Supplemental Digital Content is available in the text.
Abstract
Background:
Preoperative signs and symptoms of patients with Poly Implant Prothese (PIP) implants could be predictive of device failure. Based on clinical observation and intraoperative findings 4 hypotheses were raised: (1) Preoperative clinical signs including acquired asymmetry, breast enlargement, fullness of the lower pole, decreased mound projection, and change in breast consistency could be indicative of implant rupture. (2) Device failure correlates with a low preoperative Baker grade of capsule. (3) Brown-stained implants are more prone to implant failure. (4) The brown gel could be indicative of iodine ingression through a substandard elastomer shell.
Methods:
Preoperative clinical signs were compared with intraoperative findings for 27 patients undergoing PIP implant explantation.
Results:
Acquired asymmetry (P = 0.0003), breast enlargement (P = 0.0002), fuller lower pole (P < 0.0001), and loss of lateral projection (P < 0.0001) were all significantly predictive of device failure. Capsule Baker grade was lower preoperatively for ruptured implants. The lack of palpable and visible preoperative capsular contracture could be secondary to the elastic nature of the capsular tissue found. Brown implants failed significantly more often than white implants. Analysis of brown gel revealed the presence of iodine, suggesting povidone iodine ingression at implantation.
Conclusions:
Preoperative signs can be predictive of PIP implant failure. Brown-stained implants are more prone to rupture. The presence of iodine in the gel suggests unacceptable permeability of the shell early in the implant’s life span. A noninvasive screening test to detect brown implants in situ could help identify implants at risk of failure in those who elect to keep their implants.
The NHS Wales Guidelines on Poly Implant Prothese (PIP, France) breast implants1 has given rise to a cohort of female patients who can select a management plan based on their informed decision without limitations of personal funds impacting on their choice. The patient can elect for implant explantation, explantation and implant exchange, or diagnostic ultrasound scan (USS) and annual follow-up if the implants are deemed clinically intact.
This study outlines how the clinical presentation of a failed device, defined as either rupture or heavy gel bleed, can give rise to a different set of signs and symptoms than those attributed to implant rupture in the national guidelines.1–3 These manifestations are sufficient to raise suspicion of implant failure and can be detected by both patients and surgeon. No ruptured PIP implant presented as completely “silent” as suggested in clinical guidelines. Patients with PIP implants should be taught to recognize these signs if they elect to keep their implants in situ.
PATIENTS AND METHODS
The 27 patients in this report attended consultation during a 5-month period between October 2012 and February 2013. Their management was based on their symptoms and USS results as outlined by NHS Wales guidelines.1 Each consultation was the first patient assessment following recommendations issued by the Department of Health in January 2012.4 Patients with scan-proven implant rupture were advised to have explantation with the option of concurrent reaugmentation if it was clinically safe to do so. All other patients had the choices of explantation, reaugmentation with “like for like” silicone gel implants or to keep their PIP implants and undertake annual monitoring by examination and USS. Preoperative signs and symptoms, noted by patient and surgeon, were compared with intraoperative findings.
We defined preoperative signs of “soft rupture” as outlined in Table 1. These are compared to indicators listed in the National Guidelines for device failure. Device failure is defined as rupture or significant gel bleed.5
Table 1.
Signs and Symptoms Proposed in National Guidelines for Implant Rupture or Severe Gel Leak Compared to Those Defined by “Soft Rupture”
The first author recorded preoperative findings and performed the surgery. The patients’ preoperative appearance and the state of the explanted prostheses were recorded photographically. The preoperative capsule was categorized using Baker’s classification.6,7 Only acquired breast asymmetry subsequent to PIP augmentation was recorded. Existing breast asymmetry was present in 3 patients before PIP augmentation. Differential augmentations had been performed with a 20-cm3 volume difference in 2 cases and a 40-cm3 difference in 1 case.
At operation, the original inframammary scar was used in all cases. Chlorhexidine solution was used to prepare the surgical field in the case where gel samples were taken for analysis. The remit of NHS Wales was to remove PIP implants and replace as required. Correction of aesthetic flaws subsequent to long-term changes of breast augmentation could not be funded. All new implants were placed using the existing breast pockets. Capsulectomy was only performed where absolutely necessary. No mastopexies were undertaken.
