Abstract
The stromal vascular fraction (SVF) of human adipose tissue is a heterogeneous population, with component cell types that may or may not contribute to its regenerative potential. Recent findings indicate that single-cell mechanical biomarkers are characteristic of cell type and can be used comparably to gene and protein expressions to identify cell populations. In this study, we characterized mechanical properties and differentiation potential of cell types present in the SVF. Fluorescence-activated cell sorting was used to isolate four distinct populations based on surface markers: endothelial cells (EC), adipose-derived stem cells (ASCs), pre-adipocytes, and smooth muscle cells (SMC). Atomic force microscopy was used to mechanically characterize sorted cell populations and unsorted SVF. Differentiation capabilities of sorted and unsorted populations were evaluated by quantifying lipid production and calcified matrix deposition. Cells populating the SVF exhibited a range of mechanical properties, with ECs, ASCs, pre-adipocytes, and unsorted SVF cells being significantly more compliant than SMCs. Lineage-specific metabolite production was most robust in SVF cells, followed by ASCs, with the other cell types showing little or no potential, suggesting the unsorted populations may benefit from a paracrine response that is lacking once the cells are sorted into more uniform cell populations.
Keywords: adipose-derived stem cell, stromal vascular fraction, atomic force microscope, cell mechanics, differentiation, human lipoaspirate, mechanical biomarkers
Introduction
Adipose tissue is a promising source of mesenchymal stem cells (MSCs) for regenerative therapies and tissue engineering applications due to its abundance, relatively non-invasive surgical procedures, and high cell yields [17, 42]. The tissue is readily available from liposuction surgeries as waste tissue, known as lipoaspirate. These adipose-derived stem cells (ASCs) can be used autologously with limited negative immune response and have the ability to differentiate along mesodermal lineages as well as other lineages such as epithelial, endothelial, and neuronal [1, 33].
A major limitation in utilizing ASCs in clinical applications is the lack of a clear set of parameters that define the stem cell population, compounded by limited research in this area as well as contradicting studies. Some researchers use the entire unpurified cell population from the lipoaspirate, called the stromal vascular fraction (SVF), while others purify the SVF to acquire a more specific and pure ASC population [34]. The SVF is a heterogeneous population that includes multipotent stem cells, smooth muscle cells, endothelial cells, and other circulating cell types such as red and white blood cells and hematopoietic stem cells [14, 41].
Current ASC enrichment techniques include monolayer expansion and surface marker-based sorting. Cultured ASCs grown in monolayers are defined by their ability to adhere to plastic surfaces and fast proliferation rates, but this method is not ideal for clinical applications since expanding cell numbers to therapeutically relevant numbers can take weeks. However, this technique works well for enrichment because any non-adherent cell types (e.g., circulating red and white blood cells) are washed away, and the higher proliferation rate of ASCs helps them take over the culture with time. It should also be noted that ASCs in vitro are not immortal and eventually undergo senescence, lower proliferation rates, and decreased differentiation potential [2, 22]. Fluorescence-activated cell sorting (FACS) using surface makers is another technique utilized to sort ASCs. While arguably the gold standard approach, FACS-based enrichment of ASCs can be problematic since surface markers for mesenchymal stem cells are constantly changing with passage, overlap with other cell populations present in adipose tissue, and often lead to low cell yields since everything but the specified combination of markers are eliminated [17, 26, 27].
Recent findings from our lab and others indicate that single-cell mechanical biomarkers can be used to distinguish among diverse cell populations, disease states, and tissue sources, in a manner similar to gene and protein expression profiles [6-8, 15, 36]. These characteristics are strongly influenced by the cell’s physiological and structural functions. Specifically, mechanical properties are dependent on cytoskeletal make-up and the level of actin organization [25]. Studies using atomic force microscopy (AFM) for single-cell analysis have shown that mechanical biomarkers can indicate cell type, predict differentiation potential of stem cells, and reflect cytoskeletal reorganization [6, 15, 39].
Maintaining ASCs in a truly undifferentiated state in culture is challenging since the cells can be affected by many factors, including plating densities, protein coatings on culture dishes, substrate stiffness, and growth media compositions [2]. To eliminate the need for culturing, it would be beneficial to develop a method for immediate ASC enrichment following SVF isolation. Since mechanics play an important role in cell properties and correlate with lineage-specific differentiation potentials, our long-term hypothesis is that a mechanics-based approach may be beneficial [15, 36]. However, to determine the feasibility of such a technique, the mechanical properties of the cell types present in the SVF must first be defined.
The goal of this study was to characterize mechanical properties and differentiation potential of component cell types present in the SVF. This was accomplished by sorting non-expanded, human SVF cells into four different populations classified as ASCs, endothelial cells (ECs), smooth muscle cells (SMCs), and pre-adipocytes, followed by characterization of elastic and viscoelastic properties for each of the sorted populations and unsorted SVF cells using AFM. Differentiation potential of the sorted cell types and the unsorted SVF was assessed based on lipid production for adipogenesis and calcified matrix deposition for osteogenesis.
Materials and Methods
SVF Isolation
Human adipose tissue was obtained as lipoaspirate from collaborators at Rhode Island Hospital following an approved protocol (IRB Registration #0000396, 00004624; CMTT/PROJ: 210312). Samples were originally from the abdomen or outer thigh regions, harvested via liposuction from seven female donors with a prior diagnosis of breast cancer (mean age 51; range 34-62 years). Approximately 250 mL of adipose tissue was processed from each donor.
Lipoaspirate was processed according to published methods with minor modifications [12]. Briefly, to isolate the SVF, samples were washed 5-7 times with equal volumes of warm phosphate buffered saline (PBS) to remove blood and tumescent fluid. The tissue was then digested with equal volumes of a collagenase solution (0.1% (wt/vol) collagenase, 1% (vol/vol) Bovine Serum Albumin (BSA, Invitrogen) (Fraction V) and 2 mM calcium chloride) in PBS for 1 hour on a shaker at 37°C. Following incubation, the digested tissue was centrifuged at room temperature at 300g for 5 minutes. The supernatant containing lipids and mature, buoyant adipocytes was aspirated. The remaining pellet was resuspended and washed in stromal medium (DMEM/F-12, 10% Fetal Bovine Serum (FBS, Zen-Bio), and 1% antibiotic/antimycotic (A/A)). The resuspended cells were filtered sequentially through 100 μm and 70 μm filters, followed by a 10 minute incubation in red blood cell lysis buffer (155 mM ammonium chloride, 10 mM potassium carbonate and 0.1 mM EDTA). After centrifugation at 400g for 5 minutes, the isolated cells, identified as the SVF, were washed once in stromal media before freezing them in 80% FBS, 10% stromal media, and 10% dimethyl sulfoxide (DMSO) at −80°C in an isopropyl alcohol insulated container, and subsequently stored in liquid nitrogen.
