 2:
A 60 year old man is admitted to a medical ward with a 2 week history of cough, arthralgia and reduced appetite. He had been prescribed doxycycline and a NSAID one week previously. He had a background of type 2 diabetes and hypertension treated with ramipril. On admission he was febrile (37.5oC), hypoxic (oxygen saturations 92% on 40% oxygen). His BP was maintained at 124/80 mmHg. His white cell count was elevated at 16.9 and CRP was 279. His serum creatinine was 250 umol/L. An initial urinalysis was reported as ‘clear’. Chest X-Ray showed multiple nodular opacities. Despite treatment with antibiotics, his renal function continued to deteriorate (serum creatinine 570 umol/L by day 3). A repeat urinalysis demonstrated 4+ blood and 3+ protein. A subsequence vasculitis screen was positive (cANCA 120, PR3 > 8). His presentation was consistent with a diagnosis of Granulomatosis with Polyangiitis (previously known as Wegener's Granulomatosis) and he was transferred to Nephrology. He was empirically treated with pulse methyl prednisolone and IV cyclophosphamide. A subsequent renal biopsy confirmed vasculitis. Two months after presentation his GFR was 50 mL/min/1.73m2 and his pulmonary nodules had resolved.
Learning Points:
This patient was initially suspected to have AKI due to a combination of pneumonia and concomitant NSAID and ACEi therapy. However there were three key pointers to the possibility of an intrinsic renal insult
The lack of a hypotensive insult during his hospital admission.
The presence of dipstick blood and protein.
The failure to improve despite appropriate antibiotic therapy targeted against pneumonia.
In patients who develop AKI apparently out of proportion to the clinical insult it is important to validate the urinalysis findings. The presence of dipstick positive blood and protein should suggest a glomerulonephritis / vasculitis and an urgent renal vasculitis screen is mandated. The presence of a positive vasculitis serology in the appropriate clinical context will allow for commencement of immunosuppressive therapy prior to obtaining histological confirmation maximizing the chance of renal recovery.
Glossary
- ACEi
Angiotensin Converting Enzyme inhibitors
- AKI
Acute Kidney Injury
- ARB
Angiotensin Receptor Blockers
- ATN
Acute Tubular Necrosis
- BP
Blood Pressure
- CKD
Chronic Kidney Disease
- eGFR
estimated Glomerular Filtration Rate
- ESRD
End Stage Kidney Disease
- GAIN
Guidelines and Audit Implementation Network
- GFR
Glomerular Filtration Rate
- MAP
Mean Arterial Pressure
- NCEPOD
National Confidential Enquiry into Patient Outcome and Death
- NICE
National Institute of Clinical Excellence
- NSAIDs
Non Steroidal Antiinflamatory Drugs
- SBP
Systolic Blood pressure
References
- 1.Stewart J, Findlay G, Smith N, Kelly K, Mason M. A review ofthe care of patients who died in hospital with a primary diagnosis of acute kidney injury. London: National Confidential Enquiry into Patient Outcome and Death; 2009. Adding insult to injury. pp. 11–75. p. Available online from: http://www.ncepod.org.uk/2009report1/Downloads/AKI_report.pdf. Last accessed July 2014. [Google Scholar]
- 2.Selby NM, Crowley L, Fluck RJ, McIntyre CW, Monaghan J, Lawson N, et al. Use of electronic results reporting to diagnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephrol. 2012;7(4):533–40. doi: 10.2215/CJN.08970911. [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence. NICE clinical guideline 169. London: NICE; 2013. Acute kidney injury. Prevention, detection and management of acute kidney injury up to the point of renal replacement therapy. Available online from: http://www.nice.org.uk/guidance/CG169/chapter/introduction. Last accessed July 2014. [Google Scholar]
- 4.Hawkes N. News. Acute kidney injury is a more important safety issue than MRSA, says NICE. BMJ. 2013;13(347):f5302. doi: 10.1136/bmj.f5302. [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease Improving Global Outcomes (KDIGO). KDIGO Clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. Available online from: http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO%20AKI%20Guideline.pdf. Last accessed July 2014. [Google Scholar]
- 6.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 7.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakar C, Christianson A, Himmelfarb J, Leonard A. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6(2):2567–72. doi: 10.2215/CJN.01120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Challiner R, Ritchie JP, Fullwood C, Loughnan P, Hutchison AJ. Incidence and consequence of acute kidney injury in unselected emergency admissions to a large acute UK hospital trust. BMC Nephrology. 2014;15:84. doi: 10.1186/1471-2369-15-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchino S1, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 11.Hall JE, Guyton AC, Jackson TE, Coleman TG, Lohmeirer TE, Trippodo NC. Control of glomerular filtration rate by rennin-angiotensin system. Am J Physiol. 1977;233(5):F336–72. doi: 10.1152/ajprenal.1977.233.5.F366. [DOI] [PubMed] [Google Scholar]
- 12.Rose BD. New York: McGraw-Hill; 1987. Pathophysiology of renal diseases. pp. 84–104. [Google Scholar]
- 13.Abuelo JG. Normotensive ischaemic acute renal failure. N Engl J Med. 2007;357(8):797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
- 14.Borthwick E, Ferguson A. Perioperative acute kidney injury: risk factors, recognition, management and outcomes. BMJ. 2010;341:c3365. doi: 10.1136/bmj.c3365. [DOI] [PubMed] [Google Scholar]
- 15.Bouchard J, Soroko SB, Chertow G, Himmelfarb J, Ikizler TA, Paganini EP, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76(4):422–7. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 16.Vivino G, Antonelli M, Moro ML, Cottini F, Conti G, Bufi M, et al. Risk factors for acute renal failure in trauma patients. Intensive Care Med. 1998;24(8):808–14. doi: 10.1007/s001340050670. [DOI] [PubMed] [Google Scholar]
- 17.Liu YL, Prowle J, Licari E, Uchino S, Bellomo R. Changes in blood pressure before the development of nosocomial acute kidney injury. Nephrol Dial Transplant. 2009;24(2):504–11. doi: 10.1093/ndt/gfn490. [DOI] [PubMed] [Google Scholar]
- 18.GAIN. Guidelines and Audit Implementation Network. Belfast: GAIN; Northern Ireland guidelines for acute kidney injury. Available online from: http://www.gain-ni.org/images/GAIN_-_AKI_-_Northern_ Ireland_Guidelines_for_Acute_Kidney_Injury_PDF.PDF. Last accessed July 2014. [Google Scholar]


