Abstract
Theranostic nanoparticles hold the potential to revolutionize future disease management. Since the last decade, there has been a growing interest in the engineering of various kinds of theranostic nanoparticles for simultaneous cancer imaging and therapy in small animals. Efficient targeting of theranostic nanoparticles to the tumor site is critical for both diagnostic and therapeutic purposes. However, difficulties still exist in the engineering of biocompatible theranostic nanoparticles with highly specific in vivo tumor targeting capabilities. Here we will discuss the current status and future perspectives of actively targeted theranostic nanoparticles for tumors, as well as challenges that still exist.
Keywords: Theranostic nanoparticle, cancer, active targeting, theranostics, nanomedicine
Theranostic nanoparticles are multifunctional nanosystems, which are well-designed for more specific and personalized disease management by combining diagnostic and therapeutic capabilities into one single biocompatible and biodegradable nanoparticle (1). Ideal theranostic nanoparticles must be safe to humans and be able to (1) rapidly and selectively accumulate in target(s) of interest, (2) report biochemical and morphological characteristics of disease(s), (3) efficiently deliver a sufficient amount of drug(s) on-demand without damaging healthy organs, and (4) be cleared from body within hours or biodegraded into nontoxic byproducts (1). Although numerous types of theranostic nanoparticles, both organic and inorganic, have been developed in the last decade for treating cancers (FIGURE 1) (2,3), none of them has satisfied all of the above criteria yet.
Figure 1.
Organic and inorganic nanoplatforms for theranostic nanoparticle synthesis. NPs: nanoparticles; CNTs: carbon nanotubes; GO: graphene oxide. Images of quantum dots, carbon nanotube, and graphene were acquired from http://www.rsc.org/chemistryworld/News/2005/September/19090501.asp, http://tradingcomputersnow.com/the-future-is-set-for-carbon-nanotube-trading-computers/, and http://en.wikipedia.org/wiki/Graphene, respectively.
The value of tumor active targeting has been demonstrated by many preclinical studies and clinical trials with peptide- or antibody-conjugated imaging nanoparticles and chemotherapeutics (4,5). Active targeting can also be particularly valuable in treating poorly vascularized small metastases (<100 mm3), where enhanced permeability and retention effect alone may not be effective. So far, most previously reported uses of theranostic nanoparticles have been focused on using passive targeting strategies. It is still a major challenge to engineer biocompatible theranostic nanoparticles with highly specific in vivo tumor active targeting capabilities. Here we will discuss the status, challenges, and future outlook of tumor actively-targeted theranostic nanoparticles.
General Synthetic Rules and Translational Research
Generally, theranostic nanoparticles can be engineered in several ways. First, this may be done by conjugating (or loading) therapeutic agents (e.g. anti-cancer drugs and photosensitizers) to existing imaging nanoparticles such as quantum dots, iron oxide nanoparticles (IONPs), gold nanocages, and the like. Tagging imaging contrast agents, like fluorescent dyes, optical or magnetic nanoparticles, and various radioisotopes, to existing therapeutic nanoparticles is another option. Encapsulating both imaging and therapeutic agents together in biocompatible nanoplatforms like polymeric nanoparticles, ferritin nanocages, and porous silica nanoparticles is also effective. Finally, engineering of unique nanoparticles (e.g. Porphysomes, [64Cu]CuS, gold nanoshells/cages) with intrinsic imaging and therapeutic properties gives the desired results. For improving the blood circulation half-life and providing tumor active targeting capability, post surface modifications with polyethylene glycol and different targeting ligands will usually be performed.
Although nanoparticle-based imaging and therapeutic agents are struggling to advance into clinical trials due to toxicity concerns, progress has been made during the last decade. So far, the Food and Drug Administration (FDA) has approved over 35 imaging or therapeutic nanoparticles for clinical trials (6). Theranostic nanoparticles are still in the very early translational stages, with nearly all efforts devoted to preclinical studies and no clinical trials to date. The engineering of theranostic nanoparticles using FDA-approved imaging (or therapeutic) nanoplatforms may be a viable option. Platforms such as biodegradable polymeric nanoparticles, IONPs (currently used in clinical practice), gold nanoparticles or nanoshells (NCT00356980, NCT00848042), silica nanoparticles (NCT02106598), and silica-gold nanoparticles (NCT01270139) might hold a greater chance to speed up the translational process.