Cell sorting
SVF samples were sorted by FACS into constituent cell types according to their surface markers. SVF cells from each donor were thawed rapidly in a 37°C water bath and transferred to 10 mL of warm stem cell expansion media (DMEM/F-12, 10% FBS, 5 ng/ml human epidermal growth factor, 1 ng/ml recombinant human fibroblastic growth factor, basic, 0.25 ng/ml transforming growth factor-β1, and 1% A/A) [12]. All growth factors were purchased from R&D systems.
Cells were counted with a hemocytometer and viability determined using Trypan Blue (viability was typically >60%, consistent with other studies [27]). Cells were washed twice in ice-cold wash buffer (1× PBS, 1% bovine serum albumin (BSA)), resuspended in cold blocking buffer (1× PBS, 3% BSA), and incubated for 10 minutes on ice. Following a wash, 150,000 cells in 100 μl were aliquoted into separate tubes for negative and single color controls. The remaining cells were aliquoted for multi-antibody stains and sorting. Pre-conjugated antibodies against the following antigens were used from BD Pharmingen at recommended concentrations: CD34-FITC (#560942), CD31-PE (#560983), CD45-PE-Cy5 (#560974), CD36-PerCP-Cy5.5 (#561536), CD146-PerCP-Cy5.5 (#562134). The combination of antibodies used to sort each cell type is described in Table 1 [4, 13, 24, 26, 27, 37, 38]. The cells were incubated with antibodies on ice for 20 minutes in the dark, followed by rinsing with ice-cold wash buffer. The sort was performed on a FACSAria IIu, and data were analyzed using FlowJo (Tree Star Inc.). Sorted cells were collected into tubes containing expansion medium with 20% FBS. 100,000 sorted cells were plated on glass cover slips in 50 × 9 mm, non-tissue culture treated dishes (BD Falcon) and allowed to adhere to the glass surface for 45 minutes prior to mechanical testing.
Table 1. Summary of antibody combinations used for FACS.
| Cell Type | Positive Markers | Negative Markers |
|---|---|---|
| Adipose-derived Stem Cell (ASC) | CD34 | CD45, CD31 |
| Endothelial Cell (EC) | CD34, CD31 | --- |
| Smooth Muscle Cell (SMC) | CD146 | CD31 |
| Pre-adipocyte | CD36 | CD31 |
For the purposes of clarity, this manuscript refers to sorted cell populations by “Cell Type” rather than their surface marker designation. However, it is possible that some cells exhibiting the stated surface antigens may or may not truly be that cell type (e.g., CD34+/CD45−/CD31− may not exclusively be ASCs). The CD36+/CD31− cell population were classified as pre-adipocytes since mature adipocytes were eliminated during the cell isolation process described earlier, and CD36 expression is present upon activation of adipogenic differentiation [13].
Mechanical Characterization of sorted SVF cells
Single-cell mechanical testing was performed using an atomic force microscope (AFM, MFP-3D-Bio, Asylum Research, Santa Barbara, CA) based on previously published techniques, with minor modifications [6, 7, 15]. Briefly, spherically tipped cantilevers were made by adhering 5 μm diameter, borosilicate glass beads to the end of tipless, silicon nitride cantilevers (Bruker Corporation, MLCT-O10, k~0.03 N/m). Cantilever spring constants (average, calibrated value of 0.027 N/m) were calculated based on the power spectral density of the thermal noise fluctuations prior to each experiment [21]. These cantilevers were used to mechanically probe single cells over their perinuclear region for elastic indentation and viscoelastic stress relaxation tests. An approach velocity of 10 μm/s was used, with a 30 s relaxation period. Trigger forces ranged between 0.75-1.5 nN, which limited indentations to <10% strain based on the height of the cell. All tests were done at room temperature
Once sorted, each cell type was allowed to adhere to a glass surface for approximately 45 minutes. At this time, the cell’s spherical morphology was confirmed visually by phase contrast microscopy. Cells were mechanically characterized within 1.5 hours of adhering to the surface, such that a rounded cell shape existed but no movement occurred during the testing procedure. Five mechanical parameters were quantified: elastic modulus (Eelastic), instantaneous modulus (E0), relaxed modulus (ER), apparent viscosity (μ), and height (h). Briefly, Eelastic is the measure of a cell’s resistance to deformation; a more compliant cell has a lower Eelastic. E0 is the initial resistance to deformation, and ER denotes the stiffness of the cell at equilibrium for a stress relaxation test. Lastly, μ represents the deformation resistance of the cell over time; a higher viscosity indicates slower deformation or flow under a given load. A Hertz model appropriate for spherical indentations (Eq. 1) was used to determine the elastic modulus, Eelastic [10]:
| (1) |
where F is the applied force, δ is the indentation, R is the relative radius (Eq. 2), and υ is the Poisson’s ratio, assumed to be 0.5 for incompressible materials. C is a thin-layer correction factor relating indentation depth, tip radius, and sample thickness [10]. The relative radius accounts for the curvature of the probe tip and cell at the point of contact:
| (2) |
where h is the height of the cell. The relaxed modulus (ER, Eq. 3), instantaneous modulus (E0, Eq. 4), and apparent viscosity (μ, Eq. 5) were determined using a thin-layer, stress relaxation model of a standard linear solid, where τσ and τε are the relaxation times under constant load and deformation, respectively [7, 8].
| (3) |
| (4) |
| (5) |
Multipotency assessment
To determine the multipotency of the unsorted SVF and sorted ASCs, the cell populations were differentiated along adipogenic and osteogenic lineages. Isolated pre-adipocytes, SMCs, and ECs were terminally differentiated or lineage-committed cell types. To determine if these cell types had trans-differentiation capabilities, the pre-adipocytes, SMCs, and ECs were also introduced to adipogenic and osteogenic induction factors.