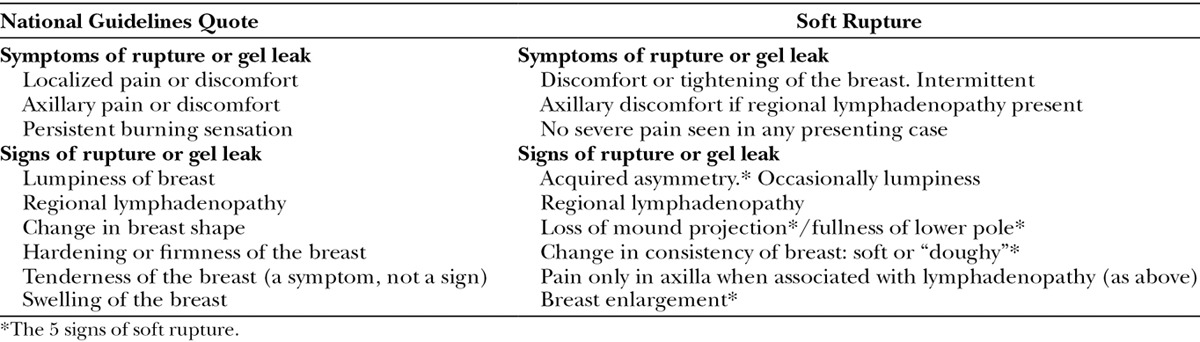
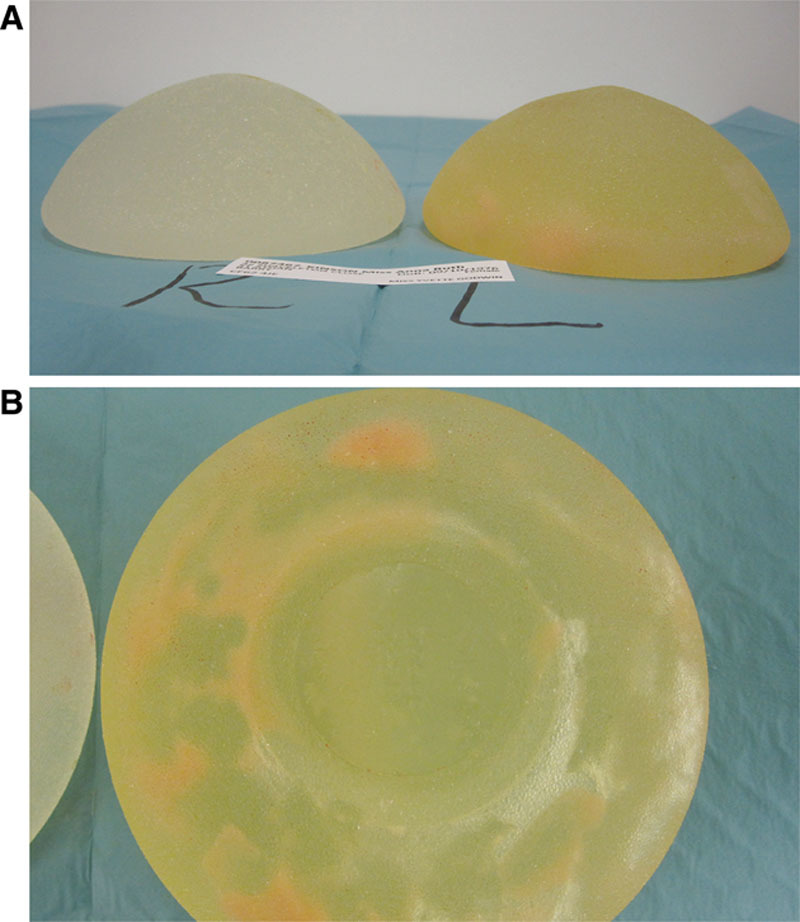
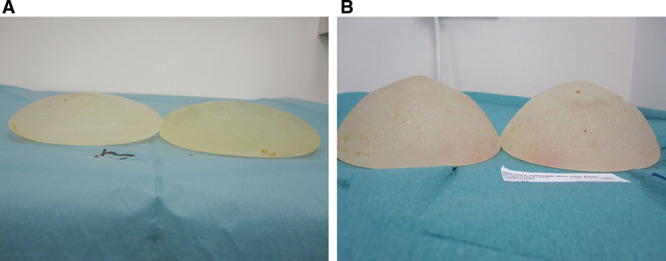
Intraoperatively, the capsule thickness was graded subjectively as mild, moderate, or severe. The breast pocket was examined for granuloma formation. The state of the implant was documented including the degree of gel bleed, the integrity of the device (ie, ruptured or intact), and the implant gel color as white or brown (Fig. 1). The profile of intact implants was assessed for loss of projection due to gel bleed (Fig. 2). The amount of intracapsular exudate was subjectively graded and its nature as cloudy or white silicone peroxidation was recorded (Fig. 3).