Tumor Active Targeting and Related Ligands
Efficient targeting of theranostic nanoparticles to the tumor site is critical for both diagnostic and therapeutic purposes. Some nanoparticles accumulate in tumor tissue based on the enhanced permeability and retention effect, whereby the leakiness of the tumor vasculature combined with poor lymphatic drainage enable nanoparticles to accumulate within the tumor matrix (7). However, because of the tremendous heterogeneity in tumor vessel leakiness over space, time, and different types of tumors, also the unpredictable tumor extravasation of nanoparticles with varied size, shape and surface charge (8), passive targeting strategy has its own limitations, and may only work in certain fast growing mouse (and human) xenograft tumors for nanoparticles with relatively long blood circulation half-life.
In contrast, tumor actively-targeted theranostic nanoparticles are developed by further conjugating different targeting ligands to recognize and selectively bind to receptors that are overexpressed on certain tumor cell (or tumor endothelial cell) surfaces. Recently, tumor angiogenesis (or vasculature) targeting has been considered a generally applicable approach for most functionalized organic and inorganic nanomaterials (9). Depending on the physical and chemical properties of a specific theranostic nanoparticle, as well as the tumor models of interest, the targeting ligands could be antibodies, small peptides or molecules, lectins, aptamers, engineered proteins, or protein fragments. To date, only very limited examples of in vivo tumor actively-targeted theranostic nanoparticles have been reported. Representative examples will be discussed in the following sections.
Targeting Folate Receptor
Folic acid (FA) is known to display high affinity (Kd≈10-10M) for the folate receptor (FR), which is a glycosylphosphatidyinositol-linked membrane protein that is overexpressed in many different kinds of cancers, including ovarian and breast cancers (10). The porphysome nanostructure is a biodegradable, liposome-mimicking, intrinsic theranostic nanoparticle. Interestingly, the disassembly of this porphysome could convert the thermal ablation mechanism (i.e. photothermal therapy [PTT]) back to the singlet oxygen (1O2) generation mechanism (i.e. photodynamic therapy [PDT]). Inspired by this, researchers were able to develop targeting-triggered activatable nanobeacons by synthesizing folate-conjugated porphysomes (11). Results showed an efficient disruption of the porphysome nanostructure and the switching of photodynamic activity after the specific internalization of folate-conjugated porphysomes into tumor cells (FIGURE 2). Other folate-conjugated theranostic nanoparticles include the heparin-folic acid-IR-780 nanoparticle (12), and folate-conjugated drug or dye-loaded IONPs (13).
Figure 2.
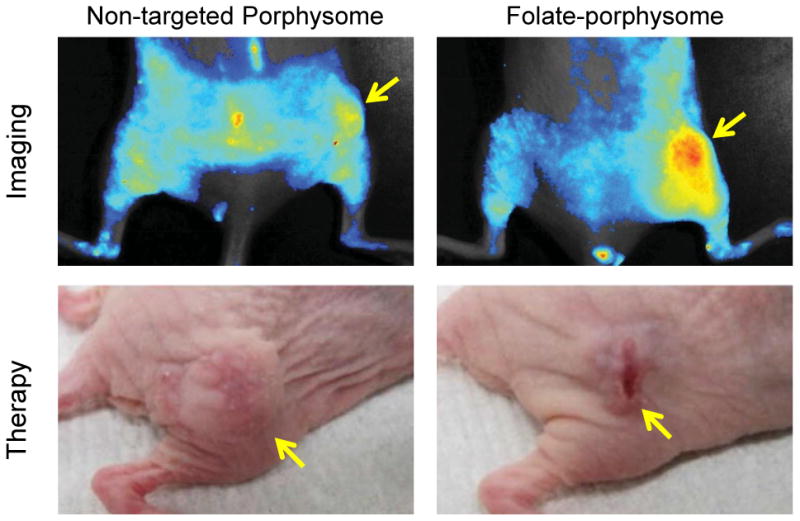
In vivo fluorescence activation imaging and PDT treatment response of non-targeted porphysome and folate-porphysome in KB tumor-bearing mice. Tumors are marked by yellow arrows.