Adipogenic Differentiation
Cells from a representative donor were used to evaluate the multipotency of individual cell types in freshly isolated SVF. Frozen cells were thawed and sorted using the markers defined in Table 1. Each sorted cell type was seeded in a 96-well plate at a density of 2,000-8,000 cells per well and allowed to grow for 5-7 days before inducing adipogenic differentiation. Unsorted SVF cells were differentiated concurrently as well. All cells were incubated for 14 days in either adipogenic medium (DMEM/F-12, 10% FBS, 10 μM insulin, 1 μM dexamethasone, 0.25 mM isobutyl-1-methylxanthine, 200 μM indomethacin (Sigma-Aldrich) and 1% A/A) or control medium (DMEM/F-12, 10% FBS, 1% A/A) [40]. After 14 days of culture, cells were fixed with 10% formalin, and differentiation was visualized with Oil Red O staining (ORO, Sigma-Aldrich) to assess lipid accumulation in induced and control samples. Images were analyzed with a custom MATLAB program using the built-in Image Processing Toolbox to detect and measure lipid droplet size. Lipid size was used as a metric to assess adipogenesis since positive differentiation is characterized by an increase in intracellular lipids, specifically large droplets. The percentage of cells in each sample exhibiting a rounded morphology was manually calculated (confluent, monolayer cultures were considered to be 0% rounded and 100% spread based on visual observation). ORO dye was eluted from each sample and measured at 500 nm using a spectrophotometer to obtain quantitative, optical density values. Lastly, cell nuclei were stained with 4′,6-diamino-2-phenylindole (DAPI, Thermo Fisher Scientific), counted, and used to normalize optical densities on a per-cell basis [5].
Osteogenic Differentiation
For osteogenic differentiation, SVF cells were thawed, sorted, and plated as for adipogenesis. Sorted ASCs, ECs, SMCs, pre-adipocytes, and unsorted SVF cells were incubated for 21 days in either osteogenic medium (DMEM/HG, 10% FBS, 10 mM β-glycerophosphate, 0.15 mM ascorbate-2-phosphate, 10 nM dexamethasone, and 1% A/A) or control medium [18]. After 21 days of culture, cells were fixed with 10% formalin, and differentiation was visualized with Alizarin Red S staining (ARS, Sigma-Aldrich) to assess calcified matrix deposition in induced and control samples. As with adipogenesis, the percentage of cells exhibiting spread versus rounded morphologies was quantified. After imaging the wells, the ARS dye was eluted from each sample and measured at 540 nm using a spectrophotometer for quantification. Lastly, cell nuclei were stained with DAPI, counted, and used to normalize optical density measurements on a per-cell basis [5].
Statistical Analysis
All statistical analysis was performed in SigmaPlot 12.3 (Systat Software Inc.). Data throughout the study are presented as arithmetic mean ± standard deviation (SD). Mechanical data collected from each of the four, cell type populations were not normal, according to the Shapiro-Wilk test. Non-parametric analysis was performed using a Kruskal-Wallis analysis of variance (ANOVA) on ranks, followed by Dunn’s test for multiple comparisons with a significance of p < 0.05. Height data collected for the cell types were found to be normal using the Shapiro-Wilk test and were analyzed using a one-way ANOVA, followed by a Fisher LSD post-hoc test with a significance of p < 0.05. Student’s t-tests were performed for differentiation data to compare induced and control conditions within cell types. A two-way ANOVA, followed by a Duncan’s Multiple Range post-hoc test was performed to compare osteogenic differentiation between the unsorted SVF and four, individual sorted cell types.
Results
Sorting of Cells in the SVF
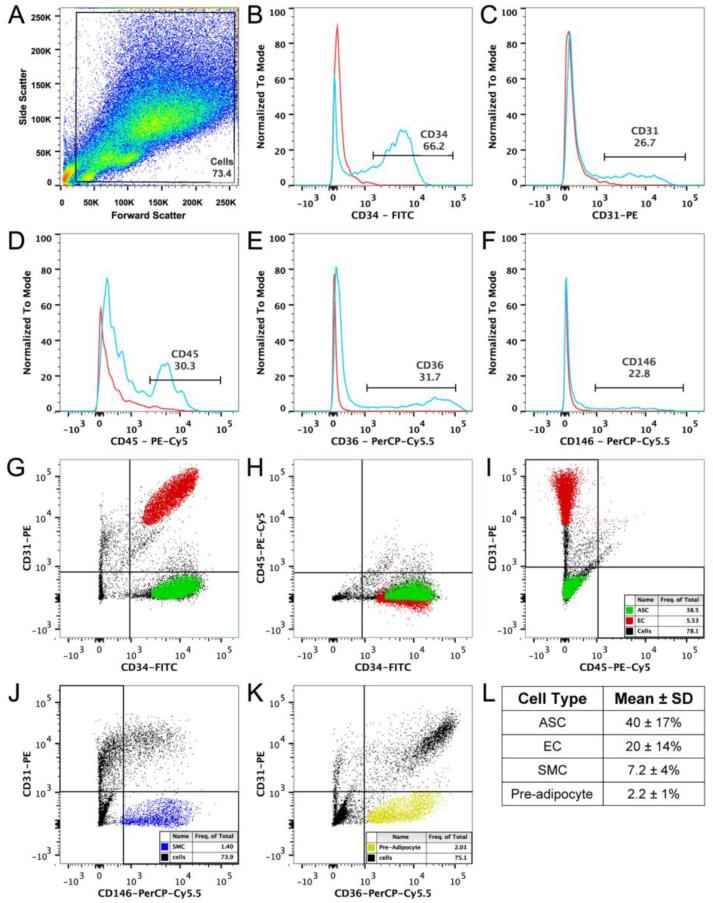
SVF cells were sorted via FACS into four distinct populations based on previously reported surface marker characteristics [23, 32, 38]. Either two- or three- color labeling was used to define each of the cell types. On average, ~75% of the entire cell population was used in downstream gating schemes to eliminate debris, dead cells, and cell aggregates based on the forward and side scatter plot (Figure 1A). Single CD marker controls were first run for CD34, CD31, CD45, CD146, and CD36 to determine expression levels of each within the SVF (Figure 1B-F). When double- and triple-stained SVF cells were sorted into four distinct populations, noticeable variation was observed in percentage of each cell types among the seven donors (Figure 1G-K). Overall, the largest fraction of cells in the SVF were ASCs, followed by ECs, pre-adipocytes, and SMCs (Figure 1L).
Figure 1.