Fig. 1.
A, Color difference between white- and brown-stained implants removed from the same patient. B, The brown implant contains white intraluminal exudate (silicone peroxidation).
Fig. 2.
A, Explanted PIP implants associated with severe gel bleed and loss of ultrahigh profile. B, Explanted ultrahigh PIP implants with minimal gel bleed and maintained projection.
Fig. 3.
Cloudy exudates around a ruptured implant (A) and white silicone peroxidation firmly adherent to the pectoral fascia (B).
Intraoperative bacterial swabs were taken of all breast pockets. Histology was performed on 2 samples of thick capsules and 1 chest wall granuloma.
The determination of iodine in the sample was performed by leaching with 5% (w/w) tetramethylammonium hydroxide followed by analysis of leachates using inductively coupled plasma mass spectrometry. Further details on the methodology used can be found in the supplementary supporting information.
Fisher’s exact test was used to determine the statistical significance of all associations, and odds ratios (ORs), 95% confidence intervals (CIs), and 2-sided P-values are reported. Observations are not significant unless specifically stated. Chi-square test was used only for the association of white/brown implants with the amount of gel bleed.
RESULTS
Table 2 outlines the demographics of the 27 patients. All 27 patients were elected for explantation and reaugmentation. When the patient choice of management is not constrained by personal funds, 89% of patients attending clinic elected for explantation and reaugmentation with only 11% of patients electing to keep their PIP implants and be monitored.
Table 2.
Demographics and USS Results of 27 Patients Who Underwent Removal of Their PIP Implants
Table 3 outlines the preoperative signs and symptoms reported by the cohort. Neither pain nor discomfort was a marked symptom. Pain was principally associated with axillary lymphadenopathy. Only 3 patients reported intermittent, mild pain in the breast mound. Acquired breast asymmetry and loss of mound projection were the 2 principal signs noted by the patients in the presence of implant failure.
Table 3.
Symptoms and Signs Reported Preoperatively by 27 Patients with PIP Breast Implants Compared with Percentage Device Failure at Explantation
Table 4 summarizes preoperative signs noted by the surgeon. Breast asymmetry (OR, 43.33; 95% CI, 3.895–482.1; P = 0.0003) and loss of mound projection (OR, 17.5; 95% CI, 4.248–72.08; P < 0.0001) were common in patients with device failure. Additional signs linked to device failure included breast enlargement (OR, 27; 95% CI, 3.063–238; P = 0.0002) and inferior pole fullness (OR, 31; 95% CI, 6.522–147.4; P < 0.0001) on the affected side. These 2 latter signs were identified by the surgeon but not noted by the patients (see Supplemental Digital Content 1, which demonstrates 4 examples of soft rupture, http://links.lww.com/PRSGO/A57).
Table 4.
Preoperative Signs Noted by the Surgeon on Examination of 27 Patients with PIP Breast Implants and Correlation of Signs with Intraoperative Condition of the Implant
The average (mode) Baker grade was II for patients with intact implants compared with I for breasts found to have implant rupture. The Baker grade of capsule is hence lower when the capsule is in contact with free silicone gel. The affected breasts were soft, without contracture or distortion, on the ruptured side. The majority of cases had submammary breast pockets (22 of 27), so capsular contracture should have been more prevalent.