Targeting Integrin αvβ3
Peptide-modified ferritin nanocage (a protein-based nanoparticle made of 24 subunits, which can be self-assembled into a cage-like nanostructure) is another attractive biocompatible nanoplatform. It may be used for the targeted delivery of both imaging (e.g. radiometal isotopes and fluorescent dyes) and therapeutic agents (e.g. photosensitizers and doxorubicin [DOX]) to tumors that overexpress integrin αvβ3.
For example, nanocages loaded with ZnF16Pc, which is a potent PS with a high 1O2 quantum yield, and modified with RGD4C were recently developed for in vivo integrin αvβ3-targeted imaging and PDT (14). By replacing ZnF16Pc with Cu-precomplexed doxorubicin (Dox-Cu) and near-infrared dye (ZW800), similar theranostic nanostructures have also been developed and shown enhanced in vivo optical imaging and chemotherapeutic effects (FIGURE 3) (15). RGD-modified BaYbF5: Er3+ nanocubes were also reported for integrin αvβ3-targeted and computed tomography (CT) image-guided radiotherapy. The PEGylated BaYbF5: Er3+ nanocubes were utilized as both the CT imaging contrast enhancer and radio-sensitizer in these studies (16). However, the potential long-term toxicity of the heavy metal contained in these nanocubes might hinder their future clinical translation.
Figure 3.
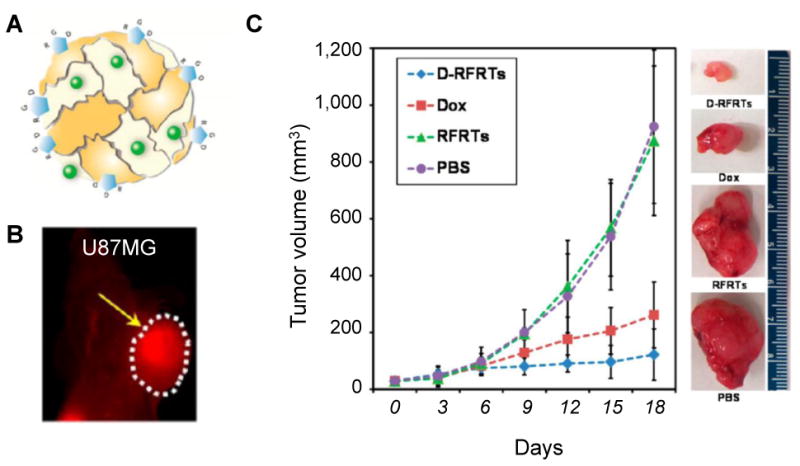
(A) A schematic illustration of Dox-Cu and ZW800 co-loaded ferritin nanocage. (B) In vivo targeted optical imaging of as-developed ferritin nanocages in U87MG tumor-bearing mice. (C) In vivo chemotherapy study in U87MG tumor bearing mice (n=5/group). RFRTs: RGD4C modified ferritins. D-RFRTs: Dox-loaded RFRTs. The tumor is marked by a yellow arrow.
Targeting Other Cancer Markers
Besides FA and integrin αvβ3, well-designed theranostic nanoparticles have also been developed for targeting other receptors, such as prostate-specific membrane antigen (PSMA) in prostate cancer and the urokinase plasminogen activator receptor (uPAR) in pancreatic cancer (17,18). For example, a theranostic nanoplex which contains multimodality imaging reporters (e.g. fluorescent dyes and radioisotopes) and a PSMA-targeting moiety was developed to deliver small interfering RNA (siRNA) and a prodrug enzyme to PSMA-expressing tumors (17). The nanoplex was carefully investigated using built-in non-invasive multimodality imaging to evaluate its diagnostic aspects of PSMA imaging, the therapeutic aspects of siRNA-mediated down-regulation of a target gene, and the conversion of prodrug to cytotoxic drug. No significant immune response or obvious toxicity to the liver or kidney was observed.