Multi-color sorts of SVF cells based on protein expression using FACS. (A) Distribution of cells, plotted as forward (cell size) vs. side scatter (granularity). The population within the gate “Cells” represented ~75% of total events and was used for downstream gating strategies for dot plots and histograms. (B-F) Cells were analyzed for expression of CD34-FITC, CD31-PE, CD45-PE-Cy5, CD36-PerCP-Cy5.5, and CD146-PerCP-Cy5.5. Each histogram plot depicts a control, unstained, SVF population in red and a stained SVF population in blue. Percent positive cells are shown. (G-I) Three-color FACS was used to sort SVF samples for ASCs (CD34+/CD31−/CD45−, green) and ECs (CD34+/CD31+, red). (J-K) Double-color analysis was used to sort SVF samples for SMCs (CD146+/CD31−, blue) and pre-adipocytes (CD36+/CD31−, yellow). (L) Mean ± SD of each sorted cell type across the seven donors.
Characterization of Mechanical Properties of Cells in SVF
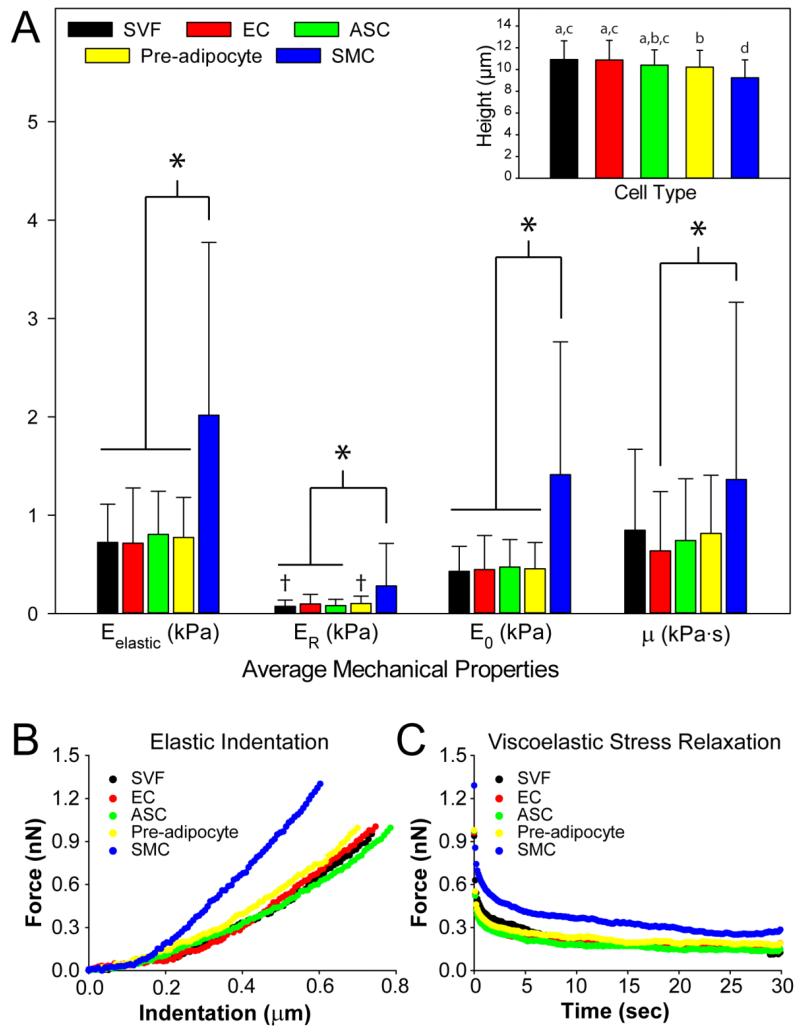
Following cell sorting, the mechanical properties of four adherent cell types in the SVF were measured using AFM. Testing was also conducted on the unsorted SVF from each donor. Five parameters were measured to obtain a complete panel of elastic and viscoelastic properties. 109 ECs, 135 ASCs, 129 pre-adipocytes, 110 SMCs, and 105 unsorted, SVF cells were tested in total from all seven donors in approximately equal proportions. As expected, different cell types that reside within adipose tissue exhibited a range of mechanical properties. The results indicated that cell populations in adipose tissue vary in compliance, apparent viscosity, and height (Figure 2). SMCs exhibited Eelastic, ER, and E0 values three times that of ASCs, ECs, and pre-adipocytes. While the apparent viscosity (μ) exhibited large variability regardless of cell type, as has been observed before [6], a statistically significant difference did exist between ECs and SMCs, with the latter being twice as viscous as the former (p < 0.05). Furthermore, SMCs were 10-15% smaller in height than ASCs, ECs, and pre-adipocytes (Figure 2 Inset, p < 0.05). Pre-adipocytes were also about 6% smaller than ECs. SVF cells showed similar mechanical properties to pre-adipocytes, ECs, and ASCs. By using the percentage splits determined from FACS among the cell populations, we can calculate a composite elastic modulus for all cells present in the SVF, 0.8 kPa. This is approximately the same as the measured value of 0.7 kPa for unsorted SVF cells. Variability in mechanical properties among the seven donors was noticeable; however, the overall trend for Eelastic, E0,ER, μ, and cell height among the cell types was consistent (Supplemental Figure 1). Eelastic was determined by fitting a modified, Hertz model to the initial indentation response of individual cells (Figure 2B). The viscoelastic parameters, E0, ER, and μ, were extracted using the 30 second relaxation phase of the single-cell test (Figure 2C).
Figure 2.
Biomechanical properties of the unsorted SVF and sorted cell types residing in adipose tissue for seven donors. (A) Mean ± SD of each of the elastic and viscoelastic properties of the four cell types are shown (*p<0.05, † p<0.05 as determined by Kruskal-Wallis ANOVA on ranks, followed by Dunn’s test for multiple comparisons). On average, SMCs displayed Eelastic, E0, and ER values three times those of ASCs, ECs, and pre-adipocytes. SMCs also exhibited μ values twice that of ECs. (Inset) Cell heights showed that SMCs were also significantly shorter than ECs, ASCs, pre-adipocytes, and unsorted SVF cells. Average height ± SD for each cell type is shown (Unmatched letters are significant from each other, p < 0.05); determined by a one-way ANOVA and Fisher LSD post hoc test. Overall, SMCs were less compliant and smaller than the other sorted cell types. Mechanical properties of SVF cells were representative of its component cells. (B, C) Representative, individual cells from the unsorted SVF and sorted cell populations illustrate the mechanical trends of the overall population, with SMCs portraying a less compliant phenotype than other cell types for elastic and viscoelastic tests.