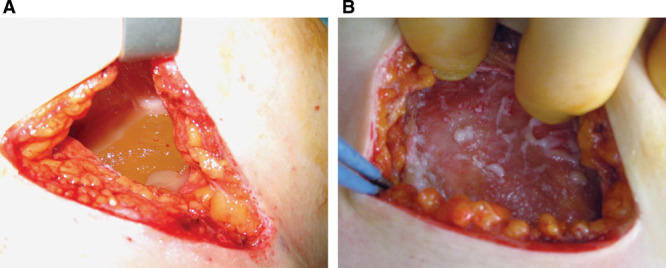
Supplemental Digital Content 2 (http://links.lww.com/PRSGO/A57) compares preoperative signs recorded by the surgeon with the intraoperative findings in the 10 patients with ruptured implants. Rupture was bilateral in 2 patients and unilateral in 8 patients. Twelve of the 54 implants removed showed macroscopic rupture. In 10 of 12 implants, the elastomer shell had completely disintegrated (Fig. 4). Two implants exhibited minor tears. Palpable axillary lymphadenopathy was confirmed by the surgeon preoperatively in 6 patients with ruptured implants. Only 3 patients were aware of their lymphadenopathy. At least 2 out of the 5 clinical signs of soft rupture were present in all patients with proven implant rupture (on average, 4 signs per patient). The majority of ruptured implants demonstrated brown staining (10 of 12). The surgeon noted breast asymmetry and loss of project in the majority of patients with ruptured implants. Intraoperative capsule thickness was described most commonly as “mild.” All intraoperative capsules were pliable and elastic (Fig. 5), with no cases of capsular calcification.
Fig. 4.
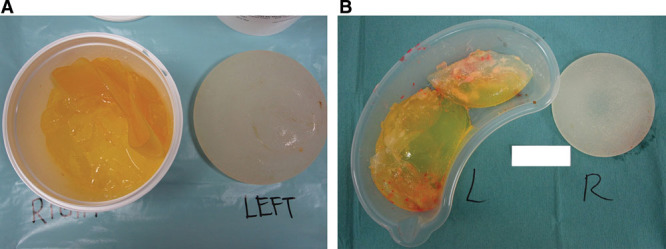
A and B, Implant rupture with complete disintegration of the elastomer shell, and in both cases, the gel is stained brown. Image B shows white silicone peroxidation.
Fig. 5.
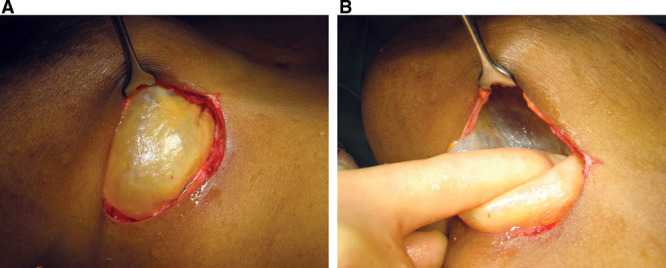
A, Appearance of an intraoperative capsule. B, Digital pressure demonstrates the pliable, elastic nature of the capsule. No calcification is present.
Breast enlargement was noted in all cases where free intracapsular fluid was present either from implant disruption or severe bleed with exudate formation. White silicone peroxidation was present in 14 breast pockets: in 1 case, this was intraluminal, and 9 of 14 cases were associated with ruptured implants (Fig. 1).
The intraoperative state of the implant and clinical implications were evaluated. Seventeen of the 54 implants removed were stained brown and 37 were white. Ten brown and 2 white implants were ruptured. Three of 17 brown and 5 of 37 white implants showed heavy gel bleed. Brown implants are more likely to be associated with rupture than white implants (OR, 25; 95% CI, 4.468–139.9; P < 0.0001). White implants show less tendency to gel bleed compared with brown implants (P = 0.0003) and hence keep their high profile projection (OR, 8.615; 95% CI, 2.086–35.58; P = 0.0028) when compared with brown implants. Implants associated with heavy gel bleed or rupture (device failure) were more likely to present with thinner intraoperative capsules (OR, 0.1364; 95% CI, 0.03708–0.5015; P = 0.002). Lymphadenopathy is associated with a higher incidence of implant failure (OR, 33; 95% CI, 3.751–290.3; P < 0.0001).
Implant failure and capsular thickness were not associated with bacterial contamination. All intraoperative breast pocket bacterial swabs were negative. Histologically, intraoperative capsules and granulomas showed the presence of foamy macrophages, multinuclear giant cells within fibrovascular tissue.
The presence of iodine in a ruptured and brown-stained PIP implant was determined by inductively coupled plasma mass spectrometry (see Supplemental Digital Content 3, which displays a mass spectrometry report, http://links.lww.com/PRSGO/A57). The results indicated that iodine was present at considerably high concentrations in brown-stained implants compared to previously reported values for “off-the-shelf” PIP implants.5 It would appear that the presence of iodine is specific to the brown implant removed.