In order to overcome the physical barrier of tumor stroma, uPAR-targeted IONPs, which also carry the chemotherapy drug gemcitabine (Gem), were designed for targeted delivery into uPAR-expressing tumor and stromal cells in another study (18). As-developed theranostic nanoparticles (i.e. ATF-IONP-Gem) enabled the intracellular release of Gem following receptor-mediated endocytosis into tumor cells, and also provided contrast enhancement in magnetic resonance imaging (MRI) of tumors.
Challenges and Future Directions
Clearly, there is a trend toward combining the diagnostic and therapeutic functions of theranostic nanoparticles, resulting in greatly improved and personalized disease management. In order for clinical translation to take place, many major challenges must be overcome, such as the selection of the best nanoplatform, improvement of ligand conjugation efficiency, and the development of an ideal synthetic technique with less steps, higher reproducibility, and lower cost. Due to the high sensitivity and accurate quantification of positron emission tomography imaging, radiolabeling of FDA-approved therapeutic nanoparticles, such as Doxil (liposomal doxorubicin), could become a highly straightforward strategy to enable the visualization and accurate assessment of biodistribution, circulation half-life, and pharmacokinetics of theranostic nanoparticles, without compromising drug loading capacity and safety. Conjugating targeting ligands to nanoparticles with intrinsic imaging and therapeutic properties, such as porphysomes and gold nanoshells and cages, will be another ideal way for developing future tumor actively-targeted theranostic nanoparticles.
Efforts in seeking better active targeting strategies will also continue. Recent research has shown that well-designed nanosystems could communicate in vivo to amplify the cancer-targeting efficacy (19). In one recent study, RGD-modified ferritin nanocages were synthesized as “smart” photosensitizer carriers, which could site-specifically deliver 1O2 to the tumor endothelium for altering the vasculature permeability. Results also demonstrated that such external stimuli could facilitate the extravasation of the second dose of imaging or therapeutic nanoparticles at the tumor site (20). Although still in the early stages of development, such concepts of in vivo cooperative targeting strategies hold great potential to improve tumor targeting capability, and may become one of the most interesting research directions over the next 5 years.
There is also a growing interest in developing activatable theranostic nanoparticles for even “smarter” cancer imaging and therapy. For example, an activatable theranostic prodrug was designed by conjugating SN-38 (a topoisomerase inhibitor) with piperazinerhodol fluorophore using a self-immolative linker based on disulfide bonds. Such a design could permit real-time monitoring of the delivery and release of the SN-38 payload in the presence of intracellular thiols (21). With activatable theranostic nanoparticles, getting vital information related to the treatment response might also be possible, which will be extremely helpful during the decision-making process, and help doctors alter treatment protocols in time.
Conclusion
The last decade has witnessed an unprecedented expansion in engineering of various kinds of theranostic nanoparticles for cancer imaging and therapy. The current status, challenges, and future perspectives of tumor actively-targeted theranostic nanoparticles were discussed in this short review. The development of theranostic nanoparticles that are tumor-specific, safer, simpler, and yet still powerful will continue to be the focus in the near future, holding a greater potential to be translated into the clinic.
Acknowledgments
This work is supported, in part, by the University of Wisconsin - Madison, the National Institutes of Health (NIBIB/NCI 1R01CA169365, P30CA014520), the Department of Defense (W81XWH-11-1-0644), and the American Cancer Society (125246-RSG-13-099-01-CCE).
Footnotes
Disclosure: No potential conflict of interest relevant to this article was reported.
Contributor Information
Feng Chen, Email: fchen@uwhealth.org.
Weibo Cai, Email: wcai@uwhealth.org.