Differentiation of Cells in SVF
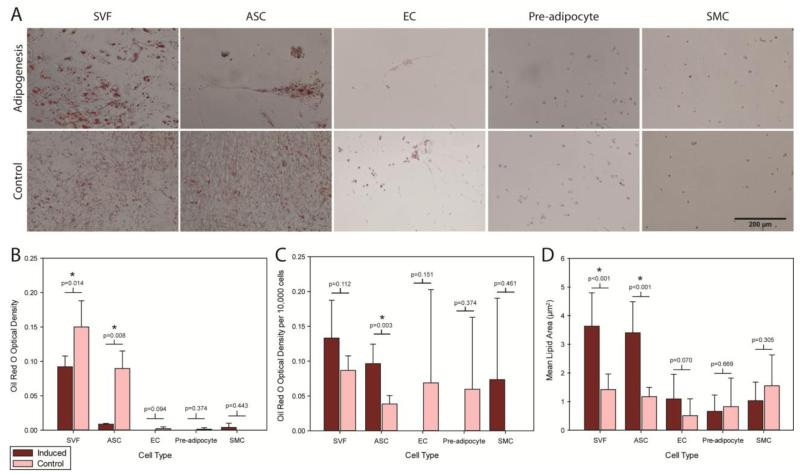
To compare the multilineage differentiation potential of the four sorted populations and the unsorted SVF, isolated cells were induced for adipogenesis and osteogenesis. Qualitatively, a clear difference was observed between induced SVF and ASC samples over their respective controls, with adipogenic conditions resulting in large lipid droplets forming in the cells compared to uniformly distributed, small, intracellular lipid droplets (Figure 3A). When comparing optical densities of eluted ORO stain of induced versus control wells as a whole, only the SVF and ASCs exhibited lipid production, with the controls displaying higher optical densities than induced wells (p<0.05, Figure 3B). To account for differences due to cell numbers, the optical densities for ORO stain were normalized to 10,000 cells per well for further interpretation (Figure 3C). Based on this measure, induced ASCs produced significantly more lipids than controls (p < 0.05), and while increased lipid production was visually observed in induced SVF cells than controls, the difference did not reach a level of statistical significance (p = 0.112). Differences in lipid production were not observed between induced and control cells for ECs, pre-adipocytes, and SMCs. Quantitative analysis of mean lipid area between induced and control samples showed a clear difference existed for SVF cells and ASCs (p<0.001, Figure 3D) but not for other cell types.
Figure 3.
Adipogenic differentiation was assessed by ORO staining of intracellular lipids. (A) Adipogenically induced samples are shown on the top row, with corresponding controls depicted on the bottom row (Scale bar = 200 μm). (B) Optical densities corresponding to eluted ORO stain indicated no significant lipid production occurred in induced samples over controls on a per sample basis. (C) When normalized to the number of cells in each sample, more robust lipid production was observed in sorted ASCs than unsorted SVF cells, ECs, pre-adipocytes, and SMCs (* p < 0.05). (D) Mean lipid droplet size measurements showed induced SVF cells and ASCs produced significantly larger lipids over control cells, which produced smaller, uniformly distributed lipids throughout the samples (* p < 0.05). Data are presented as Mean ± SD. Student’s t-test determined statistical significance.
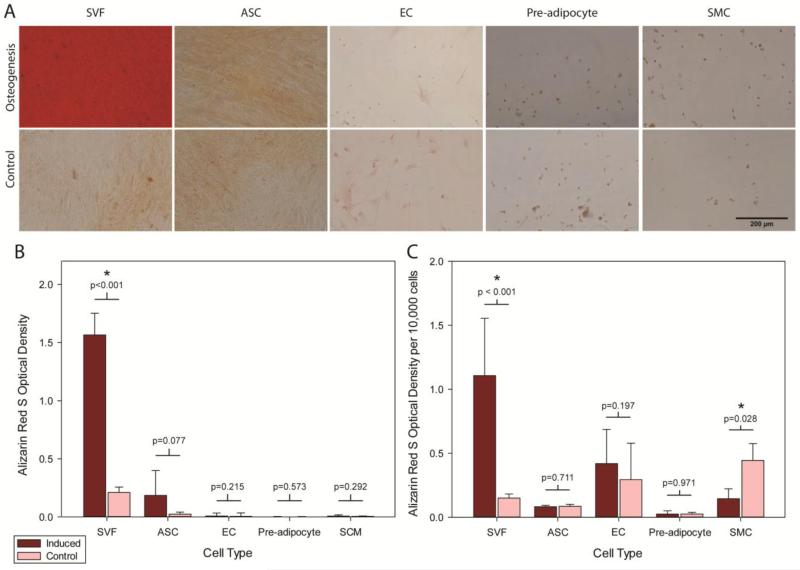
For osteogenesis, qualitatively, a noticeable difference was only observed in induced SVF samples over controls, with osteogenic conditions resulting in significant calcified matrix deposition (Figure 4A). The optical densities for ARS stain corresponding to matrix production were also compared for induced versus control wells on a per well and per cell basis. Only the induced, unsorted SVF cells produced significantly more calcified matrix over its control condition (p<0.05, Figure 4B-C). The induced, unsorted SVF cells also produced significantly more calcified matrix than the four induced, sorted cell types (p<0.001).
Figure 4.
Osteogenic differentiation was assessed by ARS staining of calcified matrix. (A) Osteogenically induced samples are shown on the top row, with corresponding controls depicted on the bottom row (Scale bar = 200 μm). (B) Optical densities corresponding to the eluted ARS stain indicated a robust osteogenic response in SVF cells compared to purified, component cell types (* p < 0.05). (C) When normalized to the number of cells in each sample, more robust calcified matrix deposition was observed in the unsorted SVF cells than sorted ASCs, ECs, pre-adipocytes, and SMCs (* p < 0.05). Data are presented as Mean ± SD. Student’s t-test determined statistical significance.
It was also visually observed that few of the SMCs or pre-adipocytes spread, produced lipid, or deposited any matrix. SVF cells undergoing osteogenesis and adipogenesis presented a spread morphology. ASCs undergoing osteogenesis were spread, but for adipogenesis, 47 ± 18% presented a rounded morphology. Regardless of differentiation condition, ECs presented a primarily rounded morphology (adipogenesis: 99 ± 1%; osteogenesis: 78 ± 31%). All SMCs and pre-adipocytes presented only a rounded morphology. The viability of the SVF cells and sorted cell types was tested after plating and prior to differentiation, with >60% being alive. Despite having a rounded morphology, SMCs and pre-adipocytes remained adhered to the culture surface throughout differentiation, which included multiple medium changes.