DISCUSSION
Nonmedical silicone gel filler was used in the manufacture of PIP breast implants.4,5,9 The shell elastomer is considered substandard secondary to variable thickness and possibly the omission of the antibleed, fluorinated barrier layer.9–11 PIP implants carry an increased risk of implant failure compared with other implant brands.5,10,12–15 Unacceptably high prevalence of rupture has been reason enough for prophylactic explantation of all PIP implants in some countries.11,13
Due to the inert nature of silicone, rupture has been previously defined as a harmless event that does not produce significant clinical symptoms or activate the humoral immune system, with explantation viewed as mandatory only if silicone migration occurs.16 This criteria cannot be applied to PIP implants due to the scandal surrounding the gel filler. Unscrupulous human behavior during the manufacture of PIP implants means the content of these prostheses cannot be absolutely guaranteed irrespective of testing off-the-shelf samples. The risks associated with tissue being in contact with a filler of unknown origin cannot be predicted although the nonmedical gel filler is believed to not represent a risk to human health.15 Immune responses have been reported.8,9,17 Very little analysis to date has taken place of “used” or explanted implants.9 Patients with asymptomatic device failure carry the gel content in direct contact with the breast parenchyma and may not present for assessment.
Clinically, implant integrity is assessed by history, examination, and diagnostic scan.18 Despite large cohort studies documenting PIP premature failure, there is little documentation of the preoperative clinical signs that are specific to PIP rupture.10,12–14 We have attempted to identify subtle clinical signs and defined them as “soft rupture.” They differ from those quoted in national guidelines and do relate to intraoperative findings in each specific patient.1–3 Detection of these clinical signs could raise the suspicion of rupture early rather than assuming that asymptomatic patients can be left to “watch and wait.” USS can then be applied to support or refute clinical suspicion of rupture.13,14,16,19
The breast consistency changes in soft rupture, becoming soft or doughy. Softening has been correlated with implant failure in previous studies16,18,20 but is not commonly quoted in guidelines as a sign of rupture. Breast enlargement, fullness in the lower pole, and loss of mound project result as free gel, either from bleed or rupture, is no longer constrained within the elastomer envelope. Free gel sitting in the lower pole of the breast pocket with gravity creates a “bottomed out” deformity. The Baker grade of capsule is low in contrast to other implant brands. The capsule constituted a pliable bag containing free intracapsular silicone gel and exudate. The loss of the exaggerated projection of an ultrahigh PIP implant may be the only sign the patient notices on device failure.
Breast enlargement is secondary to intracapsular exudate formation and possibly inflammation of breast parenchyma. The presence of inflammatory cells in the capsule, soft-tissue granulomas, and exudate formation reinforces the suggestion of an immune response.
The nature of the exudate has been discussed in other reports.5,9,12,21 Silicone peroxidation, defined as white exudate, was associated with 9 of 12 cases of implant rupture. This can be part of the liquid exudate, firmly attached to soft tissues (Fig. 3B) or intraluminal (Fig. 1B). Cloudy exudate was commonly seen around implants with severe bleed and is thought to be a suspension of silicone in water5,9 (Fig. 3A). Exudate formation was not associated with an infective process, suggesting that this inflammatory response is secondary to allergens present in the free gel or elastomer shell.
Following implant failure, the capsules around PIP implants were elastic and pliable irrespective of thickness (Fig. 5). None in this series were calcified. Strong adherence to the breast tissue and pectoral fascia was common.22 Other studies have confirmed that PIP implants are not associated with high rates of capsular contracture when compared with other brands.4,5 Multiple causal factors have been implicated in capsule contracture, including the tendency for hypertrophic scar formation.23,24 Because PIP implants have a higher incidence of rupture and gel bleed, free liquid silicone is more likely to be found in direct contact with the capsule compared with other implants.5,10–14 Topical silicone gel reduces hypertrophic scar formation.25–28 The mechanism is not fully understood, but this may be by decreasing fibroblast proliferation.25 Free silicone in direct contact with the capsule may induce the soft, pliable capsules reported.
Brown staining of PIP implants has been noted in other studies,9,12 but the implications have not been evaluated. The integrity of the elastomer shell dictates the stability of the implant. Any defect will result in an increased incidence of device failure. We raised the hypothesis that brown staining of the implant could be a warning sign that the device is more prone to rupture or gel bleed secondary to elastomer envelope failure. If the brown color is due to iodine ingression at the time of primary surgery, then permeability and, hence, integrity of the implant elastomer have to be questioned at a very early stage in the implant’s life span. The analysis of gel content in this study suggests that iodine has permeated across the elastomer. Statistical analysis suggested that there is a higher rate of bleeding and rupture among brown implants. Brown staining has been attributed to ingression of biological fluids in vivo.9,12 The actual causal agent of the discoloration is not of importance. The main point is that the elastomer shell must be inappropriately permeable to allow the gel to stain, and stained implants rupture more commonly.