References
- 1.Jokerst JV, Gambhir SS. Molecular imaging with theranostic nanoparticles. Acc Chem Res. 2011;44(10):1050–1060. doi: 10.1021/ar200106e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2010;62(11):1064–1079. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic nanomedicine. Acc Chem Res. 2011;44(10):1029–1038. doi: 10.1021/ar200019c. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol. 2012;30(21):2585–2592. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- 5.Benezra M, Penate-Medina O, Zanzonico PB, et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J Clin Invest. 2011;121(7):2768–2780. doi: 10.1172/JCI45600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakor AS, Gambhir SS. Nanooncology: the future of cancer diagnosis and therapy. CA Cancer J Clin. 2013;63(6):395–418. doi: 10.3322/caac.21199. [DOI] [PubMed] [Google Scholar]
- 7.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Smith BR, Kempen P, Bouley D, et al. Shape matters: intravital microscopy reveals surprising geometrical dependence for nanoparticles in tumor models of extravasation. Nano Lett. 2012;12(7):3369–3377. doi: 10.1021/nl204175t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen F, Cai W. Tumor vasculature targeting: a generally applicable approach for functionalized nanomaterials. Small. 2014;10(10):1887–1893. doi: 10.1002/smll.201303627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudimack J, Lee RJ. Targeted drug delivery via the folate receptor. Adv Drug Deliv Rev. 2000;41(2):147–162. doi: 10.1016/s0169-409x(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 11.Jin CS, Cui L, Wang F, Chen J, Zheng G. Targeting-triggered porphysome nanostructure disruption for activatable photodynamic therapy. Adv Healthc Mater. 2014;3(8):1240–1249. doi: 10.1002/adhm.201300651. [DOI] [PubMed] [Google Scholar]
- 12.Yue C, Liu P, Zheng M, et al. IR-780 dye loaded tumor targeting theranostic nanoparticles for NIR imaging and photothermal therapy. Biomaterials. 2013;34(28):6853–6861. doi: 10.1016/j.biomaterials.2013.05.071. [DOI] [PubMed] [Google Scholar]
- 13.Santra S, Kaittanis C, Grimm J, Perez JM. Drug/dye-loaded, multifunctional iron oxide nanoparticles for combined targeted cancer therapy and dual optical/magnetic resonance imaging. Small. 2009;5(16):1862–1868. doi: 10.1002/smll.200900389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhen Z, Tang W, Guo C, et al. Ferritin nanocages to encapsulate and deliver photosensitizers for efficient photodynamic therapy against cancer. ACS Nano. 2013;7(8):6988–6996. doi: 10.1021/nn402199g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhen Z, Tang W, Chen H, et al. RGD-modified apoferritin nanoparticles for efficient drug delivery to tumors. ACS Nano. 2013;7(6):4830–4837. doi: 10.1021/nn305791q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing H, Zheng X, Ren Q, et al. Computed tomography imaging-guided radiotherapy by targeting upconversion nanocubes with significant imaging and radiosensitization enhancements. Sci Rep. 2013;3:1751. doi: 10.1038/srep01751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Penet MF, Nimmagadda S, et al. PSMA-targeted theranostic nanoplex for prostate cancer therapy. ACS Nano. 2012;6(9):7752–7762. doi: 10.1021/nn301725w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee GY, Qian WP, Wang L, et al. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano. 2013;7(3):2078–2089. doi: 10.1021/nn3043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Maltzahn G, Park JH, Lin KY, et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat Mater. 2011;10(7):545–552. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhen Z, Tang W, Chuang YJ, et al. Tumor vasculature targeted photodynamic therapy for enhanced delivery of nanoparticles. ACS Nano. 2014;8(6):6004–6013. doi: 10.1021/nn501134q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhuniya S, Maiti S, Kim EJ, et al. An activatable theranostic for targeted cancer therapy and imaging. Angew Chem Int Ed Engl. 2014;53(17):4469–4474. doi: 10.1002/anie.201311133. [DOI] [PubMed] [Google Scholar]