Discussion
The results of this study indicate that the human SVF is heterogeneous, with subpopulations exhibiting differences in mechanical properties, a range of surface marker expression, and variations in differentiation potential. Overall, significant differences in elastic and viscoelastic properties were observed when comparing unsorted SVF, ASCs, ECs, and pre-adipocytes to SMCs. Dramatic differences in lineage-specific metabolite production between ASCs and unsorted SVF cells suggest that surface marker-based sorting may eliminate supportive cell types necessary for robust differentiation, especially for osteogenesis.
The study identified significant differences in mechanical properties between major cell types residing in adipose tissue, which is a first step towards mechanics-based enrichment of specific populations within the SVF. These properties could also provide insight on mechanical differences between terminally differentiated cell types and cells in a progenitor state. Cells were mechanically characterized in a spherical morphology to assess their properties with minimal contribution to mechanics from their microenvironment. Therefore, the properties identified can be translated more easily to a suspension state suitable for high-throughput cell sorting techniques, while limiting the influence various morphologies of spread cells have on measured properties. Furthermore, in the spherical morphology, all cells have nominally the same shape in the same conditions, providing a means to compare across cell types. Importantly, it has been previously shown that cells exhibit distinct mechanical biomarkers in the spherical morphology [15]. By allowing the cells to adhere to glass coverslips for only 45 minutes, the cells do not have enough time for substantial cytoskeletal reorganization, including formation of large actin filament bundles, and mechanical properties obtained can be more confidently related to their suspended state mechanics [7, 8].
Microfluidics-based platforms can be used to sort these cells based on their elastic properties and deformation through multiple methods, most of which capitalize on fluid shear forces and inertial focusing [11, 16, 20, 31]. The ability to sort on such a platform would also eliminate the need to immunolabel cells prior to FACS. While properties obtained through AFM will not fully translate to cells in suspension, these data still provide a comparable starting point, as shown by studies comparing micropipette aspiration, where cells are completely in suspension, to AFM testing, where cells are slightly adhered to a surface [8]. For comparison, a previously reported elastic modulus for ECs determined using micropipette aspiration is approximately 0.5 kPa, while the AFM technique used in this study calculated Eelastic as 0.7 kPa [19]. Similarly, pre-adipocytes had an Eelastic of 0.8 kPa, as reported in this study, which is similar to the previously determined 0.9 kPa, also calculated on spherical cells using AFM [6]. To our knowledge, micropipette aspiration studies have not been performed on SMCs, so a valid comparison of elastic moduli in their spherical state cannot be made. Variations are possibly due to differing techniques, conditions used from study to study, and differences in the tissue and donor source of each cell type.
The SVF isolated from lipoaspirate was also confirmed to be a heterogeneous population with relation to their cells’ surface marker profile. The sorted populations analyzed in this study accounted for ~70% of the entire SVF, and although not investigated as part of this study, we hypothesize the remaining ~30% of the SVF primarily contains a CD45+ hematopoietic lineage population based on previously published studies [24, 26]. The cellular composition of the SVF, as defined by percentage of the whole cell population, varied from donor to donor. The range for ASCs spanned from 18% to 61%, ECs 5% to 31%, pre-adipocytes 2% to 10%, and SMCs 1% to 4%. Other groups using similar surface marker profiles also reported a high degree of variability among donors [24, 26, 27, 38]. The surface marker profile for the “cell types” selected for these studies was designed to be broad to include progenitor cells that more definitive profiles would eliminate. However, as mentioned, it is likely that a fraction of cells within each group are not actually of the specified lineage, which inserts some noise into the system. Furthermore, expected surface markers are not always present since they have been shown to change with cell cycle, which could result in the possible elimination of progenitors, emphasizing the need for an alternative means to identify cell phenotype, such as cellular mechanical biomarkers [9, 29].
Tissue source and donor medical history should also be considered as potential sources of variability in cellular properties. While all donors in this study had a prior diagnosis of breast cancer, lipoaspirate was harvested from non-cancerous sites, limiting the potential impact of the disease on observed cellular phenotypes. Our reported mechanical properties and proportions of each cell type within the SVF were similar to those seen in previously published studies with healthy donors [6, 19, 24, 26, 27, 38]. Regardless of health status, cells from the non-discarded tissue were used clinically for reconstructive purposes with good success, indicating these cells are a viable option for regenerative therapies.
Based on previous studies, it was expected that the sorted ASC population would have a more significant adipogenic differentiation response compared to the unsorted SVF [23]. When considering the normalized optical density of ORO stain, only the sorted ASCs produced significantly more intracellular lipids compared to control media. However, to account for the large, mature lipid production visible in the induced SVF and ASCs, mean lipid size was determined as an alternative metric for lipid quantification. Both induced SVF cells and ASCs produced lipids over twice the size of lipids formed in control media (p < 0.001), a response not observed in other cell types. While Li et al. did not observe the same response of increased large lipid production from the unsorted SVF, the adipogenic potential of ASCs, defined in the present study as CD34+/CD31−/CD45−, was found to be comparable. The differences in the studies could be due to differing adipogenic induction media used, tissue harvest methods of abdominoplasty instead of liposuction, and donor to donor variability. The higher adipogenic potential of ASCs and the unsorted SVF could be due to an inherently higher expression of adipocyte markers such as peroxisome proliferator-activated receptor-γ and fatty acid binding protein-4 [23]. Both the unsorted SVF and sorted ASCs could therefore be viable options for soft tissue regeneration such as fat reconstruction.
The unsorted SVF cells deposited seven times more calcified matrix in induced versus control media. Conversely, the four, sorted populations showed no significant matrix deposition. This finding is also similar to Li et al., who qualitatively showed that cultured, unsorted cells differentiated better than purified, component populations [23]. Additional studies comparing osteogenic differentiation of CD34+ and CD34− populations concluded similar results, showing that the CD34+ population (describing ASCs) did not differentiate as well as CD34− populations [35]. Further study and analysis of the interaction and signaling among other cell types in the SVF, such as endothelial cells and the CD45+ populations, is necessary to fully understand the overall osteogenic potential of unsorted cells.