Outside Wales, a higher proportion of patients have elected to keep their PIP implants or have declined screening.10,14 This, in part, may be due to the fear of relinquishing implants if they are ruptured, and also, if the patients are asymptomatic, they may not appreciate the need for review. It would be beneficial to have a noninvasive diagnostic test that could identify brown implants in this population. Magnetic resonance imaging is not applicable, as no sequence is available that allows iodine detection. The application of dual-energy computed tomographic scanning for the potential detection of iodine needs further exploration.
CONCLUSIONS
We believe the symptoms of soft rupture can be clearly defined and should raise suspicion of device failure. These signs and symptoms could be added to current guidelines. A combination of recognition of symptoms of soft rupture and a diagnostic procedure to detect brown implants in situ may be a way forward to monitoring those who elect to keep their PIP implants.
ACKNOWLEDGMENTS
We thank the patients who participated in this study. We thank Professor Fiona Gilbert and Dr. Michael Middleton for their advice concerning radiological screening of PIP implants. We thank Dr. Suzie Howarth for her review of histological samples used in this study. We also thank the Department of Medical Photography at Morriston Hospital for their technical support.
Supplementary Material
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. A portion of the Article Processing Charge was paid for by PRS Global Open at the discretion of the Editor-in-Chief. The remainder of the Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.NHS Wales support for women with Poly Implant Prothèse (PIP) breast implants. Available at: http://www.wales.nhs.uk/polyimplantprothesepipsiliconebreastimplantsadvicetowomen. Accessed June 12, 2014.
- 2.PIP breast implants: Joint surgical statement on clinical guidance for patients, GPs and surgeons (Royal College of Surgeons of England) Available at: http://www.rcseng.ac.uk/publications/docs/PIP_statement_finalv2.pdf/view?searchterm=Statement+PIP+implants. Accessed June 12, 2014.
- 3.Poly Implant Protheses (PIP) Breast Implants: Interim report of the Expert group. Sir Bruce Keogh. Annex E: Clinical Guidance for GP’s and Surgeons. Available at: http://www.nhs.uk/news/2012/01january/documents/pip-report.pdf. Accessed june 12, 2014.
- 4.MHRA toxicology testing and collection of clinical findings upon removal of the implant. Available at: http://www.mhra.gov.uk/Safetyinformation/Generalsafetyinformationandadvice/Product-specificinformationandadvice/Product-specificinformationandadvice-A-F/Breastimplants/PIPbreastimplants/MHRAtoxicologytestingandcollectionofclinicalfindingsuponremovaloftheimplant/index.htm. Accessed June 12, 2014.
- 5.Poly Implant Prothese (PIP) Breast Implants: Final report of the expert group. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/146635/dh_134657.pdf.pdf. Accessed June 12, 2014.
- 6.Baker JL., Jr . Scottsdale, Arizona: Presented at the Aesthetic Breast Symposium; 1975. Classification of spherical contractures. [Google Scholar]
- 7.Spear SL, Baker JL., Jr Classification of capsular contracture after prosthetic breast reconstruction. Plast Reconstr Surg. 1995;96:1119–1123; discussion 1124. [PubMed] [Google Scholar]
- 8.Tickunas T, Howarth S, Godwin Y. Inflammatory changes of the nipple areolar complex of a patient with PIP breast implants: a possible immune response to free silicone from gel bleed? J Plast Reconstr Aesthet Surg. 2014;67:423–425. doi: 10.1016/j.bjps.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Swarts E, Kop AM, Nilasaroya A, et al. Rupture of Poly Implant Prothese silicone implants: an implant retrieval study. Plast Reconstr Surg. 2013;131:480–489. doi: 10.1097/PRS.0b013e3182818a00. [DOI] [PubMed] [Google Scholar]
- 10.Berry MG, Stanek JJ. PIP implant biodurability: a post-publicity update. J Plast Reconstr Aesthet Surg. 2013;66:1174–1181. doi: 10.1016/j.bjps.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 11.Maijers MC, Niessen FB. Prevalence of rupture in Poly Implant Prothèse silicone breast implants, recalled from the European market in 2010. Plast Reconstr Surg. 2012;129:1372–1378. doi: 10.1097/PRS.0b013e31824f0108. [DOI] [PubMed] [Google Scholar]