Purification of the SVF based on surface marker profiles may not be ideal for regenerative therapies since cells never exist in the body in a completely pure form. Supportive cell types or cells from other tissues in the local microenvironment could play a role in the proper function of the cells to effect repair [3, 28]. In a study on bone marrow transplantation, Nilsson et al. determined a purified bone marrow stem cell population had lower engraftment than unsorted bone marrow cells, further suggesting the existence of a “non-stem cell facilitator population” [28]. Alternative characteristics like mechanical biomarkers encompass many aspects of the cell and can serve as a secondary characteristic for gene and protein expressions. Other options such as selecting cells based on physical, electrical, or gene expression characteristics may also provide a broader swathe of cell types that will lessen the possibility of “over purification.” Alternatively, finding the proper combination of completely pure cell types could allow for highly controlled regenerative procedures since many cells in SVF are already committed to a terminally differentiated state.
Culturing conditions were kept consistent across cell types isolated from SVF to allow for comparative analysis. The apparent lack of growth for ECs, SMCs, and pre-adipocytes could be due partially to these culture conditions, although these cells did attach to the surface and remain adhered for the 2-3 weeks of differentiation. Customizing the culture environment for each cell type could produce different results at the expense of a uniform experiment (e.g., surface coating stimulates EC growth and maintenance [30]). It would be expected that that these terminally differentiated and lineage-committed cells should not change even when placed in a lineage-inducing environment. The lack of growth of these cell types support this. Increasing cell density could be beneficial for acquiring a differentiation response due to a larger concentration of paracrine signaling. The goal of this assay was to determine whether significant levels of “trans-differentiation” existed within the sorted cell populations, and the data suggest that this was not the case. While only one representative donor was used to evaluate the differentiation potential of component cell types within SVF, findings were consistent with previously published data using multiple donors [23, 35].
While surface markers alone may not be conclusive for isolating cell populations with robust differentiation potential, the addition of mechanical biomarkers may facilitate enrichment of a therapeutically beneficial cell source. In the current study, lack of adipogenic and osteogenic responses from CD146+ and CD36+ cells indicate they would contribute little to the differentiation response of the overall SVF population. However, it would be interesting to assess differentiation potential of specific combinations of cell types necessary to elicit optimal, regenerative responses. The addition of mechanical biomarkers to this sorting scheme could better refine populations with overlapping and transient surface marker profiles. Previous findings also suggest the cellular mechanical properties may indicate the synthetic potential of a cell and not just its differentiation potential [15]. The dramatic difference in cellular mechanical properties between SMCs and other cell types could provide a means to identify and potentially exclude this cell type. However, researchers should bear in mind that removing integral cell types might actually degrade the regenerative response of a heterogeneous cell population. In the SVF of lipoaspirate, significant variation is present in the mechanical properties of resident cells. Further experiments will be necessary to assess the effectiveness of the combined use of surface markers and mechanical biomarkers via microfluidics and mechanics-based, high-throughput platforms to determine an ideal range of properties for enriched, differentiation-capable cell populations.
Supplementary Material
Supplemental Figure 1. While variability was observed, overall trends of biomechanical properties measured using AFM across the seven donors tested was conserved. The unsorted SVF cells, ECs, ASCs, and pre-adipocytes, on average, were more compliant and viscous compared to SMCs for the (A) elastic modulus, (B) relaxed modulus, (C) instantaneous modulus, and (D) apparent viscosity. (E) SMCs were observed to be smaller in cell height across all donors compared to the unsorted SVF, ECs, ASCs, and pre-adipocytes.
Ethical standards.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5), and approved by Rhode Island Hospital’s Institutional Review Board. Informed consent was obtained from all patients providing waste tissue in this study. No animal studies were carried out by the authors for this article.
Acknowledgements
The authors would like to thank Dr. Paul Liu from Rhode Island Hospital for lipoaspirate, and Nicholas R. Labriola for assistance with AFM and development of the MATLAB program for lipid size analysis. This work was supported by awards from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR063642), National Institute of General Medical Sciences (P20GM104937), and National Science Foundation (CAREER Award, CBET1253189). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or National Science Foundation.
Abbreviations
- AFM
atomic force microscopy
- ARS
Alizarin Red S
- ASC
adipose-derived stem cell
- EC
endothelial cell
- Eelastic
elastic modulus
- E0
instantaneous modulus
- ER
relaxed modulus
- FACS
fluorescence-activated cell sorting
- h
height
- MSC
mesenchymal stem cell
- ORO
Oil Red O
- SMC
smooth muscle cell
- SVF
stromal vascular fraction
- μ
viscosity
Footnotes
Conflicts of interest
Manisha Kanthilal and Eric M. Darling declare that they have no conflicts of interest.
References
- 1.Baer PC. Adipose-derived stem cells and their potential to differentiate into the epithelial lineage. Stem Cells Dev. 2011;20(10):1805–16. doi: 10.1089/scd.2011.0086. [DOI] [PubMed] [Google Scholar]
- 2.Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012;2012:812693. doi: 10.1155/2012/812693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15(6):641–8. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7(10):R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darling EM, Topel M, Zauscher S, Vail TP, Guilak F. Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. J Biomech. 2008;41(2):454–64. doi: 10.1016/j.jbiomech.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darling EM, Zauscher S, Block JA, Guilak F. A thin-layer model for viscoelastic, stress-relaxation testing of cells using atomic force microscopy: do cell properties reflect metastatic potential? Biophys J. 2007;92(5):1784–91. doi: 10.1529/biophysj.106.083097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darling EM, Zauscher S, Guilak F. Viscoelastic properties of zonal articular chondrocytes measured by atomic force microscopy. Osteoarthritis Cartilage. 2006;14(6):571–9. doi: 10.1016/j.joca.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 9.De Francesco F, Tirino V, Desiderio V, Ferraro G, D’Andrea F, Giuliano M, Libondi G, Pirozzi G, De Rosa A, Papaccio G. Human CD34(+)/CD90(+) ASCs Are Capable of Growing as Sphere Clusters, Producing High Levels of VEGF and Forming Capillaries. Plos One. 2009;4(8) doi: 10.1371/journal.pone.0006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimitriadis EK, Horkay F, Maresca J, Kachar B, Chadwick RS. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophysical Journal. 2002;82(5):2798–2810. doi: 10.1016/S0006-3495(02)75620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekpenyong AE, Whyte G, Chalut K, Pagliara S, Lautenschlager F, Fiddler C, Paschke S, Keyser UF, Chilvers ER, Guck J. Viscoelastic Properties of Differentiating Blood Cells Are Fate- and Function-Dependent. Plos One. 2012;7(9) doi: 10.1371/journal.pone.0045237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5(7):1294–311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Festy F, Hoareau L, Bes-Houtmann S, Pequin AM, Gonthier MP, Munstun A, Hoarau JJ, Cesari M, Roche R. Surface protein expression between human adipose tissue-derived stromal cells and mature adipocytes. Histochem Cell Biol. 2005;124(2):113–21. doi: 10.1007/s00418-005-0014-z. [DOI] [PubMed] [Google Scholar]
- 14.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Cruz RD, Fonseca VC, Darling EM. Cellular mechanical properties reflect the differentiation potential of adipose-derived mesenchymal stem cells. Proc Natl Acad Sci U S A. 2012;109(24):E1523–9. doi: 10.1073/pnas.1120349109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gossett DR, Weaver WM, Mach AJ, Hur SC, Tse HT, Lee W, Amini H, Di Carlo D. Label-free cell separation and sorting in microfluidic systems. Anal Bioanal Chem. 2010;397(8):3249–67. doi: 10.1007/s00216-010-3721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189(1):54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 18.Guilak F, Lott KE, Awad HA, Cao QF, Hicok KC, Fermor B, Gimble JM. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. Journal of Cellular Physiology. 2006;206(1):229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 19.Hochmuth RM. Micropipette aspiration of living cells. J Biomech. 2000;33(1):15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- 20.Hur SC, Henderson-MacLennan NK, McCabe ERB, Di Carlo D. Deformability-based cell classification and enrichment using inertial microfluidics. Lab on a Chip. 2011;11(5):912–920. doi: 10.1039/c0lc00595a. [DOI] [PubMed] [Google Scholar]
- 21.Hutter JL, Bechhoefer J. Calibration of Atomic-Force Microscope Tips. Review of Scientific Instruments. 1993;64(7):1868–1873. [Google Scholar]
- 22.Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, Bunnell BA. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68(11):4229–38. doi: 10.1158/0008-5472.CAN-07-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Zimmerlin L, Marra KG, Donnenberg VS, Donnenberg AD, Rubin JP. Adipogenic potential of adipose stem cell subpopulations. Plast Reconstr Surg. 2011;128(3):663–72. doi: 10.1097/PRS.0b013e318221db33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin K, Matsubara Y, Masuda Y, Togashi K, Ohno T, Tamura T, Toyoshima Y, Sugimachi K, Toyoda M, Marc H, Douglas A. Characterization of adipose tissue-derived cells isolated with the Celution system. Cytotherapy. 2008;10(4):417–26. doi: 10.1080/14653240801982979. [DOI] [PubMed] [Google Scholar]
- 25.Maloney JM, Nikova D, Lautenschlager F, Clarke E, Langer R, Guck J, Van Vliet KJ. Mesenchymal stem cell mechanics from the attached to the suspended state. Biophys J. 2010;99(8):2479–87. doi: 10.1016/j.bpj.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, Kloster A, Di Halvorsen Y, Ting JP, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24(5):1246–53. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24(2):376–85. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson SK, Dooner MS, Tiarks CY, Weier HU, Quesenberry PJ. Potential and distribution of transplanted hematopoietic stem cells in a nonablated mouse model. Blood. 1997;89(11):4013–4020. [PubMed] [Google Scholar]
- 29.Reddy GPV, Tiarks CY, Pang LZ, Wuu J, Hsieh CC, Quesenberry PJ. Cell cycle analysis and synchronization of pluripotent hematopoietic progenitor stem cells. Blood. 1997;90(6):2293–2299. [PubMed] [Google Scholar]
- 30.Relou IAM, Damen CA, van der Schaft DWJ, Groenewegen G, Griffioen AW. Effect of culture conditions on endothelial cell growth and responsiveness. Tissue & Cell. 1998;30(5):525–530. doi: 10.1016/s0040-8166(98)80032-3. [DOI] [PubMed] [Google Scholar]
- 31.Sawetzki T, Eggleton CD, Desai SA, Marr DWM. Viscoelasticity as a Biomarker for High-Throughput Flow Cytometry. Biophysical Journal. 2013;105(10):2281–2288. doi: 10.1016/j.bpj.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorensen AL, Timoskainen S, West FD, Vekterud K, Boquest AC, Ahrlund-Richter L, Stice SL, Collas P. Lineage-specific promoter DNA methylation patterns segregate adult progenitor cell types. Stem Cells Dev. 2010;19(8):1257–66. doi: 10.1089/scd.2009.0309. [DOI] [PubMed] [Google Scholar]
- 33.Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54(3):132–41. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 34.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21(14):2724–52. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 35.Suga H, Matsumoto D, Eto H, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18(8):1201–10. doi: 10.1089/scd.2009.0003. [DOI] [PubMed] [Google Scholar]
- 36.Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007;3(4):413–38. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teboul L, Febbraio M, Gaillard D, Amri EZ, Silverstein R, Grimaldi PA. Structural and functional characterization of the mouse fatty acid translocase promoter: activation during adipose differentiation. Biochem J. 2001;360(Pt 2):305–12. doi: 10.1042/0264-6021:3600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varma MJ, Breuls RG, Schouten TE, Jurgens WJ, Bontkes HJ, Schuurhuis GJ, van Ham SM, van Milligen FJ. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16(1):91–104. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- 39.Yourek G, Hussain MA, Mao JJ. Cytoskeletal changes of mesenchymal stem cells during differentiation. ASAIO J. 2007;53(2):219–28. doi: 10.1097/MAT.0b013e31802deb2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng B, Cao BH, Li GH, Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Engineering. 2006;12(7):1891–1901. doi: 10.1089/ten.2006.12.1891. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26(6):664–75. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- 42.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. While variability was observed, overall trends of biomechanical properties measured using AFM across the seven donors tested was conserved. The unsorted SVF cells, ECs, ASCs, and pre-adipocytes, on average, were more compliant and viscous compared to SMCs for the (A) elastic modulus, (B) relaxed modulus, (C) instantaneous modulus, and (D) apparent viscosity. (E) SMCs were observed to be smaller in cell height across all donors compared to the unsorted SVF, ECs, ASCs, and pre-adipocytes.