- 12.Berry MG, Stanek JJ. The PIP mammary prosthesis: a product recall study. J Plast Reconstr Aesthet Surg. 2012;65:697–704. doi: 10.1016/j.bjps.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Berry MG, Stanek JJ. PIP silicone breast implants. J Plast Reconstr Aesthet Surg. 2014;67:127–128. doi: 10.1016/j.bjps.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Quaba O, Quaba A. PIP silicone breast implants: rupture rates based on the explantation of 676 implants in a single surgeon series. J Plast Reconstr Aesthet Surg. 2013;66:1182–1187. doi: 10.1016/j.bjps.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Poly Implant Prothese (PIP) Breast Implants: Final report of the expert group. Appendices. Volume 2 Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/146636/dh_134656.pdf.pdf. Accessed June 12, 2014. [Google Scholar]
- 16.Hölmich LR, Vejborg IM, Conrad C, et al. Untreated silicone breast implant rupture. Plast Reconstr Surg. 2004;114:204–214; discussion 215. doi: 10.1097/01.prs.0000128821.87939.b5. [DOI] [PubMed] [Google Scholar]
- 17.Cawrse NH, Pickford MA. Cutaneous manifestation of silicone dissemination from a PIP implant—a case for prophylactic explantation? J Plast Reconstr Aesthet Surg. 2011;64:e208–e209. doi: 10.1016/j.bjps.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Hölmich LR, Fryzek JP, Kjøller K, et al. The diagnosis of silicone breast-implant rupture: clinical findings compared with findings at magnetic resonance imaging. Ann Plast Surg. 2005;54:583–589. doi: 10.1097/01.sap.0000164470.76432.4f. [DOI] [PubMed] [Google Scholar]
- 19.Song JW, Kim HM, Bellfi LT, et al. The effect of study design biases on the diagnostic accuracy of magnetic resonance imaging for detecting silicone breast implant ruptures: a meta-analysis. Plast Reconstr Surg. 2011;127:1029–1044. doi: 10.1097/PRS.0b013e3182043630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen BE, Biggs TM, Cronin ED, et al. Assessment and longevity of the silicone gel breast implant. Plast Reconstr Surg. 1997;99:1597–1601. [PubMed] [Google Scholar]
- 21.Berry RB. Rupture of PIP breast implants. J Plast Reconstr Aesthet Surg. 2007;60:967–968. doi: 10.1016/j.bjps.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 22.Malata CM, Cunniffe NG, Blake AM, et al. A single surgeon’s experience of the PIP breast implant “saga”: indications for surgery and treatment options. J Plast Reconstr Aesthet Surg. 2013;66:e141–e145. doi: 10.1016/j.bjps.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Dancey A, Nassimizadeh A, Levick P. Capsular contracture—what are the risk factors? A 14 year series of 1400 consecutive augmentations. J Plast Reconstr Aesthet Surg. 2012;65:213–218. doi: 10.1016/j.bjps.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Berry MG, Cucchiara V, Davies DM. Breast augmentation: part II—adverse capsular contracture. J Plast Reconstr Aesthet Surg. 2010;63:2098–2107. doi: 10.1016/j.bjps.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 25.McCauley RL, Riley WB, Jr, Juliano RA, et al. In vitro alterations in human fibroblast behavior secondary to silicone polymers. J Surg Res. 1990;49:103–109. doi: 10.1016/0022-4804(90)90118-l. [DOI] [PubMed] [Google Scholar]
- 26.Wong TW, Chiu HC, Chen JS, et al. Symptomatic keloids in two children: dramatic improvement with silicone cream occlusive dressings. Arch Dermatol. 1995;131:775–77. [PubMed] [Google Scholar]
- 27.Chan KY, Lau CL, Adeeb SM, et al. A randomized, placebo-controlled, double-blind, prospective clinical trial of silicone gel in prevention of hypertrophic scar development in median sternotomy wound. Plast Reconstr Surg. 2005;116:1013–1020; discussion 1021. doi: 10.1097/01.prs.0000178397.05852.ce. [DOI] [PubMed] [Google Scholar]
- 28.Mustoe TA, Cooter RD, Gold MH, et al. International Advisory Panel on Scar Management. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–571. doi: 10.1097/00006534-200208000-00031. [DOI] [PubMed] [Google Scholar]


